INTRODUCTION
A recent American Heart Association report shows that 34% of US adults ≥ 20 years of age have hypertension (HTN) representing about 86 million adults.1 A substantial increase in the prevalence of HTN has occurred globally.2 The projected number of individuals with systolic blood pressure (SBP) of 140 mmHg or higher has doubled from 442 million in 1990 to 874 million in 2015. HTN is one of the most prevalent risk factors for cardiovascular disease (CVD).1 Results of the recently concluded Systolic Blood Pressure Intervention Trial (SPRINT) funded by the National Heart, Lung, and Blood Institute (NHLBI) showed that among older adults with HTN but without diabetes, lowering SBP to a target goal of 120 mmHg, as compared with the standard goal of 140 mmHg, resulted in significantly lower rates of fatal and nonfatal cardiovascular events and death from any cause.3
Some groups such as African Americans who display both disproportionately earlier onset and higher prevalence of HTN have increased risk of blood pressure (BP)-related cardiovascular and renal disease complications compared to non-Hispanic whites. Despite intensive attempts to influence lifestyle changes, nutritional counseling, and intensive anti-hypertensive drug treatment strategies, about 14% of all HTN patients seem to be resistant to anti-hypertensive interventions.1 Resistant HTN is defined as BP above goal (> 140/90 mmHg) on three or more BP lowering medications, or needing four or more medications prescribed at optimal dose to control BP to goal. Thus, both increased prevalence and inability to achieve BP goals in a large patient population with HTN are creating a tremendous health care burden. In addition, no novel anti-HTN drugs have been added to our formulary since 1995, when the first angiotensin receptor antagonist was approved in the US. The recent attempts to use percutaneous renal artery sympathetic denervation, as a novel means to control HTN, have been largely unsuccessful so far.4,5 Therefore, there is a crucial need to discover novel and innovative ways to address the BP control issue.
Recent studies showing a role for microbiota in BP regulation might provide promising new therapeutic approaches. Increasing evidence indicates that dysregulation of commensal microbiota is linked to a variety of chronic disorders, some of which (including diabetes, obesity, chronic kidney disease, heart failure, etc.) have an impact on BP regulation and are risk factors for HTN.6,7 Thus, it is reasonable to infer that microbiota could be a major participant in BP homeostasis. In fact, several recent studies have identified gut dysbiosis being associated with HTN and new links between brain-gut, kidney-gut, and microbial metabolites-host interactions in BP homeostasis.8–11 These results, coupled with a sense of urgency to discover novel mechanism-based therapeutic strategies for HTN, led the NHLBI to convene a Working Group (WG) on June 10, 2016 to discuss this emerging area of the role of microbiota in BP regulation. The WG brought together 16 experts from diverse backgrounds in HTN, cancer, cardiovascular, renal, nutritional, inflammatory, microbiome/microbial, and oral diseases.
The WG reviewed existing and emerging scientific evidence connecting gut and oral microbiota to BP regulation. The WG was organized into four thematic sessions: (1) The link between microbiota and HTN session included presentations on gut-brain axis and microbiota in animal models of HTN and vascular dysfunction; (2) A session on the role of microbiota in human disease, included CVD, kidney disease, and metabolic syndrome; (3) The oral microbiota session discussed the Human Oral Microbiome Database (HOMD) advances in metagenomic and metabolomic technologies and the role of nitrate in BP regulation; and (4) Discussion on microbiota as a potential therapeutic target included dietary modifications, circadian regulation, and impact of chronic stress on the gut-brain axis. Since the WG members came from diverse backgrounds, and most have not collaborated before, the meeting provided an excellent opportunity for cross-disciplinary thinking and discussions and highlighted potential for future collaborations and research topics. In this report, we provide a summary of the WG presentations, discussion, and its recommendations for future research directions.
GUT MICROBIOTA AND HTN
Indirect involvement of gut microbiota in BP regulation and HTN has been known for some time, primarily from studies involving fermented milk, probiotics, and meta-analysis of randomized trials.12–14 Evidence for a more direct link was first presented in two simultaneous reports, using several different animal models of HTN (i.e., Dahl salt-sensitive and salt-resistant rats, spontaneously hypertensive rats, and angiotensin II-infusion rat models).8,11 Significant changes in gut microbiota and alterations in Firmicutes/Bacteroidetes ratio were found to be linked with high BP.11 Furthermore, the microbiota of hypertensive animals demonstrated significant decreases in butyrate- and acetate-producing bacterial populations. Interestingly, in the angiotensin II-infusion rat models, treatment with minocycline, an anti-inflammatory antibiotic which prevented dysbiosis, expanded both acetate- and butyrate-producing bacteria and lowered high BP.11 Contributions of antibiotic and anti-inflammatory properties of minocycline on its anti-hypertensive actions remains to be investigated. These original observations have been validated with other HTN animal models strengthening the connection between gut dysbiosis and high BP.15–17
Changes in gut microbiota in HTN were associated with changes in gut inflammatory status and pathology.18 Reported structural changes in the gut of hypertensive animals include an increase in gut wall fibrosis and decreases in villi length and number of goblet cells, coupled with alterations in gastrointestinal cell tight junction proteins, in turn associated with increased gut leakiness. Increased gut permeability is an important factor in enhancing bi-directional flow of biological mediators, including microbes, microbial products, hormones, immune cells, etc., thus affecting peripheral and central BP control mechanisms.
In summary, several recent studies have provided the initial bases for the fundamental concept that the gut microbiota is involved in BP control and HTN. Of course, the story is just beginning and further research is needed to fully unravel the translational and clinical potential of these observations. Some of the questions that need to be explored include: (i) Microbiome comparisons among control, hypertensive, and resistant hypertensive patients with respect to sex, race, and anti-hypertensive therapy. (ii) Brain-gut causal relationship, i.e., is the autonomic dysregulation involved in gut pathology and dysbiosis? (iii) What is the primary site for perception of pro-hypertensive signals: the brain or the gut, or perhaps both contribute equally?
MICROBIAL-HOST INTERACTIONS IN A GENETIC MODEL OF HTN
The gut is the first organ exposed to any dietary components; thus, the role of gut microbiota in chronic diseases, including CVD and HTN, has been questioned. Based on previous evidence connecting dietary salt and BP regulation, microbial-host interactions were investigated in various models of HTN, including in the salt-sensitive rat model of HTN, i.e., the Dahl salt-sensitive (S) and salt-resistant (R) rats fed with high salt diet.8 The hypothesis was that the gut microbiota composition in the S rat would be different from that in R rats despite identical diets and housing conditions, and that changes in microbiota composition would result in BP modifications. The genome of R rats is resistant to alterations in BP, consistent with the finding that their BP was not altered by fecal microbiota transplant (FMT) from hypertensive S rats.8 On the other hand, FMT from R rats induced sustained and exacerbated high BP in S rats. These induced effects on BP were associated with post-transplantation changes in the gut microbial composition, providing the fundamental basis to explore further the host-microbiota interactions in BP regulation.
Genetics of the host as well as metagenomic (microbiota genome) factors from the microbiota may interact to confer susceptibility or resistance to the development of HTN. System biology approach for dissecting the host genomic factors as well as for dissecting the metagenomic factors through a meta-genomic, metatranscriptomic, metabolomics approaches will be required. While it is very useful to study the connection between the microbiome, genetic, and dietary conditions, analyses limited to the use of experimental animal models cannot explore all the sources for potential variations, specifically those related to inherited causal factors. Thus, population studies to track Mendelian inheritance of host-microbiota interactions in HTN will be necessary in the future.
GUT MICROBIAL METABOLITES, HOST SENSORY RECEPTORS, AND BP CONTROL
Short chain fatty acids (SCFAs) are a relatively well-studied class of gut microbial metabolites, primarily comprised of acetate, propionate, and butyrate. SCFAs and/or SCFA receptors have been implicated in two well-established areas of microbiota-host interactions: immune responses and metabolism.19–21 It is important to note that virtually all the SCFAs in the circulating bloodstream are microbial in origin, as evidenced by the nearly non-detectable levels of SCFAs in the plasma of germ-free mice.22 Therefore, changes in SCFAs would indicate an involvement of the gut microbiota.
A recent finding suggests that SCFAs play a role in BP regulation.23 This work largely relies on the analysis of phenotypes of two different SCFA receptor null mice: olfactory receptor 78 (Olfr78) and G-protein coupled receptor 41 (Gpr41). Olfr78 is localized in the renal afferent arteriole and in vascular smooth muscle cells of large vessels, whereas Gpr41 is localized in the vascular endothelium. Consistent with the localization of Olfr78 to the afferent arteriole (the site of renin storage and secretion), Olfr78 null mice have lowered plasma renin levels and lowered baseline BP.9 By contrast, Gpr41 null animals have isolated systolic HTN, consistent with an altered vascular tone in vivo which results in functionally “stiffer” vessels.24 In support of this SCFA-driven vasoactive response, studies have been reported that SCFAs can induce dilatation of resistance vessels ex vivo.23 Similarly, the use of acetate in hemodialysis buffers has been reported to cause hypotension25, consistent with a role of SCFAs in the dilatation of resistance vessels. Furthermore, population-based human studies found that interventions which alter SCFAs production correlate with changes in BP.12 Recent studies investigating a link between the gut microbiota and BP regulation have indicated potential roles of SCFAs in influencing phenotypic changes.8,11 Further investigations are needed to define the specific mechanisms and contribution of these new pathways among many other pathways which influence both the gut microbiota and BP.
GUT MICROBIOTA AND AGE-RELATED VASCULAR DYSFUNCTION
The risk of CVD of all causes increases progressively with advancing age. The primary event that drives aging-associated increases in CVD risk is related to arterial dysfunction: primarily large elastic artery (i.e., aorta and carotid artery) stiffening and vascular endothelial dysfunction.26,27 Substantial evidence indicates that the key underlying mechanisms mediating vascular aging include excessive superoxide-associated vascular oxidative stress and chronic low-grade vascular inflammation.26,27 Despite this understanding, the “upstream” events that drive vascular aging are largely unknown at present.
Remodeling of the gut microbiota has recently been linked to aging, including evidence of alterations in both diversity and abundance of specific taxa.28,29 Although the role of such changes in the gut microbiota with aging on vascular dysfunction and arterial BP has not been systematically investigated, both published and preliminary findings support this possibility. For example, increases in aortic stiffening and endothelial dysfunction with aging in C57Bl/6 mice are causally associated with increased vascular superoxide production. A recent preliminary study showed that short term (3–4 weeks) administration of broad spectrum antibiotics, a treatment that suppresses many gut bacterial species, normalized vascular superoxide production and ameliorated vascular dysfunction in old mice (Gioscia-Ryan and Seals, unpublished). Also, concentrations of plasma trimethylamine N-oxide (TMAO), a gut microbial-dependent metabolite, were increased 3-fold in healthy late-middle-aged and older adults (55+2 years) compared with young controls (22+1 year). In this regard, plasma TMAO concentrations are positively correlated with CVD in humans.30,31 Moreover, brachial artery flow-mediated dilation, a measure of vascular endothelial function, was inversely related to plasma TMAO (r=0.43), whereas systolic arterial BP was positively related to TMAO levels (r=0.47) (Gioscia-Ryan and Seals, unpublished). Together, these data support the concept that changes in the gut microbiota contribute to vascular dysfunction and increases in arterial BP with aging.
Several knowledge gaps need to be explored in this field. These include: (i) Does the gut microbiota influence arterial function and BP with primary aging (aging in the absence of clinical disease) and/or in age-related CVDs such as essential HTN? Additional cause-and-effect studies (e.g., gut microbiota transfer experiments) are needed. (ii) What specific changes to the microbiome (microbe composition and/or metabolite production) associated with aging or age-related diseases cause arterial dysfunction and effects on BP? (iii) What are the integrated physiological mechanisms connecting the gut microbiota to arterial dysfunction and elevated BP? For example, are inflammatory mediators released into the circulation via a “leaky gut”? What is the potential role of elevated circulating TMAO with aging? (iv) Do “environmental” influences (exercise, diet, sleep, stress, smoking, antibiotic use, etc.) modulate the gut microbiota to alter arterial function, BP, and health with aging? If so, how? (v) What is the potential efficacy of preventive and/or therapeutic strategies that target the gut microbiota to mitigate arterial dysfunction and elevated BP with aging?
DIET-INDUCED GUT MICROBIAL METABOLITES IN CVD AND HTN
The gut microbiota can directly influence downstream pathways leading to autonomic imbalance and immune responses. In turn, microbiota composition can also be influenced by the environment - especially nutrients from dietary sources. Indeed, one of the largest environmental exposures of the gut microbiota comes from food, and the gut microbiota produce various metabolites. Metabolites generated by certain microbes can exert both paracrine and endocrine effects on the host, leading to either favorable or unfavorable consequences. One such metabolite has recently been described as a microbial nitrogenous metabolite from dietary phosphatidylcholine or L-carnitine known as trimethylamine (TMA), which is absorbed and converted by hepatic enzymes (flavin-containing monooxygenase 3) in human to form TMAO and excreted by the kidneys. Studies have demonstrated that the gut microbiota play an obligatory role in producing TMA/TMAO, and its generation is a combined result of dietary exposure and microbial metabolism.32,33 Accumulation of circulating TMAO can lead to a wide range of adverse effects including macrophage/foam cell activation, steroid and bile acid metabolism, vascular and endothelial cell dysfunction, and platelet hyper-responsiveness, leading to atherosclerosis, cardio-renal impairment, and thrombosis.34 While TMAO does not affect BP in normotensive animals, it prolongs the hypertensive effect of angiotensin II, suggesting the possibility that TMAO increases the susceptibility to developing HTN.35 Thus, further investigations on the role of gut microbial-derived metabolites in the pathophysiology of HTN are warranted for the development of novel therapeutic approaches.
MICROBIOTA-DERIVED TOXINS AND CHRONIC KIDNEY DISEASE
HTN is an important risk factor for chronic kidney disease (CKD) and is the second leading cause of end-stage-renal disease (ESRD) in the US.36 Evidence has been accumulating in recent years as to alterations in the gut microbiota in patients with CKD and ESRD.37,38 Patients with CKD have an increase in aerobic and anaerobic bacteria in the upper intestinal tract. There are significant differences in abundance of 190 microbial operational taxonomic units between ESRD and normal control individuals.38 The most notable changes are reductions in both the Lactobacillaceae and Prevotellaceae families. The number of aerobic bacteria such as Enterobacteria and Enterococci was found to be approximately 100 times higher in patients on maintenance hemodialysis than in controls.39 Among anaerobic bacteria, hemodialysis patients had lower counts for Bifidobacteria and higher counts for Clostridium perfringens. Multiple factors contribute to the pathogenesis of dysbiosis in uremia, including secretion of urea into the gut, decreased consumption of dietary fiber, frequent use of antibiotics, slow colonic transit, metabolic acidosis, intestinal wall edema, and possibly oral iron intake. Impaired protein assimilation in uremia leads to a large influx of undigested proteins into the distal intestine, which favors the proliferation of proteolytic bacteria. Increased protein fermentation by proteolytic bacteria then results in generation of potentially toxic metabolites, such as ammonia, phenols, amines, indoles, and thiols.40 Uremia also increases intestinal permeability41, allowing translocation of intact bacteria, bacterial fragments, endotoxin and uremic toxins into the systemic circulation. Interestingly, TMAO concentrations are also found high in patients with CKD.42 In animal models, TMAO directly contributed to progressive renal fibrosis and dysfunction.42 Taken together, it is possible to hypothesize that the gut microbiota and the products generated by them, coupled with predicted leaky gut, could have major implications in the development and establishment of CKD and perhaps high BP as well.
To move this field forward, new studies will need to respond to several key questions: (i) Is alteration in the microbiome progressive in CKD? (ii) If so, is there a unique bacterial signature in CKD? (iii) Does the metabolic potential of the gut microbiota change in CKD? Novel therapeutic strategies (i.e. prebiotics, probiotics, or symbiotics) will need to be explored for CKD and ESRD and to examine any associated effects on BP. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) is conducting two studies, the “Hemodialysis Novel Therapies Consortium” (NCT02572882) and the CKD Pilot Studies, to define variability in microbiome profile and link these with metabolite profiles.
ORAL MICROBIOME AND HTN
Remarkable progress has been made to advance our understanding of the complex bacterial communities in human oral cavities, their classification, and implications in human health and diseases.43–45 The oral microbiome is comprised of nearly 700 species, and about 67% of oral bacterial species have been cultivated (Human Oral Microbiome Database, www.homd.org). Recent advances in metagenomic and metabolomic technologies, coupled with successful cultivation of oral bacterial species, have all contributed to an explosion of oral microbiome research and understanding. For example, using sequencing data combined with spectral fluorescence imaging, Mark-Welch et al.44 have recently demonstrated a direct visualization of the highly organized microbial consortium in human dental plaque at the micron scale. Since the oral cavity is the first to experience environmental, nutritional, and other external influences, a delicate balance in its microbial communities can have a major impact on human health and diseases. The significance of oral microbiome is continuously emerging, implicating oral dysbiosis in many periodontal diseases and other chronic diseases, such as CVD and HTN.46,47 For example, a direct relationship between the levels of subgingival periodontal bacteria and high BP has recently been reported.47
The WG discussed the role of oral microbiota in nitric oxide (NO) production and its implication in HTN. It is known that NO can be modulated by microbial communities in the oral cavity to effectively reduce dietary nitrates to nitrite and NO, independent of its enzymatic synthesis from L-arginine.48 Dietary nitrate from vegetable matter or from the oxidation of endogenous NO production is absorbed from the upper digestive tract into the bloodstream and concentrated in the salivary glands by active transport. About 20% of salivary nitrate is reduced to nitrite in the mouth by facultative anaerobic bacteria which are found on the surface of the tongue.49 The circulation of nitrate back to the oral cavity for reduction to nitrite/NO is termed the entero-salivary nitrate-nitrite-NO pathway.48 This pathway in humans appears to serve as an alternative mechanism that can provide an endothelium-independent source of bioactive NO compensating for insufficient host NO production (e.g., with aging). This pathway is dependent upon commensal oral bacteria to perform the first step (two-electron reduction) since mammals lack a functional nitrate reductase.46 The presence of nitrate-reducing bacteria in the oral cavity, concentrated on the tongue, has been well documented.50,51
A recent meta-analysis has shown that increasing the dietary intake of nitrate-rich food products is effective in reducing BP.52 Therapeutically, this information can offer an effective strategy to promote NO production and treat HTN by modulating the specific oral nitrate and nitrite reducing bacterial communities. Understanding and harnessing this redundant compensatory pathway may prove to be a viable and cost-effective strategy. Furthermore, this pathway may explain the biochemical and physiological link between oral health and CVD through maintenance of NO production.
DIETS, PROBIOTICS, AND HTN
Foods and dietary constituents contribute to the gut microbial community structure (i.e., the types of micro-organisms) and functional activity (i.e., microbial gene expression). Given that components of host diet serve as energy and nutrient sources for microbial growth, substrates reaching the large intestine are a key contributor to the composition of the microbial community. Epidemiological studies indicate that high fiber diet is associated with reduced BP and CVD.13,53 Also, many studies have been reported regarding beneficial effects of certain probiotic bacterial strains that can decrease circulating levels of cholesterol and BP.14 A recent meta-analysis of randomized, controlled trials has also demonstrated beneficial effects of probiotics in reducing BP.12 However, some studies have failed to find any beneficial effect of probiotics on BP, heart rate, or cardiovascular risk markers.13,14,53 Thus, further studies are needed to resolve this conundrum.
Animal studies have demonstrated the role of diet and gut microbiota in BP control and HTN. Treatment with probiotics Lactobacillus strains exerted antihypertensive effects in spontaneously hypertensive rats and improved endothelial function. This effect was associated with changes in the gut microbiota.54 Another study showed that dietary intake of fiber and supplementation with acetate could modulate renal and cardiac pathways to produce cardiovascular beneficial effects and lower BP in mice.16 Again, this treatment was associated with changes in the gut microbiota: i.e., decreased ratio of Firmicutes and Bacteroidetes to induce eubiosis, induce acetate-producing bacteria, and attenuate adverse actions of mineralocorticoid excesses on BP. The emerging story is that the diets with beneficial cardiovascular effects likely influence gut microbial communities and influence microbial metabolites with profound effects on the cardiovascular system and BP. However, answers to many more questions will be needed to support this concept. Some include: (i) Are there unique microbial communities enhanced by high fiber diet? (ii) If so, would they be beneficial as anti-hypertensive probiotics? (iii) Are there ethnic- and sex-linked differences in HTN prevalence associated with variability in diet-induced microbiome responsiveness? (iv) Are there specific dietary probiotic formulations that could be used for BP regulation, either across the board or in a patient-tailored manner?
Metabolomic profiling of patients on different dietary conditions and associations with ethnic, gender, and microbiome composition needs to be carried out. Similarly, interactions between salt and diet on gut pathophysiology and microbiome must be evaluated.
CONCLUSIONS, RECOMMENDATIONS, AND FUTURE DIRECTIONS
The WG concluded that significant evidence exists to implicate the role of microbiota in BP regulation and agreed that this is a rapidly evolving field with tremendous potential for clinical implications and translation into therapeutic interventions for HTN. The WG recognized several scientific questions, areas, challenges, and opportunities for further investigation.
-
Proof of Concept” investigations need to continue:
Use of multiple animal models and development of novel animal models
Metagenomics, metatranscriptomics, metabolomics
Identification, cultivation, and genomic and functional characterization of vascular-modifying microbial strains
Host genome-microbiome crosstalk
Role of viruses, archaea, and fungi
Involvement of oral microbiome and its implication in treatment of HTN
Brain-gut axis in HTN, mechanisms, gut pathophysiology, implications in development of HTN
Kidney-gut axis in HTN, mechanisms, gut pathophysiology, implications in development of HTN
Nutritional factors and impact on microbiota-linked BP regulation
-
Preclinical Investigations:
Large-scale metagenomic studies: sex, race, drug sensitivity
Is there a unique microbial signature linked to sex, race, drug sensitivity, etc.?
Metabolomics to identify HTN and normal microbiota-derived metabolite profiles
The therapeutic potential of fecal and oral transplant for control of HTN
Investigation of pro- and pre-biotics, alone or in combination with anti-inflammatory/antimicrobial drugs and anti-hypertensive drugs for resistant HTN
-
Translational studies in humans:
To confirm observations from preclinical investigations regarding the mechanistic role of microbiome in the etiology of increases in BP/clinical HTN and other changes in CV health with aging
To test and establish the efficacy of novel lifestyle and pharmacological interventions targeting microbiome for the prevention and treatment of clinical HTN and other CV disorders
The WG also identified the following needs to move the research field forward:
Standardized technology to measure comprehensive metabolites in blood, saliva, and stool of animals and of patients with HTN
Development of an integrated system to measure BP, hydrogen-, hydrogen sulfide (H2S)- and methane-specific electrode systems to measure gut microbiota activity and diversity in vivo
National/International forum for “Microbiota in BP regulation”
Standard protocols to measure gut blood flow
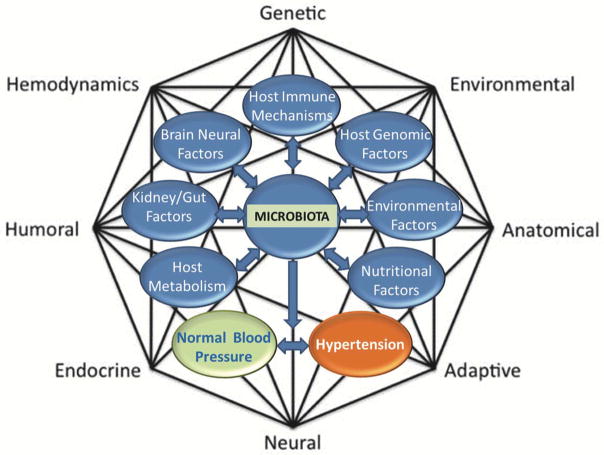
FIGURE 1.
The Revised Mosaic Theory of Hypertension showing potential influences of microbiota in BP control and hypertension.
PERSPECTIVES.
Dr. Irvin Page proposed the Mosaic Theory of HTN over 60 years ago, suggesting that a complex interplay of multiple factors, including genetics, environmental, anatomical, adaptive, neural, endocrine, humoral and hemodynamics, all of which were referred to as “forces” interdigitated to increase BP.55 This theory has been iconic in providing a framework for elucidation of various cellular, molecular, and genetic dysregulated mechanisms that occur at the level of organs assessed for HTN. These local events coordinate actions of multiple organs, including the brain, the vasculature and the kidney, to affect BP. By sheer coincidence the WG met on the day marking the anniversary of Dr. Page’s death (June 10, 1991), whereby, the opportunity arose for the panel to reflect on this theory in the context of the recent discovery of links between microbiota and etiology of HTN. The WG noted that given the compelling evidence thus far linking microbiota to HTN, it may be important to re-visit the Page model to determine if and how microbiota influences each of the “forces” that impact BP. Thus, we propose including microbiota along the previous guiding principles in the Page Theory (Figure 1).
Acknowledgments
The authors wish to thank NIH staff (Drs. M. Charette, D. Goff, P.L. Kimmel, R.D. Lunsford, C. Maric-Bilkan, G.A. Mensah, P. Srinivas, and E. Tolunay); A.T. Gewirtz (Georgia State University); K. Jamerson (University of Michigan); and J.R. Kirby (Medical College of Wisconsin), for their participation and discussion in this working group.
SOURCE OF FUNDING
The proceedings of “The Role of Microbiota in Blood Pressure Regulation” Working Group were supported through funds provided by the National Heart, Lung, and Blood Institute.
Footnotes
DISCLOSURES
The views expressed in this article are those of the authors and do not necessarily represent those of the National Institutes of Health or the United States Department of Health and Human Services. The authors have no financial disclosures that would be a potential conflict of interest with the current manuscript.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forouzanfar MH, Liu P, Roth GA, et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317:165–182. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 3.Group SR, Wright JT, Jr, Williamson JD, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahfoud F, Bakris G, Bhatt DL, Esler M, Ewen S, Fahy M, Kandzari D, Kario K, Mancia G, Weber M, Bohm M. Reduced blood pressure-lowering effect of catheter-based renal denervation in patients with isolated systolic hypertension: data from SYMPLICITY HTN-3 and the Global SYMPLICITY Registry. Eur Heart J. 2017;38:93–100. doi: 10.1093/eurheartj/ehw325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosa J, Widimsky P, Waldauf P, et al. Renal denervation in comparison with intensified pharmacotherapy in true resistant hypertension: 2-year outcomes of randomized PRAGUE-15 study. J Hypertens. 2017;35:1093–1099. doi: 10.1097/HJH.0000000000001257. [DOI] [PubMed] [Google Scholar]
- 6.Aron-Wisnewsky J, Clement K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol. 2016;12:169–181. doi: 10.1038/nrneph.2015.191. [DOI] [PubMed] [Google Scholar]
- 7.Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. 2013;11:639–647. doi: 10.1038/nrmicro3089. [DOI] [PubMed] [Google Scholar]
- 8.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47:187–197. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santisteban MM, Kim S, Pepine CJ, Raizada MK. Brain-Gut-Bone Marrow Axis: Implications for Hypertension and Related Therapeutics. Circ Res. 2016;118:1327–1336. doi: 10.1161/CIRCRESAHA.116.307709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong MH, Qi YF, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut Dysbiosis Is Linked to Hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 13.Borghi C, Cicero AF. Nutraceuticals with a clinically detectable blood pressure-lowering effect: a review of available randomized clinical trials and their meta-analyses. Br J Clin Pharmacol. 2017;83:163–171. doi: 10.1111/bcp.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ettinger G, MacDonald K, Reid G, Burton JP. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes. 2014;5:719–728. doi: 10.4161/19490976.2014.983775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM, Jr, Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49:96–104. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, Tan JK, Kuruppu S, Rajapakse NW, El-Osta A, Mackay CR, Kaye DM. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation. 2017;135:964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 17.Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, Hollister EB, Bryan RM., Jr Role of the Gut Microbiome in Obstructive Sleep Apnea-Induced Hypertension. Hypertension. 2016;67:469–474. doi: 10.1161/HYPERTENSIONAHA.115.06672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ, Raizada MK. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ Res. 2017;120:312–323. doi: 10.1161/CIRCRESAHA.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pluznick JL. Microbial Short-Chain Fatty Acids and Blood Pressure Regulation. Curr Hypertens Rep. 2017;19:25. doi: 10.1007/s11906-017-0722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiological Genomics. 2016;48:826–834. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagel MD, Ahmad S, Vizzo JE, Scribner BH. Acetate and bicarbonate fluctuations and acetate intolerance during dialysis. Kidney Int. 1982;21:513–518. doi: 10.1038/ki.1982.54. [DOI] [PubMed] [Google Scholar]
- 26.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 27.Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol. 2016;594:2001–2024. doi: 10.1113/jphysiol.2014.282665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Claesson MJ, Cusack S, O’Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015;350:1214–1215. doi: 10.1126/science.aac8469. [DOI] [PubMed] [Google Scholar]
- 30.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Roberts AB, Buffa JA, et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang WH, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circ Res. 2017;120:1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ufnal M, Jazwiec R, Dadlez M, Drapala A, Sikora M, Skrzypecki J. Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can J Cardiol. 2014;30:1700–1705. doi: 10.1016/j.cjca.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 36.CDC. [Accessed April 12, 2017];National Chronic Kidney Disease Facts. https://www.cdc.gov/diabetes/pubs/pdf/kidney_factsheet.pdf [Internet]
- 37.Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25:657–670. doi: 10.1681/ASN.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 39.Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron. 1996;74:349–355. doi: 10.1159/000189334. [DOI] [PubMed] [Google Scholar]
- 40.Wing MR, Patel SS, Ramezani A, Raj DS. Gut microbiome in chronic kidney disease. Exp Physiol. 2016;101:471–477. doi: 10.1113/EP085283. [DOI] [PubMed] [Google Scholar]
- 41.Magnusson M, Magnusson KE, Sundqvist T, Denneberg T. Impaired intestinal barrier function measured by differently sized polyethylene glycols in patients with chronic renal failure. Gut. 1991;32:754–759. doi: 10.1136/gut.32.7.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mark Welch JL, Rossetti BJ, Rieken CW, Dewhirst FE, Borisy GG. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci U S A. 2016;113:E791–800. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker JL, Bor B, Agnello M, Shi W, He X. Ecology of the Oral Microbiome: Beyond Bacteria. Trends Microbiol. 2017;25:362–374. doi: 10.1016/j.tim.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bryan NS, Tribble G, Angelov N. Oral Microbiome and Nitric Oxide: the Missing Link in the Management of Blood Pressure. Curr Hypertens Rep. 2017;19:33. doi: 10.1007/s11906-017-0725-2. [DOI] [PubMed] [Google Scholar]
- 47.Desvarieux M, Demmer RT, Jacobs DR, Jr, Rundek T, Boden-Albala B, Sacco RL, Papapanou PN. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST) J Hypertens. 2010;28:1413–1421. doi: 10.1097/HJH.0b013e328338cd36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 49.Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Nitrate, bacteria and human health. Nat Rev Microbiol. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 50.Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci. 2005;113:14–19. doi: 10.1111/j.1600-0722.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 51.Hyde ER, Andrade F, Vaksman Z, Parthasarathy K, Jiang H, Parthasarathy DK, Torregrossa AC, Tribble G, Kaplan HB, Petrosino JF, Bryan NS. Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PLoS One. 2014;9:e88645. doi: 10.1371/journal.pone.0088645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siervo M, Lara J, Ogbonmwan I, Mathers JC. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and meta-analysis. J Nutr. 2013;143:818–826. doi: 10.3945/jn.112.170233. [DOI] [PubMed] [Google Scholar]
- 53.Kim Y, Je Y. Dietary fibre intake and mortality from cardiovascular disease and all cancers: A meta-analysis of prospective cohort studies. Arch Cardiovasc Dis. 2016;109:39–54. doi: 10.1016/j.acvd.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Gomez-Guzman M, Toral M, Romero M, Jimenez R, Galindo P, Sanchez M, Zarzuelo MJ, Olivares M, Galvez J, Duarte J. Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol Nutr Food Res. 2015;59:2326–2336. doi: 10.1002/mnfr.201500290. [DOI] [PubMed] [Google Scholar]
- 55.Page IH. The mosaic theory 32 years later. Hypertension. 1982;4:177. doi: 10.1161/01.hyp.4.2.177. [DOI] [PubMed] [Google Scholar]