We will argue here that there is net lactate transfer from astrocytes to neurons and that this transfer is important for brain function. Following CrossTalk guidelines, we will focus on data published over the last decade.
Experiments in cultured cells
The astrocyte‐to‐neuron lactate shuttle (ANLS) hypothesis was proposed based on glutamate experiments with cultured cells (Pellerin & Magistretti, 1994). Later on, comparative NMR spectroscopy confirmed that cultured astrocytes are more glycolytic than neurons (Bouzier‐Sore et al. 2006), a metabolic divergence that has later been explained by constitutive inhibition of phosphofructokinase in neurons but not in astrocytes, which diverts the neuronal glucose flux towards the pentose phosphate pathway (Herrero‐Mendez et al. 2009). Since then, other neuronal signals have been found to be capable, like glutamate, of commanding the production and/or release of lactate by astrocytes, specifically potassium and ammonium (Bittner et al. 2011; Choi et al. 2012; Lerchundi et al. 2015; Sotelo‐Hitschfeld et al. 2015). In all cases, the findings in culture were confirmed in slices or in vivo (see below). In contrast, we could not find any reports of lactate release by neurons in the presence of physiological lactate, either at rest or during electrical stimulation. But to what extent are cells in culture representative of cells in vivo? According to transcriptomic analysis of adult brain cells, the metabolic difference between astrocytes and neurons described in culture is also found in vivo (Zhang et al. 2014), a difference that becomes accentuated if astrocytes and neurons are cultured together and even more so upon induction of neuronal activity (Mamczur et al. 2015; Hasel et al. 2017).
Glucose flux experiments in brain tissue
Because lactate is made from glucose, the uptake of glucose informs on the question in hand. Measured in tissue slices, Bergmann glia and astrocytes were found to transport and metabolise fluorescent glucose analogues, NBDGs, faster than neighbouring neurons (Barros et al. 2009; Jakoby et al. 2014). In a separate study in vivo, whisker stimulation caused a stronger increase in NBDG accumulation in astrocytes than in neurons of the somatosensory cortex (Chuquet et al. 2010). These results are in line with the much higher cytosolic NADH/NAD+ of hippocampal astrocytes relative to neurons (Mongeon et al. 2016), indicative of stronger astrocytic glycolysis, and with the decrease in neuronal cytosolic NADH/NAD+ after blocking the neuronal monocarboxylate transporter MCT2 (Diaz‐Garcia et al. 2017). Also in hippocampal slices, astrocytic glucose consumption could be induced by neuronal stimulation, a phenomenon mediated by the sodium/bicarbonate cotransporter NBCe1 (Ruminot et al. 2017). Astrocytic uptake of glucose in vivo is further supported by an increased fluoro‐deoxyglucose (FDG) uptake in response to pharmacological stimulation of the astrocytic glutamate transporter GLT‐1 (Zimmer et al. 2017). Glial support for neuronal energy metabolism was demonstrated in compact white matter (Saab et al. 2016). Activity‐dependent glutamate release from axons leads to increased oligodendroglial glucose uptake and energetic support of spiking axons in the form of lactate. As it is widely accepted that most glucose metabolised by brain tissue ends up as CO2 in neurons and not in glial cells, the preferential uptake of glucose by glial cells implies net carbon transfer from astrocytes to neurons in the form of lactate. Furthermore, direct evidence of lactate consumption by orexinergic neurons in the hypothalamus was shown very recently (Clasadonte et al. 2017).
A contrasting conclusion was reached from two other studies. In one of them, forebrains of animals injected with FDG were used to prepare nerve terminal vesicles. The radioactivity present in the vesicles was compared with the concentration of the neuronal marker N‐acetylaspartate (NAA) and found to be similar to that of the starting tissue homogenate (Patel et al. 2014). Taken at face value, this similarity would mean that glial cells do not consume any glucose, a rather extreme proposition. Without information about isotope and NAA leakage and degradation during membrane disruption/resealing and prolonged density gradient centrifugation, the meaning of the FDG/NAA ratio does not seem straightforward to us. In the second study, the glucose analogue IR2DG800 was found to preferentially stain neurons over astrocytes (Lundgaard et al. 2015) when administered into the cerebrospinal fluid, thus bypassing the physiological and more efficient pathway of glucose entry from the circulation via astrocytic endfeet. Critically, IR2DG800 may not be a transported substrate because of its size (molecular mass 1300 Da), which is larger than the GLUT blocker cytochalasin B (molecular mass 480 Da) and much larger than NBDGs (342 Da). Based on its inhomogeneous subcellular distribution, it was concluded that IR2DG800 probably enters cells by endocytosis (Kovar et al. 2009). Because of these technical issues, we are doubtful that these two articles provide compelling evidence against ANLS.
Lactate studies in vivo
Given the choice, neurons in vivo prefer lactate over glucose, as shown by equicaloric substitution of glucose‐ by lactate‐consumption during intravenous infusion of lactate (Van Hall et al. 2009; Wyss et al. 2011). A similar conclusion was reached based on the rapid use of tissue lactate upon withdrawal of anaesthesia, which was quantified by NMR spectroscopy to be approx. 5 μm/s (Funfschilling et al. 2012). As the metabolism of lactate to CO2 is strictly coupled to the use of oxygen and most oxygen consumption in brain tissue is neuronal, it follows that in these three studies lactate was oxidised by neurons. But what is the source of lactate for neurons under normal conditions, when blood lactate and tissue lactate are low? Neural activation triggers a surge in brain tissue lactate, which is detected in humans and rodents by multiple techniques, including NMR spectroscopy, microdialysis, enzyme‐based microprobes and genetically encoded sensors. For example, a memory task caused a rapid increase in interstitial lactate (Newman et al. 2011) that, assuming a resting lactate level of 1 mm, may be estimated to be about 10 μm/s. This means that some cells released lactate at a speed commensurate with the rate of glucose consumption of the tissue. Our interpretation is that the lactate was released by astrocytes, which have preferential access to blood‐borne glucose and also contain glycogen that may be metabolised to lactate. Moreover, astrocytes maintain high resting levels of intracellular lactate, a dynamic reservoir that can be quickly mobilised upon neuronal demand via a lactate‐permeable ion channel gated by extracellular potassium (Sotelo‐Hitschfeld et al. 2015; Ruminot et al. 2017). In contrast, neurons, which are separated from blood glucose by astrocytes and do not possess glycogen stores, are poised to import lactate, as they maintain lower resting lactate levels (Machler et al. 2016) and lower NADH/NAD+ than astrocytes, thus favouring lactate to pyruvate conversion (Mongeon et al. 2016). Stimulation of neuronal glycolysis by electrical activity was inferred from a rise in neuronal NADH/NAD+ that was insensitive to MCT2 blockage, which was interpreted as evidence against ANLS (Diaz‐Garcia et al. 2017). However, the same study reported a parallel rise in neuronal lactate that was also unaffected by MCT2 blockage, which implies lack of lactate release. Thus, if neurons do not contribute to the activity‐dependent interstitial lactate surge, the surge may only come from glial cells. Worthy of note is that neurons remained much more oxidised than resting astrocytes even at the peak of their activity‐dependent NADH/NAD+ rise (Mongeon et al. 2016; Diaz‐Garcia et al. 2017), which also conspires against reversal of pre‐stimulation ANLS. Furthermore, astrocytes are likely to become even more reduced during activity, as judged by their NADH/NAD+ response to high extracellular potassium (Sotelo‐Hitschfeld et al. 2015).
Genetic and pharmacological evidence
In between the lactate pools of glial cells and neurons are the monocarboxylate transporters (MCTs), which would be redundant were there no intercellular lactate transfer. However several studies have reported perturbation of neuronal function and viability in response to pharmacological or genetic disruption of MCTs in astrocytes, oligodendrocytes or neurons (Newman et al. 2011; Suzuki et al. 2011; Funfschilling et al. 2012; Lee et al. 2012; Mazuel et al. 2017). Significantly, the deletion of MCTs in glial cells but not in neurons could be rescued by lactate, meaning that maintaining function requires neurons to have access to extracellular lactate. In the same vein, inhibition of glycogen degradation resulted in memory deficits that were also rescued by exogenous lactate (Newman et al. 2011; Suzuki et al. 2011). Even more dramatic are the effects of genetic inhibition of mitochondrial respiration in mice, which is lethal for neurons and innocuous for astrocytes (Funfschilling et al. 2012; Supplie et al. 2017), and also the genetic deletion of glycolytic enzymes in fruit fly, which is deleterious in glia but innocuous in neurons (Volkenhoff et al. 2015).
In summary, while some of the jurors on ANLS may still be out, we are of the opinion that fresh evidence from numerous laboratories using diverse techniques and experimental models, in vitro and in vivo, supports an important transfer of lactate from astrocytes and other glial cells to neurons (Fig. 1). We look forward to quantitative measurement of these fluxes and their dependence on brain states in the near future.
Figure 1. Working model of glucose and lactate exchange between astrocytes and neurons.
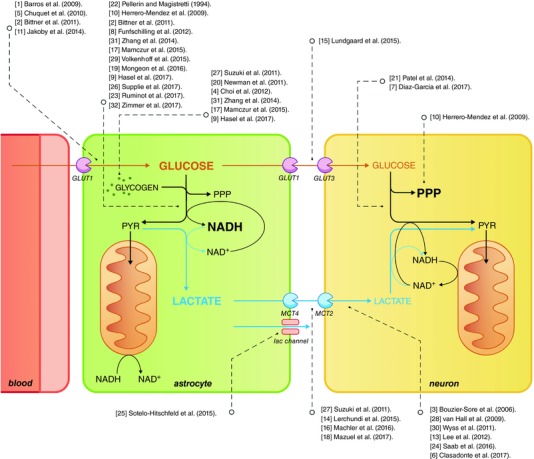
Dashed arrows connect the relevant pathways with the respective published work. For the sake of simplicity, only the main findings of the papers are considered. Please refer to the main text for details. We apologise to authors of relevant work that had to be omitted because of the maximum 30 references allowed by CrossTalk guidelines. GLUT1, GLUT3: glucose transporters GLUT1 and GLUT3; lac channel: lactate channel; MCT2, MCT4: monocarboxylate transporters MCT2 and MCT4; PPP: pentose phosphate pathway; PYR: pyruvate.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief (250 word) comment. Comments may be submitted up to 6 weeks after publication of the article, at which point the discussion will close and the CrossTalk authors will be invited to submit a ‘LastWord’. Please email your comment, including a title and a declaration of interest, to jphysiol@physoc.org. Comments will be moderated and accepted comments will be published online only as ‘supporting information’ to the original debate articles once discussion has closed.
Additional information
Competing interests
None declared.
Author contributions
Both authors have contributed to the conception or design of the work, acquisition or analysis or interpretation of data for the work, and drafting the work or revising it critically for important intellectual content. Both authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
L.F.B. is funded by Fondecyt Grant 1160317 and is supported by the Chilean Government through the Centers of Excellence Basal Financing Program of CONICYT PB‐01 granted to the Centro de Estudios Científicos (CECs). B.W. is supported by the University of Zurich, by the Swiss National Science Foundation, and the European Community Horizon 2020 GLINT project. He is a member of the Molecular Imaging Network Zurich.
Acknowledgements
We thank the members of the Barros and Weber labs for helpful discussions, and Karen Everett for critical reading of the manuscript.
Biographies
L. Felipe Barros qualified as a Medical Doctor in 1988 and obtained his PhD in Sciences in 1993 at the Universidad de Chile, advised by David Yudilevich. From 1993 to 1996 he was a Wellcome Trust Fellow in Steve Baldwin's lab in Leeds, UK. In 1996 he became Assistant Professor and then Associate Professor at the University of Chile. In 2000, he joined the Centro de Estudios Científicos as Principal Investigator. How can organisms manage to balance the flux of matter and energy? To address this question, his team are developing new molecular tools, capable of measuring metabolic parameters with high spatiotemporal resolution.

Bruno Weber studied and obtained his PhD in Neuroscience in Zurich, Switzerland. He was a postdoctoral fellow at the Max Planck Institute in Tübingen, Germany and is now a professor at the Institute of Pharmacology and Toxicology at the University of Zurich. His group uses a wide range of imaging tools to study the cell‐to‐cell communication pathways involved in energy metabolism and information processing in cerebral cortex. He is working on dissecting the interaction of neurons and astrocytes with the vascular system, which is responsible for maintaining adequate delivery of oxygen and energy substrates to the brain. As well as studying these systems, the development of imaging systems for in vivo research is an additional research focus of the group.
Linked articles This article is part of a CrossTalk debate. Click the links to read the other articles in this debate: https://doi.org/10.1113/JP274945, https://doi.org/10.1113/JP275506 and https://doi.org/10.1113/JP275507.
Edited by: Francisco Sepúlveda & Ian Forsythe
References
- Barros LF, Courjaret R, Jakoby P, Loaiza A, Lohr C & Deitmer JW (2009). Preferential transport and metabolism of glucose in Bergmann glia over Purkinje cells: a multiphoton study of cerebellar slices. Glia 57, 962–970. [DOI] [PubMed] [Google Scholar]
- Bittner CX, Valdebenito R, Ruminot I, Loaiza A, Larenas V, Sotelo‐Hitschfeld T, Moldenhauer H, San Martín A, Gutiérrez R, Zambrano M & Barros LF (2011). Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J Neurosci 31, 4709–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzier‐Sore AK, Voisin P, Bouchaud V, Bezancon E, Franconi JM & Pellerin L (2006). Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur J Neurosci 24, 1687–1694. [DOI] [PubMed] [Google Scholar]
- Choi HB, Gordon GR, Zhou N, Tai C, Rungta RL, Martinez J, Milner TA, Ryu JK, McLarnon JG, Tresguerres M, Levin LR, Buck J & MacVicar BA (2012). Metabolic communication between astrocytes and neurons via bicarbonate‐responsive soluble adenylyl cyclase. Neuron 75, 1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuquet J, Quilichini P, Nimchinsky EA & Buzsaki G (2010). Predominant enhancement of glucose uptake in astrocytes versus neurons during activation of the somatosensory cortex. J Neurosci 30, 15298–15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasadonte J, Scemes E, Wang Z, Boison D & Haydon PG (2017). Connexin 43‐mediated astroglial metabolic networks contribute to the regulation of the sleep‐wake cycle. Neuron 95, 1365–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz‐Garcia CM, Mongeon R, Lahmann C, Koveal D, Zucker H & Yellen G (2017). Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metab 26, 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S & Nave KA (2012). Glycolytic oligodendrocytes maintain myelin and long‐term axonal integrity. Nature 485, 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasel P, Dando O, Jiwaji Z, Baxter P, Todd AC, Heron S, Markus NM, McQueen J, Hampton DW, Torvell M, Tiwari SS, McKay S, Eraso‐Pichot A, Zorzano A, Masgrau R, Galea E, Chandran S, Wyllie DJA, Simpson TI & Hardingham GE (2017). Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat Commun 8, 15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero‐Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S & Bolanos JP (2009). The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C‐Cdh1. Nat Cell Biol 11, 747–752. [DOI] [PubMed] [Google Scholar]
- Jakoby P, Schmidt E, Ruminot I, Gutierrez R, Barros LF & Deitmer JW (2014). Higher transport and metabolism of glucose in astrocytes compared with neurons: a multiphoton study of hippocampal and cerebellar tissue slices. Cereb Cortex 24, 222–231. [DOI] [PubMed] [Google Scholar]
- Kovar JL, Volcheck W, Sevick‐Muraca E, Simpson MA & Olive DM (2009). Characterization and performance of a near‐infrared 2‐deoxyglucose optical imaging agent for mouse cancer models. Anal Biochem 384, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ & Rothstein JD (2012). Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerchundi R, Fernandez‐Moncada I, Contreras‐Baeza Y, Sotelo‐Hitschfeld T, Machler P, Wyss MT, Stobart J, Baeza‐Lehnert F, Alegria K, Weber B & Barros LF (2015). NH4 + triggers the release of astrocytic lactate via mitochondrial pyruvate shunting. Proc Natl Acad Sci USA 112, 11090–11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Li B, Xie L, Kang H, Sanggaard S, Haswell JD, Sun W, Goldman S, Blekot S, Nielsen M, Takano T, Deane R & Nedergaard M (2015). Direct neuronal glucose uptake heralds activity‐dependent increases in cerebral metabolism. Nat Commun 6, 6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machler P, Wyss MT, Elsayed M, Stobart J, Gutierrez R, von Faber‐Castell A, Kaelin V, Zuend M, San Martin A, Romero‐Gomez I, Baeza‐Lehnert F, Lengacher S, Schneider BL, Aebischer P, Magistretti PJ, Barros LF & Weber B (2016). In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab 23, 94–102. [DOI] [PubMed] [Google Scholar]
- Mamczur P, Borsuk B, Paszko J, Sas Z, Mozrzymas J, Wisniewski JR, Gizak A & Rakus D (2015). Astrocyte‐neuron crosstalk regulates the expression and subcellular localization of carbohydrate metabolism enzymes. Glia 63, 328–340. [DOI] [PubMed] [Google Scholar]
- Mazuel L, Blanc J, Repond C, Bouchaud V, Raffard G, Deglon N, Bonvento G, Pellerin L & Bouzier‐Sore AK (2017). A neuronal MCT2 knockdown in the rat somatosensory cortex reduces both the NMR lactate signal and the BOLD response during whisker stimulation. PLoS One 12, e0174990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongeon R, Venkatachalam V & Yellen G (2016). Cytosolic NADH‐NAD+ redox visualized in brain slices by two‐photon fluorescence lifetime biosensor imaging. Antioxid Redox Signal 25, 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, Korol DL & Gold PE (2011). Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One 6, e28427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, Lai JC, Chowdhury GM, Hyder F, Rothman DL, Shulman RG & Behar KL (2014). Direct evidence for activity‐dependent glucose phosphorylation in neurons with implications for the astrocyte‐to‐neuron lactate shuttle. Proc Natl Acad Sci USA 111, 5385–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L & Magistretti PJ (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91, 10625–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruminot I, Schmälzle J, Leyton B, Barros LF & Deitmer JW (2017). Tight coupling of astrocyte energy metabolism to synaptic activity revealed by genetically encoded FRET nanosensors in hippocampal tissue. J Cereb Blood Flow Metab (in press; https://doi.org/10.1177/0271678X17737012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, Mobius W, Goetze B, Jahn HM, Huang W, Steffens H, Schomburg ED, Perez‐Samartin A, Perez‐Cerda F, Bakhtiari D, Matute C, Lowel S, Griesinger C, Hirrlinger J, Kirchhoff F & Nave KA (2016). Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo‐Hitschfeld T, Niemeyer MI, Machler P, Ruminot I, Lerchundi R, Wyss MT, Stobart J, Fernandez‐Moncada I, Valdebenito R, Garrido‐Gerter P, Contreras‐Baeza Y, Schneider BL, Aebischer P, Lengacher S, San Martin A, Le Douce J, Bonvento G, Magistretti PJ, Sepulveda FV, Weber B & Barros LF (2015). Channel‐mediated lactate release by K+‐stimulated astrocytes. J Neurosci 35, 4168–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supplie LM, Duking T, Campbell G, Diaz F, Moraes CT, Gotz M, Hamprecht B, Boretius S, Mahad D & Nave KA (2017). Respiration‐deficient astrocytes survive as glycolytic cells in vivo. J Neurosci 37, 4231–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ & Alberini CM (2011). Astrocyte‐neuron lactate transport is required for long‐term memory formation. Cell 144, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hall G, Stromstad M, Rasmussen P, Jans O, Zaar M, Gam C, Quistorff B, Secher NH & Nielsen HB (2009). Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab 29, 1121–1129. [DOI] [PubMed] [Google Scholar]
- Volkenhoff A, Weiler A, Letzel M, Stehling M, Klambt C & Schirmeier S (2015). Glial glycolysis is essential for neuronal survival in Drosophila . Cell Metab 22, 437–447. [DOI] [PubMed] [Google Scholar]
- Wyss MT, Jolivet R, Buck A, Magistretti PJ & Weber B (2011). In vivo evidence for lactate as a neuronal energy source. J Neurosci 31, 7477–7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA & Wu JQ (2014). An RNA‐sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer ER, Parent MJ, Souza DG, Leuzy A, Lecrux C, Kim HI, Gauthier S, Pellerin L, Hamel E & Rosa‐Neto P (2017). [18F]FDG PET signal is driven by astroglial glutamate transport. Nat Neurosci 20, 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]


