Abstract
Carrion beetles, Nicrophorus vespilloides, are reared on decomposing carrion where larvae are exposed to high populations of carcass‐derived bacteria. Larvae do not become colonized with these bacteria but instead are colonized with the gut microbiome of their parents, suggesting that bacteria in the beetle microbiome outcompete the carcass‐derived species for larval colonization. Here, we test this hypothesis and quantify the fitness consequences of colonization with different bacterial symbionts. First, we show that beetles colonized by their endogenous microbiome produce heavier broods than those colonized with carcass‐bacteria. Next, we show that bacteria from the endogenous microbiome, including Providencia rettgeri and Morganella morganii, are better colonizers of the beetle gut and can outcompete nonendogenous species, including Serratia marcescens and Escherichia coli, during in vivo competition. Finally, we find that Providencia and Morganella provide beetles with colonization resistance against Serratia and thereby reduce Serratia‐induced larval mortality. This effect is eliminated in larvae first colonized by Serratia, suggesting that while competition within the larval gut is determined by priority effects, these effects are less important for Serratia‐induced mortality. Our work suggests that an unappreciated benefit of parental care in N. vespilloides is the social transmission of the microbiome from parents to offspring.
Keywords: bacterial symbionts, colonization resistance, microbiome, Nicrophorus vespilloides
1. INTRODUCTION
Animals are colonized by a diverse array of bacterial symbionts, the microbiome, that provide essential functions to their hosts (Flórez et al., 2015; McFall‐Ngai et al., 2013; Rajagopal, 2009). Animal microbiomes can alter nutrient uptake (Hacquard et al., 2015), development (Sommer & Bäckhed, 2013), parasite susceptibility (Schwarz, Moran, & Evans, 2016), and even behaviors like mate choice (Sharon et al., 2010). In addition, symbionts that reside within animal guts can provide their hosts with resistance to bacterial pathogens via a process called colonization resistance (Buffie & Pamer, 2013). For example, the gut bacterial community of locusts, Schistocerca gregaria, prevents invasion and disease from the insect pathogen Serratia marcescens, an outcome that depends in part on the diversity of the gut microbial community (Dillon et al., 2005). Similarly, honeybees became more susceptible to Serratia infection following treatment with antibiotics that altered the structure of their endogenous microbiota (Raymann, Shaffer, & Moran, 2017). These results support the idea that gut bacteria can provide protection against pathogens while also highlighting the importance and timing of symbiont transmission in juvenile animals (Macke et al., 2017). However, it is often unclear if colonization resistance results from specific inhibition of invading pathogens or whether it results from the simple fact that symbionts get there first (Dillon & Dillon, 2004; Dillon et al., 2005; Jarosz, 1979). In other words, is colonization resistance the result of specificity or priority?
To address this question, we focus on the role of the endogenous microbiota of the burying beetle, Nicrophorus vespilloides. This system is especially well suited to this work given the high exposure of larval beetles to environmental bacteria (Duarte, Welch, Swannack, Wagner, & Kilner, 2017; Hall et al., 2011; Rozen, Engelmoer, & Smiseth, 2008; Scott, 1998), together with extensive data on the composition and transmission of the beetle microbiome from parents to offspring (Duarte et al., 2017; Kaltenpoth & Steiger, 2014; Shukla, Vogel, Heckel, Vilcinskas, & Kaltenpoth, 2017; Vogel et al., 2017; Wang & Rozen, 2017). Nicrophorus vespilloides larvae are reared on small vertebrate carcasses where they feed directly from the carcass and are provided regurgitated food from parent beetles that care for developing broods (Eggert et al., 1998; Scott, 1998; Smiseth, Darwell, & Moore, 2003). Parental beetles dramatically increase larval growth and fitness during brood rearing by investing in pre‐ and posthatch care (Capodeanu‐Nägler et al., 2016; Eggert et al., 1998; Rozen et al., 2008; Trumbo, Sikes, & Philbrick, 2016). During prehatch care, parents remove the fur and guts of the carcass and coat its surface in oral and anal secretions that have antimicrobial activity (Arce et al., 2012; Cotter & Kilner, 2010; Hoback et al., 2004; Scott, 1998). Posthatch, parents defend their developing larvae from other insect species and also feed larvae with regurgitated food (Milne & Milne, 1976; Smiseth et al., 2003). In a recent study, we found that parents transmit their gut microbiome to their larvae by direct feeding. In addition, we found that the core members of this microbiota could even be transmitted to larvae indirectly, by bacteria deposited onto the carcass by parents (Wang & Rozen, 2017). This unexpected result suggested that these core bacterial species were outcompeting the numerous microbes living on and inside the carcass within the larval gut, thus giving rise to the stable endogenous Nicrophorus microbiome (Vogel et al., 2017; Wang & Rozen, 2017). However, the mechanisms of their increased competitiveness remained unclear as were the consequences of their colonization.
Here, we carry out invasion experiments into sterile larvae to directly quantify competitive interactions taking place between endogenous and nonendogenous microbes from the Nicrophorus gut. We first quantify bacterial growth rates within the larval gut and then directly determine the competitive interactions between species during mixed inoculations and in different orders. Finally, we quantify whether members of the core microbiome provide colonization resistance against S. marcescens, a known insect pathogen (El Sanousi, El Sarag, & Mohamed, 1987; Nehme et al., 2007; Renoz et al., 2015). Briefly, we show that native gut species significantly outcompete foreign species within the host gut, irrespective of infection order. In addition, we find that the endogenous microbiota increases beetle fitness, both in terms of brood size and in terms of pathogen resistance in larvae. Our results provide strong evidence that an important benefit of parental care in N. vespilloides is the social transmission of the microbiome from caring parents to their offspring.
2. METHODS AND MATERIALS
2.1. Beetle collection and rearing
Experimental beetles were taken from an outbred laboratory population derived from wild‐caught N. vespilloides individuals trapped near Leiden in the Netherlands, between May and June 2015. Beetles were maintained in the laboratory at 20°C with a 15:9‐hr light/dark cycle and fed fresh chicken liver twice a week. Mating pairs were established by placing a male and female in a small plastic container containing ~1 cm soil overnight. Mated females were provided with a fresh carcass (20–23 g) the following morning to initiate egg laying.
To examine the impact of different microbial communities on N. vespilloides fitness, we established independent treatment populations containing endogenous‐ or carcass‐derived gut bacteria, designated FC (full care) and NC (no care) beetles, respectively. Whereas parents and larvae in the FC treatment were reared in the presence of parental care and thus acquired their microbiota primarily from their parents, larvae in the NC group were reared in the absence of parental care (with an unprepared carcass that we opened using a sterile scalpel) and acquired their microbiota from the carcass and surrounding soil (Wang & Rozen, 2017). Ten‐day‐old adults that had eclosed from FC and NC broods were paired within treatments for mating (n = 15/treatment) and subsequently provided with a fresh mouse carcass (22–24 g) for breeding. To control for the possible effects of female size on reproductive output (Steiger, 2013), females from each treatment were size‐matched prior to establishing their own broods (mean ± SD: NC: 0.25 +/‐ 0.009, FC: 0.25 ± 0.026; t 25 = 0.759, p = .455). The fitness of both parental treatment groups (NC and FC) was determined by quantifying total brood size, total larval weight, and mean larval mass.
2.2. Experimental bacterial inoculation of N. vespilloides larvae
To generate germ‐free larvae, we collected eggs 15 hr after FC females were provided with a fresh carcass. These were surface sterilized twice for 15 min in an antimicrobial solution containing hen egg‐white lysozyme (1 mg/ml), streptomycin (500 μg/ml), and ampicillin (100 μg/ml) and followed by a sterile water wash. Next, treated eggs were transferred onto 1% water agar plates to hatch. Previous experiments have shown that eggs thus treated are free of bacteria (Jacobs et al., 2014). Zero‐ to 24‐hr‐old first‐instar larvae were transferred onto new sterile 1% water agar petri dishes (100 mm × 15 mm) in groups of a maximum of seven larvae. Larvae on each plate were derived from independent breeding pairs. Larvae were fed a sterile diet developed using pasteurized chicken liver prepared via a “Sous vide” cooking approach. Fresh chicken liver was sliced into 3‐g chunks using aseptic technique and transferred in individual pieces to a 1.5‐ml Eppendorf tube containing 100 μl sterile water. These were then placed in a water bath at 65°C for 8 min, followed by immediate cooling at −20°C. We determined the effectiveness of this method by plating liver samples before and after pasteurization onto both one‐third strength Tryptic Soy agar and LB agar. The initial CFU of unpasteurized liver was ~1e6/g CFU while following treatment the CFU was reduced to 0 (with a limit of detection of ~10 CFU/ml). Larvae were offered this sterile diet, alone or coated with different bacterial inocula, on new 1% water agar plates daily.
2.3. In vivo competition within larvae
To determine if “endogenous” bacteria can outcompete foreign strains during larval colonization, we competed bacterial strains against one another within the larval gut, focusing on four different bacterial species. The bacterial species Providencia rettgeri and Morganella morganii are abundant N. vespilloides gut symbionts throughout development and are considered “endogenous” species (Vogel et al., 2017; Wang & Rozen, 2017). By contrast, S. marcescens and Escherichia coli, which are found commonly in both soil and on decomposing carcasses, colonize larvae that are reared without parental care in NC broods (Wang & Rozen, 2017). Serratia marcescens is also a known insect pathogen in several insect species (El Sanousi et al., 1987; Nehme et al., 2007; Renoz et al., 2015), including N. vespilloides. P. rettgeri (P) and M. morganii (M) were isolated from N. vespilloides adults guts while S. marcescens (S) and E. coli (E) were isolated from decomposing mouse carcasses (Wang & Rozen, 2017).
Bacteria for inoculations were cultured overnight at 30°C in 1/3 TSB medium. Overnight cultures of each species were pelleted and washed two times in sterile phosphate‐buffered saline (PBS, pH = 7.2) and diluted to an optical density at 600 nm (OD600) of 0.2 measured using a BIO‐RAD SmartSpec™ Plus spectrophotometer. Ten microliters of this solution, containing ~106 cells total, was used to coat sterile liver prepared as above. Inoculations with two species contained the same total bacterial density, with each species present at a 1:1 ratio. Larvae were provided with inoculated diet for 6 hr on a sterile water agar plate, after which they were transferred to a new agar plate containing new sterile diet. Subsequent transfers to plates containing fresh sterile food took place every 24 hr for 7 days, or until larvae were destructively sampled. In experiments where larvae were sequentially challenged with different bacterial species, we treated larvae the same as above, but larvae were inoculated with target strains in series: the first as above and the second 6 hr later on a new plate containing diet coated with the second bacterial strain. As with the first exposure, larvae were exposed to bacteria in the second inoculum for 6 hr, after which they were returned to a sterile plate with sterile diet.
To examine competitive interactions within the Nicrophorus gut, larvae were inoculated either simultaneously or in series with two of the four species in the following pairings (Strain 1 vs Strain 2): P versus S; M versus S; P versus E; M versus E; P versus M; and S versus E. Within each treatment, larvae from independent families (n = 6) were inoculated as outlined above, and then six larvae were destructively sampled for plating 6 hr or 24 hr later. These values were taken as estimates of input and final densities, respectively. Competition indices (CI) were calculated using the following equation: CI = (Strain 1output/Strain 2output)/(Strain 1input/Strain 2input), where input and output values refer to initial and final densities of each competitor, respectively. CI was log transformed, so that a CI of 0 indicates equal competitiveness, while CI > 0 indicates that the strain 1 is a stronger in vivo competitor. A two‐tailed t test was used to test if CI values for each strain differed significantly from 0.
At each time point, larvae were sampled by sterilely dissecting individual larval guts with fine forceps and suspending these in 0.7 ml sterile PBS. Gut contents were serially diluted in PBS and plated to quantify CFU on a chromogenic medium (CHROMagar™ Orientation), which can distinguish our experimental strains based on both color and morphology (Figure 1). Mortality rates for each treatment were as follows: P versus S (38.1%); M versus S (47.6%) and P versus M (35%), P versus E (54.5%); M versus E (64.7%) and S versus E (71.4%).
Figure 1.
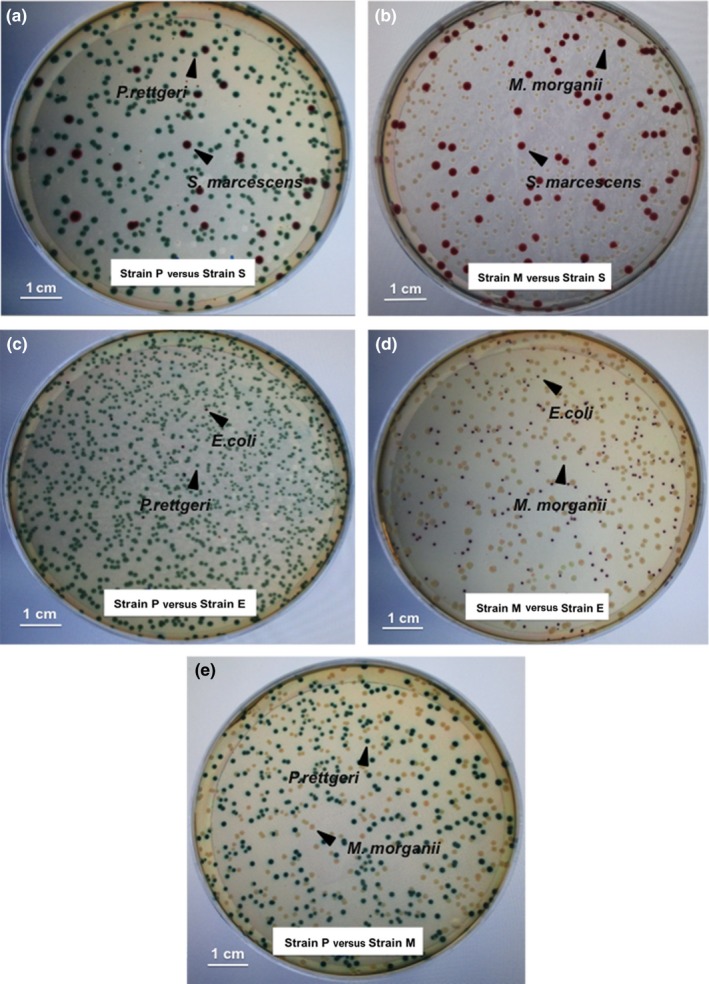
Color and morphology of experimental strains on chromogenic agar plates (CHROMagar™ Orientation) used for bacterial competition assays. Bacterial combinations shown are: P versus S (a); M versus S (b); P versus E (c); M versus E (d); and P and M (e)
2.4. Larval fitness with different bacterial colonizers
To determine the impact of different bacterial symbionts on larval survival, larvae were inoculated as above and then monitored for survival through time. Larvae exposed to sterile PBS (pH = 7.2) were used as a control in this experiment. A minimum of 40 larvae from 9 to 15 families were collected for each bacterial treatment. We monitored larval survival every 24 hr after inoculation. To reduce the high rates of mortality in larvae reared on liver, all the experimental larvae were transferred daily into a fresh petri dish containing fresh sterile diet.
2.5. Statistical analysis
Parental fitness and bacterial colonization data were analyzed using ANOVA. Larval survival data were analyzed by fitting a Cox proportional hazard model; this model was constructed by fitting a saturated model using treatment, block, and treatment*brood interactions as covariates. The Wald's test was used to compare mortality between treatments. All analyses were conducted using SPSS version 24 (IBM SPSS Inc., Chicago, IL, U.S.A.).
3. RESULTS
3.1. The effects of gut microbiota on parental fitness
To examine the role of the parental gut microbiome on beetle fitness, we reared larvae either with (FC) or without parental care (NC) and then mated the dispersed adults within treatments and allowed them to rear broods on fresh carcasses. Through this treatment, all parents were given the opportunity to rear offspring under identical conditions, and there were neither differences in carcass or maternal weight (both NS). Previous results have shown that parents from these different rearing conditions differ significantly in microbiome composition (Wang & Rozen, 2017), the FC individuals containing an endogenous symbiont population and the NC individuals a microbial population derived from the soil and the decomposing carcass. Our results show that gut microbiomes have a significant influence on parental fitness (Figure 2). FC parents produced significantly heavier broods than NC parents, irrespective of brood size (two‐tailed ANCOVA: F 1,27 = 6.09, p = .021).
Figure 2.
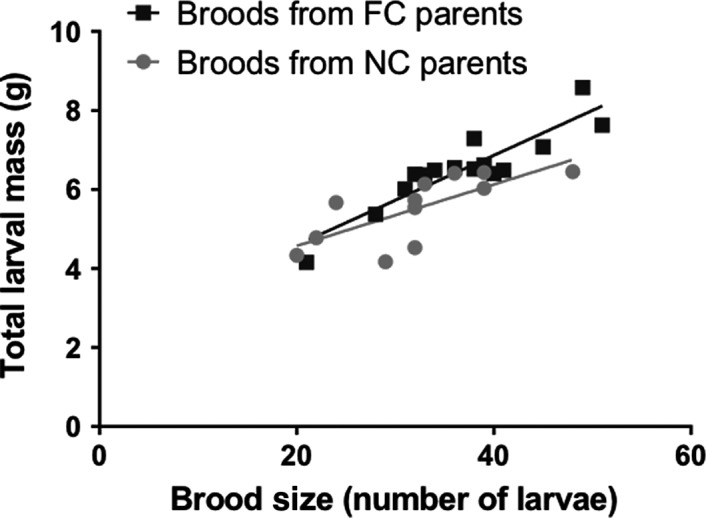
Total larval mass as a function of brood size for maternal beetles that were reared with either Full Care (FC) or No Care (NC)
3.2. Competitive interactions in vivo
To study competitive interactions between bacteria during larval colonization, we selected four focal species to examine in detail. Two species, Providencia rettgeri and Morganella morganii, are common endogenous colonizers of the beetle gut (Duarte et al., 2017; Vogel et al., 2017; Wang & Rozen, 2017), while the other two, S. marcescens and E. coli, are found more commonly in the guts of beetles reared without parental care (Wang & Rozen, 2017). We first inoculated beetles with each species alone to measure growth and colonization. Figure 3 shows that while all four species are able to colonize the larval gut, their ability to increase in density in vivo varies significantly between strains (two‐tailed ANOVA: F 3,12 = 43.13, p < .001).
Figure 3.
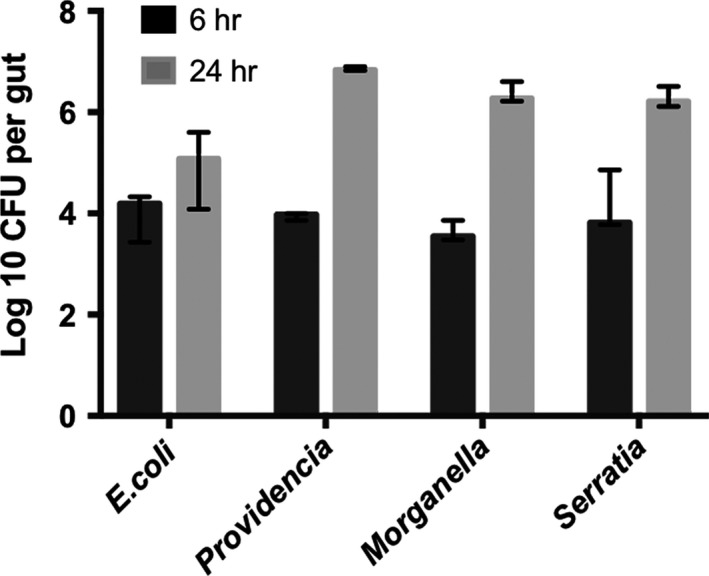
Growth and colonization of Nicrophorus vespilloides symbionts within the larval gut over 24 hr. Values correspond to the mean ±95% CI
Competitive interactions between species were next determined using in vivo pairwise assays where two species were simultaneously inoculated into 1‐day‐old larvae. Consistent with expectations based on mono‐associated larvae, we observed clear competitive differences between the strains. Providencia and Morganella significantly outcompeted both Serratia (P vs S: t 5 = 2.52, p = .053; M vs S: t 3 = 4.42, p = .022) and E. coli (P vs E: t 2 = 11.26, p < .001; M vs E: t 3 = 5.89, p = .01), although to different degrees. By contrast, there were no significant competitive differences between Providencia and Morganella (P vs M: t 4 = 2.16, p = .097) (Figure 4a).
Figure 4.
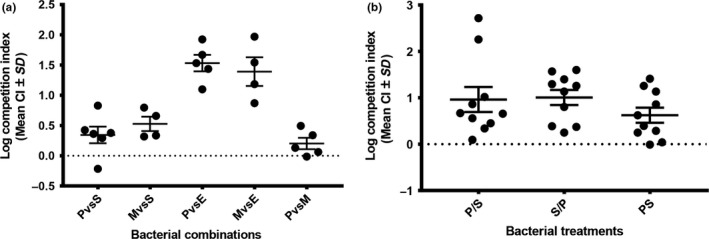
Competitive differences between different bacterial species in vivo within the larval gut. Competition indices (CI) are given in reference to the first species listed on the x‐axis for (a) and with respect to Providencia for (b). Strains were either inoculated simultaneously (a) or in series (b) in cases where strains are separated with a/(e.g., P/S: Providencia was inoculated first and then followed with Serratia, whereas in PS both strains were coinoculated). The dashed black line illustrates a CI of 0, which indicates equal competitiveness of two strains. Values >0 indicate that strain 1 is a stronger in vivo competitor
We next determined if competitive interactions between Providencia and Serratia were influenced by the order of inoculation. Specifically, we were interested in determining if the outcome of competition was reversed in larvae that were first inoculated with Serratia. Our results in Figure 4b clarify that order is not an important determinant of competitive fitness (two‐tailed ANOVA: F 2,29 = 0.59, p = .56). Providencia outcompetes Serratia in all cases to a similar degree regardless of the order of inoculation.
3.3. Larval survival with different bacterial colonizers
Our results show that the endogenous microbiome provides likely benefits to Nicrophorus by increasing total brood mass and also that key members of this microbiome can outcompete species that are predominantly found in larvae that do not receive parental care. To test if these competitive interactions translate into differences in larval fitness, we measured the survival of larvae inoculated with single or multiple strains, as above. Results in Figure 5a show that larval mortality varies significantly as a function of their bacterial colonists (χ2 = 11.364, df = 3, p < .01), with increased mortality in larvae inoculated with Serratia compared to either Morganella, Providencia, or a PBS control (Wald statistic = 6.274; 4.794; 9.202, respectively, all p < .05). This result is consistent with the known pathogenic effects of Serratia. By contrast, there were no significant differences in mortality between larvae inoculated with either Providencia or Morganella and the PBS control (χ2 = 0.156, df = 2, p = .925).
Figure 5.
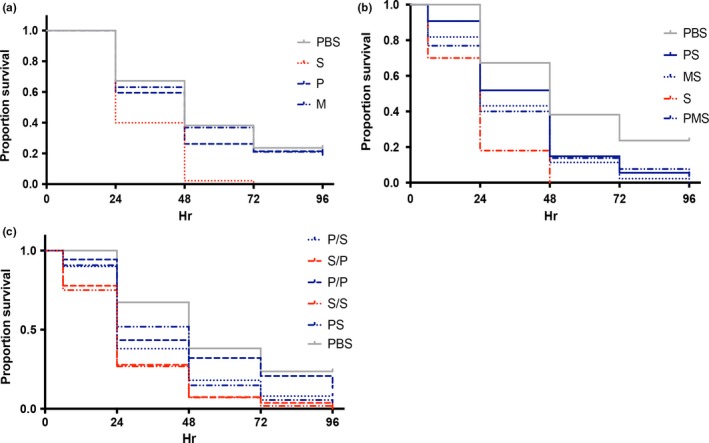
Larval survival when inoculated with different bacterial species. Larvae were inoculated with (a) single bacterial species in monoculture, (b) >1 species in coculture simultaneously, or (c) bacteria either simultaneously or in series. Bacteria inoculated simultaneously are designated with the first letter of the species name (e.g., PS = Providencia with Serratia), while species inoculated in series are given in the same way with a slash (e.g., P/S = Providencia followed by Serratia). A PBS (phosphate‐buffered saline) control was set up for all the experiments
We also observed significant differences in survival when larvae were simultaneously inoculated with Serratia and either of the endogenous species compared to survival when Serratia is inoculated alone (χ2 = 38.767, df = 4, p < .001). Most importantly, we found that coinoculating Serratia with Providencia and/or Morganella significantly increased larval survival, suggesting that these species provide protection via colonization resistance for larvae (Wald statistic of PS; MS; PMS = 8.188; 3.697; 5.102, respectively, all p < .05, Figure 5b). However, this benefit of colonization resistance disappeared when Serratia was able to become established prior to inoculation with Providencia; there were no survival differences between larvae inoculated with Serratia twice in series and larvae first inoculated with Serratia and then followed by Providencia (Wald statistic of S/P = 0.077, p = .782, Figure 5c). In light of results in Figure 4b showing that order of inoculation does not affect Providencia competitiveness, these survival results indicate that Serratia‐induced larval mortality is insensitive to bacterial competitiveness, thus supporting the idea that initial establishment of the endogenous microbiota is crucial for colonization resistance.
4. DISCUSSION
Nicrophorus larvae are exposed to a highly diverse microbiota in their breeding environment, first from the soil where they hatch and next from the microbes proliferating on and within their carrion resource. In the absence of parental care, larvae become colonized with these bacteria (Wang & Rozen, 2017) which reduces their weight and survival (Rozen et al., 2008) and also leads to reduced brood mass when these larvae reproduce as parents (Figure 2). However, when larvae are reared with parental care, their gut microbiome resembles that of their parent, even if parental care is limited to carcass preparation prior to larval hatch (Wang & Rozen, 2017). These results suggested that the bacteria within the parental gut are better competitors for the larval gut, but our earlier work neither tested the colonization potential and competitiveness of the constituent species nor determined the consequences of colonization with the Nicrophorus “endogenous” microbiome. Our aims here were therefore to address these questions experimentally by inoculating different endogenous or nonendogenous bacterial species into the guts of developing larvae. We focus specifically on four species: Providencia rettgeri and Morganella morganii, which are dominant members of the larval microbiome (Vogel et al., 2017; Wang & Rozen, 2017), and E. coli and S. marcescens, which are nonendogenous species, but which are either observed in the larval gut (Serratia) or have the potential to colonize it through exposure on the mouse carcass (E. coli) (Bäumler & Sperandio, 2016; Wang & Rozen, 2017).
Using this approach, we first determined that there are clear differences in the colonization potential of different bacterial species. While Providencia, Morganella, and Serratia increase in density more than 100‐fold in 24 hr within the larval gut, E. coli was a poor colonizer and only increased by ~10‐fold over the same time interval (Figure 3). In addition to clarifying these differences, these experiments also established that it is feasible to experimentally colonize larval beetles via diet manipulation. The growth differences between strains in monoculture were reflected in their interactions in vivo during coculture. Specifically, we saw competitive dominance of Providencia and Morganella over E. coli and Serratia when pairs of strains were simultaneously fed to larvae (Figure 4a). Moreover, in competition experiments between Providencia and Serratia, we found that the order of inoculation did not affect the competitive outcome between strains (Figure 4b). This latter result suggested that priority effects are not realized in this system because Serratia could be displaced even after a 24‐hr head start in colonization. By contrast, another recent study found that the colonization competitiveness of Borrelia strains within the mouse gut is significantly determined by their order of presentation to the host mouse (Devevey et al., 2015).
At present, we have limited understanding of the factors that mediate the competitive differences between strains within the Nicrophorus larval gut. Differences in in vivo growth rates are sufficient to explain the competition results during simultaneous inoculation. However, the fact that Providencia can still invade an established Serratia‐colonized larva (Figure 4b) suggests the possibility that competitive interactions are in part mediated by the host. For instance, host innate immunity could be a direct factor in determining the competitive outcome and final population density of bacterial species within hosts (Portal‐Celhay & Blaser, 2012). Equally, commensal bacteria could prime the host immune response to limit pathogen colonization by causing an up‐regulation of antimicrobial peptides, such as AMP molecules in Aedes aegypti mosquitoes and islet‐derived protein 3γ in mice (Kamada et al., 2013; Kambris & Al, 2009); however, these would need to be specifically targeted to nonsymbiont species. Host involvement in this system is further suggested by other experimental results showing that in vitro, Serratia is able to outcompete Providencia (Y. Wang, unpublished data), a result likely attributed to the faster growth of this strain during in vitro culture. An important aim for future work will be to clarify the factors that drive the competitive interactions between bacterial strains during colonization.
To understand the consequences of the Nicrophorus microbiome for beetle fitness, we quantified larval mortality following inoculation of monocultures or cocultures of different bacterial species. Consistent with results on colonization resistance in other systems (Dillon et al., 2005; Sant'Anna et al., 2014), these experiments showed that P. rettgeri and M. morganii both provide protection against Serratia infection, but with no added protection if both endogenous species are present (Figure 5b). This is expected given the results from in vivo competition assays. By contrast, when Serratia is inoculated first, the protection provided by Providencia is abolished, in spite of the fact that Providencia can outcompete Serratia in these conditions (Figure 5c). Interestingly, these results indicate that the pathogenesis of Serratia is separate from its in vivo competitive ability, perhaps owing to toxin production or invasion through the gut into the hemocoel within the first 24 hr (Andrejko, 1999; Lauzon et al., 2003; Tan, Jackson, & Hurst, 2006). Thus, initial establishment of the endogenous microbiota is apparently crucial for colonization resistance.
Although the mechanism of Serratia‐induced mortality remains unknown in this species, the fact that colonization resistance requires the prior or simultaneous establishment of the Nicrophorus endogenous microbiota has important implications for our understanding of the functions of parental care. Parents protect larvae and provide nutrition in the form of regurgitated food (Eggert et al., 1998). In addition, they transfer their gut microbiome to larvae by direct feeding and via contamination of the carcass surface (Duarte et al., 2017; Shukla et al., 2017; Wang & Rozen, 2017). The present results indicate that larvae benefit directly from the acquisition of these bacteria (Figures 2 and 5b) and suggest that two of the key members of the N. vespilloides microbiome are mutualists. Thus, while Nicrophorus adults ensure transmission of these and other species (Duarte et al., 2017; Vogel et al., 2017) from generation to generation, the bacteria provide direct benefits to beetles within the highly contaminated carcass environment (Vogel et al., 2017). It remains possible that other advantages exist, for example, improved nutrient acquisition (Wilkinson, Koga, & Fukatsu, 2007) or changes in the composition of the decomposer microbial community on the carcass (Duarte et al., 2017), but as yet these possibilities have not been measured. In addition, it is important to note that parents may transmit other factors, besides microbes, to offspring during development that may directly or indirectly affect their parental abilities. Larval size tends to be reduced in the absence of parental care, and smaller larvae give rise to smaller adults that are less competent parents (Steiger, 2013). Although we excluded this confounding factor using only size‐matched female adults (see Section 2), it was not possible to fully exclude the potential influence of other transmitted factors, for example, those affecting immunity or social development, which in turn altered parental care (see Thesing, Kramer, Koch, & Meunier, 2015 for a clear example of such transgenerational effects in earwigs). Using direct microbial inoculations may therefore be worthwhile in future studies.
Our results provide strong evidence that members of the Nicrophorus microbiome provide direct advantages to larvae and adults; however, it is important to note that these advantages were not measured in the natural context of the carcass itself. While this was necessary for the current work, it does mean that we may be underestimating larval exposure to potential bacterial pathogens (Reavey, Silva, & Cotter, 2015). In addition, our assay clearly suffers from extremely high rates of larval mortality, irrespective of treatment. Although the approach we used was essential to avoid reinfection of otherwise sterile larvae, it is possible that the heat treatment in our Sous Vide method rendered the liver diet less nutritious, or possibly modified the competitive environment of the larval gut. Artificial diets, or more ideally germ‐free mice as presented by Prof. Rebecca Kilner at the recent meeting for the European Society for Evolutionary Biology, that better mimic the larval environment and that improve larval nutrition and survival are thus needed to more fully elucidate the functions of the Nicrophorus microbiome. However, despite these limitations, our results point toward yet another role of parental care in N. vespilloides and argue for further comparative studies in other congeners that vary in their requirements for parental care.
CONFLICT OF INTERESTS
None declared.
AUTHOR CONTRIBUTIONS
Both authors were involved in experimental design and analysis and codrafted the manuscript. YW conducted all experimental work. Both authors approve of this final submitted manuscript.
ACKNOWLEDGMENTS
We acknowledge the helpful comments of Chris Jacobs and Andres Arce on an earlier version of this manuscript. YW was supported by a graduate scholarship from the China Scholarship Council, and DER was supported by funds from Leiden University.
Wang Y, Rozen DE. Gut microbiota in the burying beetle, Nicrophorus vespilloides, provide colonization resistance against larval bacterial pathogens. Ecol Evol. 2018;8:1646–1654. https://doi.org/10.1002/ece3.3589
REFERENCES
- Andrejko, M. (1999). Exoproteinases of the type A in pathogenesis of insect bacterial diseases. Folia Biologica, 47(3–4), 135–141. [PubMed] [Google Scholar]
- Arce, A. N. , Johnston, P. R. , Smiseth, P. T. , & Rozen, D. E. (2012). Mechanisms and fitness effects of antibacterial defences in a carrion beetle. Journal of Evolutionary Biology, 25(5), 930–937. https://doi.org/10.1111/jeb.2012.25.issue-5 [DOI] [PubMed] [Google Scholar]
- Bäumler, A. J. , & Sperandio, V. (2016). Interactions between the microbiota and pathogenic bacteria in the gut. Nature, 535(7610), 85–93. https://doi.org/10.1038/nature18849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffie, C. G. , & Pamer, E. G. (2013). Microbiota‐mediated colonization resistance against intestinal pathogens. Nature Reviews Immunology, 13(11), 790–801. https://doi.org/10.1038/nri3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capodeanu‐Nägler, A. , Keppner, E. M. , Vogel, H. , Ayasse, M. , Eggert, A.‐K. , Sakaluk, S. K. , & Steiger, S. (2016). From facultative to obligatory parental care: Interspecific variation in offspring dependency on post‐hatching care in burying beetles. Scientific Reports, 6(April), 29323 https://doi.org/10.1038/srep29323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter, S. C. , & Kilner, R. M. (2010). Sexual division of antibacterial resource defence in breeding burying beetles, Nicrophorus vespilloides . Journal of Animal Ecology, 79(1), 35–43. https://doi.org/10.1111/jae.2009.79.issue-1 [DOI] [PubMed] [Google Scholar]
- Devevey, G. , Dang, T. , Graves, C. J. , Murray, S. , & Brisson, D. (2015). First arrived takes all: Inhibitory priority effects dominate competition between co‐infecting Borrelia burgdorferi strains. BMC Microbiology, 15(1), 381 https://doi.org/10.1186/s12866-015-0381-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon, R. J. , & Dillon, V. M. (2004). The gut bacteria of insects: Nonpathogenic interactions. Annual Review of Entomology, 49(1), 71–92. https://doi.org/10.1146/annurev.ento.49.061802.123416 [DOI] [PubMed] [Google Scholar]
- Dillon, R. J. , Vennard, C. T. , Buckling, A. , & Charnley, A. K. (2005). Diversity of locust gut bacteria protects against pathogen invasion. Ecology Letters, 8(12), 1291–1298. https://doi.org/10.1111/ele.2005.8.issue-12 [Google Scholar]
- Duarte, A. , Welch, M. , Swannack, C. , Wagner, J. , & Kilner, R. M. (2017). Strategies for managing rival bacterial communities: Lessons from burying beetles. Journal of Animal Ecology, 00, 1–14. https://doi.org/10.1111/1365-2656.12725 [Accessed September 8, 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert, A.‐K. , Reinking, M. , & Muller, J.K. (1998). Parental care improves offspring survival and growth in burying beetles. Animal Behavior, 55, 97–107. https://doi.org/10.1006/anbe.1997.0588 [DOI] [PubMed] [Google Scholar]
- El Sanousi, S. M. , El Sarag, M. S. A. , & Mohamed, S. E. (1987). Properties of Serratia marcescens isolated from diseased honeybee (Apis mellifera) larvae. Microbiology, 133(1), 215–219. https://doi.org/10.1099/00221287-133-1-215 [Google Scholar]
- Flórez, L. V. , Biedermann, P. H. W. , Engl, T. , & Kaltenpoth, M. (2015). Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms. Natural Products Reports, 32(7), 904–936. https://doi.org/10.1039/C5NP00010F [DOI] [PubMed] [Google Scholar]
- Hacquard, S. , Garrido‐Oter, R. , Gonzalez, A. , Spaepen, S. , Ackermann, G. , Lebeis, S. , … Schulze‐Lefert, P. (2015). Microbiota and host nutrition across plant and animal kingdoms. Cell Host & Microbe, 17(5), 603–616. https://doi.org/10.1016/j.chom.2015.04.009 [DOI] [PubMed] [Google Scholar]
- Hall, C. L. , Wadsworth, N. K. , Howard, D. R. , Jennings, E. M. , Farrell, L. D. , Magnuson, T. S. , & Smith, R. J. (2011). Inhibition of microorganisms on a carrion breeding resource: The antimicrobial peptide activity of burying beetle (Coleoptera: Silphidae) oral and anal secretions. Environmental Entomology, 40(3), 669–678. https://doi.org/10.1603/EN10137 [DOI] [PubMed] [Google Scholar]
- Hoback, W. W. , Bishop, A. A. , Kroemer, J. , Scalzitti, J. , & Shaffer, J. J. (2004). Differences among antimicrobial properties of carrion beetle secretions reflect phylogeny and ecology. Journal of Chemical Ecology, 30(4), 719–729. https://doi.org/10.1023/B:JOEC.0000028427.53141.41 [DOI] [PubMed] [Google Scholar]
- Jacobs, C. G. C. , Wang, Y. , Vogel, H. , Vilcinskas, A. , van der Zee, M. , & Rozen, D. E. (2014). Egg survival is reduced by grave‐soil microbes in the carrion beetle, Nicrophorus vespilloides . BMC Evolutionary Biology, 14, 208 https://doi.org/10.1186/s12862-014-0208-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz, J. (1979). Gut flora of Galleria mellonella suppressing ingested bacteria. Journal of Invertebrate Pathology, 34(2), 192–198. https://doi.org/10.1016/0022-2011(79)90101-0 [DOI] [PubMed] [Google Scholar]
- Kaltenpoth, M. , & Steiger, S. (2014). Unearthing carrion beetles' microbiome: Characterization of bacterial and fungal hindgut communities across the Silphidae. Molecular Ecology, 23(6), 1251–1267. https://doi.org/10.1111/mec.12469 [DOI] [PubMed] [Google Scholar]
- Kamada, N. , Seo, S. U. , Chen, G. Y. , & Núñez, G. (2013). Role of the gut microbiota in immunity and inflammatory disease. Nature Reviews Immunology, 13(5), 321–335. https://doi.org/10.1038/nri3430 [DOI] [PubMed] [Google Scholar]
- Kambris, Z. , & Al, E. (2009). Immune activation by life‐shortening. Science, 134(October), 134–136. https://doi.org/10.1126/science.1177531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzon, C. , Bussert, T. G. , Sjogren, R. E. , & Prokopy, R. J. (2003). Serratia marcescens as a bacterial pathogen of Rhagoletis pomonella flies (Diptera: Tephritidae). European Journal of Entomology, 100(1), 87–92. https://doi.org/10.14411/eje.2003.017 [Google Scholar]
- Liarzi, O. , Bar, E. , Lewinsohn, E. , & Ezra, D. (2016). Use of the endophytic fungus Daldinia cf. concentrica and its volatiles as bio‐control agents J. Jones, ed. PLoS ONE, 11(12), e0168242 https://doi.org/10.1371/journal.pone.0168242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macke, E. , Tasiemski, A. , Massol, F. , Callens, M. , & Decaestecker, E. (2017). Life history and eco‐evolutionary dynamics in light of the gut microbiota. Oikos, 126(4), 508–531. https://doi.org/10.1111/oik.2017.v126.i4 [Google Scholar]
- McFall‐Ngai, M. , Hadfield, M. G. , Bosch, T. C. , Carey, H. V. , Domazet‐Lošo, T. , Douglas, A. E. , … Wernegreen, J. J. (2013). Animals in a bacterial world, a new imperative for the life sciences. Proceedings of the National Academy of Sciences of the United States of America, 110(9), 3229–3236. https://doi.org/10.1073/pnas.1218525110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne, L. J. , & Milne, M. (1976). The social behavior of burying beetles. Scientific American, 235(2), 84–89. https://doi.org/10.1038/scientificamerican0876-84 [Google Scholar]
- Nehme, N. T. , Liégeois, S. , Kele, B. , Giammarinaro, P. , Pradel, E. , Hoffmann, J. A. , … Ferrandon, D. (2007). A model of bacterial intestinal infections in Drosophila melanogaster . PLoS Pathogens, 3(11), 1694–1709. https://doi.org/10.1371/journal.ppat.0030173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portal‐Celhay, C. , & Blaser, M. J. (2012). Competition and resilience between founder and introduced bacteria in the Caenorhabditis elegans gut. Infection and Immunity, 80(3), 1288–1299. https://doi.org/10.1128/IAI.05522-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal, R. (2009). Beneficial interactions between insects and gut bacteria. Indian Journal of Microbiology, 49(2), 114–119. https://doi.org/10.1007/s12088-009-0023-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymann, K. , Shaffer, Z. , & Moran, N. A. (2017). Antibiotic exposure perturbs the gut microbiota and elevates mortality in honeybees. PLoS Biology, 15(3), 1–22. https://doi.org/10.1371/journal.pbio.2001861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reavey, C. E. , Silva, F. W. S. , & Cotter, S. C. (2015). Bacterial infection increases reproductive investment in burying beetles. Insects, 6(4), 926–942. https://doi.org/10.3390/insects6040926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoz, F. , Noël, C. , Errachid, A. , Foray, V. , & Hance, T. (2015). Infection dynamic of symbiotic bacteria in the pea aphid Acyrthosiphon pisum gut and host immune response at the early steps in the infection process. PLoS ONE, 10(3), 1–18. https://doi.org/10.1371/journal.pone.0122099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen, D. E. , Engelmoer, D. J. P. , & Smiseth, P. T. (2008). Antimicrobial strategies in burying beetles breeding on carrion. Proceedings of the National Academy of Sciences of the United States of America, 105(46), 17890–17895. https://doi.org/10.1073/pnas.0805403105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant'Anna, M. R. , Diaz‐Albiter, H. , Aguiar‐Martins, K. , Al Salem, W. S. , Cavalcante, R. R. , Dillon, V. M. , … Dillon, R. J. (2014). Colonisation resistance in the sand fly gut: Leishmania protects Lutzomyia longipalpis from bacterial infection. Parasites & Vectors, 7(1), 329 https://doi.org/10.1186/1756-3305-7-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, R. S. , Moran, N. A. , & Evans, J. D. (2016). Early gut colonizers shape parasite susceptibility and microbiota composition in honey bee workers. Proceedings of the National Academy of Sciences of the United States of America, 113(33), 9345–9350. https://doi.org/10.1073/pnas.1606631113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, M. P. (1998). The ecology and behaviour of burying beetles. Annual Review of Entomology, 43, 595–618. https://doi.org/10.1146/annurev.ento.43.1.595 [DOI] [PubMed] [Google Scholar]
- Sharon, G. , Segal, D. , Ringo, J. M. , Hefetz, A. , Zilber‐Rosenberg, I. , & Rosenberg, E. (2010). Commensal bacteria play a role in mating preference of Drosophila melanogaster . Proceedings of the National Academy of Sciences of the United States of America, 107(46), 20051–20056. https://doi.org/10.1073/pnas.1009906107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla, S. P. , Vogel, H. , Heckel, D. G. , Vilcinskas, A. , & Kaltenpoth, M. (2017). Burying beetles regulate the microbiome of carcasses and use it to transmit a core microbiota to their offspring. Mol Ecol, 00, 1–12. https://doi.org/10.1111/mec.14269 [Accessed September 11, 2017]. [DOI] [PubMed] [Google Scholar]
- Smiseth, P. T. , Darwell, C. T. , & Moore, A. J. (2003). Partial begging: An empirical model for the early evolution of offspring signalling. Proceedings. Biological Sciences/The Royal Society, 270(1526), 1773–1777. https://doi.org/10.1098/rspb.2003.2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer, F. , & Bäckhed, F. (2013). The gut microbiota–masters of host development and physiology. Nature Reviews Microbiology, 11(4), 227–238. https://doi.org/10.1038/nrmicro2974 [DOI] [PubMed] [Google Scholar]
- Steiger, S. (2013). Bigger mothers are better mothers: Disentangling size‐related prenatal and postnatal maternal effects. Proceedings of the Royal Society of London B: Biological Sciences, 280(1766), 20131225 https://doi.org/10.1098/rspb.2013.1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, B. , Jackson, T. A. , & Hurst, M. R. H. (2006). Virulence of Serratia strains against Costelytra zealandica . Applied and Environmental Microbiology, 72(9), 6417–6418. https://doi.org/10.1128/AEM.00519-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesing, J. , Kramer, J. , Koch, L. K. , & Meunier, J. (2015). Short‐term benefits, but transgenerational costs of maternal loss in an insect with facultative maternal care. Proceedings of the Royal Society of London B: Biological Sciences, 282(1817), 20151617 https://doi.org/10.1098/rspb.2015.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbo, S. T. , Sikes, D. S. , & Philbrick, P. K. B. (2016). Parental care and competition with microbes in carrion beetles: A study of ecological adaptation. Animal Behaviour, 118, 47–54. https://doi.org/10.1016/j.anbehav.2016.06.001 [Google Scholar]
- Vogel, H. , Shukla, S. P. , Engl, T. , Weiss, B. , Fischer, R. , Steiger, S. , … Vilcinskas, A. (2017). The digestive and defensive basis of carcass utilization by the burying beetle and its microbiota. Nature Communications, 8(May), 15186 https://doi.org/10.1038/ncomms15186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , & Rozen, D. E. (2017). Gut microbiota colonization and transmission in the burying beetle Nicrophorus vespilloides throughout development. Applied and Environmental Microbiology, 83(9), e03250‐16 https://doi.org/10.1128/AEM.03250-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, T. L. , Koga, R. , & Fukatsu, T. (2007). Role of host nutrition in symbiont regulation: Impact of dietary nitrogen on proliferation of obligate and facultative bacterial endosymbionts of the pea aphid Acyrthosiphon pisum . Applied and Environmental Microbiology, 73(4), 1362–1366. https://doi.org/10.1128/AEM.01211-06 [DOI] [PMC free article] [PubMed] [Google Scholar]


