Abstract
A correlation between endoplasmic reticulum (ER) stress and laxative effects was first reported in a constipation model treated with an aqueous extract of Liriope platyphylla (AEtLP) roots. To investigate the correlation between the laxative effect of uridine (Urd) and ER stress response, alterations in the key parameters for ER stress were measured in loperamide (Lop) induced constipation Sprague Dawley (SD) rats treated with Urd. The efficacy of the laxative effect of Urd was notable on the symptoms of chronic constipation, including alteration of stool parameters and structure of the transverse colon, in Lop induced constipated SD rats. In the PERK/eIF2-ATF4 pathway of ER stress response, the levels of eukaryotic initiation factor 2 alpha (eIF2α) phosphorylation and DNA damage-inducible protein (GADD34) transcripts were significantly enhanced in the Lop+Vehicle treated group. However, the levels were restored in the Lop+Urd treated group, although few differences were detected in the decrease rate. Similar changes were observed for levels of inositol-requiring enzyme 1 beta (IRE1β) phosphorylation and X-box binding protein 1 (XBP-1) transcript in the IRE1α/XBP pathway. Furthermore, the number of ER stress-induced apoptotic cells and Bax and Bcl-2 expression were recovered in the Lop+Urd treated group compared to the Lop+Vehicle treated group. The results of the present study therefore provide first evidence that the laxative effects of Urd may be tightly correlated with the recovery of ER stress response in constipation models.
Keywords: Uridine, ER stress response, Constipation, Laxative effects, Loperamide, apoptosis
Among the several cellular organelles of eukaryotic cells, ER plays a key role in protein synthesis and folding, lipid biosynthesis and calcium storage [1]. However, these functions get interrupted by various factors, including intracellular metabolic changes (calcium and redox imbalance), microenvironmental conditions (hypoxia, hypoglycemia and acidosis), abnormal diet, effect of natural products (thapsigargin and tunicamycin), and prescription drugs (nelfinavir, bortezomib and celecoxib) [2]. Disturbances lead to several impairments of the ER function, including an enhancement in demand for secretory protein, a decrease of folding capacity and an increase of misfolding proteins [3]. The degradation of ER functions finally results in an ER stress response, which includes various molecular mechanisms such as attenuation of protein synthesis, induction of ER chaperone genes, increased ER-associated degradation (ERAD), and induction of apoptosis to safely dispose cells injured by ER stress [3]. These processes are accompanied by accumulation of unfolded or misfolded proteins, leading to the activation of the unfolded protein response (UPR) [3].
Alterations in the ER stress response has also been observed in various pathological conditions associated with aging, cancer, inflammation, diabetes, atherosclerosis, Alzheimer's disease, liver disease, Parkinson's disease and constipation [4,5,6,7]. The chronic activation of the UPR is an important factor during pathogenesis of the above diseases [6]. Especially, ER stress response on constipation is recently being investigated using the constipation model. The alterations of key indicators associated with ER stress significantly increased in the Lop-induced constipation SD rats compared with that of No treated group. These levels were restored in the Lop+AEtLP treated group, although the decrease rate was varied [7]. However, no studies have provided scientific evidence of the correlation between laxative drugs and ER stress, even though the laxative effects of Urd was well investigated on the excretion parameters, histological structure and molecular mechanisms [8].
The present study was therefore conducted to verify the correlation between laxative effects of Urd and recovery of the ER stress response in a Lop-induced constipation model. These results provide the first evidence that the laxative effects of Urd improve the ER stress induced during Lop treatment.
Materials and Methods
Design of animal experiment
The animal protocol used in this study was based on the ethical procedures for scientific care, and reviewed and approved by the Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC; Approval Number PNU-2015-0949). Adult SD rats were purchased from SamTacho BioKorea (Osan, Korea), and handled at the Pusan National University Laboratory Animal Resources Center accredited by the Korea Food and Drug Administration (FDA)(Accredited Unit Number-000231) and AAALAC International (Accredited Unit Number; 001525). All rats were provided with standard irradiated chow (Purina Mills, Seoungnam, Korea) ad libitum, and were maintained in a specific pathogen-free (SPF) state under a strict light cycle (lights on at 06:00 h and off at 18:00 h) at 22±2℃ and a relative humidity of 50±10%.
The model for constipation was followed as previously described [7,9]. Briefly, the 8-week-old SD rats (Samtako BioKorea Co., Osan, Korea) (n=28) were assigned to either a non-constipation group (No group, n=14) or a constipation group (n=14). Constipation was induced by subcutaneous injection of Lop (4 mg/kg weight) prepared in 0.5% Tween 20 in saline, twice a day for 3 days, whereas the non-constipation group was injected with 0.5% Tween 20 in saline. The non-constipation group was further divided into a No treated group (n=7) and a Urd treated group (n=7). The No treated group was untreated during the experimental period, whereas the Urd treated group received a single dose of 100 mg/kg Urd (Sigma-Aldrich Co., Saint Louis, MO, USA) (Figure 1A). Additionally, the constipation group was further divided into a Lop+Vehicle treated group (n=7) and Lop+Urd treated group (n=7). The Lop+Urd treated groups were orally administered a single dose of 100 mg/kg body weight Urd, while the Lop+Vehicle treated group received the same volume of 1× PBS under the same pattern. At 24 h after Urd treatment, all SD rats were euthanized using CO2 gas, and the tissue samples were acquired and stored in Eppendorf tubes at 70℃ until assay.
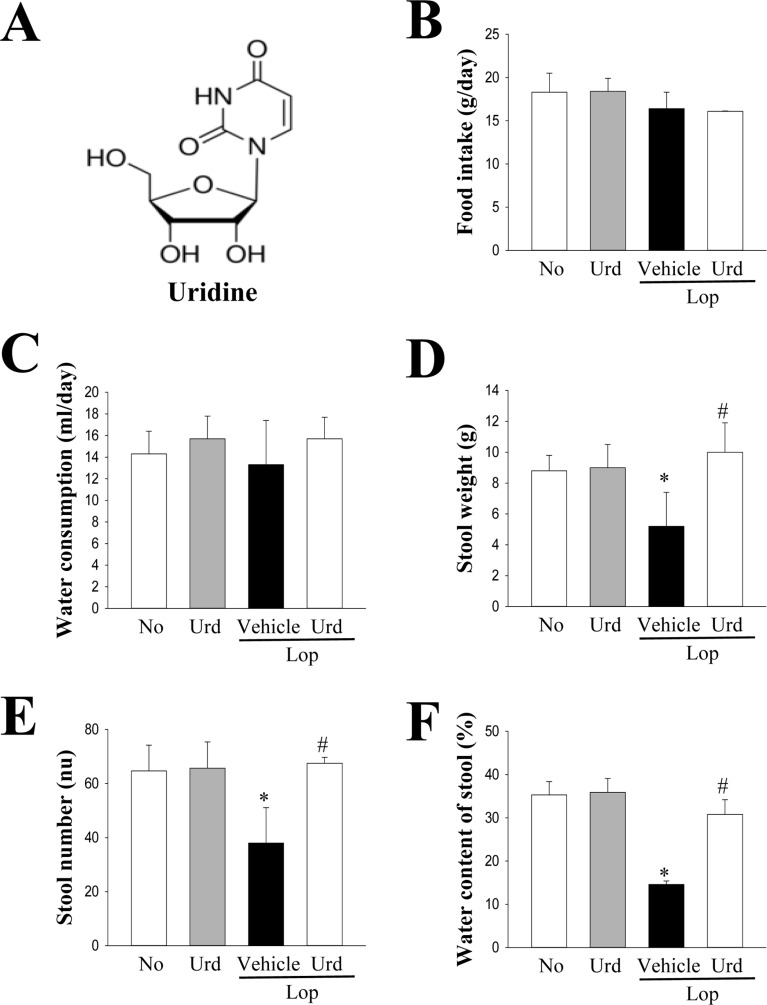
Figure 1. Evaluation of the feeding behavior and stool parameters in Lop+Urd treated SD rats. (A) Chemical structure of Urd. (B and C) Food intake and water consumption were measured at every day. (D–F) Key stool parameters, including weight, number and water contents, were analyzed as described in Materials and Methods. Data represents the mean±SD from three replicates. *P<0.05 compared to the No treated group. #P<0.05 compared to the Lop+Vehicle treated group.
Analysis of feeding behavior
Throughout the experimental period, alterations in food intake and water intake were measured daily at 10:00 am, using an electrical balance (METTLER-TOLEDO, Greifensee, Switzerland) and a measuring cylinder, respectively. All measurements were performed three times to ensure accuracy.
Measurement of stool parameters
To avoid any contamination, all SD rats in the subset groups were bred in individual metabolic cages (Daejong Ltd., Seoul, Korea) during the experimental period. The stools and urine excreted from each SD rat were collected at 10:00 am. Stool weight was measured three times per sample using an electric balance (METTLER-TOLEDO), whereas the water content was determined as the difference between the wet and dry weights of the stool, as previously described [8,9].
Histological analysis
Transverse colons were prepared for histological analysis as previously described [9]. Briefly, transverse colons collected from SD rats were fixed with 10% formalin for 12 h, embedded in paraffin wax, and sectioned into 4 ìm thick slices that were then stained with hematoxylin & eosin (H&E, Sigma-Aldrich Co.). Morphological features of these sections were observed by light microscopy, after which the mucosa layer thickness and muscle thickness were measured using the Leica Application Suite (Leica Microsystems, Heerbrugg, Switzerland).
RT-PCR
To detect the transcript level of ER stress related genes, frozen transverse colons were chopped with scissors and homogenized in RNAzol B solution, according to the manufacturer's protocol (Tet-Test Inc., Friend-swood, TX, USA). The concentration of isolated RNA was then determined by UV-spectroscopy. The expression of the genes was subsequently examined by RT-PCR using 5 µg of the total RNA from each tissue. Briefly, 500 ng of oligo-dT primer [Gibco BRL, Uxbridge, Middlesex, UK] was annealed with template RNA at 70℃ for 10 min. The comple-mentary DNA, which served as the template for subsequent amplification, was then synthesized by adding dATP, dCTP, dGTP and dTTP with 200 units of reverse transcriptase and 10 pmole of sense and antisense primers. Next, the amplification was conducted by subjecting the samples to 28 cycles of 30 sec at 94℃, 30 sec at 62℃, and 45 sec at 72℃, in a T100TM Thermal Cycler (BIORAD, Hercules, CA, USA). In each case, negative-RT controls were included to differentiate the DNA and RNA products. RT-PCR was also performed using primers specific to β-actin to ensure the RNA integrity. The primer sequences used to evaluate XBP-1 expression were as follows: sense primer, 5′-AAACA GAGTA GCAGC ACAGA CTGC-3′; antisense primer, 5′-CTTCC AGCTT GGCTG ATGAG GTCC-3′. Additionally, the primer sequences used to evaluate GADD34 expression were as follows: sense primer, 5′-TAGAG AGCAA GAAGT GGAGC ACACA GC-3′; antisense primer, 5′-CGTCA TCTTC TTCTT CTGTG TCCTC-3′. The sequences of the β-actin sense and antisense primers were 5′-TGGAA TCCTG TGGCA TCCAT GAAAC-3′ and 5′-TAAAA CGCAG CTCAG TAACA GTCCG-3′, respectively. The level of the PCR products was quantified using a Kodak Electrophoresis Documentation and Analysis System 120 and 1% agarose gels.
Western blotting
To investigate an alteration in the expression of ER stress marker proteins, total proteins collected from the transverse colons of No-, Lop+Vehicle and Lop+Urd treated rats were separated by 4–20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for 3 h, after which the resolved proteins were transferred to nitrocellulose membranes for 2 h at 40 V. Each membrane was subsequently separately incubated overnight at 4℃, with the following primary antibodies: eIF2α (Cell Signaling Technology Inc., Cambridge, MA, USA), p-eIF2α (Cell Signaling Technology Inc.), IRE1β (Santa Cruz Biotechnology, Santa Cruz, CA, USA), p- IRE1β (Santa Cruz Biotechnology) and anti-β-actin (Cell Signaling Technology Inc.). The membranes were then washed with washing buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, and 0.05% Tween 20) and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Zymed Laboratories, South San Francisco, CA, USA) at a dilution of 1:1,000 at room temperature for 2 h. Finally, the membrane blots were developed using Chemiluminescence Reagent Plus kits (Pfizer, New York, NY, USA and Pharmacia, New York, NY, USA). The chemiluminescence signals that originated from specific bands were detected using FluorChemi®FC2 (Alpha Innotech Co., San Leandro, CA, USA).
Tunnel assay
The number of cells with DNA fragmentation was measured in situ using the BrdU-Red DNA fragmentation (TUNEL) detection kit (BD Bioscience, Franklin Lakes, NJ, USA), according to the manufacturer's protocol (with minor modifications). After the collection of transverse colon from SD rats, tissues were embedded in paraffin; serial sections 4 µm thick were placed onto slides and stained with hematoxylin and eosin. For the TUNEL assay, sections of the transverse colon were deparaffinized, and apoptotic cells were detected using the specific antibody for fragmented DNA assay kit. The percentage of FITC-BrdU positive cells and mean green fluorescence intensity were detected using Moticam pro 285A (Motic, Xiamen, China). All experiments were performed in duplicate.
Statistical analysis
One-way ANOVA was used to identify significant differences between the No and Lop treated groups (SPSS for Windows, Release 10.10, Standard Version, Chicago, IL, USA). Additionally, differences between the Lop+Vehicle treated group and the Lop+Urd treated group were evaluated by a post hoc test (SPSS for Windows, Release 10.10, Standard Version) of the variance and significance levels. All values are expressed as the means±SD. A P value of <0.05 is considered significant.
Results
Laxative effects of Urd in Lop-induced constipation model
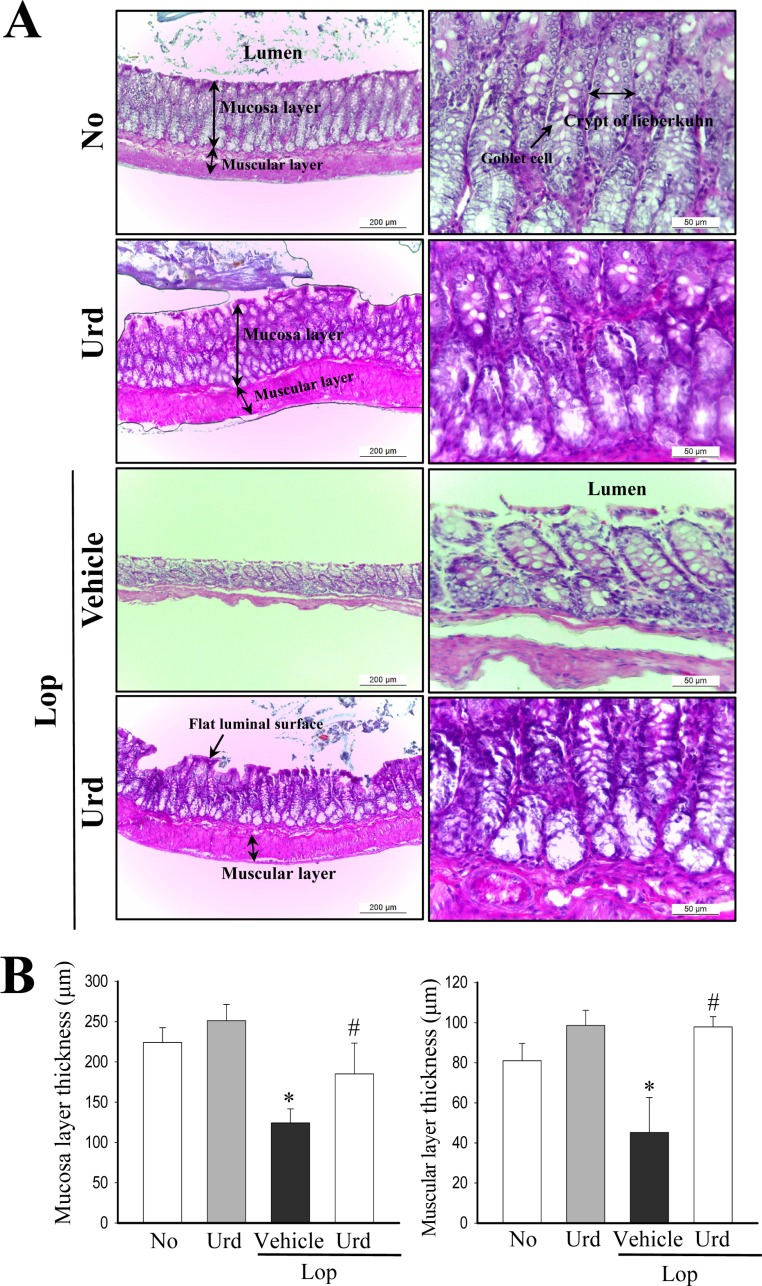
To confirm the laxative effects of Urd in a Lopinduced constipation model, an alteration in the feeding behaviors, excretion parameters and histological structure were measured in No, Lop+Vehicle and Lop+Urd treated SD rats. Although the level of food intake and water consumption were maintained constant after Urd treatment, the decrease in the number, weight and water contents of stools in the Lop+Vehicle treated group were almost recovered in the Lop+Urd treated groups, relative to those in the No treated group (Figure 1B-F). A significant recovery was also observed in the histological structure of the transverse colon. The thickness of the mucosa layer and muscle significantly decreased in the Lop+Vehicle treated group, relative to the No treated group. However, these levels were dramatically enhanced by 44 and 121% after Lop+Urd co-treatment, as compared with the Lop+Vehicle treated group (Figure 2). Taken together, these results indicate that Urd treatment symptomatically improves chronic constipation in Lop-induced SD rats, without any changes in the feeding behavior.
Figure 2. Alteration of the histological structure in transverse colons. (A) Hematoxylin and eosin (H&E) stained sections of transverse colons from the No treated group, Lop+Vehicle treated group or Lop+Urd treated group were observed at two different magnifications (100× and 400×) using a light microscope. (B) The thickness of the mucosa and muscular layer are presented as graphs. Data represents the mean±SD from three replicates. *P<0.05 compared to the No treated group. #P<0.05 compared to the Lop+Vehicle treated group.
Relive effect of Urd treatment on the ER stress response
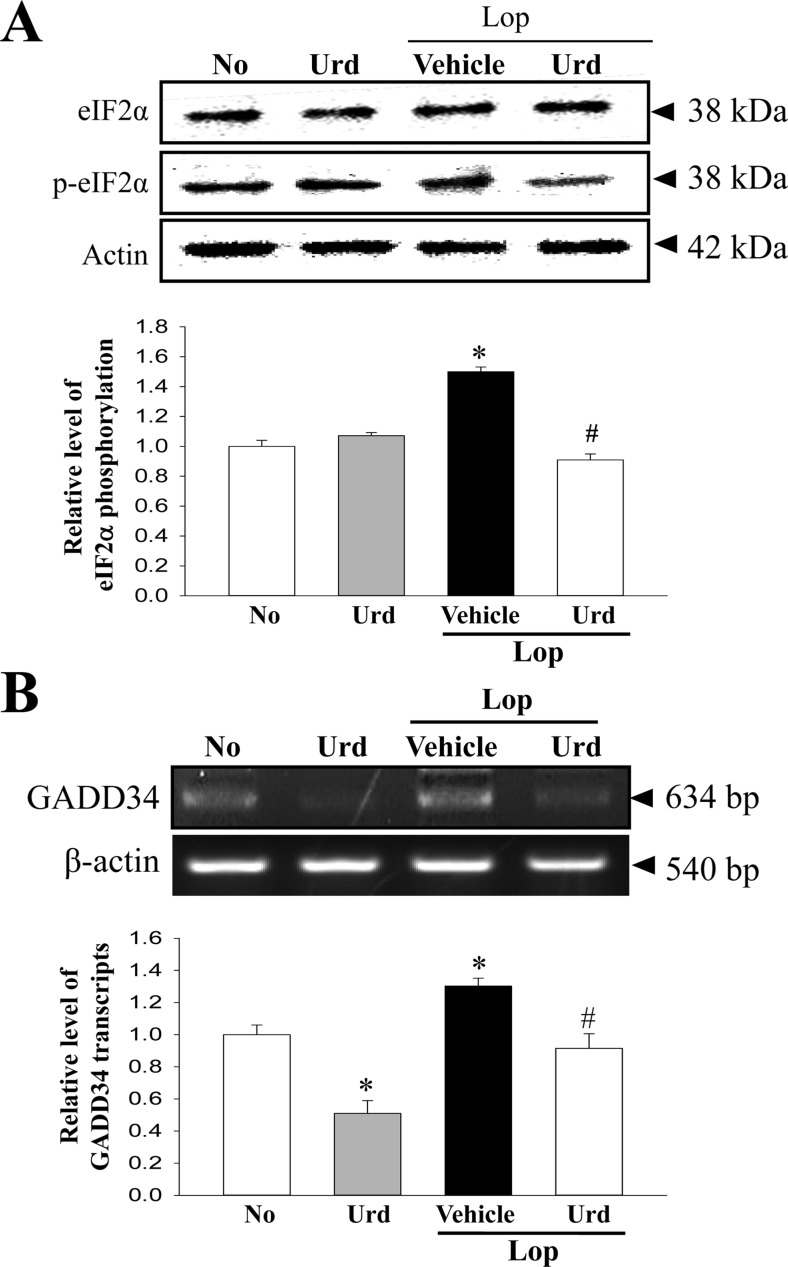
We next investigated whether the laxative effects of Urd are correlated with the subsequent abolition of the ER stress response. To achieve this, an alteration in the levels of key proteins of the PERK/eIF2-ATF4 pathway and IRE1α/XBP pathway were evaluated in the transverse colon of Lop-induced constipation rats treated with Urd. In the PERK/eIF2-ATF4 pathway, the levels of eIF2α phosphorylation and GADD34 transcripts were higher (43 and 29%, respectively) in the Lop+Vehicle treated group than the No treated group. However, these levels were almost restored in the Lop+Urd treated group, although the decreased levels were less than those of No treated group (Figure 3).
Figure 3. Expression of marker proteins in the PERK/eIF2-ATF4 pathway. (A) The expression levels of eIF2α and p-eIF2α in total proteins were detected with specific antibodies. The level of β-actin is also shown as an endogenous control. The band intensity of these proteins was determined using an imaging densitometer, and the relative levels of each protein were calculated based on the intensity of actin protein as an endogenous control. (B) The levels of GADD34 transcripts in total mRNA of transverse colons were measured by RT-PCR using specific primers. After the intensity of each band was determined using an imaging densitometer, the relative levels of GADD34 transcripts were calculated based on the intensity of actin transcripts. Data represent the means±SD from three replicates. *P<0.05 compared to the No treated group. #P<0.05 compared to the Lop+Vehicle treated group.
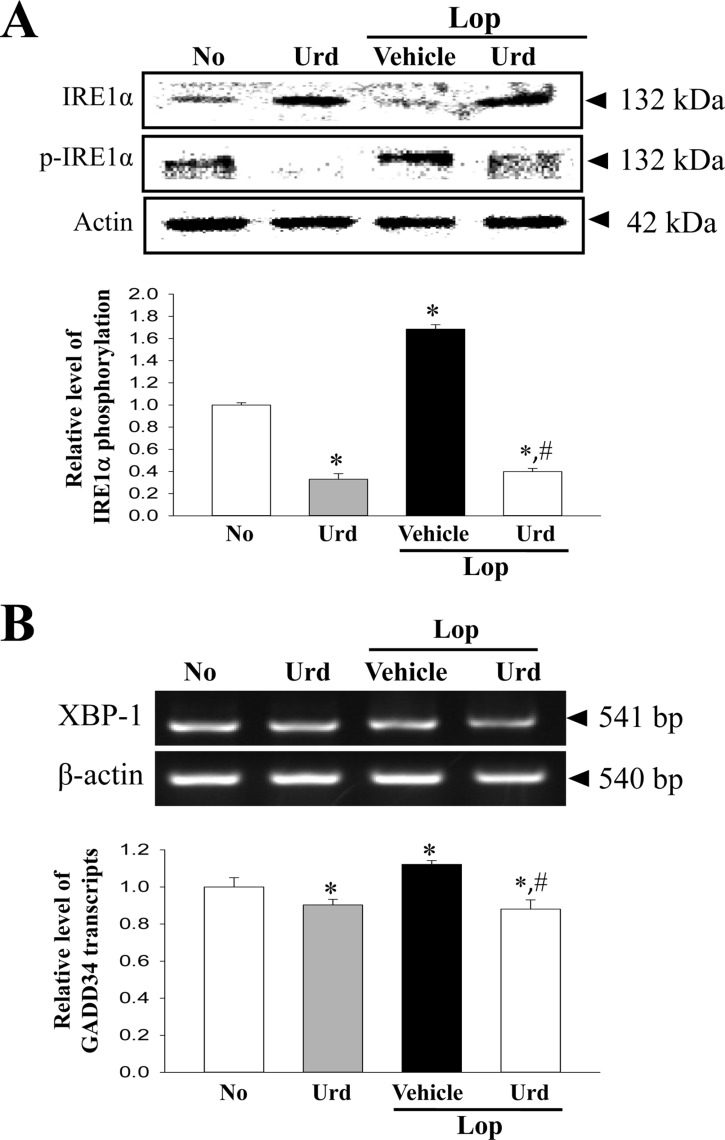
A similar change was observed in the IRE1α/XBP pathway. The greatest increase in the phosphorylation of IRE1α and XBP-1 transcript was observed in the Lop+Urd treated group, when compared with the Lop+Vehicle treated group (Figure 4). These results indicate that Lop-induced ER stress can be successfully abolished by the laxative effect of Urd treatment in the chronic constipation model.
Figure 4. Expression of marker proteins in IRE1α/XBP pathway. (A) The expression levels of IRE1α and p- IRE1α in total proteins were detected with specific antibodies. The level of β-actin is also shown as an endogenous control. The band intensity of the proteins was determined using an imaging densitometer, and the relative levels of each protein were calculated based on the intensity of actin protein as an endogenous control. (B) The levels of XBP-1 transcripts in total mRNA of transverse colons were measured by RT-PCR using specific primers. After the intensity of each band was determined using an imaging densitometer, the relative levels of XBP-1 transcripts were calculated based on the intensity of actin transcripts. Data represents the means±SD from three replicates. *P<0.05 compared to the No treated group. #P<0.05 compared to the Lop+Vehicle treated group.
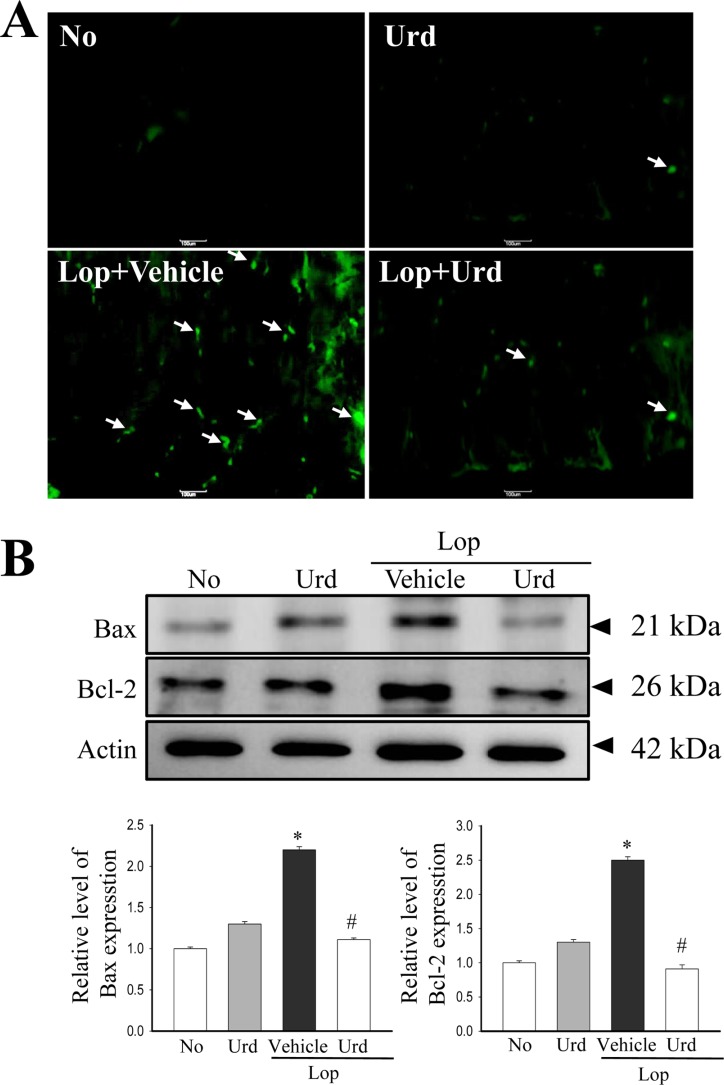
Effect of Urd treatment on the ER stress-induced apoptosis
The prolonged unfolded protein response (UPR) activation is known to promote apoptosis [10]. In order to investigate the correlation between the laxative effects of Urd and ER stress-induced apoptosis, an alteration in the number of apoptotic cells and protein expression were analyzed in Lop+Urd treated rats. After Lop treatment, an increase in the number of apoptotic cells with high fluorescence was observed in tissue sections of the transverse colon. However, there was a significant disappearance in the fluorescence in the Lop+Urd treatment group when compared to the Lop+Vehicle treated group (Figure 5A). Similar results were observed for Bax/Bcl-2 expression; the increase of Bax and Bcl-2 expression levels in the Lop+Vehicle treated group recovered in the Lop+Urd treated group (Figure 5B). These results suggest that the laxative effect of Urd improves the ER stress-induced apoptosis.
Figure 5. Level of apoptotic cells and proteins. (A) Representative images show staining of TUNEL in transverse colon treated with Lop+Vehicle or Lop+Urd as indicated. The transverse colon of normal (No) rats were used as controls. (B) Total tissue lysates were prepared from the transverse colon of Lop-injected SD rats treated with Vehicle or Urd, as described in the Materials and Methods. A total of 50 µg of protein per sample was immunoblotted with antibodies for each protein. Three samples were assayed in triplicate by western blotting. Data represents the means±SD of three replicates. *P<0.05 compared to the No treated group. #P<0.05 compared to the Lop+Vehicle treated group.
Discussion
ER stress is considered as an attractive target for therapeutic discovery, since ER stress-induced cellular dysfunction and cell death is commonly detected in numerous diseases including neurodegenerative diseases, ophthalmology disorders, inflammation, viral infection, constipation, cancer, metabolic diseases and atherosclerosis [11]. The current study especially focused on the applicability of this target in the constipation model during treatment with a laxative drug. The results of the present study suggest first evidences that disturbance of the ER stress response can be revived by the laxative effects of Urd treatment, in SD rats with Lop-induced constipation.
Gastointestinal tract consist of four different layers: the innermost layer is the mucosa, underneath this is the submucosa, followed by the muscularis propria and finally, the outermost layer - the adventitia although the structural variances are observed in different regions of the digestive system [12]. Among these, mucosa layer and muscularis propria plays an important role in gastrointestinal function. Mucosa layer can directly contact with digested food and is occurred a digestive, absorptive and secretory processes [13]. Muscularis propria regulate two fundamental patterns (peristalsis and segmentation contractions) of gastrointestinal motility in digestive tube [13]. However, normal thickness of these layers can be significantly reduced in various gastrointestinal diseases including dystrophin [14], colonic inertia [15], mesenteric ischemia [16], Alzheimer's disease [17] and ulcerative colitis [18]. Also, these alterations on the thickness of mucosa and muscles layer are similar to those observed in mice treated with Lop as showed in Figure 2. Therefore, our results provide additional evidence that an recovery on the structural change of colon layer in constipation can seriously consider as one of key markers for laxative effect.
Our previous study was the first to report that ER stress response was tightly correlated with chronic constipation induced by Lop treatment; we further reported the laxative effects of AEtLP, although several studies had provided some clues for the molecular mechanism of constipation drugs [7]. In the above mentioned study, we reported that the level of key indicators for ER stress, such as eIF2α phosphorylation and IRE1β expression, significantly recovered in the Lop+AEtLP treated group, but the phosphorylation level of c-Jun N-terminal protein kinase (JNK) remained constant. Also, the level of their downstream signaling molecules, including of XBP-1 and GADD34, was slightly or completely recovered after AEtLP treatment [7]. Although we had used another laxative drug, similar results were observed in the currently presented Urd treated constipation model. As shown in Figure 3 and 4, the alterations of several key proteins in the PERK/eIF2α-ATF4 and IRE1α/XBP pathways were significantly recovered after co-treatment with Lop and Urd. However, the decrease rate was greater in the Lop+Urd treated group than in the Lop+AEtLP treated group. The differences between these studies can be attributed to the composition and concentration of several compounds in the treatment materials. More studies are therefore required to understand what other factors in the laxative drugs will determine the changes in the ER stress response.
Prolonged activation of URP stimulates the IRE1 and CHOP-mediated pro-apoptotic signaling pathway [10]. This process involves at least one member of the Bcl-2 family that includes both anti-apoptotic and pro-apoptotic members regulating the cross talk between ER and mitochondria [19,20]. In gadd153/MEF cell lines, the elevation of Gadd153 expression induces the down-regulation of Bcl2 expression [21]. However, there are no studies correlating the laxative drug and ER stress-induced apoptosis in the constipation model. Our study provides the first results that the laxative effects of Urd can revive the ER stress-induced apoptosis through regulation of Bax and Bcl-2 expression.
Taken together, our study examined the correlation between the laxative effect of Urd and ER stress response in Lop-induced constipation SD rats. The alteration of several key proteins in the PERK/eIF2α-ATF4 and IRE1α/XBP pathways as well as ER stress-induced apoptosis were dramatically recovered in Lop+Urd treated rats. The results presented herein are the first to suggest that the laxative effects of Urd are closely correlated with the improvement of ER stress response and ER stress-induced apoptosis in the Lop-induced constipation model.
Acknowledgments
We thank Jin Hyang Hwang, the animal technician, for directing the care and use of animals at the Laboratory Animal Resources Center. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A3B03032631).
Footnotes
Conflict of interests: The authors declare that there is no financial conflict of interests to publish these results.
References
- 1.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. 5th ed. New York: Garland Science; 2008. pp. 723–748. [Google Scholar]
- 2.Schonthal AH. Endoplasmic reticulum stress: Its role in disease and novel prospects for therapy. Scientifica (Cairo) 2012;2012:857516. doi: 10.6064/2012/857516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida H. ER stress and disease. FEBS J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 5.Forman MS, Lee VMY, Trojanowski JQ. ‘Unfolding’ pathways in neurodegenerative disease. Trends Neurosci. 2003;26(8):407–410. doi: 10.1016/S0166-2236(03)00197-8. [DOI] [PubMed] [Google Scholar]
- 6.Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012;63:317–328. doi: 10.1146/annurev-med-043010-144749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JE, Go J, Sung JE, Lee HA, Seo EJ, Yun WB, Hwang DY. Laxative effects of Liriope platyphylla are tightly correlated with suppression of endoplasmic reticulum stress in loperamide-induced constipation of SD rats. Lab Anim Res. 2016;32(1):16–23. doi: 10.5625/lar.2016.32.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JE, Go J, Sung JE, Lee HA, Yun WB, Hong JT, Hwang DY. Uridine stimulate laxative effect in the loperamide-induced constipation of SD rats through regulation of the mAChRs signaling pathway and mucin secretion. BMC Gastroenterol. 2017;17:21. doi: 10.1186/s12876-017-0576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JE, Lee YJ, Kwak MH, Ko J, Hong JT, Hwang DY. Aqueous extracts of Liriope platyphylla induced significant laxative effects on loperamide-induced constipation of SD rats. BMC Complement Altern Med. 2013;13:333. doi: 10.1186/1472-6882-13-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scull CM, Tabas I. Mechanisms of ER stress-induced apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(12):2792–2797. doi: 10.1161/ATVBAHA.111.224881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833(12):3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doughty D. Structure and function of the gastrointestinal tract in infants and children. J Wound Ostomy Continence Nurs. 2004;31(4):207–212. doi: 10.1097/00152192-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Shen L. Functional morphology of the gastrointestinal tract. Curr Top Microbiol Immunol. 2009;337:1–35. doi: 10.1007/978-3-642-01846-6_1. [DOI] [PubMed] [Google Scholar]
- 14.Alves GA, Silva LR, Rosa EF, Aboulafia J, Freymüller-Haapalainen E, Souccar C, Nouailhetas VL. Intestine of dystrophic mice presents enhanced contractile resistance to stretching despite morphological impairment. Am J Physiol Gastrointest Liver Physiol. 2014;306(3):G191–G199. doi: 10.1152/ajpgi.00314.2013. [DOI] [PubMed] [Google Scholar]
- 15.Chan OT, Chiles L, Levy M, Zhai J, Yerian LM, Xu H, Xiao SY, Soffer EE, Conklin JL, Dhall D, Kahn ME, Balzer BL, Amin MB, Wang HL. Smoothelin expression in the gastrointestinal tract: implication in colonic inertia. Appl Immunohistochem Mol Morphol. 2013;21(5):452–459. doi: 10.1097/PAI.0b013e31827387c9. [DOI] [PubMed] [Google Scholar]
- 16.Tóth S, Jonecová Z, Varga J, Staško P, Kovavalèinová B, Maretta M, Veselá J. Mesenteric ischemia-reperfusion injury: specific impact on different cell populations within the jejunal wall in rats. Acta Histochem. 2012;114(3):276–284. doi: 10.1016/j.acthis.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Karri S, Acosta-Martinez V, Coimbatore G. Effect of dihydrotestosterone on gastrointestinal tract of male Alzheimer's disease transgenic mice. Indian J Exp Biol. 2010;48(5):453–465. [PubMed] [Google Scholar]
- 18.Cao W, Fiocchi C, Pricolo VE. Production of IL-1beta, hydrogen peroxide, and nitric oxide by colonic mucosa decreases sigmoid smooth muscle contractility in ulcerative colitis. Am J Physiol Cell Physiol. 2005;289(6):C1408–C1416. doi: 10.1152/ajpcell.00073.2005. [DOI] [PubMed] [Google Scholar]
- 19.Oakes SA, Lin SS, Bassik MC. The control of endoplasmic reticulum-initiated apoptosis by the BCL-2 family of proteins. Curr Mol Med. 2006;6:99–109. doi: 10.2174/156652406775574587. [DOI] [PubMed] [Google Scholar]
- 20.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21(4):1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]