Abstract
The ovaries are often thought of as the main and only source of estrogens involved in the regulation of female behavior. However, aromatase, the key enzyme for estrogen synthesis, although it is more abundant in males, is expressed and active in the brain of females where it is regulated by similar mechanisms as in males. Early work had shown that estrogens produced in the ventromedial hypothalamus are involved in the regulation of female sexual behavior in musk shrews. But the question of the role of central aromatase in general had not received much attention until recently. Here I will review the emerging concept that central aromatization plays a role in the regulation of physiological and behavioral endpoints in females. The data support the notion that in females brain aromatase is not simply a non-functional evolutionary vestige and provide support for the importance of locally produced estrogens for brain function in females. These observations should also have an impact for clinical research.
Keywords: neuroestrogens, reproduction, sexual behavior, cognition, learning and memory
Introduction
Estrogens regulate reproductive and non-reproductive functions in both males and females including nociception, brain plasticity and learning (Cornil et al. 2012a; Micevych and Meisel 2017; Azcoitia et al. 2017). They exert these pleiotropic effects through a combination of nuclear- and membrane-initiated signaling which are often associated with different latencies of action (Vasudevan and Pfaff 2007; Cornil et al. 2015; Micevych et al. 2015).
Estrogens are synthesized by the aromatization of androgens, a reaction catalyzed by the enzyme aromatase. The ovaries constitute a major source of estrogens, but many other tissues express active aromatase whose products act locally (Boon et al. 2010). In particular, the brain expresses high amounts of aromatase in discrete hypothalamic regions, the medial preoptic area, the bed nucleus of the stria terminalis and the medial amygdala, while lower amounts are also found in the hippocampus and cortical regions (Lauber and Lichtensteiger 1994; Foidart et al. 1995; Wagner and Morrell 1996; Roselli et al. 1998b; Saldanha et al. 2000; Roselli et al. 2001). In physiological conditions, aromatase is constitutively expressed in neurons of reptiles, birds and mammals, while it seems to be only expressed in radial glia in fish with the notable exception of the goldfish (Callard et al. 2013; Pellegrini et al. 2013).
The role of brain aromatase has mostly been studied in males in which it is known to play a critical role in the expression of sexual, aggressive and parental behavior both in the short and long-term (Trainor and Marler 2002; Balthazart et al. 2004; Trainor et al. 2006; Hull and Rodriguez-Manzo 2009; Heimovics et al. 2015; Cornil and de Bournonville 2017). In parallel, aromatase activity in the male brain is regulated through distinct mechanisms in these two timeframes corresponding to the two modes of action of estrogens (Charlier et al. 2013).
Aromatase is a microsomal enzyme expressed mainly in the endoplasmic reticulum. In the brain, it is also expressed and active in synaptic terminals (Schlinger and Callard 1989; Roselli 1995; Naftolin et al. 1996; Peterson et al. 2005) where it is acutely regulated (Remage-Healey et al. 2011; Cornil et al. 2012b). Along with the discovery of membrane-initiated actions of estrogens, this led to the idea that locally produced estrogens, also called neuroestrogens, can act in a neurotransmitter-like manner (Balthazart and Ball 2006; Saldanha et al. 2011; Cornil et al. 2015; Rudolph et al. 2016).
Finally, it should be noted that, for reasons that remain unclear but likely result from a lower expression potentially limited to specific subcellular compartments, aromatase is notoriously difficult to immunostain in mammals and its activity is also relatively more difficult to measure. A great deal of information regarding aromatase expression and regulation has thus been gained from studies conducted in birds where it is expressed in much higher amounts.
This article will review what we know regarding the expression, regulation and function of brain aromatase in females. Because ovarian estrogens are circulating at high concentrations in females, the study of the role of brain aromatase has been somewhat neglected in this sex. However, females express substantial amounts of active brain aromatase that is regulated in similar ways as in males. I will first review what is known about its expression and regulation in females. Then, I will discuss the results of studies that investigated the functional role of locally produced estrogens in the brain of females.
The brain of females expresses substantial amounts of active aromatase
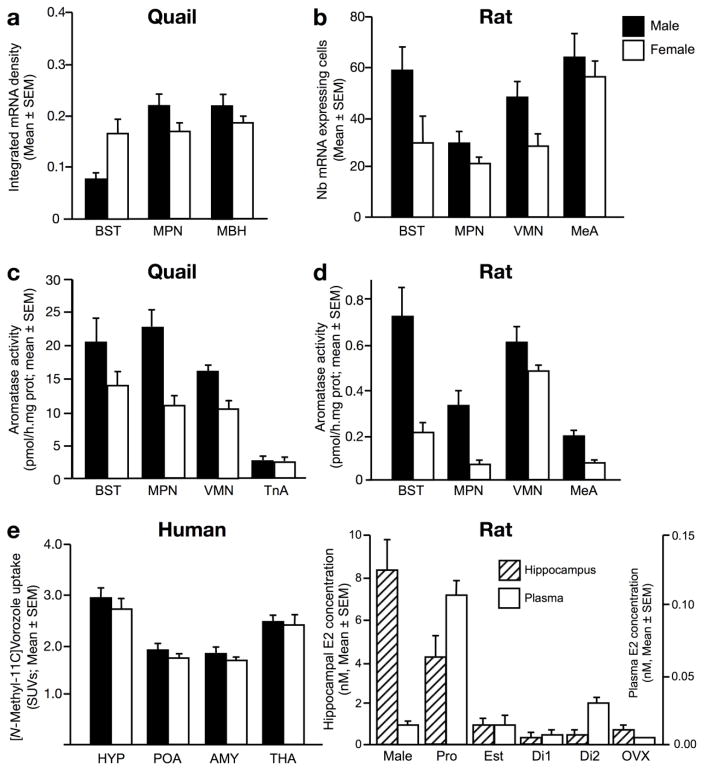
As the ovary constitutes one of the major sources of estrogens, it is often assumed that the physiological and behavioral effects of estrogens reflect effects of fluctuations in circulating estrogen concentrations and thus that the active estrogens are derived from the ovary. However, although aromatase tends to be expressed in larger amounts in males than females, adult females do express aromatase in many brain regions (Figure 1). Interestingly, the affinity of brain aromatase for its substrate is the same in males and females (Konkle and Balthazart 2011), but seems to be higher than the affinity of ovarian aromatase (Hutchison et al. 1992).
Figure 1.
The brain of females expresses lower, but non-negligible, amounts of aromatase compared to males, as evidenced by the expression of its mRNA in Japanese quail (a) and rats (b), of its enzymatic activity in Japanese quail (c) and rats (d) and the uptake of the radiolabeled aromatase inhibitor Vorozole in humans (e). Interestingly, local estrogen synthesis translates in a higher concentration of estradiol (E2) in the hippocampus relative to plasma in both sexes (f). Note that panel A represents levels of optical density of autoradiograms corrected for the volume of the examined nucleus, while panel a represents the number of stained cells per 0.35 mm2. In panel f, note also that the scales are dramatically different between hippocampus and plasma levels. Abbreviations : AMY, Amygdala ; BST, Bed nucleus of the stria terminalis ; Di1, females in diestrus ; Di2, females in diestrus 2; Est, estrus females; HYP, hypothalamus ; MeA, Medial amygdala; MPN, medial preoptic nucleus ; OVX, ovariectomized females; Pro, proestrus females; POA, Preoptic area ; SUVs, Standardized uptake values ; THA, Thalamus ; TnA, Nucleus Taeniae of the Amygdala ; VMN, Ventromedial nucleus of the hypothalamus. Adapted from (Roselli et al. 1985; Wagner and Morrell 1996; Cornil et al. 2011; Kato et al. 2013; Biegon et al. 2015).
In birds, female Japanese quail (Coturnix japonica) show similar levels of aromatase mRNA as males in most brain regions, including the medial preoptic area (MPOA) and the mediobasal hypothalamus (MBH) (Fig.1a) (Voigt et al. 2007), but show a slightly lower number of aromatase-immunoreactive cells than males in the MPOA (Balthazart et al. 1996). In the hypothalamus-preoptic area (HPOA) and the caudomedial nidopallium (NCM), female zebra finches (Taeniopygia guttata) exhibit a similar number of aromatase-positive cell bodies as males, but have fewer immunopositive fibers and synapses (Saldanha et al. 2000; Peterson et al. 2005).
In mammals, since the visualization of the protein product of the aromatase gene is difficult, comparative data across sexes derive almost exclusively from the analysis of its transcript or the expression of detectable proteins whose expression was engineered to reflect the expression of aromatase mRNA. In rodents, females exhibit between one quarter and two thirds of the transcript levels detected in males depending on the species, the region and the method used to measure transcripts (Fig.1b) (Wagner and Morrell 1996; Roselli et al. 1998a; Wu et al. 2009; Stanic et al. 2014). The highest levels of expression in females were reported in the bed nucleus of the stria terminalis (BST), the MPOA, the ventromedial hypothalamus (VMH), the medial amygdala (MeA) and the olfactory tubercles. Interestingly, in humans (Fig.1e), the radiolabeled aromatase inhibitor Vorozole only shows a slightly higher level of uptake in the hypothalamus, preoptic area and amygdala of men compared to women (Biegon et al. 2015).
Similar to what was described for the transcript and protein, females display a lower enzymatic activity than males in all regions where it was measured, and show particularly high aromatase activity in the HPOA, BST and MeA in a variety of avian and mammalian species (Reddy et al. 1973; Weisz and Gibbs 1974; Naftolin et al. 1975; Selmanoff et al. 1977; Roselli et al. 1985; Hutchison and Steimer 1986; Connolly et al. 1990; Steimer and Hutchison 1990b; Rissman et al. 1996; Corbin et al. 2009; Cornil et al. 2011; Dickens et al. 2014). In zebra finches, females show a lower aromatase activity than males in synaptic terminals (Rohmann et al. 2007). Finally, a higher estradiol content was measured in discrete brain regions of the female rat and monkey brain compared to plasma suggesting that local estrogen synthesis may provide another source of estrogens in addition to estrogens of ovarian origin (Fig.1f) (Kato et al. 2013; Kenealy et al. 2013; Kenealy et al. 2016).
Brain aromatase is regulated in a similar fashion in females as in males
Long-term regulation of aromatase expression
In males, the concentration of brain aromatase is under the control of testosterone and its metabolites that up-regulate its expression, a process detected within a few hours and reaching a maximum after several days depending on the species considered (Roselli et al. 1987; Balthazart et al. 1990a). The regulation of brain aromatase expression has mostly been studied in rats (Rattus norvegicus), ring doves (Streptopelia risoria) and Japanese quail. In male rats, brain aromatase expression is up-regulated by testosterone in a region-specific manner. Transcription of the gene coding for aromatase is up-regulated by testosterone in the POA and the ventromedial nucleus of the hypothalamus (VMN), but not in the amygdala (Abdelgadir et al. 1994). This up-regulation of the transcript is mirrored by changes in enzymatic activity measured in these regions and other brain regions, including the hippocampus, the suprachiasmatic nucleus, the arcuate nucleus and the median eminence region (Roselli et al. 1984; Roselli et al. 1985; Abdelgadir et al. 1994).
Work in birds showed similar testosterone-induced changes in aromatase activity in the same brain regions (Balthazart et al. 1990b). However, most studies mainly focused on changes occurring in the POA, particularly in the medial preoptic nucleus (POM), where the up-regulation following testosterone treatment was evidenced at all three levels of investigation: the transcript, the protein and the enzymatic activity levels (Steimer and Hutchison 1981; Schumacher and Balthazart 1986; Balthazart et al. 1990a; Harada et al. 1992; Foidart et al. 1994; Balthazart et al. 1996; Voigt et al. 2011).
In all three species, the estrogenic and androgenic metabolites of testosterone act in synergy to up-regulate aromatase activity, with a predominance of androgenic action in rats (Roselli et al. 1987; Abdelgadir et al. 1994; Roselli et al. 1997), but of estrogenic action in quail and doves (Hutchison and Steimer 1986; Schumacher et al. 1987; Hutchison et al. 1991; Balthazart and Foidart 1993; Harada et al. 1993).
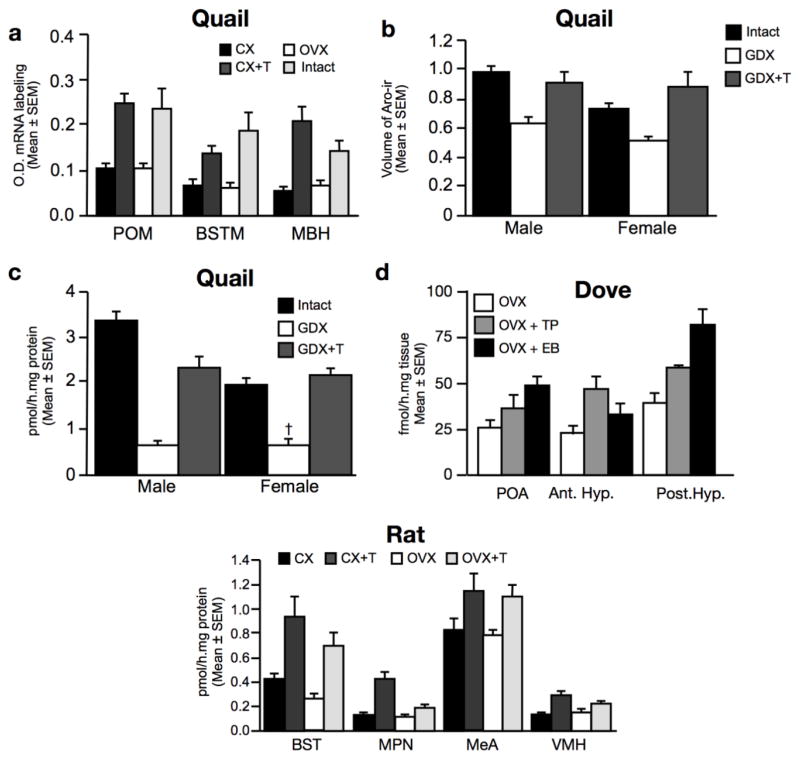
As illustrated in figure 2, in females, aromatase mRNA, protein and activity levels are up-regulated by testosterone in a region-specific manner in both rats (Roselli et al. 1984; Steimer and Hutchison 1990a; Roselli et al. 1996a; Roselli et al. 1996b; Roselli and Klosterman 1998) and birds (Schumacher and Balthazart 1986; Balthazart et al. 1990a; Balthazart et al. 1990b; Steimer and Hutchison 1990a; Hutchison et al. 1992; Balthazart et al. 1996; Voigt et al. 2011). To the best of my knowledge, the effect of estrogens on female aromatase was only investigated in doves (Hutchison et al. 1992). In this species, treatment of gonadally intact and ovariectomized females with estrogens increases aromatase activity in the preoptic area and hypothalamus (Fig.2d). Moreover, HPOA estrogen formation is positively correlated with follicular development in the ovary of females paired with a male, thus supporting a role for estrogens in the control of brain aromatase expression in females. Importantly, the highest levels of aromatase activity in doves were measured in females that had been paired for several days with a male, suggesting that other factors than sex steroids are involved in the regulation of aromatase activity (see also below). In female rats, however, brain aromatase does not seem to vary significantly across the estrous cycle (Roselli et al. 1984). Although brain aromatase is also regulated in a region-specific manner in male rhesus macaques (Macaca mulatta; Roselli and Resko 2001), nothing is known regarding its regulation in females in this species. However, using a measure of uptake of the aromatase inhibitor Vorozole, Pareto and colleagues suggested that the expression of aromatase fluctuates across the estrous cycle in female baboons (Pareto et al. 2013; as reviewed in Biegon et al. 2015). Together, these data support the notion that the expression of aromatase in the brain of females is regulated by testosterone, and possibly by estrogens, in a manner similar to males.
Figure 2.
The expression of brain aromatase in females is regulated by sex steroids in several species as evidenced by investigation of the level of its mRNA (a), protein (b–c) and enzymatic activity (d–e). A. Optical density levels from autoradiograms. b. Volume of tissue covered by cells that are immunoreactive for aromatase in the quail medial preoptic nucleus (POM). c–e, Aromatase activity measured in quail, doves and rats. Note that the enzymatic activity was assessed in quail and dove through the estrogen formation assay, where the amount of radioactive estradiol was produced following incubation with a radioactive androgen substrate. By contrast, in rats, aromatase activity was measured by the tritiated water assay, in which the conversion of each molecule of tritiated androstenedione gives rise to the release of one molecule of tritiated water. Abbreviations: Ant. Hyp., anterior hypothalamus; BSTM, medial portion of the bed nucleus of the stria terminalis; CX, castrated males; EB, estradiol benzoate; GDX, gonadectomized; MBH, mediobasal hypothalamus; MeA, medial amygdala; MPN, medial preoptic nucleus; OVX, ovariectomized females; POA, preoptic area; Post. Hyp., posterior hypothalamus; POM, medial preoptic nucleus; T, testosterone; TP, testosterone propionate; VMH, ventromedial hypothalamus. Adapted from (Balthazart et al. 1990b; Balthazart et al. 1996; Roselli et al. 1996b; Hutchison et al. 1992; Voigt et al. 2011).
Short-term regulation of aromatase enzymatic activity
Besides the transcriptional control of aromatase concentration described above, work performed in male birds showed that the enzymatic efficiency of brain aromatase is also rapidly modulated by neuronal activity. This is evidenced by a rapid and transient down-regulation in aromatase activity elicited in quail HPOA explants maintained in vitro by potassium-induced depolarizations or exposure to glutamatergic agonists (Balthazart et al. 2001; Balthazart et al. 2003; Balthazart et al. 2006). Similarly, in vivo injection of the glutamate agonist kainate in the POM of male quail results in a rapid down-regulation of aromatase activity measured ex vivo in preoptic microdissections (de Bournonville et al. 2017a). Finally, estradiol concentration rapidly decreases in the dialysate collected from the caudomedial nidopallium of zebra finches after the retrodialysis of potassium or glutamate (Remage-Healey et al. 2008; Remage-Healey et al. 2011). These effects presumably result from calcium-dependent phosphorylations of the enzyme itself with subtle region and species differences (Balthazart et al. 2001; Balthazart et al. 2003; Charlier et al. 2011; Cornil et al. 2012b; Comito et al. 2015). As opposed to what was seen in birds, glutamate increases estradiol concentration in the hippocampus of male rats (Hojo et al. 2004; Sato and Woolley 2016). Whether this discrepancy relates to a species or a region difference is not clear yet.
Importantly, in male birds, rapid changes in aromatase activity or estradiol concentration occur in behaviorally relevant contexts in different brain regions and these changes are associated to the control of known behavioral or physiological processes (Heimovics et al. 2015; Cornil and de Bournonville 2017; Remage-Healey et al. 2017).
In the brain of females, aromatase activity is also regulated in vitro by calcium-depend phosphorylations. Indeed, aromatase activity measured in HPOA homogenates derived from female quail is significantly inhibited by high, but physiological, concentration of Ca2+, Mg2+ and ATP and this effect is blocked by protein kinase inhibitors. Aromatase activity is also up-regulated by calcium chelation. Interestingly, compared to males, brain aromatase activity in females appears to be less sensitive to changes in calcium concentrations, but more sensitive to protein kinase inhibition, suggesting that the mechanisms underlying these changes may be slightly different between sexes (Konkle and Balthazart 2011). Similar investigations in homogenates derived from the hypothalamus, the hippocampus and the nidopallium of female zebra finches showed that aromatase activity showed similar responses to phosphorylating conditions and calcium chelation as in males in all regions, except the hippocampus, where a sex difference in sensitivity to ATP was detected (Cornil et al. 2012b; Comito et al. 2015). Hence, these data suggest that brain aromatase activity is also acutely regulated by calcium-dependent phosphorylation in females with subtle differences compared to males.
Moreover, the same discrepancy regarding the direction of these effects between birds and mammals seems to exist in female as in males. Indeed, in vivo microdialysis revealed a rapid rise in hippocampal estrogen levels in response to treatment with the glutamate agonist kainate in female rats, that is similar to the increase observed in males (Sato and Woolley 2016). Similarly, in ovariectomized Rhesus monkeys, estradiol concentration (80–400pg/ml) measured in the median eminence stalk by in vivo microdialysis is superior to circulating estradiol levels (±8pg/ml) and is rapidly modulated following a 5 min electrical stimulation, supposed to induce local glutamate release (Fig. 3d). Following an initial trend of reduction, local estradiol concentration then transiently peaks within 40–60 min of stimulation (Kenealy et al. 2013).
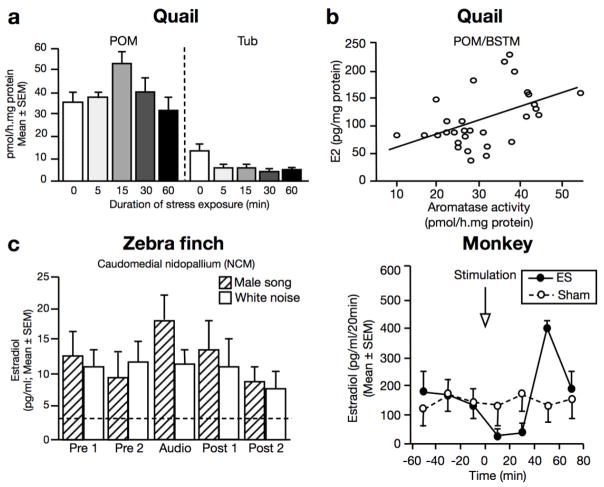
Figure 3.
Aromatase activity is acutely regulated in the brain of females. a. Effect of acute restraint stress in the medial preoptic nucleus (POM) and tuberal hypothalamus (Tub) of female quail (a region probably homologous to the arcuate nucleus of mammals). b. Positive correlation between preoptic content in estradiol and the aromatase activity measured in the same female sample using the tritiated water assay (see fig.2). c. Rapid elevation of estradiol levels induced by male song playback in the caudomedial nidopallium of female zebra finches. d. Effect of an electrical stimulation (ES) of the median eminence stalk of female monkeys on the concentration of estradiol measured nearby by in vivo microdialysis. Abbreviations: BSTM, medial portion of the nucleus of stria terminalis; ES, electrical stimulation; Tub, tuberal hypothalamus. Adapted from (Dickens et al. 2011; Remage-Healey et al. 2012; Kenealy et al. 2013).
As illustrated in figure 3, aromatase activity in the brain of females also fluctuates acutely in a site-specific manner in response to changes in the environment. In Japanese quail, such changes have been described in the POM and the tuberal hypothalamus (a region thought to be homologous to the mammalian arcuate nucleus) in response to stress and sexual interactions. Aromatase activity is up-regulated in the POM and down-regulated in the tuberal hypothalamus of females within minutes of acute restraint stress (Fig.3a) (Dickens et al. 2011; Dickens et al. 2012). By contrast, an interaction with a sexually active male results in a decreased activity in the POM (de Bournonville et al. 2017b). Importantly, aromatase activity positively correlates with tissue content in estradiol in both the POA and the MBH, regardless of whether females had been stressed or paired with a male partner (Fig. 3b). This observation suggests that, at the individual level, local estradiol concentration is regulated independently of ovarian production (Dickens et al. 2014). Finally, in zebra finches, playback of male songs elicits a rapid elevation in local estradiol concentration in the caudomedial nidopallium (NCM) of females, a region homologous to the mammalian auditory cortex and involved in the processing of conspecific sounds (Remage-Healey et al. 2012). These local changes are independent of circulating estradiol concentration, as evidenced by the absence of fluctuation in blood estradiol in the same animals and by the absence of similar changes in the neighboring mesopallium, where aromatase is not, or minimally, expressed in females. No similar changes were detected in response to male visual stimuli. Together, these data demonstrate that aromatase activity and subsequent local estrogen availability rapidly fluctuate in the brain of females in a stimulus- and site-specific manner. These changes are also sex-specific as they show different neuroanatomical localization or dynamics as compared to males.
Collectively, the observations presented here indicate that brain aromatase activity is regulated in females both in the long- and the short-term by mechanisms similar to those identified in males. Hence, estrogens produced in the brain could act along with ovarian derived estrogens and potentially independently of them. The next section will review studies supporting the hypothesis that these estrogens do exert a functional role in females.
Funtional role of neuroestrogens in behavior and physiology
Early experiments indicated that female sexual behavior could be elicited in female of different species by treatments with testosterone alone (Beach 1942; Beyer et al. 1970b; Beyer et al. 1970a; Beyer and Komisaruk 1971) or combined with progesterone (Pfaff 1970; Whalen and Hardy 1970; Whalen et al. 1972; Luttge et al. 1975; Gladue et al. 1978; Hsu 1990). This effect was not mimicked by non-aromatizable androgens (Beyer and Komisaruk 1971), but was blocked by the estrogen antagonist CI-628 (Whalen et al. 1972; Luttge et al. 1975) or the aromatase inhibitor ATD (Gladue et al. 1978; Hsu 1990). This indicated that this effect resulted from the conversion of the androgen into an estrogen. However, where this conversion occurs remained unknown.
Female sexual behavior in the musk Shrew
Although the first suggestions of a possible role of brain aromatase in females were formulated earlier (Beyer et al. 1970b; Pfaff 1970), to my knowledge, the first piece of direct evidence for a role of neuroestrogens in females was identified in the musk shrew (Suncus murinus). In this species, a key role for extra-gonadal aromatization was suspected based on the observation that 1) testosterone is the main circulating sex steroid (Rissman and Crews 1988), 2) sexual receptivity is induced in ovariectomized females by aromatizable androgens or estradiol benzoate, but not non-aromatizable androgens, administered systemically (Rissman et al. 1990) and finally 3) systemic blockade of estrogen synthesis, or action, impairs sexual behavior in gonadally intact females or ovariectomized females treated with testosterone (Rissman et al. 1990; Rissman 1991).
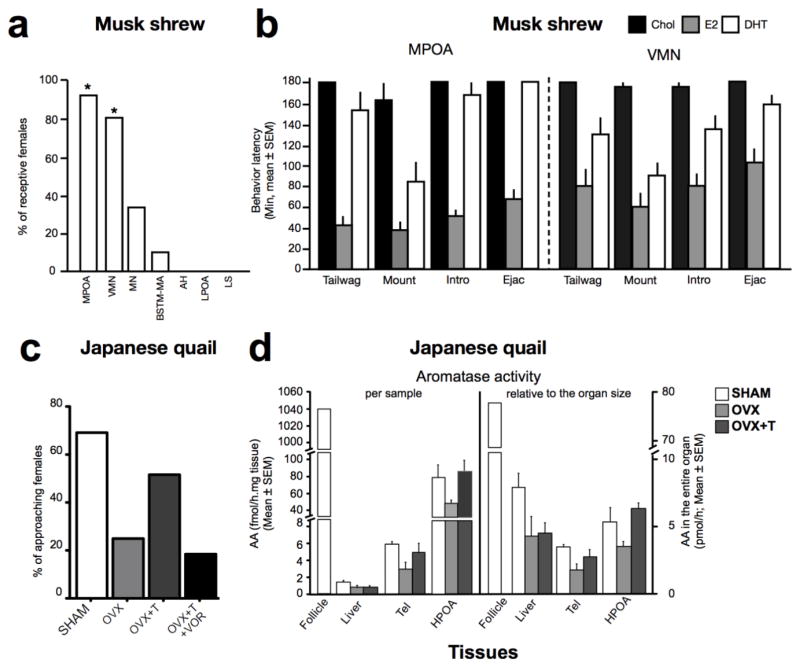
Like females of other species, the female musk shrew expresses lower levels of brain aromatase activity than males, with the highest levels detected in the POA, BST and VMH (Rissman et al. 1996). Implants filled with testosterone propionate were placed in these three areas in ovariectomized females to directly test the contribution of central aromatization to female sexual behavior (Veney and Rissman 2000). Implants that fell within the boundaries of the MPOA and VMN resulted in reduced copulatory latencies (Fig. 4a) and in most females displaying sexual receptivity, while implants in the BST or in the neighboring regions of MPOA and VMH did not induce such behavioral effects. In the MPOA, similar effects were observed when testosterone was replaced by estradiol, but not by the non-aromatizable androgen dihydrotestosterone (DHT) (Fig.4b). In the VMH, estradiol produced a slightly less dramatic effect and behavioral latencies induced by DHT implantation were intermediate between controls and estradiol-treated females. Together these results thus suggested that brain derived estrogens contribute significantly to the expression of female behavior.
Figure 4.
Role of neuroestrogens in the regulation of female sexual behavior in Musk shrew (a–b) and Japanese quail (c–d). a. In musk shrew, testosterone increases the percentage of ovariectomized females showing sexual receptivity, as assessed by the number of females receiving an ejaculation, when implanted in the medial preoptic area (MPOA) or ventromedial nucleus of the hypothalamus (VMN). b. In this species, the behavioral effect of implants filled with estradiol (E2), dihydrotestosterone (DHT) or cholesterol (Chol, as a control) targeted to the MPOA or VMN was assessed on the latency of females to wag their tail and to receive their first mount, intromission (Intro) and ejaculation (Ejac). c. In quail, sham operated or ovariectomized (OVX) females systemically treated or not with testosterone (T) alone or in combination with the aromatase inhibitor vorozole (VOR) were tested for approaching a tethered male in a partner preference test when given the choice to spend time with a male or a female. d. Aromatase activity in the third ovarian follicle, the liver, the telencephalon and HPOA of sham operated females or ovariectomized females treated or not with testosterone. Data are expressed per mg of tissue (left) or in the entire organ (right). Besides the ovary, the highest aromatase activity in females is contained in the brain and the liver (d). Abbreviations: AH, anterior hypothalamus; BNST-MA, medial anterior division of the bed nucleus of the stria terminalis; LPOA, lateral preoptic area; LS, lateral septum; MN, mammillary nucleus; MPOA, medial preoptic area; VMN, ventromedial nucleus of the hypothalamus. Adapted from (Veney and Rissman 2000; de Bournonville et al. 2016).
However, musk shrews are induced ovulators. They display sexual behavior prior to follicular development and, as opposed to spontaneous ovulators, ovulation is induced by somatosensory stimuli provided during coitus. Cats and rabbits are other induced ovulators in which testosterone was found to facilitate female sexual behavior in ovariectomized females (Beyer et al. 1970a; Whalen and Hardy 1970). Moreover, female sexual behavior is also restored in ovariectomized rabbits (Oryctolagus cuniculus) by testosterone implants targeting the VMH, but not by systemic DHT treatment (Palka and Sawyer 1966; Beyer et al. 1970a; Beyer et al. 1970b). Although these studies raise the interesting possibility that central aromatization plays a role in the regulation of female behavior, it is also possible that this feature is a common trait to reflex ovulators, in which sexual interactions occur independently of the ovarian cycle, that cannot be generalized to spontaneous ovulators such as rats, mice, sheep, monkeys, and humans.
Female sexual behavior in Japanese quail
Estrogens also play a key role in the induction of female sexual behavior in quail (Adkins and Adler 1972; Noble 1973; Adkins and Nock 1976; Delville and Balthazart 1987; de Bournonville et al. 2016). As female receptivity covaries with fluctuations of ovarian secretion in quail (Adkins and Adler 1972; Noble 1972; Delville et al. 1986), it is thus logically assumed that all active estrogens originate from the ovary. However, a recent study showed that female sexual behavior is partially restored by a systemic treatment with testosterone in ovariectomized quail. This is evidenced by an increased amount of time spent near the male and an increased percentage of females approaching a male in a partner prefer choice test (Fig.4c) as well as by the increased number of receptive postures and the decreased number of avoiding behaviors exhibited by approaching females (de Bournonville et al. 2016). This effect of testosterone is prevented by concurrent pharmacological blockade of aromatase activity, thus indicating that an extra-gonadal source of estrogens contributes to the activation of female sexual behaviors.
To evaluate this possibility and determine the source of estrogens, aromatase activity was measured in different tissues from gonadally intact females or ovariectomized females treated or not with testosterone. Non-surprisingly, the HPOA and the telencephalon displayed the highest aromatase activities, as expressed per weight of fresh tissue (Fig.4d). Similar to female sexual behavior, this activity was regulated by testosterone in agreement with the idea that neuroestrogens partially contribute to the regulation of female sexual behavior in Japanese quail (de Bournonville et al. 2016). However, the samples from the liver showed a low, but detectable, aromatase activity, which was equivalent to that of the HPOA, when corrected for the size of the entire organ (Fig.4d). The liver could thus constitute a non-negligible source of estrogens as well. Liver aromatase activity was however not affected by testosterone and locally produced estrogens are presumably subjected to intense catabolism. It is thus likely that the estrogens contributing to the activation of female sexual behavior in ovariectomized female quail treated with testosterone arise from the brain. If confirmed, this would support the idea that the contribution of brain estrogen synthesis to the regulation of female sexual behavior is not an exclusive feature of reflex ovulators. Future studies involving the specific blockade of brain aromatase and the identification of the brain site(s) involved in this effect of testosterone in ovariectomized females will be necessary to definitively confirm this hypothesis.
Regulation of the reproductive axis in primates
Estrogens control female reproductive physiology at multiple levels of the hypothalamic-pituitary-gonadal axis. Classically, the hypothalamic component consists of the gonadotropin-releasing hormone (GnRH), produced by neurons scattered throughout the POA, whose pulsatile release in the portal vein system triggers the release of gonadotropins (LH and FSH) from the anterior pituitary into the circulation. In turn, gonadotropins control ovarian steroidogenesis and folliculogenesis (Clarke et al. 2012). During most of the reproductive cycle, estradiol inhibits gonadotropin secretion by suppressing the activity of GnRH neurons (negative feedback). At mid-cycle, estradiol action switches from negative to positive feedback to generate the pre-ovulatory GnRH and subsequent LH surges (Herbison 1998). Other neuronal populations have been identified that act as upstream regulators of GnRH release. In particular, kisspeptin (Kp) neurons are considered as major modulators of the activity of GnRH neurons and are involved in both the induction of the negative and positive feedbacks of estrogens on LH secretion (Goodman and Lehman 2012; Piet et al. 2015). This model of central regulation of ovarian physiology is by essence considered to rely on the sensing by the brain and the pituitary of circulating levels of ovarian steroids.
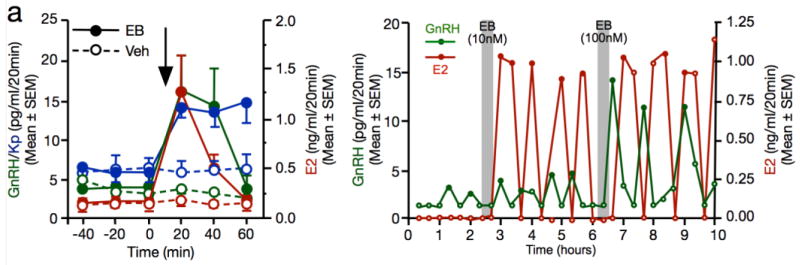
Yet, recent work conducted in female Rhesus monkeys indicates that estrogens produced by the hypothalamus contribute to the regulation of GnRH secretion (Kenealy et al. 2013; Kenealy et al. 2015; Kenealy et al. 2016). As alluded to earlier, electrical stimulation of the median eminence stalk elicits a rise in local estradiol concentration in ovariectomized female monkeys (See Fig. 3d). This stimulation also elicits a rapid rise in the amount of GnRH released per pulse. This effect is mimicked by a short (20 min) and local infusion of estradiol benzoate (EB) and is accompanied by an oscillatory release of estradiol whose properties, in terms of peak duration and inter-pulse interval, closely resembled those of GnRH (Fig. 5) (Kenealy et al. 2013). Importantly, both estradiol and GnRH release are blocked by local aromatase inhibition.
Figure 5.
Role of neuroestrogens in the regulation of hypothalamus-pituitary-gonadal axis in female rhesus monkeys. a. Acute infusion of estradiol benzoate (EB) within the median eminence stalk results in a rapid elevation of local levels of gonadotropin-releasing hormone (GnRH, green lines), estradiol (E2, red lines) and kisspeptin (Kp, blue lines) as measured over time in live animals by in vivo microdialysis. The arrow indicates when the infusion of EB or its vehicle was (Veh) performed. b. Illustration of the pattern of fluctuations in GnRH (green lines) and E2 levels (red lines) in the median eminence of an individual female in response to infusion with two concentrations of EB. Adapted from (Kenealy et al. 2013; Kenealy et al. 2016).
Intriguingly, both a short and a prolonged infusion of EB in the median eminence stalk also induce the release of kisspeptin. By contrast, systemic EB treatment suppresses the release of both GnRH and kisspeptin (Kenealy et al. 2015). It thus seems that the origin of estradiol impacts the physiological response induced. In particular, the effect of local EB infusion in the median eminence stalk of ovariectomized females induces a surge of LH reminiscent of the pre-ovulatory GnRH surge, while systemic EB decreases LH levels, an expected effect of negative feedback of estrogens in ovariectomized females (Kenealy et al. 2015). Importantly, both of these studies revealed that the increased release of GnRH and Kp as well as the induction of pulsatile estradiol levels in the median eminence of ovariectomized monkeys are site specific, thus potentially explaining the difference in the effects of locally infused vs. systemic EB treatments (Kenealy et al. 2013; Kenealy et al. 2015).
These effects are surprising in many respects. Notably, they are observed within latencies that are much shorter than those typically associated with the induction of the negative and positive feedback. Moreover, they occur at a site that was largely overlooked by past studies. They suggest that local estrogen synthesis takes place in the median eminence as 1) basal estradiol levels detected in the median eminence exceed its circulating concentrations; 2) both electrical stimulation and EB treatment induce pulsatile changes in local estradiol levels, while a conversion from EB to estradiol would have been expected to translate into a unique peak of estradiol; and 3) this effect is blocked by local aromatase inhibition (Kenealy et al. 2013). Importantly, these effects also indicate that neuroestradiol contributes to the regulation of GnRH release, as aromatase blockade ablates EB-induced GnRH release which concurs with oscillatory estradiol changes.
Finally, higher levels of estradiol were also measured in the median eminence than in the plasma of pre-pubertal female (Kenealy et al. 2016). Interestingly, local estradiol concentration is markedly lower in the median eminence of early pubertal compared to pre-pubertal females suggesting that its local production may play a role in the central inhibition of GnRH release characteristic of the pre-pubertal stage. Therefore, although numerous questions remain regarding the mechanisms underlying these local changes in neuroestradiol and the impact of local aromatase blockade on reproductive physiology of gonadally intact females, these data bring support to the idea that locally produced estradiol is involved in the regulation of GnRH secretion at different life stages.
Regulation of auditory processing in songbirds
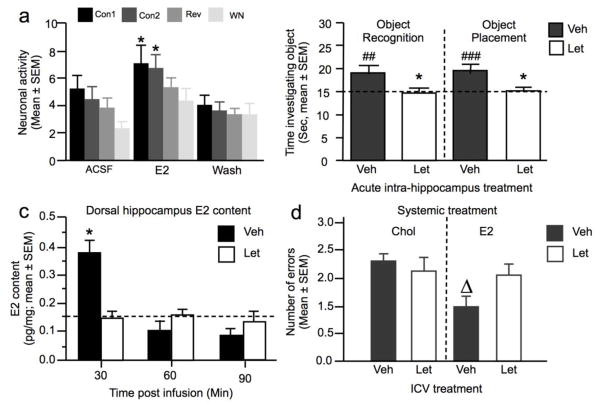
Singing behavior is used for attraction and recognition of sexual partners in songbirds. The caudomedial nidopalium (NCM), a nucleus of the songbird telencephalon analogous to the mammalian auditory cortex (Pinaud and Terleph 2008), shows neural selectivity to auditory cues from individual conspecific songs or calls and is considered to play an important role in this recognition process (Mello et al. 1992; Chew et al. 1996). High amounts of aromatase are expressed in the NCM of males (Metzdorf et al. 1999; Saldanha et al. 2000), where its enzymatic activity is the highest during breeding season of seasonal species (Fusani et al. 2000; Soma et al. 2003). In male zebra finches, the local estradiol concentration in the NCM fluctuates rapidly in response to male conspecific songs (Remage-Healey et al. 2008). These local and rapid changes in estradiol bioavailability are associated with a strengthening of the auditory encoding of conspecific stimuli by NCM neurons through the activation of membrane associated estrogen receptors (Fig.6a) (Tremere et al. 2009; Remage-Healey et al. 2010; Tremere and Pinaud 2011; Remage-Healey and Joshi 2012) and were shown to influence song preference in an acute manner (Remage-Healey et al. 2010).
Figure 6.
Role of neuroestrogens in the regulation of cognitive processes. a. Neuronal activity in response to auditory stimulation with playback of conspecific songs (Con 1 and 2), a conspecific song played in reverse (REV) and white noise (WN) in birds treated locally with artificial cerebro-spinal fluid (ACSF), E2 or during the wash period with ACSF. b. Time spent investigating the object in a novel object recognition test or an object placement test, in which treatment with vehicle (Veh) or letrozole (Let, 0.05μg) occurred immediately after training and was tested respectively 24 (object recognition) and 4 hours (object placement) later. c. Estradiol (E2) content in the dorsal hippocampus of animals collected 30, 60 or 90 min after training and bilateral injection of Vehicle or Let (0.025μg). d. Mean number of errors in the first eight arm choices in a hippocampus-dependent special memory task following systemic chronic treatment with estradiol or cholesterol combined to a chronic intracerebroventricular treatment with Let or its vehicle. * p <0.05 vs control; #, ##, ### p < 0.5, 0.1 or 0.001 vs chance; Δ p< 0.05 vs all other groups. Adapted from (Remage-Healey et al. 2012; Nelson et al. 2016; Tuscher et al. 2016).
Females also need to learn and recognize songs that are specific to their species (Riebel et al. 2002). Central auditory processing involves the same brain regions in males and females although slight sex differences have been reported (Brenowitz and Remage-Healey 2016). The NCM of female songbirds also expresses aromatase whose expression is up-regulated by testosterone in canaries (Fusani et al. 2001). As alluded to previously, similar changes in estradiol concentration were detected in the NCM of female zebra finches exposed to the playback of conspecific male songs (Remage-Healey et al. 2012). Coupling in vivo electrophysiological recordings with local steroid delivery, it was also shown that estradiol, or its membrane impermeable analog estradiol-biotin, rapidly enhances NCM neuronal activity in response to male songs in female zebra finches (Remage-Healey et al. 2012). Conversely, blocking local estrogen synthesis reduced neuronal responses elicited by song playback. Together, these data show that rapid fluctuations in the bioavailability of neuroestradiol within NCM of females serve the same purpose in auditory processing as in males and provide another example where locally produced estrogens play a functional role in females independently of ovarian secretion.
Learning and memory
A wealth of data supports the role of estrogens in female learning and memory (Daniel 2006; Choleris et al. 2012; Luine 2014; Frick et al. 2015; Barha and Galea 2010). The role of ovarian estrogens in modulating these processes is supported by fluctuations of the number of dendritic spines and proliferating neurons in the hippocampus across the estrous cycle (Woolley and McEwen 1992, 1993; Tanapat et al. 1999) which are mirrored by similar changes in ovariectomized females treated with estrogens (Gould et al. 1990; Woolley and McEwen 1993; Tanapat et al. 1999). In parallel, learning abilities or strategies were also shown to vary across the estrous cycle (Frick and Berger-Sweeney 2001; Korol et al. 2004). However, a massive amount of studies also demonstrated the existence of membrane-initiated effects of estrogens on synaptic transmission or plasticity (Teyler et al. 1980; Gu and Moss 1998; Kramar et al. 2009; Smejkalova and Woolley 2010; Huang and Woolley 2012; for review, see Woolley 2007; Srivastava et al. 2013) as well as on learning and memory (Packard and Teather 1997; Luine et al. 2003; Gresack and Frick 2006; Fernandez et al. 2008; Liu et al. 2008; Harburger et al. 2009; Barha et al. 2010; Phan et al. 2011; Inagaki et al. 2010; Boulware et al. 2013; for review see, Luine and Frankfurt 2012; Ervin et al. 2015; Frick et al. 2015) that are probably too rapid to be explained by changes in circulating estrogen concentrations.
As mentioned earlier, aromatase is expressed in the hippocampus and cortex of birds and mammals, including humans (Saldanha et al. 1998; Yague et al. 2006; Yague et al. 2008). Evidence from in vitro and in vivo work indicates that hippocampal estrogen synthesis plays a role in the maintenance of dendritic spines and long-term potentiation (Kretz et al. 2004; Zhou et al. 2010; Vierk et al. 2012). Importantly, the female hippocampus contains higher amounts of estradiol than plasma at all stages of the estrous cycle and this difference is not altered by ovariectomy (Kato et al. 2013). In the brain, active aromatase is found in cell bodies and synaptic boutons (Schlinger and Callard 1989; Roselli 1995; Naftolin et al. 1996; Peterson et al. 2005). The telencephalon of songbirds is particularly enriched in synaptic aromatase (Rohmann et al. 2007), which was suggested to be more prone to rapid modulation than perikaryal aromatase (Cornil et al. 2012b; Remage-Healey et al. 2011), thus suggesting that the cortex and hippocampus might be privileged sites of local estrogen synthesis to regulate behavior independently from ovarian secretions.
The first indication that aromatization plays an important role in learning and memory had been brought about by the effect of systemic aromatase blockade on fear extinction in male rats (Graham and Milad 2014), spatial memory in female zebra finches (Rensel et al. 2013) and in both intact and ovariectomized female rats (Aydin et al. 2008). However, whether brain estrogen synthesis is critical to the behavioral effects had not been demonstrated yet.
This gap was first filled in male zebra finches in which the inhibition of hippocampal aromatase was shown to impair the acquisition and performance in a spatial memory task (Bailey 2013). Two studies recently reached a similar conclusion in female rodents. The first study investigated the effect of acute aromatase inhibition in the hippocampus on memory consolidation in young ovariectomized female mice (Tuscher et al. 2016). A single bilateral infusion of letrozole in the dorsal hippocampus immediately after training impaired the learning of object recognition and object placement (Fig.6b), while delayed local estrogen depletion was without effect. Thus, even in the absence of ovarian estrogens, neuroestrogens are produced in the hippocampus and play a role in memory consolidation occurring immediately after training. Importantly, estradiol content measured 30 min after object placement training was markedly higher in the dorsal hippocampus of control than of letrozole females, while estradiol concentration measured 1 or 2 hours after training was low in both the control and letrozole group (Fig.6c), thus suggesting that training is immediately followed by a transient elevation of local estradiol levels which seem to contribute to learning. Finally, the beneficial effects observed following acute treatment with exogenous estradiol were not blocked by local aromatase blockade. Together, this work provided clear evidence that aromatization persists in the hippocampus despite ovariectomy and that locally produced estradiol acutely facilitates hippocampal memory consolidation immediately after training.
The second study investigated the effect of the chronic inhibition of central aromatase on spatial working memory in ovariectomized rats (Nelson et al. 2016). The aromatase inhibitor delivered in the third ventricle via an osmotic minipump did not affect learning compared to vehicle treated ovariectomized females. However, this treatment blocked the beneficial effect of a long-term estrogen treatment (evidenced by a reduction in the number of errors) (Fig.6d).
The seemingly contradictory results of these two studies likely arise from the fact that they were conducted in different time frames and are thus addressing different questions. In the first one, the acute inhibition of estrogen synthesis only impacted transcription-independent effects of estrogens and is thus investigating the role of neurotransmitter-like actions of neuroestrogens (Tuscher et al. 2016). On the other end, the chronic depletion of central estrogens employed in the second study likely reduced estrogen-mediated transcription (Nelson et al. 2016). In this case, the beneficial effect of exogenous estradiol is likely explained by the activation of both transcription-dependent and independent signaling. Together, these two studies thus provide complementary information suggesting that the positive effects of neuroestradiol on learning and memory rely on both genomic and non-genomic actions of estrogens, as described for other processes (Vasudevan and Pfaff 2007; Cornil et al. 2015; Micevych et al. 2015; Cornil and de Bournonville 2017). Alternatively, the discrepancy between these two studies might arise from the fact that they are investigating different memory processes using different tasks. In this case, these seemingly conflicting data might thus indicate that neuroestradiol modulates different learning processes through different mechanisms. Of course, these two hypotheses are not mutually exclusive.
What is the source of androgens aromatized in the brain?
These findings raise the question of the source of the androgenic substrate(s) of brain aromatization. Although females have on average lower circulating androgen concentrations than males, average systemic testosterone concentrations in females of numerous species are not negligible and the distribution of individual concentrations overlaps with that of males (Cornil et al. 2006). Given the very high affinity of the enzyme for androgens (in the low nM range), even low levels of circulating androgens secreted from the gonads, adrenals or any other source are thus likely to act as substrates for local estrogen synthesis in the female brain (Roselli et al. 1996b; Baillien and Balthazart 1997). In addition, dehydroepiandrosterone (DHEA) is produced in large amounts by the adrenals in both sexes and is a known androgen precursor (Maninger et al. 2009). In male song sparrows, DHEA secreted by the adrenals plays a key role in the activation of singing activity outside the breeding season (Soma et al. 2008). Evidence shows that DHEA can serve as a substrate for aromatase following its conversion in androstenedione by 3β-HSD in the brain (Vanson et al. 1996; Soma et al. 2004). Moreover, the activity of brain 3β-HSD is also rapidly regulated by estrogens (Pradhan et al. 2008; Pradhan et al. 2010). Local production of DHEA would thus constitute a likely candidate to permit a dissociation of brain estrogen synthesis and action from the regulation by peripheral hormones. Finally, it is also possible that the androgens serving as precursors for estrogen synthesis are produced de novo in the brain. Indeed, all the enzymes required for the synthesis of androgens from cholesterol are expressed in the brain (Do Rego et al. 2009; London et al. 2009) and changes in androgen levels have been described to occur in the brain independently from changes in circulating concentrations (Remage-Healey et al. 2008; Pradhan et al. 2010).
Summary and Emerging questions
Although they are still limited in number, the data reviewed here bring strong support to the idea that brain-derived estrogens contribute to the normal regulation of female physiology and behavior. Not only is aromatase expressed in the brain of females, but its expression and activity are regulated in different ways leading to measurable changes in region-specific neuroestradiol production in response to specific stimuli. In turn, manipulations mimicking changes in local estrogen concentrations translate in the modulation of physiological and behavioral responses ranging from reproduction to cognition. Finally, these phenomena have been described in several vertebrate taxa suggesting that these processes may be conserved across species.
Obviously, these observations are raising numerous questions. One might wonder why a local supply of estrogens would be necessary when large amounts are available in the periphery. Based on their lipophilic nature steroids can virtually access any tissue sufficiently supplied with blood and it is thus usually considered that the local concentration of a hormone in a given tissue is similar to its concentration in the periphery. However, this is not always the case as exemplified by the higher concentration of estrogens in the jugular blood leaving the brain of male zebra finches compared to the concentration measured in the carotid arteries entering the brain (Schlinger and Arnold 1992) or by the higher levels of estrogens measured in discrete brain regions compared to plasma (Kato et al. 2013; Kenealy et al. 2013; Kenealy et al. 2016). It thus seems that the brain is able to regulate the concentration of estrogens locally independently from the periphery. Although changes in local estrogen levels were largely presented here as depending on changes in synthesis, changes in catabolism should also be considered (Cornil et al. 2006).
In 1992, Hutchison and colleagues suggested that such local hormonal production might be beneficial when circulating levels are low, for example at the early stages of the reproductive cycle (Hutchison et al. 1992). Along the same line, brain synthesis of estradiol in the female musk shrew could compensate for the relatively low peripheral availability in estradiol given that sexual behavior occurs when ovarian follicles are still underdeveloped (Veney and Rissman 2000). This might also constitute a compensatory mechanism able to maintain neural function when ovarian secretions drop across the ovarian cycle, outside the breeding season or at menopause. Such a mechanism may have evolved to allow some dissociation of specific aspects of neural regulation by hormones from the reproductive cycle.
Another likely function of this local control of estrogen production or catabolism could be to allow reaching higher concentrations locally. The affinity for estradiol of all estrogen receptors characterized so far is around 5 nM (Cornil et al. 2006) which is superior to the average circulating concentration reported for estradiol in females of various taxa (high picomolar to low nanomolar) (Cornil et al. 2006). Raising the concentration locally would enhance the number of receptors that are occupied and thus strengthen the activated response. Moreover, higher estradiol concentrations may be necessary to activate membrane-associated receptors than nuclear receptors. In many studies, the rapid actions of estrogens have indeed been observed only after administration of very high doses of the steroid (Cornil et al. 2006; Herbison 2009). Whether this refers to the need to establish a high gradient of concentrations to ensure a rapid access to the receptors or to a requirement of membrane receptors possibly related to a lower affinity for the ligand is not always clear.
Finally, local estrogen production may be a mechanism allowing for faster modulation of the responses by neural processes compared to slower modulations by bodily factors. Although rapid shifts in circulating concentrations of sex steroids have been described in response to changes in the social environment, these changes do not seem to occur fast enough to take advantage of the rapidity offered by membrane-initiated actions on physiology and behavior (Cornil et al. 2006). By contrast, acute blockade of local estrogen synthesis has been shown to induce rapid changes in behavior (Remage-Healey et al. 2010; Remage-Healey et al. 2012; Seredynski et al. 2013; Seredynski et al. 2015; Tuscher et al. 2016).
When considering the potential offered by local estrogen synthesis to dissociate local neural regulation from the ovarian control of reproduction, it is somewhat counterintuitive that female sexual behavior (Veney and Rissman 2000; de Bournonville et al. 2016) and GnRH secretion (Kenealy et al. 2013; Kenealy et al. 2016) rely in part on central estrogen synthesis. Indeed, one would think that such key processes to insure the synchronization of gamete transfer with ovulation and the maturation of the reproductive system would be exclusively regulated by ovarian hormones. Moreover, it should be kept in mind that the effects described here were observed in ovariectomized females. Further research should thus determine the impact of local estrogen depletion to the regulation of sexual behavior and reproduction in gonadally intact females.
Interestingly, local progesterone synthesis also plays a functional role in the control of reproduction. In vitro and in vitro work converge to support the notion that, in hypothalamic astrocytes, estradiol activates the synthesis of neuroprogesterone which is necessary for the release of kisspeptin and the subsequent induction of the preovulatory LH surge (Micevych et al. 2003; Sinchak et al. 2003; Kuo et al. 2010; Micevych and Sinchak 2011; Micevych et al. 2015; Mittelman-Smith et al. 2015; Mittelman-Smith et al. 2017). The involvement in the regulation of physiological processes of a brain synthesis of steroids that are classically produced in the ovary is thus not exclusive to estrogens.
How brain-derived estrogens cooperate with estrogens produced in the periphery also remains an open question. The fact that sustained central estrogen depletion produces effects on learning and memory that are alleviated by chronic systemic estrogen treatments suggests that ovarian estrogens may be required to activate local circuits (through the regulation of transcription-dependent pathways), such that they are able to modulate and/or respond to a local estrogen production in response to environmental stimuli (Nelson et al. 2016; Tuscher et al. 2016). Further investigations will thus also be needed to answer this question.
Finally, recognition of the implication of central aromatization in the regulation of female behavior and physiology might impact clinical research and ultimately improve women’s well-being. For example, menopause is associated with cognitive decline (Sherwin 2012). While ovaries cease to secrete estrogens at menopause, circulating androgen levels increase and could serve as substrates for brain aromatization (Judd et al. 1974; Laughlin et al. 2000; Fogle et al. 2007). Yet, to my knowledge, whether post-menopausal cognitive decline is associated with a lower brain estrogen synthesis, due to lower circulating androgens and/or a failure of brain estrogen synthesis or action, is unknown and deserves further attention. In addition, aromatase inhibitors, which are a common treatment in breast cancer, have been associated with cognitive impairment (Frank et al. 2015), psychiatric effects (Goodwin 2006; Rocha-Cadman et al. 2012) or sexual dysfunction (Derzko et al. 2007). As chemotherapy is provided systemically, aromatase blockade concerns all potential sources of estrogens. Knowing that brain-derived estrogens play a role in the regulation of processes affected by chemotherapy should promote research leading to the development of molecules that do not cross the blood brain barrier to mitigate the side-effects of these treatments.
In conclusion, the data presented here support the notion that female brain aromatase is not simply a non-functional evolutionary vestige and provide support for a role of locally produced estrogens in brain function in females. This conclusion is important in and of itself, but should also interest clinicians. It is thus hoped that this review will stimulate both basic science investigators and clinicians to further study the role and impact of central aromatization in females.
Acknowledgments
I would like to thank Dr Jacques Balthazart for his careful reading of a previous version of this manuscript. C.A.C is Senior Research Associate from the Belgian Funds for Research (Fonds de la Recherche Nationale or F.R.S.-FNRS) and her work reviewed here was supported by the National Institutes for Health (R01 NIH/MH50388).
Abbreviation list
- BST
Bed nucleus of the stria terminalis
- DHEA
dehydroepiandrosterone
- DHT
Dihydrotestosterone
- E2
Estradiol
- EB
Estradiol benzoate
- FSH
Follicle stimulating hormone
- GnRH
Gonadotropin releasing hormone
- HPOA
Hypothalamus-preoptic area
- Kp
Kisspeptin
- LH
Luteinizing hormone
- MBH
Mediobasal hypothalamus
- MeA
Medial amygdala
- MPOA
Medial preoptic area
- NCM
Caudomedial nidopalium
- POA
Preoptic area
- POM
Medial preoptic nucleus
- VMH
Ventromedial hypothalamus
- VMN
Ventromedial nucleus of the hypothalamus
References
- Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology. 1994;135:395–401. doi: 10.1210/endo.135.1.8013375. [DOI] [PubMed] [Google Scholar]
- Adkins EK, Adler NT. Hormonal control of behavior in the japanese quail. J Comp Physiol Psychol. 1972;81:27–36. doi: 10.1037/h0033315. [DOI] [PubMed] [Google Scholar]
- Adkins EK, Nock BL. The effects of the antiestrogen CI-628 on sexual behavior activated by androgen and estrogen in quail. Horm Behav. 1976;7:417–429. doi: 10.1016/0018-506x(76)90013-1. [DOI] [PubMed] [Google Scholar]
- Aydin M, Yilmaz B, Alcin E, Nedzvetsky VS, Sahin Z, Tuzcu M. Effects of letrozole on hippocampal and cortical catecholaminergic neurotransmitter levels, neural cell adhesion molecule expression and spatial learning and memory in female rats. Neuroscience. 2008;151:186–194. doi: 10.1016/j.neuroscience.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Arevalo MA, Garcia-Segura LM. Neural-derived estradiol regulates brain plasticity. J Chem Neuroanat. 2017 doi: 10.1016/j.jchemneu.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Baillien M, Balthazart J. A direct dopaminergic control of aromatase activity in the quail preoptic area. J Steroid Biochem Molec Biol. 1997;63:99–113. doi: 10.1016/s0960-0760(97)00080-0. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol. 2001;13:63–73. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur J Neurosci. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A. Brain aromatase and the control of male sexual behavior. J Steroid Biochem Molec Biol. 1993;44:521–540. doi: 10.1016/0960-0760(93)90256-v. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Hendrick C. The induction by testosterone of aromatase activity in the preoptic area and activation of copulatory behavior. Physiol Behav. 1990a;47:83–94. doi: 10.1016/0031-9384(90)90045-6. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Schumacher M, Evrard L. Sex differences and steroid control of testosterone-metabolizing enzyme activity in the quail brain. J Neuroendocrinol. 1990b;2:675–683. doi: 10.1111/j.1365-2826.1990.tb00464.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Tlemcani O, Harada N. Localization of testosterone-sensitive and sexually dimorphic aromatase-immunoreactive cells in the quail preoptic area. J Chemical Neuroanat. 1996;11:147–171. doi: 10.1016/0891-0618(96)00149-4. [DOI] [PubMed] [Google Scholar]
- Barha CK, Dalton GL, Galea LA. Low doses of 17alpha-estradiol and 17beta-estradiol facilitate, whereas higher doses of estrone and 17alpha- and 17beta-estradiol impair, contextual fear conditioning in adult female rats. Neuropsychopharmacology. 2010;35:547–559. doi: 10.1038/npp.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Galea LA. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim Biophys Acta. 2010;800:1056–1067. doi: 10.1016/j.bbagen.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Beach FA. Male and female mating behavior in prepubertally castrated female rats treated with androgens. Endocrinology. 1942;31:673–678. [Google Scholar]
- Beyer C, Komisaruk B. Effects of diverse androgens on estrous behavior, lordosis reflex, and genital tract morphology in the rat. Horm Behav. 1971;2:217–225. [Google Scholar]
- Beyer C, McDonald P, Vidal N. Failure of 5-alpha-dihydrotestosterone to elicit estrous behavior in the ovariectomized rabbit. Endocrinology. 1970a;86:939–941. doi: 10.1210/endo-86-4-939. [DOI] [PubMed] [Google Scholar]
- Beyer C, Vidal N, Mijares A. Probable role of aromatization in the induction of estrous behavior by androgens in the ovariectomized rabbit. Endocrinology. 1970b;87:1386–1389. doi: 10.1210/endo-87-6-1386. [DOI] [PubMed] [Google Scholar]
- Biegon A, Alexoff DL, Kim SW, Logan J, Pareto D, Schlyer D, Wang GJ, Fowler JS. Aromatase imaging with [N-methyl-11C]vorozole PET in healthy men and women. J Nucl Med. 2015;56:580–585. doi: 10.2967/jnumed.114.150383. [DOI] [PubMed] [Google Scholar]
- Boon WC, Chow JD, Simpson ER. The multiple roles of estrogens and the enzyme aromatase. Progr Brain Res. 2010;181:209–232. doi: 10.1016/S0079-6123(08)81012-6. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA, Remage-Healey L. It takes a seasoned bird to be a good listener: communication between the sexes. Cur Opin Neurobiol. 2016;38:12–17. doi: 10.1016/j.conb.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callard GV, Greytak SR, Novillo A, Cotter KA, Meyer RK. Brain aromatase in fishes: historical perspectives and comparative approaches. In: Balthazart J, Ball GF, editors. Brain aromatase, estrogens and behavior. Oxford university press; 2013. pp. 13–42. [Google Scholar]
- Charlier TD, Cornil CA, Balthazart J. Rapid modulation of aromatase activity in the vertebrate brain. J Exp Neurosci. 2013;7:31–37. doi: 10.4137/JEN.S11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Harada N, Balthazart J, Cornil CA. Human and quail aromatase activity is rapidly and reversibly inhibited by phosphorylating conditions. Endocrinology. 2011;152:4199–4210. doi: 10.1210/en.2011-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Vicario DS, Nottebohm F. A large-capacity memory system that recognizes the calls and songs of individual birds. Proc Natl Acad Sci U S A. 1996;93:1950–1955. doi: 10.1073/pnas.93.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Valsecchi P, Kavaliers M. Estrogenic involvement in social learning, social recognition and pathogen avoidance. Front Neuroendocrinol. 2012;33:140–159. doi: 10.1016/j.yfrne.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Campbell R, Smith JT, Prevot V, Wray S. Neuroendocrine control of reproduction. In: Fink G, Pfaff DW, Levine JE, editors. Handbook of Neuroendocrinology. 1. Academic press; 2012. pp. 197–236. [Google Scholar]
- Comito D, Pradhan DS, Karleen BJ, Schlinger BA. Region-specific rapid regulation of aromatase activity in zebra finch brain. J Neurochem. 2015 doi: 10.1111/jnc.13513. [DOI] [PubMed] [Google Scholar]
- Connolly PB, Roselli CE, Resko JA. Aromatase activity in adult guinea pig brain is androgen dependent. Biol Reprod. 1990;43:698–703. doi: 10.1095/biolreprod43.4.698. [DOI] [PubMed] [Google Scholar]
- Corbin CJ, Berger T, Ford JJ, Roselli CE, Sienkiewicz W, Trainor BC, Roser JF, Vidal JD, Harada N, Conley AJ. Porcine hypothalamic aromatase cytochrome P450: isoform characterization, sex-dependent activity, regional expression, and regulation by enzyme inhibition in neonatal boars. Biol Reprod. 2009;81:388–395. doi: 10.1095/biolreprod.109.076331. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. Rapid control of male typical behaviors by brain-derived estrogens. Front Neuroendocrinol. 2012a;33:425–446. doi: 10.1016/j.yfrne.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. The dual action of estrogen hypothesis. Trends Neurosci. 2015;38:408–416. doi: 10.1016/j.tins.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J, Charlier TD. Organizing effects of sex steroids on brain aromatase activity in quail. PloS one. 2011;6:e19196. doi: 10.1371/journal.pone.0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, de Bournonville C. Dual action of neuro-estrogens in the regulation of male sexual behavior. Gen Comp Endocrinol. 2017 doi: 10.1016/j.ygcen.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Leung CH, Pletcher ER, Naranjo KC, Blauman SJ, Saldanha CJ. Acute and specific modulation of presynaptic aromatization in the vertebrate brain. Endocrinology. 2012b;153:2562–2567. doi: 10.1210/en.2011-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- de Bournonville C, Ball GF, Balthazart J, Cornil CA. Rapid changes in brain aromatase activity in the female quail brain following expression of sexual behavior. J Neuroendocrinol. 2017b doi: 10.1111/jne.12542. [DOI] [PubMed] [Google Scholar]
- de Bournonville C, Balthazart J, Ball GF, Cornil CA. Non-ovarian aromatization is required to activate female sexual motivation in testosterone-treated ovariectomized quail. Horm Behav. 2016;83:45–59. doi: 10.1016/j.yhbeh.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bournonville C, Smolders I, Van Eeckhaut A, Ball GF, Balthazart J, Cornil CA. Glutamate released in the preoptic area during sexual behavior controls local estrogen synthesis in male quail. Psychoneuroendocrinology. 2017a;79:49–58. doi: 10.1016/j.psyneuen.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delville Y, Balthazart J. Hormonal control of female sexual behavior in the Japanese quail. Horm Behav. 1987;21:288–309. doi: 10.1016/0018-506x(87)90016-x. [DOI] [PubMed] [Google Scholar]
- Delville Y, Sulon J, Balthazart J. Diurnal variations of sexual receptivity in the female Japanese quail (Coturnix coturnix japonica) Horm Behav. 1986;20:13–33. doi: 10.1016/0018-506x(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Derzko C, Elliott S, Lam W. Management of sexual dysfunction in postmenopausal breast cancer patients taking adjuvant aromatase inhibitor therapy. Curr Oncol. 2007;14(Suppl 1):S20–40. doi: 10.3747/co.2007.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens MJ, Balthazart J, Cornil CA. Brain aromatase and circulating corticosterone are rapidly regulated by combined acute stress and sexual interaction in a sex-specific manner. J Neuroendocrinol. 2012;24:1322–1334. doi: 10.1111/j.1365-2826.2012.02340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens MJ, Cornil CA, Balthazart J. Acute stress differentially affects aromatase activity in specific brain nuclei of adult male and female quail. Endocrinology. 2011;152(11):4242–4251. doi: 10.1210/en.2011-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens MJ, de Bournonville C, Balthazart J, Cornil CA. Relationships between rapid changes in local aromatase activity and estradiol concentrations in male and female quail brain. Horm Behav. 2014;65:154–164. doi: 10.1016/j.yhbeh.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 2009;30:259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Ervin KS, Lymer JM, Matta R, Clipperton-Allen AE, Kavaliers M, Choleris E. Estrogen involvement in social behavior in rodents: rapid and long-term actions. Horm Behav. 2015;74:53–76. doi: 10.1016/j.yhbeh.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92:3040–3043. doi: 10.1210/jc.2007-0581. [DOI] [PubMed] [Google Scholar]
- Foidart A, de Clerck A, Harada N, Balthazart J. Aromatase-immunoreactive cells in the quail brain: effects of testosterone and sex dimorphism. Physiol Behav. 1994;55:453–464. doi: 10.1016/0031-9384(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical reexamination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J Chem Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- Frank JS, Vance DE, Triebel KL, Meneses KM. Cognitive deficits in breast cancer survivors after chemotherapy and hormonal therapy. J Neurosci Nurs. 2015;47:302–312. doi: 10.1097/JNN.0000000000000171. [DOI] [PubMed] [Google Scholar]
- Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav Neurosci. 2001;115:229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- Frick KM, Kim J, Tuscher JJ, Fortress AM. Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn Mem. 2015;22:472–493. doi: 10.1101/lm.037267.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusani L, Hutchison JB, Gahr M. Testosterone regulates the activity and expression of aromatase in the canary neostriaturn. J Neurobiol. 2001;49:1–8. doi: 10.1002/neu.1061. [DOI] [PubMed] [Google Scholar]
- Fusani L, Van't Hof T, Hutchison JB, Gahr M. Seasonal expression of androgen receptors, estrogen receptors, and aromatase in the canary brain in relation to circulating androgens and estrogens. J Neurobiol. 2000;43:254–268. [PubMed] [Google Scholar]
- Gladue BA, Dohanich GP, Clemens LG. Hormonally mediated lordosis in female rats: actions of flutamide and an aromatization inhibitor. Pharmacol Biochem Behav. 1978;9:827–832. doi: 10.1016/0091-3057(78)90363-5. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN. Kisspeptin neurons from mice to men: similarities and differences. Endocrinology. 2012;153:5105–5118. doi: 10.1210/en.2012-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM. Aromatase inhibitors and bipolar mood disorder: a case report. Bipolar disord. 2006;8:516–518. doi: 10.1111/j.1399-5618.2006.00367.x. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Inhibition of estradiol synthesis impairs fear extinction in male rats. Learn Mem. 2014;21:347–350. doi: 10.1101/lm.034926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gu Q, Moss RL. Novel mechanism for non-genomic action of 17b-oestradiol on kainate-induced in isolated rat CA1 hippocampal neurones. J Physiol. 1998;506:745–754. doi: 10.1111/j.1469-7793.1998.745bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Abe-Dohmae S, Loeffen R, Foidart A, Balthazart J. Synergism between androgens and estrogens in the induction of aromatase and its messenger RNA in the brain. Brain Res. 1993;622:243–256. doi: 10.1016/0006-8993(93)90825-8. [DOI] [PubMed] [Google Scholar]
- Harada N, Yamada K, Foidart A, Balthazart J. Regulation of aromatase cytochrome P-450 (estrogen synthetase) transcripts in the quail brain by testosterone. Mol Brain Res. 1992;15:19–26. doi: 10.1016/0169-328x(92)90146-3. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Saadi A, Frick KM. Dose-dependent effects of post-training estradiol plus progesterone treatment on object memory consolidation and hippocampal extracellular signal-regulated kinase activation in young ovariectomized mice. Neuroscience. 2009;160:6–12. doi: 10.1016/j.neuroscience.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Trainor BC, Soma KK. Rapid effects of estradiol on aggression in birds and mice: the fast and the furious. Integr and Comp Biol. 2015;55(2):281–293. doi: 10.1093/icb/icv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Rapid actions of oestrogen on gonadotropin-releasing hormone neurons; from fantasy to physiology? J Physiol. 2009;587:5025–5030. doi: 10.1113/jphysiol.2009.179838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C-H. Blockade of lordosis by androst-1,4,6-triene-3,17-dione (ATD) and tamoxifen in female hamsters primed with testosterone propionate. Horm Behav. 1990;24:14–19. doi: 10.1016/0018-506x(90)90023-q. [DOI] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Rodriguez-Manzo G. Male sexual behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain and behavior. Vol. 1. Academic Press; San Diego, CA: 2009. pp. 5–65. [Google Scholar]
- Hutchison JB, Steimer T. Formation of behaviorally effective 17b-estradiol in the dove brain: steroid control of preoptic aromatase. Endocrinology. 1986;118:2180–2187. doi: 10.1210/endo-118-6-2180. [DOI] [PubMed] [Google Scholar]
- Hutchison JB, Steimer T, Hutchison RE. Area-specific hormonal regulation of brain aromatase. Brain Res. 1991;550:95–100. doi: 10.1016/0006-8993(91)90409-o. [DOI] [PubMed] [Google Scholar]
- Hutchison RE, Wozniak AW, Hutchison JB. Regulation of female brain aromatase activity during the reproductive cycle of the dove. J Endocrinol. 1992;134:385–396. doi: 10.1677/joe.0.1340385. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd HL, Judd GE, Lucas WE, Yen SS. Endocrine function of the postmenopausal ovary: concentration of androgens and estrogens in ovarian and peripheral vein blood. J Clin Endocrinol Metab. 1974;39:1020–1024. doi: 10.1210/jcem-39-6-1020. [DOI] [PubMed] [Google Scholar]
- Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, Kawato S. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circuits. 2013;7:149. doi: 10.3389/fncir.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Kapoor A, Guerriero KA, Keen KL, Garcia JP, Kurian JR, Ziegler TE, Terasawa E. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J Neurosci. 2013;33:19051–19059. doi: 10.1523/JNEUROSCI.3878-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Garcia JP, Richter DJ, Terasawa E. Prolonged infusion of estradiol benzoate into the stalk median eminence stimulates release of GnRH and kisspeptin in ovariectomized female rhesus macaques. Endocrinology. 2015;156:1804–1814. doi: 10.1210/en.2014-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Kapoor A, Terasawa E. Neuroestradiol in the stalk median eminence of female rhesus macaques decreases in association with puberty onset. Endocrinology. 2016;157:70–76. doi: 10.1210/en.2015-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle AT, Balthazart J. Sex differences in the rapid control of aromatase activity in the quail preoptic area. J Neuroendocrinol. 2011;23:424–434. doi: 10.1111/j.1365-2826.2011.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30:12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber ME, Lichtensteiger W. Pre- and postnatal ontogeny of aromatase cytochrome P450 messenger ribonucleic acid expression in the male rat brain studied by in situ hybridization. Endocrinology. 1994;135:1661–1668. doi: 10.1210/endo.135.4.7925130. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Muhlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85:645–651. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- London SE, Remage-Healey L, Schlinger BA. Neurosteroid production in the songbird brain: a re-evaluation of core principles. Front Neuroendocrinol. 2009;30:302–314. doi: 10.1016/j.yfrne.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66:602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and alterations in spine density. Front Neuroendocrinol. 2012;33:388–402. doi: 10.1016/j.yfrne.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luttge WG, Hall NR, Wallis CJ, Campbell JC. Stimulation of male and female sexual behavior in gonadectomized rats with estrogen and androgen therapy and its inhibition with concurrent anti-hormone therapy. Physiol Behav. 1975;14:65–73. doi: 10.1016/0031-9384(75)90143-2. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. The J Compr Neurol. 1999;407:115–129. [PubMed] [Google Scholar]
- Micevych P, Sinchak K. The neurosteroid progesterone underlies estrogen positive feedback of the LH surge. Front Endocrinol. 2011;2:90. doi: 10.3389/fendo.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78:29–35. doi: 10.1159/000071703. doi:71703. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Meisel RL. Integrating Neural Circuits Controlling Female Sexual Behavior. Front Systems Neurosci. 2017;11:42. doi: 10.3389/fnsys.2017.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Wong AM, Mittelman-Smith MA. Estradiol Membrane-Initiated Signaling and Female Reproduction. Comprehensive Physiol. 2015;5:1211–1222. doi: 10.1002/cphy.c140056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelman-Smith MA, Wong AM, Kathiresan AS, Micevych PE. Classical and membrane-initiated estrogen signaling in an in vitro model of anterior hypothalamic kisspeptin neurons. Endocrinology. 2015;156:2162–2173. doi: 10.1210/en.2014-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelman-Smith MA, Wong AM, Micevych PE. Estrogen and progesterone integration in an in vitro model of RP3V kisspeptin neurons. Neuroendocrinology. 2017 doi: 10.1159/000471878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. Neuroendocrinology. 1996;63:149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Rec Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- Nelson BS, Black KL, Daniel JM. Circulating Estradiol Regulates Brain-Derived Estradiol via Actions at GnRH Receptors to Impact Memory in Ovariectomized Rats. eNeuro. 2016;3(6) doi: 10.1523/ENEURO.0321-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble R. The effects of estrogen and progesterone on copulation in female quail (Coturnix coturnix japonica) housed in continuous dark. Horm Behav. 1972;3:199–204. doi: 10.1016/0018-506x(72)90032-3. [DOI] [PubMed] [Google Scholar]
- Noble R. Hormonal control of receptivity in female quail (Coturnix coturnix japonica) Horm Behav. 1973;4:61–72. [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport. 1997;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- Palka YS, Sawyer CH. Induction of estrous behavior in rabbits by hypothalamic implants of testosterone. Am J Physiol. 1966;211:225–228. doi: 10.1152/ajplegacy.1966.211.1.225. [DOI] [PubMed] [Google Scholar]
- Pareto D, Biegon A, Alexoff D, Carter P, Shea C, Muench L, Xu Y, Fowler JS, Kiw SW, Logan J. In vivo imaging of brain aromatase in female baboons : 511C]vorozole kinetics and effect of the menstrual cycle. Mol Imaging. 2013;12:518–524. doi: 10.2310/7290.2013.00068. [DOI] [PubMed] [Google Scholar]
- Pellegrini E, Vaillant C, Diotel N, Benquet P, Brion F, Kah O. Expression, regulation and potential functions of aromatase in radial glial cells of the fish brain. In: Balthazart J, Ball GF, editors. Brain aromatase, estrogens and behavior. Oxford university press; 2013. pp. 115–137. [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D. Nature of sex hormone effects on rat sex behavior: specificity of effects and individual patterns of response. J Comp Physiol Psychol. 1970;73:349–358. doi: 10.1037/h0030242. [DOI] [PubMed] [Google Scholar]
- Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor alpha and beta selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–1502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- Piet R, de Croft S, Liu X, Herbison AE. Electrical properties of kisspeptin neurons and their regulation of GnRH neurons. Front Neuroendocrinol. 2015;36:15–27. doi: 10.1016/j.yfrne.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Pinaud R, Terleph TA. A songbird forebrain area potentially involved in auditory discrimination and memory formation. J Biosci. 2008;33:145–155. doi: 10.1007/s12038-008-0030-y. [DOI] [PubMed] [Google Scholar]
- Pradhan DS, Lau LY, Schmidt KL, Soma KK. 3beta-HSD in songbird brain: subcellular localization and rapid regulation by estradiol. J Neurochem. 2010;115(3):667–675. doi: 10.1111/j.1471-4159.2010.06954.x. [DOI] [PubMed] [Google Scholar]
- Pradhan DS, Yu Y, Soma KK. Rapid estrogen regulation of DHEA metabolism in the male and female songbird brain. J Neurochem. 2008;104:244–253. doi: 10.1111/j.1471-4159.2007.04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VV, Naftolin F, Ryan KJ. Aromatization in the central nervous system of rabbits: effects of castration and hormone treatment. Endocrinology. 1973;92:589–594. doi: 10.1210/endo-92-2-589. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci. 2011;31:10034–10038. doi: 10.1523/JNEUROSCI.0566-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012;107:1621–1631. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Heimovics SA, Soma KK, Cornil CA. Rapid effects of estrogens on avian brain and social behavior. In: Pfaff DW, Joëls M, editors. Hormones, brain, and behavior. Vol. 2. Academic Press; Oxford: 2017. pp. 291–303. [Google Scholar]
- Remage-Healey L, Joshi NR. Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. J Neurosci. 2012;32:8231–8241. doi: 10.1523/JNEUROSCI.1114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensel MA, Salwiczek L, Roth J, Schlinger BA. Context-specific effects of estradiol on spatial learning and memory in the zebra finch. Neurobiol Learn Mem. 2013;100:41–47. doi: 10.1016/j.nlm.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebel K, Smallegange IM, Terpstra NJ, Bolhuis JJ. Sexual equality in zebra finch song preference: evidence for a dissociation between song recognition and production learning. Proc R Soc Lond [Biol ] 2002;269:729–733. doi: 10.1098/rspb.2001.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF. Evidence that neural aromatization of androgen regulates the expression of sexual behaviour in female musk shrews. J Neuroendocrinol. 1991;3:441–448. doi: 10.1111/j.1365-2826.1991.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Clendenon AL, Krohmer RW. Role of androgens in the regulation of sexual behavior in the female musk shrew. Neuroendocrinology. 1990;51:468–473. doi: 10.1159/000125376. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Crews D. Hormonal correlates of sexual behavior in the female musk shrew: the role of estradiol. Physiol Behav. 1988;44:1–7. doi: 10.1016/0031-9384(88)90338-1. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Harada N, Roselli CE. Effect of vorozole, an aromatase enzyme inhibitor, on sexual behavior, aromatase activity and neural immunoreactivity. J Neuroendocrinol. 1996;8:199–210. doi: 10.1046/j.1365-2826.1996.04505.x. [DOI] [PubMed] [Google Scholar]
- Rocha-Cadman X, Massie MJ, Du Hamel K. Aromatase inhibitors and mood disturbances. Palliat Support Care. 2012;10:225–227. doi: 10.1017/S1478951512000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol. 2007;67:1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]
- Roselli CE. Subcellular localization and kinetic properties of aromatase activity in rat brain. J Steroid Biochem Mol Biol. 1995;52:469–477. doi: 10.1016/0960-0760(94)00192-o. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Abdelgadir SE, Jorgensen E, Resko JA. Sex differences in androgen-regulated cytochrome P450 aromatase mRNA in the rat brain. Endocrine. 1996a;5:59–65. doi: 10.1007/BF02738657. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Abdelgadir SE, Resko JA. Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull. 1997;44:351–357. doi: 10.1016/s0361-9230(97)00214-1. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Abdelgadir SE, Ronnekleiv OK, Klosterman SA. Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol Reprod. 1998a;58:79–87. doi: 10.1095/biolreprod58.1.79. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Ellinwood WE, Resko JA. Regulation of brain aromatase activity in rats. Endocrinology. 1984;114:192–200. doi: 10.1210/endo-114-1-192. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Horton LE, Resko JA. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology. 1985;117:2471–2477. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Horton LE, Resko JA. Time-course and steroid specificity of aromatase induction in rat hypothalamus-preoptic area. Biol Reprod. 1987;37:628–633. doi: 10.1095/biolreprod37.3.628. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Klosterman SA. Sexual differentiation of aromatase activity in the rat brain: effect of perinatal steroid exposure. Endocrinology. 1998;139:3193–3201. doi: 10.1210/endo.139.7.6101. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Klosterman SA, Fasasi TA. Sex differences in androgen responsiveness in the rat brain: regional differences in the induction of aromatase activity. Neuroendocrinology. 1996b;64:139–145. doi: 10.1159/000127111. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Klosterman SA, Resko JA. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J Comp Neurol. 2001;439:208–223. doi: 10.1002/cne.1343. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA. Cytochrome P450 aromatase (CYP19) in the non-human primate brain: distribution, regulation, and functional significance. J Ster Biochem Molec Biol. 2001;79:247–253. doi: 10.1016/s0960-0760(01)00141-8. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Stormshak F, Resko JA. Distribution and regulation of aromatase activity in the ram hypothalamus and amygdala. Brain Res. 1998b;811:105–110. doi: 10.1016/s0006-8993(98)00995-0. [DOI] [PubMed] [Google Scholar]
- Rudolph LM, Cornil CA, Mittelman-Smith MA, Rainville JR, Remage-Healey L, Sinchak K, Micevych PE. Actions of steroids: new neurotransmitters. J Neurosci. 2016;36(45):11449–11458. doi: 10.1523/JNEUROSCI.2473-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Popper P, Micevych PE, Schlinger BA. The passerine hippocampus is a site of high aromatase: inter- and intraspecies comparisons. Horm Behav. 1998;34(2):85–97. doi: 10.1006/hbeh.1998.1447. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev. 2011;32:532–549. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Sato SM, Woolley CS. Acute inhibition of neurosteroid estrogen synthesis suppresses status epilepticus in an animal model. eLife. 2016:5. doi: 10.7554/eLife.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP. Circulating estrogens in a male songbird originate in the brain. Proc Natl Acad Sci U S A. 1992;89:7650–7653. doi: 10.1073/pnas.89.16.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Callard GV. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinology. 1989;49:434–441. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Alexandre C, Balthazart J. Interactions des androgènes et des oestrogènes dans le contrôle de la reproduction. C R Acad Sci Paris. 1987;305:569–574. [PubMed] [Google Scholar]
- Schumacher M, Balthazart J. Testosterone-induced brain aromatase is sexually dimorphic. Brain Res. 1986;370:285–293. doi: 10.1016/0006-8993(86)90483-x. [DOI] [PubMed] [Google Scholar]
- Selmanoff MK, Brodkin LD, Weiner RI, Siiteri PK. Aromatization and 5a-reduction of androgens in discrete hypothalamic and limbic regions of the male and female rat. Endocrinology. 1977;101:841–848. doi: 10.1210/endo-101-3-841. [DOI] [PubMed] [Google Scholar]
- Seredynski AL, Balthazart J, Ball GF, Cornil CA. Estrogen receptor beta activation rapidly modulates male sexual motivation through the transactivation of metabotropic glutamate receptor 1a. J Neurosci. 2015;35:13110–13123. doi: 10.1523/JNEUROSCI.2056-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seredynski AL, Balthazart J, Christophe VJ, Ball GF, Cornil CA. Neuroestrogens rapidly regulate sexual motivation but not performance. J Neurosci. 2013;33:164–174. doi: 10.1523/JNEUROSCI.2557-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women: the critical period hypothesis. In: Fink G, Pfaff DW, Levine JE, editors. Handbook in neuroendocrinology. Academic press; 2012. pp. 535–550. [Google Scholar]
- Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK, Micevych P. Estrogen induces de novo progesterone synthesis in astrocytes. Dev Neurosci. 2003;25:343–348. doi: 10.1159/000073511. [DOI] [PubMed] [Google Scholar]
- Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30:16137–16148. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Alday NA, Hau M, Schlinger BA. Dehydroepiandrosterone metabolism by 3β-hydrosteroid dehydrogen se/Δ5-Δ4 isomerase in adult zebra finch brain: sex difference and rapid effect of stress. Endocrinology. 2004;145:1668–1677. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]
- Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ. Brain aromatase, 5alpha-reductase, and 5beta-reductase change seasonally in wild male song sparrows: Relationship to aggressive and sexual behavior. J Neurobiol. 2003;56:209–221. doi: 10.1002/neu.10225. [DOI] [PubMed] [Google Scholar]
- Soma KK, Scotti MA, Newman AE, Charlier TD, Demas GE. Novel mechanisms for neuroendocrine regulation of aggression. Front Neuroendocrinol. 2008;29:476–489. doi: 10.1016/j.yfrne.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Penzes P. Insights into rapid modulation of neuroplasticity by brain estrogens. Pharmacol Rev. 2013;65:1318–1350. doi: 10.1124/pr.111.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic D, Dubois S, Chua HK, Tonge B, Rinehart N, Horne MK, Boon WC. Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors alpha and beta, and androgen receptors. PloS one. 2014;9:e90451. doi: 10.1371/journal.pone.0090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer T, Hutchison JB. Androgen increases formation of behaviourally effective oestrogen in dove brain. Nature. 1981;292:345–347. doi: 10.1038/292345a0. [DOI] [PubMed] [Google Scholar]
- Steimer T, Hutchison JB. Is androgen-dependent aromatase activity sexually differentiated in the rat and dove preoptic area? J Neurobiol. 1990a;21:787–795. doi: 10.1002/neu.480210512. [DOI] [PubMed] [Google Scholar]
- Steimer T, Hutchison JB. Is preoptic aromatase sexually differentiated in the avian and rodent brain. J Neurobiol. 1990b;21:787–795. doi: 10.1002/neu.480210512. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler TJ, Vardaris RM, Lewis D, Rawitch AB. Gonadal steroids: effects on excitability of hippocampal pyramidal cells. Science. 1980;209:1017–1018. doi: 10.1126/science.7190730. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Kyomen HH, Marler CA. Estrogenic encounters: How interactions between aromatase and the environment modulate aggression. Front Neuroendocrinol. 2006 doi: 10.1016/j.yfrne.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc Biol Sci. 2002;269:823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci. 2011;31:3271–3289. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, Frick KM. Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm Behav. 2016;83:60–67. doi: 10.1016/j.yhbeh.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanson A, Arnold AP, Schlinger BA. 3 beta-hydroxysteroid dehydrogenase/isomerase and aromatase activity in primary cultures of developing zebra finch telencephalon: dehydroepiandrosterone as substrate for synthesis of androstenedione and estrogens. Gen Comp Endocrinol. 1996;102:342–350. doi: 10.1006/gcen.1996.0077. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Membrane initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- Veney SL, Rissman EF. Steroid implants in the medial preoptic area or ventromedial nucleus of the hypothalamus activate female sexual behaviour in the musk shrew. J Neuroendocrinol. 2000;12:1124–1132. doi: 10.1046/j.1365-2826.2000.00567.x. [DOI] [PubMed] [Google Scholar]
- Vierk R, Glassmeier G, Zhou L, Brandt N, Fester L, Dudzinski D, Wilkars W, Bender RA, Lewerenz M, Gloger S, Graser L, Schwarz J, Rune GM. Aromatase inhibition abolishes LTP generation in female but not in male mice. J Neurosci. 2012;32:8116–8126. doi: 10.1523/JNEUROSCI.5319-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt C, Ball GF, Balthazart J. Neuroanatomical specificity of sex differences in expression of aromatase mRNA in the quail brain. J Chem Neuroanat. 2007;33:75–86. doi: 10.1016/j.jchemneu.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Voigt C, Ball GF, Balthazart J. Effects of sex steroids on aromatase mRNA expression in the male and female quail brain. Gen Comp Endocrinol. 2011;170:180–188. doi: 10.1016/j.ygcen.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CK, Morrell JI. Distribution and steroid hormone regulation of aromatase mRNA expression in the forebrain of adult male and female rats: a cellular-level analysis using in situ hybridization. J Comp Neurol. 1996;370:71–84. doi: 10.1002/(SICI)1096-9861(19960617)370:1<71::AID-CNE7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Weisz J, Gibbs C. Conversion of testosterone and androstenedione to estrogens in vitro by the brain of female rats. Endocrinology. 1974;94:616–620. doi: 10.1210/endo-94-2-616. [DOI] [PubMed] [Google Scholar]
- Whalen RE, Battie C, Luttge WG. Anti-estrogen inhibition of androgen induced sexual receptivity in rats. Behav Biol. 1972;7:311–320. doi: 10.1016/s0091-6773(72)80103-2. [DOI] [PubMed] [Google Scholar]
- Whalen RE, Hardy DF. Induction of receptivity in female rats and cats with estrogen and testosterone. Physiol Behav. 1970;5:529–533. doi: 10.1016/0031-9384(70)90262-3. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda SI, Harada N, Shah NM. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yague JG, Munoz A, de Monasterio-Schrader P, Defelipe J, Garcia-Segura LM, Azcoitia I. Aromatase expression in the human temporal cortex. Neuroscience. 2006;138(2):389–401. doi: 10.1016/j.neuroscience.2005.11.054. [DOI] [PubMed] [Google Scholar]
- Yague JG, Wang AC, Janssen WG, Hof PR, Garcia-Segura LM, Azcoitia I, Morrison JH. Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res. 2008;1209:115–127. doi: 10.1016/j.brainres.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Fester L, von Blittersdorff B, Hassu B, Nogens H, Prange-Kiel J, Jarry H, Wegscheider K, Rune GM. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology. 2010;151:1153–1160. doi: 10.1210/en.2009-0254. [DOI] [PubMed] [Google Scholar]