Abstract
Lusutrombopag, a small molecule thrombopoietin receptor agonist, has been approved for the treatment of chronic liver disease-associated thrombocytopenia due to hypersplenism in patients scheduled to undergo elective invasive procedures in Japan. We performed partial splenic embolization (PSE) after administration of lusutrombopag in two patients with thrombocytopenia due to cirrhosis. Case 1 involved a 50-year-old man who developed cirrhosis due to hepatitis B virus (HBV) infection and alcohol consumption. Case 2 involved a 30-year-old woman who developed cirrhosis due to HBV infection only. Lusutrombopag administration led to an increase in platelet count in both patients, and PSE was performed safely. However, in Case 2, the patient developed disseminated intravascular coagulation. Further study with a larger population is required to investigate the indications for and risks of the use of lusutrombopag.
Keywords: liver cirrhosis, thrombocytopenia, lusutrombopag, partial splenic embolization, disseminated intravascular coagulation
Introduction
Partial splenic embolization (PSE) is performed in patients with hypersplenism and symptoms caused by liver cirrhosis (e.g. pancytopenia, ascites and varices)1). Platelet transfusions are often required prior to PSE, as hypersplenism causes thrombocytopenia in patients with chronic liver disease (CLD).
The development of thrombocytopenia can be attributed to multiple factors2), one of which is reduced thrombopoietin (TPO) production or activity3). TPO is a hormone that regulates megakaryocyte and platelet production. It is synthesized in the liver; thus, TPO production or activity can reduce the development of thrombocytopenia.
In September 2015, the first approval of lusutrombopag (Mulupleta®, Shionogi, Osaka, Japan), a small molecule TPO receptor agonist, was obtained in Japan for the treatment of CLD-associated thrombocytopenia (<50,000/µL) in patients scheduled to undergo elective invasive procedures3,4).
We performed PSE with a preoperative administration of lusutrombopag for seven days in two patients who had thrombocytopenia due to liver cirrhosis. To the best of our knowledge, this report is the first to describe cases in which PSE was performed after treatment with lusutrombopag. We herein discuss the effects of lusutrombopag, including increased platelet counts, as well as adverse events associated with lusutrombopag.
Case Report
Case 1
In January 2015, a 50-year-old man, who had been diagnosed with liver cirrhosis (Child-Pugh score 6, Class A) due to hepatitis B virus (HBV) infection and alcohol (more than 80 g of ethanol daily) consumption in 2013, was found to have four esophageal varices (Ls, F3, Cb, and RC2) and two gastric varices (Lg-c, F1, Cb, and RC0) by esophagogastroduodenoscopy (EGD). He was admitted to our hospital in March 2015, and endoscopic injection sclerotherapy (EIS) was performed in April. However, in February 2016, EGD revealed recurrence of varices, and EIS was performed again the following month. The patient was scheduled to undergo PSE to prevent further recurrence of varices due to portal hypertension, as well as to improve thrombocytopenia (20,000/µL), and was started on 3 mg/day lusutrombopag for seven days, on day −11. He was readmitted to our hospital on day −1.
On admission, laboratory findings (Table, A) showed an increase in platelet count (40,000/µL). PSE was performed on day 0 (Fig. 1) according to the Takatsuka method using microcoils (C-stopper®; Piolax Medical Devices, Yokohama, Japan)5,6), as follows:(1) selective splenic arteriography was performed;(2) a micro catheter (Masters Parkway®, Asahi Intecc, Nagoya, Japan) was inserted into a branch of splenic artery, and microcoils were placed; and (3) gelatin sponges (Spongel®, Astellas, Tokyo, Japan) soaked with antibiotics (minocycline hydrochloride) were implanted proximal to the microcoils (Fig. 2 A, B). After PSE, we applied pressure manually to the right inguinal region for 15 minutes. The patient was kept in bed until the morning of day +1, and no bleeding was observed. We administered 125 mg/day methylprednisolone and antibiotics (cefmetazole 2 g/day) for three days postoperatively to prevent fever and infection. On day +6, EGD was performed, revealing that the varices had disappeared. On day +7, the patient developed a fever (grade 1, Common Terminology Criteria for Adverse Events; CTCAE, version 4.0) and was administered antibiotics (cefmetazole 2 g/day);his fever then subsided slowly. On day +9, abdominal CT imaging showed an embolization rate of 34.3%, but no focal infections, such as splenic abscess, were observed (Fig. 2 C-E). On day +17, the patient’s fever had subsided completely, and he was discharged from our hospital.
Table.
Laboratory findings on admission in Case 1 (A) and Case 2 (B).
| (A) | (B) | ||
|---|---|---|---|
| Hematologic test | Hematologic test | ||
| White Blood Cells | 2,000/μL | White Blood Cells | 4,400/μL |
| Red Blood Cells | 393×104/μL | Red Blood Cells | 410×104/μL |
| Hemoglobin | 9.4 g/dl | Hemoglobin | 11.9 g/dL |
| Platelet Count | 40,000/μL | Platelet Count | 68,000/μL |
| Coagulation | Coagulation | ||
| PT | 47.50% | PT | 67.90% |
| APTT | 34.1 sec | APTT | 34.1 sec |
| Fibrinogen | 136 mg/dL | Fibrinogen | 231 mg/dL |
| FDP | 4.6 μg/mL | FDP | <2.5 μg/mL |
| D-dimer | 2.2 μg/mL | D-dimer | <0.5 μg/mL |
| TAT | 4.23 ng/mL | TAT | 4.57 ng/mL |
| PIC | 0.40 μg/mL | PIC | 0.30 μg/mL |
| AT | 54% | AT | 68% |
PT: prothrombin time activity, APTT: activated partial thromboplastin time, FDP: fibrinogen degradation product, TAT: thrombin-antithrombin complex, PIC: plasmin-α2 plasmin inhibitor complex, AT: antithrombin
Fig. 1.
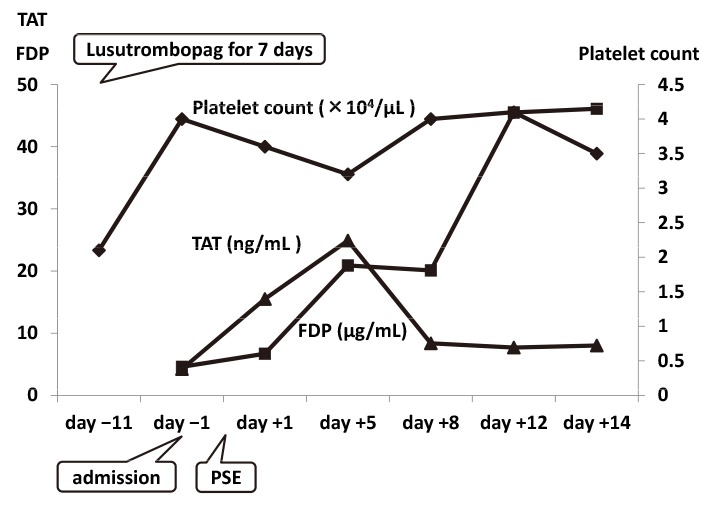
Clinical course in Case 1.
Lusutrombopag led to an increase in platelet count, which remained at 40,000/µL throughout the duration of hospitalization. After partial splenic embolization, an increase in fibrinogen degradation products was observed. However, a temporary increase in thrombin-antithrombin complex levels was noted.
Fig. 2.
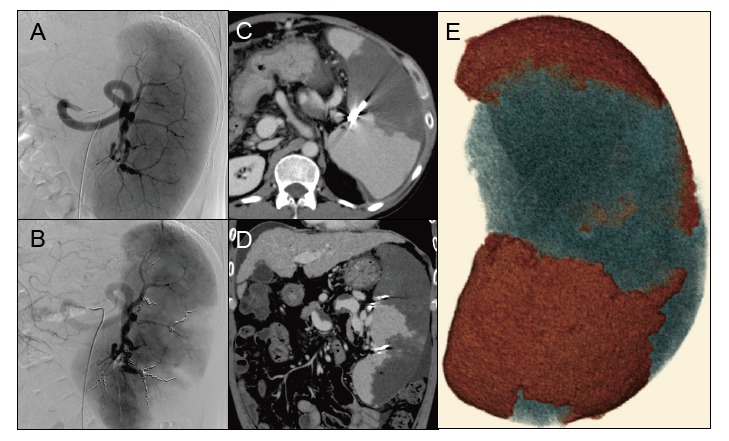
Partial splenic embolization (PSE) in Case 1.
A: Severe splenomegaly was detected on selective splenic arteriography.
B: Microcoils were placed (two metallic coils in the superior branch 2, one in the mid branch 1, three in the mid branch 2, and three in the inferior branch.
C, D: Computed tomography (CT) on day +9 showed less intense enhancement of the embolized area than the non-embolized area. Small amounts of ascites were observed around the liver and spleen.
E: Three-dimensional image of the spleen. The red and blue areas indicate non-embolized (1,150.0 cc) and embolized (600.32 cc) areas, respectively. Post-PSE CT imaging revealed the embolization rate to be 34.3%.
Case 2
In January 2016, a 30-year-old woman, who had been diagnosed with liver cirrhosis due to HBV infection and had undergone treatment with monoammonium glycyrrhizinate, glycine, aminoacetic acid, L-cysteine hydrochloride hydrate and entecavir, was found to have no tumor by abdominal CT; however, splenomegaly and portal hypertension were present. In April of the same year, EGD revealed esophageal varices (Lm, F2, Cw, and RC0), and the patient was referred to our hospital. On admission, endoscopic ultrasonography showed that her varices measured 2 mm in diameter. We decided to take a wait-and-see approach. However, she developed pancytopenia with a platelet count of 41,000/µL due to liver cirrhosis (Child-Pugh score 6, Class A);therefore, PSE was scheduled to improve her thrombocytopenia and a seven-day treatment with 3 mg/day lusutrombopag was initiated on day −12. She was admitted to our hospital on day −1.
On admission, laboratory findings (Table, B) revealed that platelet count increased to 68,000/µL. PSE was performed on day 0 (Fig. 3), according to the Takatsuka method, as described above (Fig. 4 A, B). After PSE, we applied pressure using the same method described in Case 1. The patient was kept in bed until the morning of day +1, and no bleeding was observed. On day +2, the patient developed severe thrombocytopenia (3,000/µL, grade 4, CTCAE v4.0) and coagulation parameters were abnormal (prothrombin time activity 38.5%, fibrinogen 36 mg/dl, fibrinogen degradation product 792 µg/ml, antithrombin 59%), indicating the presence of disseminated intravascular coagulation (DIC). She was treated with fibrinogen, antithrombin, recombinant thrombomodulin7), and platelet transfusions. However, due to subcutaneous bleeding on day +3 and menstrual bleeding on day +5, she developed anemia (grade 3, CTCAE v4.0) and received a transfusion of concentrated red cells. On day +7, thrombomodulin was changed to danaparoid sodium. On day +16 and thereafter, her platelet count remained high at 60,000-80,000/µL, and CT imaging showed an embolization rate of 23% (Fig. 4 C-E). Mild splenic abscess was suspected, although no fever was present. On day +24, the patient was discharged from our hospital. In October of the same year (four month after PSE), EGD revealed an improvement of the patient’s esophageal varices (Lm, F1, Cw, and RC0).
Fig. 3.
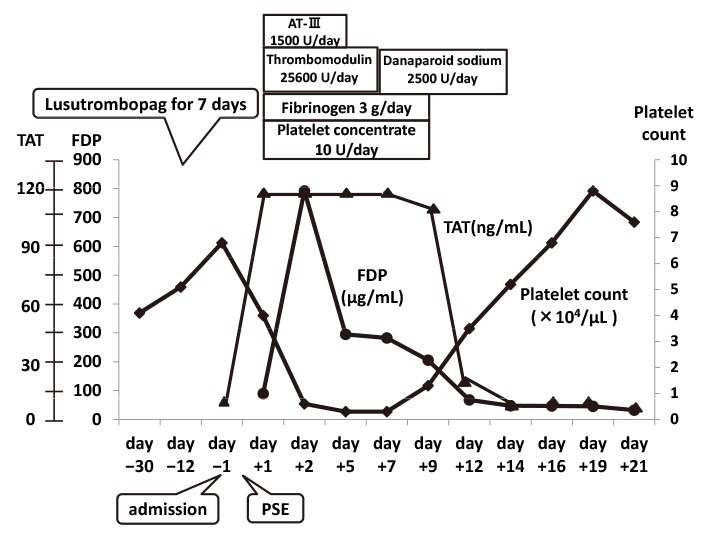
Clinical course in Case 2.
Lusutrombopag led to an increase in platelet count. The patient developed severe disseminated intravascular coagulation (DIC) post-partial splenic embolization (PSE), and severe thrombocytopenia and increases in fibrinogen degradation products and thrombin-antithrombin complex (TAT) levels were noted. TAT increased from 4.5 ng/ml to more than 120 ng/ml on day +1 and remained this high until day +7. DIC was resolved with medication and platelet transfusion. After cure of DIC, the platelet count became higher than the value obtained on admission.
Fig. 4.
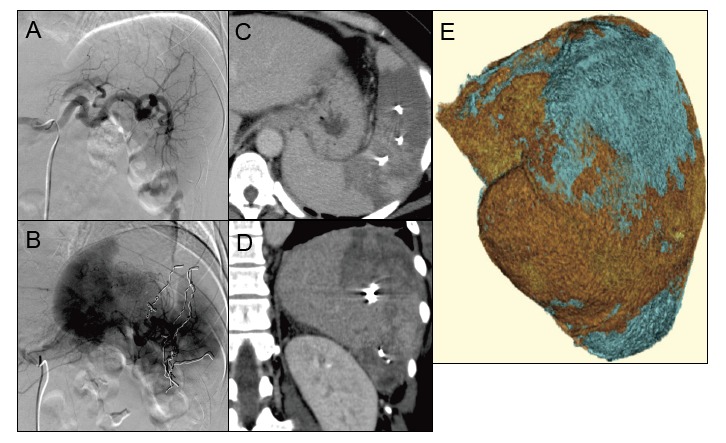
Partial splenic embolization (PSE) in Case 2
A: Before PSE
B: Microcoils were placed (two metallic coils in the superior branch 2, one in the mid branch 1, three in the mid branch 2, and three in the inferior branch).
C, D: Computed tomography (CT) on day +16 showed less intense enhancement of the embolized area than the non-embolized area. Mild splenic abscess was detected.
E: Three-dimensional image of the spleen. The red and blue areas indicate non-embolized (706.35 cc) and embolized (211.41 cc) areas, respectively. Post-PSE CT imaging revealed the embolization rate to be 23%.
Discussion
PSE, first reported by Spigos in 19798), is an effective procedure for pancytopenia and portal hypertension (esophageal and gastric varices) due to liver cirrhosis9,10). After PSE, the blood flow of the splenic vein decreases because of a decrease of the blood flow of the splenic artery; thus, portal hypertension is improved by this procedure. There have been recent reports of PSE performed for pancytopenia prior to hepatocellular carcinoma treatment and interferon therapy for hepatitis C virus infection11,12). However, platelet transfusions are often required prior to PSE, as patients with CLD often develop thrombocytopenia due to hypersplenism. Severe thrombocytopenia (<50,000/µL) with CLD can significantly increase the risk of bleeding. A retrospective analysis of 608 paracentesis or thoracentesis procedures reported that hemoglobin decreased in 8% of patients with severe thrombocytopenia, compared with decreases in only 3% of patients with platelet counts >50,000/µL13). In the hepatitis C antiviral long-term treatment against cirrhosis (HALT-C) trial, 75% of liver biopsy-related haemorrhages occurred in patients with platelet counts below 60,000/µL2,14). Studies from the United States, England, and Italy have reported that platelet transfusions are necessary before invasive treatment in patients with a platelet count of <50,000/µL15-17);however, it is often associated with complications such as alloimmunization, infections, and febrile reactions18).
Lusutrombopag is useful because it can reduce the frequency of platelet transfusion. Lusutrombopag acts selectively on human TPO receptors and activates signaling pathways that promote the proliferation and differentiation of bone marrow progenitor cells into megakaryocytes, consequently increasing the blood platelet count19,20). TPO has been reported to affect megakaryocyte colony-forming cells and megakaryocytic precursors, and enhances their ability to survive and grow21,22). Lusutrombopag can be administered in CLD patients undergoing bleeding procedures such as liver biopsy, trasnarterial chemoembolization, endoscopic submucosal dissection, or odontectomy. However, lusutrombopag cannot be administered to patients undergoing surgical operations, such as celiotomy, thoracotomy, or craniotomy23). Lusutrombopag needs to be administered for approximately eight to 13 days before the above-mentioned procedures. The effects of lusutrombopag last for approximately seven to 21 days, reducing the risk of bleeding. Shionogi & Co., Ltd. reported that platelet transfusion could be avoided in 79.2% of patients with CLD receiving lusutrombopag, when compared to a placebo (12.5%). On the other hand, in patients with portal and mesenteric vein thrombosis, care should be taken for rapid thrombocytosis when administrating lusutrombopag. In such patients, a blood test should be performed five days after the first administration, and treatment should be discontinued if the patient platelet count exceeds 50,000/µL, or increases by more than 20,000/µL.
In the present study, we performed PSE in two patients to improve thrombocytopenia and portal hypertension. Both patients had platelet counts lower than 50,000/µL; thus, lusutrombopag was started preoperatively. Their platelet counts remained lower than 50,000/µL for five days after starting lusutrombopag, so administration was continued for two more days. In Case 2, the patient showed an increase in platelet count from 41,000/µL on admission to 68,000/µL. In Case 1, his platelet count on admission was 40,000/µL, which was significantly higher compared to the value obtained before lusutrombopag administration (20,000/µL). Neither of the patients had bleeding from the point of puncture after PSE.
In Case 2, the patient developed severe thrombocytopenia (3,000/µL) post-PSE, and received a diagnosis of DIC. She was treated in accordance with DIC guidelines24), with administration of fibrinogen, antithrombin, recombinant thrombomodulin7), and platelet transfusions. She subsequently recovered. It is likely that regional DIC occurred in Case 2 because there was no general bleeding5). Fibrinolytic-inhibited-DIC, in which fibrinolytic activation is mild while coagulation activation is severe, is characterized by highly-increased thrombin-antithrombin complex levels, as well as mildly increased plasmin-α2 plasmin inhibitor complex levels and fibrinogen degradation products25). In Case 2, laboratory findings were similar to those of fibrinolytic-inhibited-DIC, so we believe that post-PSE pathological condition is similar to that of fibrinolytic-inhibited-DIC.
Lusutrombopag led to an increase in platelet count in both cases, but also promoted the risk of thrombosis. Thrombosis has been reported to occur in 2% of patients taking lusutrombopag. With other TPO receptor agonists, eltrombopag, romiplostim, and avatrombopag, thrombosis has been reported to occur in 4%, 2.4% and 20% of patients, respectively26-28). In the current report, the patient in Case 1 had no complications after PSE, but the patient in Case 2 developed DIC, which was more severe than is normally observed when no lusutrombopag is administered. There have been no reported cases of DIC that developed after administration of TPO receptor agonists, including not only lusutrombopag but also elrombopag and romiplostim, so we considered that DIC occurred due to PSE in Case 2. However, we could not completely exclude the involvement of lusutrombopag in aggravation of DIC as it promotes the secondary coagulation disorder. We administered lusutrombopag because the benefit of not needing a platelet infusion outweighed the risk of thrombosis. However, careful consideration must be given regarding the dose adjustment of lusutrombopag.
This is the first report describing the safe and efficient use of lusutrombopag in two patients with thrombocytopenia due to liver cirrhosis prior to PSE. Lusutrombopag is effective for increasing the platelet count of CLD patients undergoing bleeding procedures except for surgical operations. In fact, the platelet count of our cases increased, and no bleeding was observed until the morning of day +1. However, further accumulation of cases is required to investigate the indications for and risks of the use of lusutrombopag.
The authors state that they have no conflicts of interest (COI).
References
- 1.Yoshida H, Mamada Y, Taniai N, Tajiri T. Partial splenic embolization. Hepatology Res, 38: 225-233, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Bissonnette J, Valla D, Rautou PE. Managing periprocedural thrombocytopenia in cirrhosis: Aiming for a safety window. J Hepatol, 61: 1199-1201, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Kim ES. Lusutrombopag: First Global Approval. Drugs, 76: 155-158, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Katsube T, Ishibashi T, Kano T, Wajima T. Population pharmacokinetic and pharmacodynamic modeling of lusutrombopag, a newly developed oral thrombopoietin receptor agonist, in healthy subjects. Clin Phamacokinet, 55: 1423-1433, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu H, Takatsuka K, Yoshida A, Yoshimatsu E, Matsui K, Iwabuchi S. Partial splenic embolization reverses insulin resistance in patients with liver cirrhosis. Intern Med, 48: 747-751, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu H, Takatsuka K, Nakano H, et al. Long-term evaluation of partial splenic embolization followed by interferon therapy in patients with hepatitis C virus (HCV) cirrhosis and thrombocytopenia. Intern Med, 53: 925-931, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Saito H, Maruyama I, Shimazaki S, et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost, 5: 31-41, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Spigos DG, Jonasson O, Mozes M, Capek V. Partial splenic embolization in the treatment of hypersplenism. AJR Am J Roentgenol, 132: 777-782, 1979. [DOI] [PubMed] [Google Scholar]
- 9.Sangro B, Bilbao I, Herrero I, et al. Partial splenic embolization for the treatment of hypersplenism in cirrhosis. Hepatology, 18: 309-314, 1993. [PubMed] [Google Scholar]
- 10.Noguchi H, Hirai K, Aoki Y, Sakata K, Tanikawa K. Changes in platelet kinetics after a partial splenic arterial embolization in cirrhotic patients with hypersplenism. Hepatology, 22: 1682-1688, 1995. [PubMed] [Google Scholar]
- 11.Hidaka H, Kokubu S, Nakazawa T, et al. Therapeutic benefits of partial splenic embolization for thrombocytopenia in hepatocellular carcinoma patients treated with radiofrequency ablation. Hepatol Res, 39: 772-778, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Miyake Y, Ando M, Kaji E, Toyokawa T, Nakatsu M, Hirohata M. Partial splenic embolization prior to combination therapy of interferon and ribavirin in chronic hepatitis C patients with thrombocytopenia. Hepatol Res, 38: 980-986, 2008. [DOI] [PubMed] [Google Scholar]
- 13.McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion, 31: 164-171, 1991. [DOI] [PubMed] [Google Scholar]
- 14.Seeff LB, Everson GT, Morgan TR, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol, 8: 887-883, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiffer CA, Anderson KC, Bennett CL, et al. Platelet transfusion for patients with cancer: Clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol, 19: 1519-1538, 2001. [DOI] [PubMed] [Google Scholar]
- 16.British Committee for Standards in Haematology. Guidelines for the use of platelet transfusions. Br J Haematol, 122: 10-23, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Liumbruno G, Bennardello F, Lattanzio A, Piccoli P, Rossetti G. Recommendations for the transfusion of plasma and platelets. Blood Transfus, 7: 132-150, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poordad F. Thrombocytopenia in chronic liver disease. Aliment Pharmacol Ther, 26: 5-11, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Nurden AT, Viallard JF, Nurden P. New-generation drugs that stimulate platelet production in chronic immune thrombocytopenic purpura. Lancet, 373: 1562-1569, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Sakamaki A, Watanabe T, Abe S, et al. Lusutrombopag increases hematocytes in a compensated liver cirrhosis patient. Clin J Gastroenterol, 10: 261-264, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuter DJ, Begley CG. Recombinant human thrombopoietin: basic biology and evaluation of clinical studies. Blood, 100: 3457-3469, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Kuter DJ. New thrombopoetic growth factors. Blood, 109: 4607-4616, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izumi N, Osaki Y, Yamamoto K, Imawari M. A phase 3, randomized, double-blind, placebo-controlled study of lustrombopag for thrombocytopenia in patients with chronic liver disease undergoing elective invasive procedures in Japan (L-PLUS 1). [abstract no. LB-30]. In the AASLD liver meeting; 2015. [Google Scholar]
- 24.Wada H, Thachil J, Di Nisho M. Guidance for diagnosis and treatment of disseminated intravascular coagulation from harmonization of the recommendations from three guidelines. J Thromb Haemost, 11: 761-767, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Asakura H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J Intensive Care, 2: 20, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afdhal N, Giannini E, Tayyab G, et al. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med, 367: 716-724, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Kuter DJ, Mufti GJ, Bain BJ, Hasserjian RP, Davis W, Rutstein M. Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood, 114: 3748-3756, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Tarrault NA, Hassanein T, Howell CD, et al. Phase II study of avatrombopag in thrombocytopenic patients with cirrhosis undergoing an elective procedure. J Hepatol, 61: 1253-1259, 2014. [DOI] [PubMed] [Google Scholar]


