Abstract
The research examining the association between quality of diet and abdominal aortic aneurysm (AAA) is scarce. The aim of the present study was to explore the association between diet quality and development of AAA for middle-aged individuals in the Malmö Diet and Cancer Study (MDCS), a prospective cohort study with baseline data collection carried out between 1991 and 1996. At baseline, the study participants who were eligible for this study (n = 26133) documented their dietary habits in a food diary and questionnaire. Incident AAA cases during an average of 20.7 years of follow-up were identified by using registers. A diet quality index consisting of six components, saturated fat, polyunsaturated fat, fibre, sucrose, fruits and vegetables and fish and shellfish, was used to assess the diet quality. After adjusting for potential confounders, the diet quality index was not associated with incident AAA. However, a tendency of decreased risk was observed among individuals adhering to recommendations for fruit and vegetables compared with non-adherence. When comparing the risk of more extreme intake groups, high intakes of both fruits and vegetables were associated with decreased risk.
Introduction
Abdominal Aortic Aneurysm (AAA) is most commonly defined as an aortic diameter of 3.0 centimetres or more1. The pathogenesis of AAA is not clearly understood, and an AAA usually grows slowly and remains asymptomatic until rupture occurs. The overall mortality of ruptured AAA is still very high, at approximately 81%2.
Smoking is a strong and established risk factor of AAA, and aneurysm growth rate in current smokers was 0.35 mm/year faster than in ex- or never smokers3. Therefore, smoking cessation is the most established and effective method for diminishing growth rate4. Male sex is another risk factor for AAA and it was assessed that AAA is four to six times more common in men compared to women3,5. Most clinical AAA cases are aged above 656. Because of these risk factors, United Kingdom and Sweden have implemented screening with ultrasound for men aged 65 and beyond7.
Maintaining a high-quality diet has been shown to reduce the risk of many conditions, such as cardiovascular disease and diabetes8,9. However, studies examining the association between dietary habits and risk of developing AAA is very limited. A retrospective cross-sectional cohort study conducted in the United States, comprising over 3 million individuals, showed that a high consumption of fruits, vegetables and nuts decreased the risk of AAA10. In a prospective cohort study conducted in Sweden it was reported that a high intake of fruits, but not vegetables, reduces the risk of AAA11. The association between diet quality and incident cardiovascular disease in the Malmö Diet and Cancer Study (MDCS) has previously been explored12, but no research has focused on diet quality and incident AAA.
The aim of the present population-based prospective study on middle-aged individuals in the MDCS was to explore the association between diet quality, specific dietary components and development of AAA.
Method
Study population and data collection
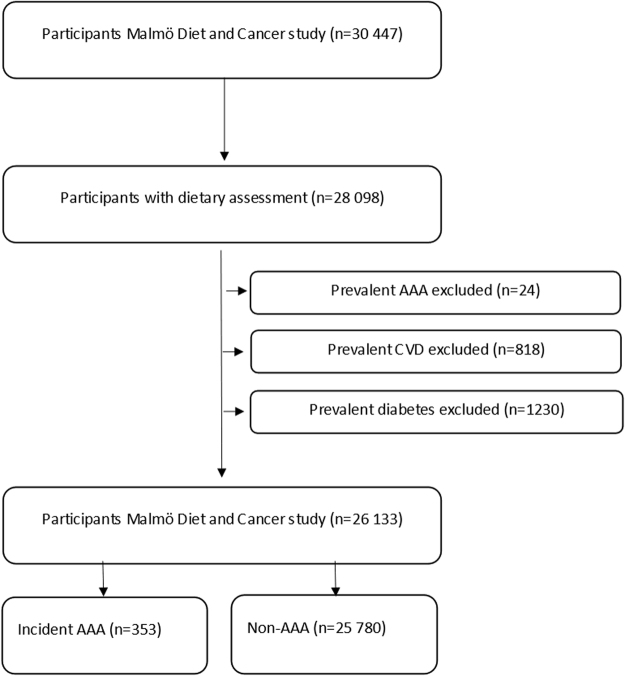
The Malmö Diet and Cancer Study (MDCS) was a prospective cohort study and baseline sampling was carried out between 1991 and 1996. Men born between 1923 and 1945 and women born between 1923 and 1950 were eligible to participate in the study. The participants had to be proficient in Swedish and reside in Malmö, Sweden. In total 30446 participants were recruited, out of which 28098 participated in diet assessment, anthropometric measurements and answered a comprehensive questionnaire. More detailed descriptions of the recruitment and data collection process can be found elsewhere13,14. Participants with prevalent AAA, cardiovascular disease (prior myocardial infarction and stroke) or diabetes mellitus (higher likelihood to change dietary habits) were excluded from the study population. This resulted in a total study population of 26133 (Fig. 1). All participants gave informed consent and the study had ethical clearance from the Regional Ethical Review Board in Lund, Sweden (Dnr §LU5190). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Figure 1.
Descriptive flow diagram of study participants, dietary data and exclusions. AAA indicates abdominal aortic aneurysm and CVD indicates cardiovascular disease. Some individuals had multiple exclusion criteria.
Diet assessment method and construction of the diet quality index
Information on dietary habits was collected through a seven-day food diary where the participant recorded their food intake at lunch and dinner meals and intake of cold beverages, together with a 168-item food frequency questionnaire where frequency and portion-size of foods regularly consumed during the past year were documented15. The food diary was complemented with a one-hour interview which collected detailed data on for instance serving sizes and cooking practises16. The methodology used when developing the diet quality index have been described in detail previously16. Briefly, candidate components had to be available within the MDCS cohort. Trans fatty acids and salt intake had to be excluded, since information on these dietary factors either was lacking or had poor validity. Second, dietary components considered most important in assuring overall diet quality (i.e. food groups and macronutrients) in relation to chronic disease were primarily considered. Third, inter-correlation between components were investigated to assess the mutual independence between components. Considering these three aspects, six dietary components were selected: Saturated fat, polyunsaturated fat, fish and shellfish, dietary fibre, fruit and vegetables, and sucrose. Individuals who reached the recommendations for each of the dietary component outlined in the Swedish nutrition recommendations and the Swedish dietary guidelines, were given one point. For saturated fat, an adjusted cut-off point was used, since only 2% of the study population reached the recommendation levels. The cut-offs for reaching the recommendations were therefore: polyunsaturated fat 5–10 E%, fish and shellfish ≥300 g/week, sucrose ≤10 E%, dietary fibre ≥2.4 g/MJ, saturated fat ≤14 E%, fruit and vegetables (fruit juices excluded) ≥400 g/d. A maximum score of six was attainable in the index, given that the individual adheres to the recommendations for each dietary component. The quality index was also categorised as low (0–1 points), medium (2–4 points) and high (5–6 points). Previous research has found that maintaining a high score on the diet quality index leads to decreased mortality17 and to lower rates of systemic inflammations, which can be directly associated with incidence of cancer and cardiovascular disease18.
Endpoint ascertainment
The personal number of the participants in the MDCS were linked with registries logging first diagnosis of AAA, ruptured AAA or surgical procedure for AAA. The included registries are the Cause of Death Register and the Inpatient and Outpatient Register. The cause of Death Registers included all registered deaths in Sweden as well as the cause of death as noted in the death certificate. The Inpatient and Outpatient Register contained records of all hospitalizations in Sweden as well as diagnostic and procedural codes. A revised version of the International Classification of Disease (ICD), version 8, 9 and 10, was used for both registers. The surgical codes are based on a Swedish classification system.
Other variables
Age and sex was determined by procuring the civic registration numbers of each participant. The weight and height of all participants was registered by nurses and body mass index (BMI) was calculated using the formula kg divided by m2 expressed in kg per m2. Hypertension was defined as systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mmHg DBP or antihypertensive drug treatment. Lifestyle variables were assessed through a self-administered questionnaire. Smoking habits were categorised as never, former and current smoker. Time since smoking cessation was stratified in tertiles and used to define a smoking score. A smoking score ranging from 0 to 4 was established: 0 = never smokers; 1 = no smoking since 25–51 years; 2 = no smoking since 12–24 years; 3 = no smoking since 1–11 years; 4 = currently smoking. In a subgroup of individuals in the present study, this smoking score was highly associated with pack-years smoked19. The participants were divided into five categories based on the highest education level attained, i.e. less than nine years, elementary school (9–10 y), upper secondary school (11–13 y), university without a degree and university degree. Leisure-time physical activity level was expressed as Metabolic equivalent of task (MET) hours per week based on intensity level and time spent at 17 different activities and was divided into five groups. Alcohol habits was divided into six groups. Those who had not consumed alcohol in the last year were categorised as zero-consumers and the rest were divided into gender-specific quintiles according to their intake as reported in the seven-day food diary. Total energy intake was kcal/day from diet and supplement, including alcohol and fibre. Season refers to time of year that the data collection took place and dietary assessment method refers to the change in coding practice after 1 September 199415. Non-adequate reporters of energy were identified by comparing their reported energy intake with their total energy expenditure (i.e., estimated from their calculated basal metabolic rate and self-reports of leisure-time physical activity, work activity, household work, and sleep hours). Individuals with reported energy intake above or below the 95% confidence interval (CI) for total energy expenditure were categorized as “misreporters”. Individuals answering yes to the questionnaire item “Have you substantially changed your dietary habits in the past?” were classified as “dietary changers”20.
Statistical analysis
The baseline characteristics for sex, age, BMI, diet and lifestyle variables were presented as median and interquartile range (IQR) for the continuous variables and as total count and percentage for the categorical variables. For these variables, we also calculated hazard ratio (HR) by using the Cox proportional hazards regression model with years of follow-up as the time scale. For age, BMI and dietary variables, HR per one standard deviation (SD) was calculated. HR for diet variables were adjusted for age, sex, diet assessment method, season and total energy intake and HR for lifestyle factors, hypertension and medication were adjusted for age and sex. The HR for diet quality index categories (low, medium and high) and according to non-adherence and adherence to each dietary recommendation was calculated and adjusted for sex, total energy intake, diet assessment method and season in the basic model, and alcohol consumption, physical activity, smoking status, education level and BMI were added in the extended multivariable model. The mutually adjusted model with the six dietary components had, in addition to the adjusted variables in the multivariable model, been mutually adjusted for the other five diet quality index components. The statistical analyses were carried out in IBM SPSS, version 22 (SPSS Inc, Chicago, IL). The chosen level of statistical significance was 0.05.
Results
Demographic baseline characteristics
In the study population of 26133, 353 (1.4%) had been diagnosed with AAA, during a median follow-up time of 20.7 years (IQR 19.7–21.4). Baseline characteristics of diet variables and life-style factors for AAA individuals and non-AAA individuals are outlined in Table 1. Individuals that developed AAA during follow-up were older, were more often males and had higher BMI than non-AAA individuals. Current smoking at baseline was strongly associated with higher incidence of AAA (HR 9.26; 95% CI 6.81–13.57) compared with never smoking. Individuals with incident AAA were less educated and had a lower degree of leisure-time physical activity than non-AAA individuals (Table 1). Intakes of fibre and fruit and vegetables were lower, and intake of polyunsaturated fat higher among individuals that were diagnosed with AAA compared to the rest of the study population.
Table 1.
Baseline characteristics of incident AAA cases (n = 353) and non-AAA cases (n = 25780) in the Malmö Diet and Cancer cohort.
| AAA cases | Non-cases | HR (95% CI) | |
|---|---|---|---|
| Males (%) | 260 (73.7) | 9616 (37) | 4.68 (3.69–5.93) |
| Age (years) | 62.14 (9.45) | 57.22 (12.91) | 2.20 (1.94–2.50) |
| BMI (kg/m2) | 25.56 (4.59) | 25.15 (4.83) | 1.12 (1.00–1.25) |
| Hypertension | 318 (90.1) | 19646 (76.2) | 1.80 (1.27–2.57) |
| Saturated fat (E%) | 15.75 (5.14) | 15.82 (4.73) | 0.96 (0.87–1.07) |
| Polyunsaturated fat (E%) | 6.07 (2.15) | 5.73 (1.97) | 1.11 (1.00–1.22) |
| Fish and shellfish (g/week) | 290.81 (290.87) | 276.67 (287.76) | 1.03 (0.93–1.14) |
| Fibre (g/MJ) | 1.87 (0.84) | 2.13 (0.78) | 0.86 (0.76–1.00) |
| Fruits and vegetables (g/day) | 274.25 (197.30) | 347.83 (228.72) | 0.77 (0.67–0.88) |
| Sucrose (E%) | 7.76 (5.25) | 8.12 (4.21) | 1.01 (0.91–1.11) |
| Alcohol consumption | |||
| Zero-consumption | 23 (6.5) | 1574 (6.1) | 1.00 |
| Quintile 1 | 70 (19.8) | 4728 (18.3) | 0.83 (0.51–1.32) |
| Quintile 2 | 65 (18.4) | 4772 (18.5) | 0.71 (0.44–1.15) |
| Quintile 3 | 68 (19.3) | 4889 (19) | 0.75 (0.47–1.20) |
| Quintile 4 | 62 (17.6) | 4892 (19) | 0.71 (0.44–1.16) |
| Quintile 5 | 65 (18.4) | 4925 (19.1) | 0.86 (0.54–1.40) |
| Smoking status | |||
| Never | 39 (11) | 10037 (38.9) | 1.00 |
| Current | 214 (60.6) | 7223 (28) | 9.26 (6.81–13.57) |
| Former | 100 (28.3) | 8510 (33) | 2.46 (1.70–3.58) |
| Smoking score (0–4) | 3.03 (1.42) | 1.77 (1.68) | 1.66 (1.54–1.79) |
| Educational level | |||
| Less than 9 years | 196 (55.7) | 10537 (40.9) | 1.00 |
| Elementary school (9–10 y) | 68 (19.3) | 6836 (26.5) | 0.72 (0.54–0.94) |
| Upper secondary school (11–13 y) | 25 (7.1) | 2303 (8.9) | 0.57 (0.38–0.87) |
| University without a degree | 27 (7.7) | 2274 (8.8) | 0.72 (0.48–1.08) |
| University degree | 35 (10.2) | 3772 (14.6) | 0.67 (0.47–0.95) |
| Leisure-time physical activity | |||
| 0–7.5 MET-h/week | 51 (14.6) | 2394 (9.4) | 1.00 |
| 7.5–15 MET-h/week | 60 (17.1) | 3819 (14.9) | 0.72 (0.35–1.04) |
| 15–25 MET-h/week | 66 (18.9) | 5896 (23) | 0.50 (0.35–0.72) |
| 25–50 MET-h/week | 120 (34.3) | 9397 (36.7) | 0.54 (0.39–0.74) |
| >50 MET-h/week | 53 (15.1) | 4098 (16) | 0.46 (0.31–0.68) |
| Use of lipid lowering drugs | 26 (7.4) | 534 (2.1) | 2.52 (1.68–3.76) |
| Use of statins | 17 (4.8) | 344 (1.3) | 2.42 (1.48–3.94) |
| Use of acetyl salicylic acid for cardiovascular disease | 14 (4.0) | 249 (1.0) | 2.91 (1.70–5.00) |
Data are median (IQR), n (%) or mean (SD).
HR for diet variables adjusted for age, sex, diet assessment method, season, energy intake.
HR per 1 SD increment was calculated for age, BMI and dietary intakes.
HR for lifestyle factors, hypertension and medications adjusted for age and sex.
Diet quality index and AAA risk
In the basic model, the diet quality index was associated with a decreased AAA risk (p = 0.03). The association was attenuated in the full multivariable model adjusting for several potential confounders (p = 0.68) (Table 2) and remained the same in a sensitivity analysis after including hypertension, acetyl salicylic acid (ASA) and statins into the full multivariable model (p = 0.70). The risk estimates associated with a 1-point increase in the dietary score was for the basic model 0.89 (95% CI 0.82–0.96) and multi-variable model 0.98 (95% CI 0.91–1.07).
Table 2.
HR and 95% CI for incident AAA by categories of diet quality index (low, medium, high) among participants in the Malmö Diet and Cancer cohort.
| Low (0–1 points) | Medium (2–4 points) | High (5–6 points) | P trend | |
|---|---|---|---|---|
| Cases/Non-cases | 61/4045 | 257/18425 | 35/3310 | |
| Basic modela | 1.00 | 0.82 (0.62–1.09) | 0.62 (0.41–1.95) | 0.03 |
| Multivariable modelb | 1.00 | 0.96 (0.72–1.27) | 0.96 (0.63–1.47) | 0.68 |
aAdjustments for age, sex, total energy intake, diet assessment method, and season.
bAdjustments for age, sex, total energy intake, diet assessment method, season, alcohol consumption, physical activity, smoking, education, and BMI.
Adherence to components of diet index quality recommendations and AAA risk
In the basic model, individuals that adhered to the recommended intake of fibre (HR 0.68; 95% CI 0.53–0.88) and fruits and vegetables (HR 0.59; 95% CI 0.46–0.76) had significantly lower risk of AAA (Table 3). In the multivariable and mutually adjusted multivariable model, a trend remained for adherence to fruits and vegetables and a lower AAA risk (p = 0.07). In a sensitivity analysis, including hypertension, ASA and statins in the mutually adjusted multivariable model, the trend remained (p = 0.07). To compare more extreme intakes, fruits and vegetables were separately divided into four groups (<100 g/day, 100–200 g/day, 200–300 g/day, >300 g/day). Both high fruit and vegetable intake was associated with a lower AAA risk in the multi-variable model (p = 0.017 and 0.006, respectively) with the following HR and 95% CI for fruit intakes: 1.00 (ref), 0.76 (0.59–0.98), 0.63 (0.46–0.88), 0.67 (0.46–0.98) and for vegetable intakes: 1.00 (ref), 0.81 (0.63–1.05), 0.74 (0.53–1.03), 0.60 (0.37–0.98). The associations were virtually unchanged when adjusted for vegetable or fruit intake, respectively.
Table 3.
HR and 95% CI for incident AAA by adherence to diet quality index components among participants in the Malmö Diet and Cancer cohort.
| Dietary components | Non-adherence | Adherence |
|---|---|---|
| Saturated fat | ≥14 E% | ≤14 E% |
| Cases/Non-cases | 250/18401 | 103/7379 |
| Basic modela | 1.00 | 0.96 (0.76–1.22) |
| Multivariable modelb | 1.00 | 1.16 (0.92–1.48) |
| Mutually adjusted multivariable modelc | 1.00 | 1.24 (0.95–1.61) |
| Polyunsaturated fat | <5 E% or >10 E%) | 5–10 E% |
| Cases/Non-cases | 92/8106 | 261/17674 |
| Basic modela | 1.00 | 1.13 (0.89–1.43) |
| Multivariable modelb | 1.00 | 1.10 (0.86–1.40) |
| Mutually adjusted multivariable modelc | 1.00 | 1.09 (0.86–1.40) |
| Fish and shellfish | ≤300 g/week | ≥300 g/week |
| Cases/Non-cases | 187/14095 | 166/11685 |
| Basic modela | 1.00 | 0.89 (0.72–1.11) |
| Multivariable modelb | 1.00 | 0.93 (0.75–1.15) |
| Mutually adjusted multivariable modelc | 1.00 | 0.93 (0.75–1.15) |
| Fibre | ≤2.4 g/MJ | ≥2.4 g/MJ |
| Cases/Non-cases | 271/17266 | 82/8514 |
| Basic modela | 1.00 | 0.68 (0.53–0.88) |
| Multivariable modelb | 1.00 | 0.94 (0.73–1.22) |
| Mutually adjusted multivariable modelc | 1.00 | 0.97 (0.71–1.32) |
| Fruits and vegetables | ≤400 g/day | ≥400 g/day |
| Cases/Non-cases | 269/15880 | 84/9900 |
| Basic modela | 1.00 | 0.59 (0.46–0.76) |
| Multivariable modelb | 1.00 | 0.79 (0.61–1.02) |
| Mutually adjusted multivariable modelc | 1.00 | 0.78 (0.59–1.03) |
| Sucrose | ≥10 E% | ≤10 E % |
| Cases/Non-cases | 102/7415 | 251/18365 |
| Basic modela | 1.00 | 0.89 (0.70–1.12) |
| Multivariable modelb | 1.00 | 0.97 (0.77–1.23) |
| Mutually adjusted multivariable modelc | 1.00 | 0.99 (0.78–1.27) |
aAdjusted for age, sex, total energy intake, diet assessment method, and season.
bAdjusted for age, sex, total energy intake, diet assessment method, season, alcohol consumption, physical activity, smoking, education and BMI.
cAdjusted for age, sex, total energy intake, diet assessment method, season, alcohol consumption, physical activity, smoking, education, BMI and mutual adjustment for the six diet quality index components.
Sensitivity analysis
A sensitivity analysis was performed after removing misreporters (n = 4799) and dietary changers (n = 5623). The association between diet quality index, the six dietary components and incident AAA was virtually unchanged (supplementary Tables 4 and 5).
Discussion
After adjusting for several confounders, Individuals with the highest diet quality score did not have a decreased risk of incident AAA. A tendency of decreased risk was observed among individuals adhering to recommendations for fruit and vegetables compared with non-adherence. When comparing the risk of more extreme intake groups, the highest intake group of fruits (<300 g/day) was associated with 33% decreased risk. The corresponding group for vegetable intake was associated with 40% decreased risk.
The finding regarding fruit consumption is consistent with the findings from another Swedish cohort study, consisting of 80 446 individuals out of which 1086 were incident AAA cases (cumulative AAA incidence 1.3%)11. That study reported that when comparing the group with the highest intake of fruits (>2 servings/day) with the lowest intake group (<0.7 servings/day), the highest group had a 25% decreased risk of incident AAA. A finding consistent with the current study. However, in contrast to that report, which did not identify an association between vegetable consumption and AAA risk11, the present study found that vegetable consumption was associated with lower AAA risk. A potential explanation to the deviations in results can be that the current study has a different diet assessment method compared to the other Swedish cohort study. In addition, it is possible that high consumers of fruit and vegetables has other way of life style than low consumers, that not has been taken into account in the multi-variable analysis, that may explain the protective effect towards AAA development.
The role of antioxidants and their ability to reduced oxidative stress in the aortic wall is one plausible theory that has been suggested as a protective factor of fruit and vegetables in AAA pathogenesis, but there is no hard evidence as there is a paucity of conducted experimental studies on this topic21. In this context, it should also be kept in mind that fruit and vegetables contains numerous types and varying concentrations of antioxidants and other bioactive compounds, for instance flavonoids such as procyanidins22,23 and quercetin24.
A diet with low quality was in the basic statistical model associated with increased AAA risk, which is in line with the large retrospective cross-sectional cohort study reported by Kent et al.10. The study conducted by Kent et al. investigated AAA risk for individuals, in total approximately 3 million, who had previously filled in a medical and lifestyle questionnaire and were screened for AAA. The study did not have an explicit focus on diet but analysed a plethora of potential risk factors, including for instance excess weight, ethnicity and family history. It was concluded that smoking cessation and a healthy lifestyle, including consumption of nuts, fruits and vegetables decreased AAA risk. The present study had detailed information on a large number of confounders that were included in the adjusted models. The association between a low-quality diet and AAA was attenuated in the multivariable model, which indicated that other lifestyle factors impact AAA risk. This is contemporaneous with the study by Kent et al.
In the basic model, fibre intake lowered risk of AAA, but the association disappeared when further adjustments were made. A positive strong correlation between fruit and vegetable and fibre intake in the MDCS has been previously identified16, and the association between fibre and AAA risk was clearly attenuated after adjusting for the other diet components, including fruit and vegetables. Further analysis of other food sources of fibre intake, including fibre rich cereal products25 and incident AAA in MDCS cohort is warranted. It is noteworthy that intake of dietary fibre, fruit and vegetables is higher for women than for men26 possibly contributing to the, in general, lower incidence and later development of AAA in women compared to men7.
The present study design with AAA ascertainment by hospital registers, reflects identification of large-sized AAA and AAA risk in its later stages. Therefore, one of the limitations of the study is the absence of ultrasound measurement of AAA diameter at baseline and we can therefore not exclude the possibility of erroneously including small AAA at study entry. To minimize this bias, however, patients with cardiovascular disease, a co-morbidity highly correlated with concomitant AAA27–29 at baseline were excluded. Moreover, the non-randomized, observational study design does not allow causal interpretations, which is another limitation to the present study.
The cohort was designed to study dietary habits, specifically fibre and fat quality, in a middle-aged and older population. However, self-reported dietary intake data are likely to be associated with some degree of misclassification and may have changed during follow up. Individuals that might have changed the dietary habits, such as patients with known diabetes mellitus, myocardial infarction or stroke, were excluded at baseline. Of note, lower extremity artery disease, a less common atherosclerotic cardiovascular morbidity, than both coronary artery disease and stroke30, was not excluded. On the other hand, individuals with lower extremity artery disease are less exposed to and aware of benefits of dietary interventions compared to individuals with coronary artery disease and stroke31. Hence, it is likely that there will be few dietary changers among the low number of individuals with lower extremity artery disease without other atherosclerotic manifestations in the MDCS cohort. In addition, a sensitivity analysis was performed, excluding misreporters and dietary changers. This analysis showed that when these individuals were excluded, the associations between the diet quality index, dietary components and incident AAA were unchanged, which confirms the findings from the whole population on diet and AAA risk. Excluding misreporters and dietary changers has been shown to result in a stable population on food habits throughout the follow-up period20.
One might argue that a supplementary evaluation of other established healthy indices such as the Mediterranean Diet score32 or Dietary Approach to Stop Hypertension (DASH)33 in relation to development of AAA would have strengthened the present study. The present diet quality index was developed to particularly assess adherence to the Swedish nutrition recommendations and the Swedish dietary guidelines16. This diet quality index was found to be valid and showed that a high diet quality was associated with a decreased risk of cardiovascular events12 in the MDCS. As a next step, it was considered warranted to evaluate the utility of this, in comparison to the above-mentioned indices, simple tool for assessment of incident AAA, a disease related to the same risk factors as those with cardiovascular events12. It was not possible to show that high-quality diet was associated with decreased AAA risk, which probably may be due to a too low number of individuals with AAA during follow up, leading to a type 2 statistical error. Perhaps a future analysis with a higher cumulative incidence of AAA would yield another result.
Besides baseline measurement, repeated measurements of life style modifiable risk factors for AAA would have been valuable, since for instance smoking prevalence has decreased7 and intakes of fruit, berries and vegetables may have increased in recent times34. The cumulative AAA incidence of 1.4% in the present cohort study is relatively high in view of the predominance of female individuals. This can be compared with the current AAA prevalence of 1.5% among 65-year old ultrasound screened Swedish men35. The large sample size, the long follow up time and the high relative validity of the dietary intake assessment34,36 are other factors that strengthen the present study. Finally, the individuals were middle-aged or older at baseline, which means that the extension of follow-up period ensured evaluation of most males beyond 80 years, an age where AAA incidence for men peaks37.
In conclusion, while individuals with the highest diet quality score did not reduce their AAA risk, high intakes of vegetables and fruits decreased the risk of incident AAA in the present study.
Electronic supplementary material
Acknowledgements
The authors wish to thank all participants in the Malmö Diet and Cancer Study for making this study possible.
Author Contributions
S.N., E.S., and S.A. had full access to all data, taking responsibility for its integrity and the accuracy of the data analyses, and had final responsibility for the decision to submit for publication. All authors have read and agree to the manuscript as written.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
A correction to this article is available online at https://doi.org/10.1038/s41598-018-25616-0.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20415-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/15/2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
- 1.Steinberg I, Stein HL. Arteriosclerotic abdominal aneurysms. Report of 200 consecutive cases diagnosed by intravenous aortography. Jama. 1966;195:1025–1029. doi: 10.1001/jama.1966.03100120093023. [DOI] [PubMed] [Google Scholar]
- 2.Reimerink JJ, van der Laan MJ, Koelemay MJ, Balm R, Legemate DA. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. British Journal of Surgery. 2013;100:1405–1413. doi: 10.1002/bjs.9235. [DOI] [PubMed] [Google Scholar]
- 3.Sweeting MJ, Thompson SG, Brown LC, Powell JT. Meta‐analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. British Journal of Surgery. 2012;99:655–665. doi: 10.1002/bjs.8707. [DOI] [PubMed] [Google Scholar]
- 4.MacSweeney ST, Ellis M, Worrell PC, Greenhalgh RM, Powell JT. Smoking and growth rate of small abdominal aortic aneurysms. Lancet (London, England) 1994;344:651–652. doi: 10.1016/S0140-6736(94)92087-7. [DOI] [PubMed] [Google Scholar]
- 5.Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force. Annals of internal medicine. 2005;142:203–211. doi: 10.7326/0003-4819-142-3-200502010-00012. [DOI] [PubMed] [Google Scholar]
- 6.Norman PE, Powell JT. Site specificity of aneurysmal disease. Circulation. 2010;121:560–568. doi: 10.1161/CIRCULATIONAHA.109.880724. [DOI] [PubMed] [Google Scholar]
- 7.Svensjo S, et al. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation. 2011;124:1118–1123. doi: 10.1161/CIRCULATIONAHA.111.030379. [DOI] [PubMed] [Google Scholar]
- 8.Jonasson L, Guldbrand H, Lundberg AK, Nystrom FH. Advice to follow a low-carbohydrate diet has a favourable impact on low-grade inflammation in type 2 diabetes compared with advice to follow a low-fat diet. Annals of medicine. 2014;46:182–187. doi: 10.3109/07853890.2014.894286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu E, et al. Diet, Lifestyle, Biomarkers, Genetic Factors, and Risk of Cardiovascular Disease in the Nurses’ Health Studies. American Journal of Public Health. 2016;106:1616–1623. doi: 10.2105/AJPH.2016.303316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kent KC, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. Journal of Vascular Surgery. 2010;52:539–548. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 11.Stackelberg O, Bjorck M, Larsson SC, Orsini N, Wolk A. Fruit and vegetable consumption with risk of abdominal aortic aneurysm. Circulation. 2013;128:795–802. doi: 10.1161/CIRCULATIONAHA.112.000728. [DOI] [PubMed] [Google Scholar]
- 12.Hlebowicz J, et al. A high diet quality is associated with lower incidence of cardiovascular events in the Malmo diet and cancer cohort. PLoS One. 2013;8:e71095. doi: 10.1371/journal.pone.0071095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manjer J, et al. The Malmo Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP) 2001;10:489–499. doi: 10.1097/00008469-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Manjer J, Elmstahl S, Janzon L, Berglund G. Invitation to a population-based cohort study: differences between subjects recruited using various strategies. Scandinavian journal of public health. 2002;30:103–112. doi: 10.1177/14034948020300020401. [DOI] [PubMed] [Google Scholar]
- 15.Wirfalt E, et al. A methodological report from the Malmo Diet and Cancer study: development and evaluation of altered routines in dietary data processing. Nutrition journal. 2002;1:3. doi: 10.1186/1475-2891-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drake I, et al. Development of a diet quality index assessing adherence to the Swedish nutrition recommendations and dietary guidelines in the Malmo Diet and Cancer cohort. Public Health Nutrition. 2011;14:835–845. doi: 10.1017/S1368980010003848. [DOI] [PubMed] [Google Scholar]
- 17.Drake I, et al. Scoring models of a diet quality index and the predictive capability of mortality in a population-based cohort of Swedish men and women. Public Health Nutrition. 2013;16:468–478. doi: 10.1017/S1368980012002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dias JA, et al. A high quality diet is associated with reduced systemic inflammation in middle-aged individuals. Atherosclerosis. 2015;238:38–44. doi: 10.1016/j.atherosclerosis.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Fagerberg B, et al. Circulating cadmium concentration and risk of aortic aneurysms: a nested case-control study within the Malmö Diet and Cancer cohort. Atherosclerosis. 2017;261:37–43. doi: 10.1016/j.atherosclerosis.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Sonestedt, E. et al. Diet Quality and Change in Blood Lipids during 16 Years of Follow-up and Their Interaction with Genetic Risk for Dyslipidemia. Nutrients8, 10.3390/nu8050274 (2016). [DOI] [PMC free article] [PubMed]
- 21.McCormick ML, Gavrila D, Weintraub NL. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:461–469. doi: 10.1161/01.ATV.0000257552.94483.14. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen SE, Frederiksen H, Struntze Krogholm K, Poulsen L. Dietary proanthocyanidins: occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Molecular nutrition & food research. 2005;49:159–174. doi: 10.1002/mnfr.200400082. [DOI] [PubMed] [Google Scholar]
- 23.Gu L, et al. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. The Journal of nutrition. 2004;134:613–617. doi: 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- 24.Griffiths, K. et al. Food Antioxidants and Their Anti-Inflammatory Properties: A Potential Role in Cardiovascular Diseases and Cancer Prevention. Diseases (Basel, Switzerland)4, 10.3390/diseases4030028 (2016). [DOI] [PMC free article] [PubMed]
- 25.Vulcan A, et al. Fibre intake and incident colorectal cancer depending on fibre source, sex, tumour location and Tumour, Node, Metastasis stage. British Journal of Nutrition. 2015;114:959–969. doi: 10.1017/S0007114515002743. [DOI] [PubMed] [Google Scholar]
- 26.Amcoff, E. et al. Livsmedels- och näringsintag bland vuxna i Sverige. (Uppsala, 2012).
- 27.Benson RA, Poole R, Murray S, Moxey P, Loftus IM. Screening results from a large United Kingdom abdominal aortic aneurysm screening center in the context of optimizing United Kingdom National Abdominal Aortic Aneurysm Screening Programme protocols. Journal of Vascular Surgery. 2016;63:301–304. doi: 10.1016/j.jvs.2015.08.091. [DOI] [PubMed] [Google Scholar]
- 28.Hernesniemi JA, Vanni V, Hakala T. The prevalence of abdominal aortic aneurysm is consistently high among patients with coronary artery disease. Journal of Vascular Surgery. 2015;62:232–240 e233. doi: 10.1016/j.jvs.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 29.Flessenkaemper IH, et al. Screening of COPD patients for abdominal aortic aneurysm. International Journal of Chronary Obstructive Pulmononary Disease. 2015;10:1085–1091. doi: 10.2147/COPD.S81439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fowkes FG, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet (London, England) 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Canela M, Martinez-Gonzalez MA. Lifestyle and dietary risk factors for peripheral artery disease. Circulation journal: official journal of the Japanese Circulation Society. 2014;78:553–559. doi: 10.1253/circj.CJ-14-0062. [DOI] [PubMed] [Google Scholar]
- 32.Mattioli AV, et al. Relationship between Mediterranean diet and asymptomatic peripheral arterial disease in a population of pre-menopausal women. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2017;27:985–990. doi: 10.1016/j.numecd.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Cohen JFW, Lehnerd ME, Houser RF, Rimm EB. Dietary Approaches to Stop Hypertension Diet, Weight Status, and Blood Pressure among Children and Adolescents: National Health and Nutrition Examination Surveys 2003–2012. Journal of the Academy of Nutrition and Dietetics. 2017;117:1437–1444.e1432. doi: 10.1016/j.jand.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Riboli E, Elmstahl S, Saracci R, Gullberg B, Lindgarde F. The Malmo Food Study: validity of two dietary assessment methods for measuring nutrient intake. International journal of epidemiology. 1997;26(Suppl 1):S161–173. doi: 10.1093/ije/26.suppl_1.S161. [DOI] [PubMed] [Google Scholar]
- 35.Wanhainen A, et al. Outcome of the Swedish Nationwide Abdominal Aortic Aneurysm Screening Program. Circulation. 2016;134:1141–1148. doi: 10.1161/CIRCULATIONAHA.116.022305. [DOI] [PubMed] [Google Scholar]
- 36.Elmstahl S, Riboli E, Lindgarde F, Gullberg B, Saracci R. The Malmo Food Study: the relative validity of a modified diet history method and an extensive food frequency questionnaire for measuring food intake. European journal of clinical nutrition. 1996;50:143–151. [PubMed] [Google Scholar]
- 37.Bengtsson H, Sonesson B, Bergqvist D. Incidence and prevalence of abdominal aortic aneurysms, estimated by necropsy studies and population screening by ultrasound. Annals of the New York Academy of Sciences. 1996;800:1–24. doi: 10.1111/j.1749-6632.1996.tb33294.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.