Abstract
Double infections of Wolbachia and Spiroplasma are frequent in natural populations of Tetranychus truncatus, a polyphagous mite species that has been a dominant species in China since 2009. However, little is known about the causes and ecological importance of such coexistences. In this study, we established T. truncatus strains with different infection types and then inferred the impact of the two endosymbionts on host reproduction and fitness. Double infection induced cytoplasmic incompatibility, which was demonstrated by reduction in egg hatchability of incompatible crosses. However, doubly infected females produced more eggs relative to other strains. Wolbachia and Spiroplasma did not affect host survival, whereas doubly infected females and males developed faster than other strains. Such reproduction and fitness benefits provided by double infections may be associated with the lower densities of each symbiont, and the quantitative results also confirmed competition between Wolbachia and Spiroplasma in doubly infected females. These symbiont‐conferred beneficial effects maintain stable prevalence of the symbionts and also help drive T. truncatus outbreaks in combination with other environmental factors.
Keywords: double infection, fitness, reproduction, symbiont, Tetranychus truncatus
1. INTRODUCTION
Heritable bacterial endosymbionts are widespread in arthropods. They utilize diverse strategies to spread within host populations, such as provision of essential nutrients (Gündüz & Douglas, 2009; Hosokawa, Koga, Kikuchi, Meng, & Fukatsu, 2010), defense against parasites (Hedges, Brownlie, O'Neill, & Johnson, 2008; Vorburger, Gehrer, & Rodriguez, 2010), and reproductive manipulation (Ma, Vavre, & Beukeboom, 2014).
Endosymbionts have traditionally been grouped into primary and secondary symbionts. Primary symbionts are mutualists that tend to have a nutritional value, which is the case in the typical example of aphids and Buchnera aphidicola (Baumann, 2005). In contrast, secondary symbionts are often facultative symbionts from the host's perspective and have a much broader array of effects. Most symbionts are exclusively or predominantly vertically transmitted; however, the often‐found incongruence between host and symbiont phylogenies indicates that these infections are generally short‐lived on macro‐evolutionary timescales. In theory, once a symbiont invades a novel host, it must immediately be capable of replication and colonization of the female germ line. Hurst and Darby (2009) also speculated that high rates of horizontal transmission, reproductive manipulation, and direct fitness benefits to the host will favour maintenance of secondary symbionts within the host population. The high prevalence of several secondary symbionts without obvious parasitic phenotypes can sometimes be linked to such direct fitness effects; for example, increased performance and sex ratio bias of infected whiteflies sufficiently explain the spread of Rickettsia symbionts (Himler et al., 2011).
Among the secondary symbionts, Wolbachia is by far the most widely documented and infects a large proportion of arthropod species (Werren, Baldo, & Clark, 2008). Wolbachia is notorious for its reproductive parasitism, which ensures its spread despite lowering host fitness. However, even for reproductive parasites, it can be beneficial to enhance host fitness. Indeed, recent years have seen a growing body of evidence on Wolbachia‐associated fitness benefits (reviewed in Zug & Hammerstein, 2015). Spiroplasma is a wall‐less bacterium associated with diverse arthropods and plants in which it has commensal, pathogenic, or mutualistic effects (Cisak et al., 2015). Some Spiroplasma species and strains are known as male killers in fruit flies, ladybird beetles, planthoppers, and butterflies, wherein infected females produce all‐female or female‐biased offspring (Jiggins, Hurst, Jiggins, VD Schulenburg, & Majerus, 2000; Sanada‐Morimura, Matsumura, & Noda, 2013; Tinsley & Majerus, 2006; Williamson et al., 1999). Independent studies have shown that the two symbionts simultaneously infect some arthropods (Duron et al., 2008; Enigl & Schausberger, 2007; Goodacre, Martin, Thomas, & Hewitt, 2006; Jaenike, Stahlhut, Boelio, & Unckless, 2010; Shokal et al., 2016). In Drosophila neotestacea, recent spread and mutualism account for the positive association between Wolbachia and Spiroplasma (Jaenike et al., 2010). However, the ecological and evolutionary importance of such coexistences remains underexplored.
Tetranychus truncatus is a polyphagous species that infests more than 60 host plant species (Bolland, Gutierrez, & Flechtmann, 1998). It is mainly distributed in East and Southeast Asia, and there is report of its presence in the USA (Migeon & Dorkeld, 2006, 1996). T. truncatus have already become the dominant pest in China since 2009 based on analysis of spider mite sampling data from 2002 to 2015. T. truncatus are host to several symbionts; for example, previous studies revealed that T. truncatus is infected with diverse Wolbachia strains (Zhang et al., 2013), and double infections of Wolbachia and Cardinium are frequent in some natural populations (Zhao, Zhang, & Hong, 2013). Recently, high prevalence of Spiroplasma was identified in T. truncatus, and there was a substantial positive association between Wolbachia and Spiroplasma in some populations (Zhang, Chen, Yang, Qiao, & Hong, 2016).
Given the high rate of co‐transmission of these two symbionts and recent T. truncatus outbreaks, we investigated the mutualistic phenotypes of these two symbionts within T. truncatus. We established different T. truncatus strains with the same host genetic background and investigated the effect of Wolbachia alone or in combination with Spiroplasma on T. truncatus reproduction and fitness. Furthermore, the densities of the two symbionts were monitored and compared during host development to infer their competition relationship.
2. MATERIALS AND METHODS
2.1. Wolbachia and Spiroplasma prevalence in T. truncatus
Adult T. truncatus were collected from six populations in northern China (Figure 1). Individual mites were either immediately analyzed in the laboratory to assess infection frequency (see below) or individual females were reared as isofemale lines on leaves of common bean (Phaseolus vulgaris L.) placed on a water‐saturated sponge mat in Petri dishes (dia. 9) at 25 ± 1°C, 60% humidity, and under L16–D8 conditions.
Figure 1.
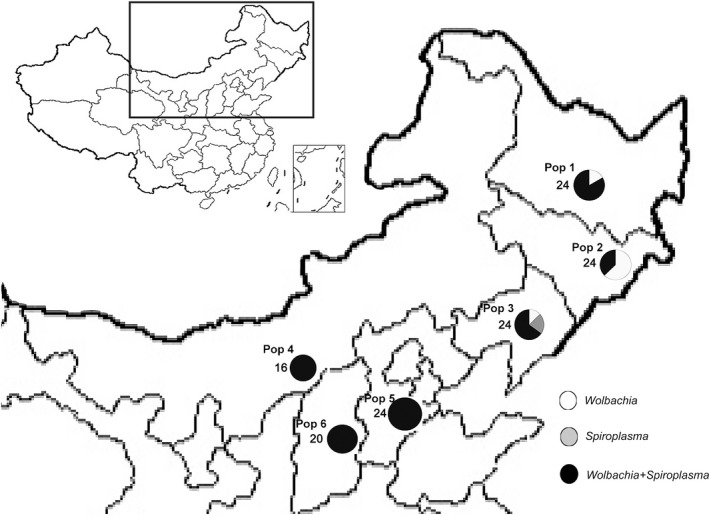
Infection frequencies of Wolbachia and Spiroplasma in six Tetranychus truncatus populations. Numbers indicate sample size and proportions of circles indicate infection frequencies. Individuals of Pop 1–6 were collected from Harbin Heilongjiang (N46.19–E125.0), Yanji Jilin (N43.13–E129.03), Shenyang Liaoning (N41.52–E123.22), Hohhot Inner Mongolia (N40.47–E111.47), Cangzhou Hebei (N38.18–E116.52) and Changzhi Shanxi (N35.55–E113.34), respectively, in August 2014
Subsequently, 16–24 field‐collected individual mites were screened for infection with Wolbachia and/or Spiroplasma. DNA was extracted from single mites using a genomic DNA extraction kit (TaKaRa, Dalian, China). All DNA samples were first PCR‐screened for the mitochondrial gene COI as a control for quality (Navajas, Gutierrez, Lagnel, & Boursot, 1996). Wolbachia and Spiroplasma presence was detected using PCR amplifications of wsp and 16S rRNA, respectively. wsp was amplified using the primers wspF1 and wspR1 (Baldo et al., 2006), and 16S rRNA was amplified using the primers SpitsJ04 and SpitsN55 (Jaenike, Polak, Fiskin, Helou, & Minhas, 2007). In addition, to confirm the presence of other two reproductive endosymbionts, Cardinium and Rickettsia, in T. truncates. PCR amplifications were performed using primers Ch‐F and Ch‐R (Zchori‐Fein & Perlman, 2004), RICS741F and RCIT1197R (Davis, Ying, Brunner, Pantoja, & Ferwerda, 1998) that were widely used to detect Cardinium and Rickettsia, respectively. Each reaction was carried out on a Veriti machine (ABI Biosystems, USA) in a 25 μl volume containing 12.5 μl 2× Taq Master Mix (Vazyme Biotech, China), 0.5 μl primer (20 μmol/L each), and 1 μl of DNA extract. Positive and negative controls were included in PCRs.
2.2. Establishment of experimental T. truncatus strains
Experimental T. truncates strains with different infection patterns were established to determine the effects of endosymbiont on host biology. In this study, we firstly established four T. truncates strains: mites infect with both Wolbachia and Spiroplasma (designated as W+S+), Wolbachia only (W+S−), Spiroplasma only (W−S+), and no symbionts (W−S−). To eliminate the effects of host genetic background, the above‐mentioned four T. truncatus strains were derived from lines collected in Shenyang, Liaoning (Pop 3 in Figure 1). The original field females included three strains, W+S+, W+S−, and W‐S+. The W−S− strains were obtained by raising infected mites on the common bean placed on a cotton bed soaked in tetracycline solution (0.1%, w/v) for three generations. These lines were maintained in a mass‐rearing environment without antibiotics for approximately six generations to allow recovery from tetracycline treatment. For each of these lines, infection status was checked during the course of the experiments.
2.3. Crossing experiments
To test whether Wolbachia and Spiroplasma cooperate to cause cytoplasmic incompatibility (CI) in T. truncatus, crossing experiments were performed (listed as male × female, Figure 2). Single females in the teleiochrysalis stage (the last developmental stage before adult emergence) were placed with a 1‐day‐old adult virgin male from the same culture on the same leaf disk. Each cross used 16 leaf disks. Males were discarded 2 days after the females reached adulthood. The mated females were allowed to oviposit for 5 days. The eggs on the leaf disks were checked daily to determine hatchability and the sex ratio (% females). Prior to analyses, data were first tested for normality (Kolmogorov–Smirnov test, SPSS 17.0) and homogeneity of group variances (Levene's test, SPSS 17.0). Which possible, square root, logarithmic or arcsine transformations were performed to attain normality and homogeneity of variance. In this study, log transformation was used for the number of eggs laid per female, and arcsine square root transformation was used for egg hatchability and sex ratio. Data were then analyzed with one‐way analysis of variance (ANOVA), and the means were compared using the Tukey HSD test (SPSS 17.0).
Figure 2.

Results of crosses between different Tetranychus truncatus strains. Number of eggs (a), egg hatch percent (b), and female proportion of offspring (c) are shown. Results are mean ± SEM, and a and b represent statistically different groups (Tukey‐HSD test, p < .05)
The male‐killing phenotype of Spiroplasma in arthropods results in the production of female‐biased offspring sex ratios. Here, the sex ratio data of the four T. truncatus strains were analyzed to determine if male‐killing occurred.
2.4. Survival assay
To test for potential effects of symbionts on host survival, we measured the survival of different T. truncatus strains by placing eight virgin females and eight virgin males from the same strains on the same leaf. Three leaves were used for each line, and females were transferred to fresh leaves every 3 days. The number of dead females was recorded daily. Survivor curves for individual hosts were compared using the Kaplan–Meier method and log‐rank test (Dobson, Rattanadechakul, & Marsland, 2004).
2.5. Development assay
The effect of symbionts on mite development was assessed. Thirty virgin and 30 mated females (which produce male and female offspring, respectively) were placed on a leaf disk and allowed to lay eggs for 8 hr. The eggs were individually moved to new small leaf disks. The small disks were monitored every 8 hr, and the stage of mite was recorded until adulthood. The development time of every stage was calculated. Log transformation was used for the total development time of each mite line, and differences were analyzed using the Mann–Whitney test (SPSS 17.0).
2.6. Wolbachia and Spiroplasma densities
The Wolbachia and Spiroplasma densities in individual mites were estimated by real‐time quantitative PCR (QPCR). QPCR was carried out with the ABI PRISM 7300 Sequence Detection System (Applied Biosystems). We used primers designed to amplify a 141‐bp fragment of wsp from Wolbachia (wQF1, 5′‐GAGCAGCGAATGTAAGCAATC‐3′, and wQR1, 5′‐AATAACGAGCACCAGCATAAAG‐3′) and a 141‐bp fragment of 16S rRNA from Spiroplasma (sQF1, 5′‐TGTAGTTCTCAGGGATTGTTTTCTC‐3′, and sQR1, 5′‐CGCTTCCACCATCGCTCTT‐3′). The PCR products of primers specific for wsp for Wolbachia and 16S rRNA for Spiroplasma were amplified by conventional PCR; then, the PCR products were purified using the AxyPrep TM DNA Gel Extraction kit (AXYGEN) and cloned into a pEASY‐T1 vector (TransGen Biotech, Beijing, China). A series of DNA standards prepared from plasmid DNA was used, and standard curves were plotted using a 10‐fold dilution series from 104 to 108 copy numbers. Ct values in each dilution were measured by QPCR to generate the standard curves for wsp and 16S rRNA. The 20 μl reaction mixture consisted of 10 μl 2× SYBR Premix Ex Taq (Applied Biosystems), 0.4 μl 10 mmol/L of each primer, 0.4 μl 50× ROX Reference Dye, 2 μl DNA template, and 6.8 μl H2O in single wells of a 96‐well plate (PE Applied Biosystems). The QPCR cycling conditions included one cycle (10 s at 95°C) followed by 40 cycles (5 s at 95°C and 31 s at 60°C), and finally one cycle (15 s at 95°C, 1 min at 60°C and 15 s at 95°C).
Eight each of singly and doubly infected females of 2‐, 4‐, 6‐ 8‐, and 12‐days‐old were collected. DNA of individual mites was extracted as described above. Three replicates were run and averaged for each DNA sample. Negative controls were included in all amplification reactions. From the slopes, a high amplification efficiency of 0.95 was determined for both wsp and 16S rRNA in the investigated range. The number of molecules in all samples is determined from the threshold cycles in the PCR based on a standard curve. Statistical analysis was performed using the Mann–Whitney U test.
3. RESULTS
3.1. Double infections of Wolbachia and Spiroplasma are frequent in T. truncatus
Among 132 wild‐caught adult mites that were collected from six sites, 16.7% and 3.8% were infected with only Wolbachia or Spiroplasma, respectively, and 79.5% were infected with both symbionts. Double infections with high prevalence were present in all screened populations (Figure 1). The infections of Cardinium and Rickettsia were not detected in these examined samples.
Sequencing of Wolbachia wsp identified two haplotypes that differed by one base (wTru1 in Pop 2, 4, 5, and 6; wTru5 in Pop 1 and 3). However, identical Spiroplasma 16S rRNA sequences were observed among infected individuals.
3.2. Wolbachia and Spiroplasma have diverse effects on host reproduction
To clarify the effects of Wolbachia and Spiroplasma on host reproduction, egg number, egg hatchability, and offspring sex ratio of the 16 crosses were compared (Figure 2 A, B, C). Among the four crosses in which we tested possible Wolbachia CI effects, the egg hatchability of the predicted compatible crosses (W+S− × W+S−) was significantly lower than that in other crosses, whereas the female proportion was significantly higher in this cross. Doubly infected males appeared to induce CI, which was inferred based on significant reductions of egg hatchability and female proportion of offspring in the predicted incompatible crosses. However, no significant effects were found for other crosses in which singly Spiroplasma‐infected males and Spiroplasma‐uninfected males were investigated. In addition, nonsignificant differences in female proportion of the four strains indicated that Spiroplasma does not cause male killing in T. truncatus.
The effects of Wolbachia and Spiroplasma on host fecundity were also measured. The doubly infected females mostly showed the highest egg production, except for the crosses that involved the singly Wolbachia‐infected males, in which singly Spiroplasma‐infected females produced more eggs. In general, egg production of singly infected females and uninfected females did not significantly differ across all crosses.
3.3. Wolbachia and Spiroplasma do not affect host survival
The longevities of four female lines were compared to discover the effects of infection. As shown in Figure 3, there was no significant effect of Wolbachia and Spiroplasma on female host survival (log‐rank test, p = .87).
Figure 3.
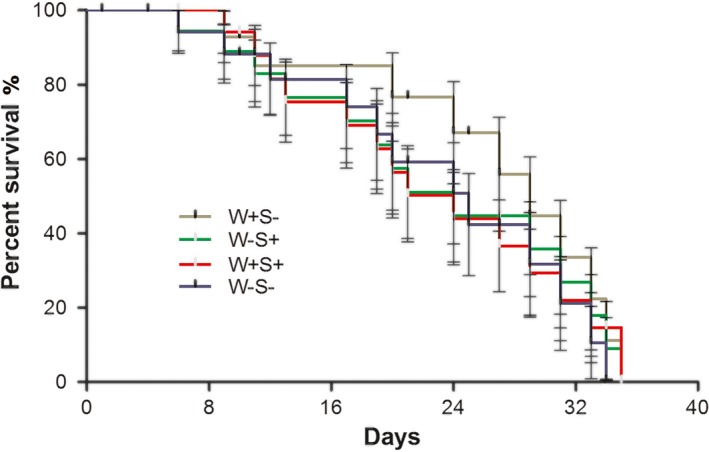
Comparison of Wolbachia and Spiroplasma effects on female Tetranychus truncatus survival. Survival curves for individual hosts were compared using the Kaplan–Meier method and log‐rank test
3.4. Double infections can shorten host developmental rate
The development times of females and males from different strains are presented in Figure 4A,B, respectively. Doubly infected females reached adulthood (10.8 ± 0.004) significantly earlier than singly infected females by 1–2 days (W+S−: 12.38 ± 0.13, W−S+: 12.11 ± 0.13) and uninfected females (11.81 ± 0.003) (p < .0001). Additionally, time to adulthood was significantly shorter in uninfected than singly infected females (p < .05), and the latter two had no significant difference (p = .97). Similarly, doubly infected (7.27 ± 0.19) and uninfected males (6.99 ± 0.25) developed faster than singly infected males (W+S−: 7.83 ± 0.15, W−S+: 8.13 ± 0.11) (p < .05), whereas no significant difference was detected between doubly infected and uninfected males (p = .41), singly Wolbachia‐ and singly Spiroplasma‐infected males (p = .31).
Figure 4.
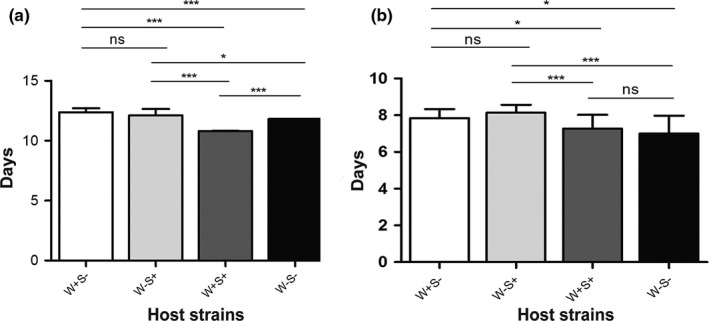
Development times of different female (a) and male (b) Tetranychus truncatus strains. Differences were analyzed using Mann–Whitney test (*p < .05; ***p < .001; NS, not significant)
3.5. Wolbachia and Spiroplasma densities are higher in singly than doubly infected females
The Wolbachia density steadily increased (except for 2‐day‐old to 4‐day‐old adults of the singly infected strain) as adult females developed in both strains. At three developmental stages, 2, 4, and 6 days after emergence, the Wolbachia densities in singly infected females were significantly higher in doubly infected females (Figure 5A, p < .01). A similar pattern was observed for Spiroplasma, which revealed that Spiroplasma density dynamics were significantly affected by coinfecting Wolbachia (Figure 5B, p < .001).
Figure 5.

Density dynamics of Wolbachia and Spiroplasma during host development in singly and doubly infected female Tetranychus truncatus. Copy numbers per ml were determined by quantitative PCR. Asterisks indicate statistically significant differences (Mann–Whitney U test, *p < .05; **p < .01; ***p < .001; NS, not significant)
4. DISCUSSION
In this study, we found that double infections of Wolbachia and Spiroplasma within the same individuals are frequent in natural T. truncatus populations. Among the six examined populations, 79.5% of mites were doubly infected, and double infection was fixed in three populations. Wolbachia and Spiroplasma are both maternally transmitted symbionts and act as reproductive manipulators in many arthropods. Symbiont infection frequency is theoretically determined by several factors, including reproductive manipulation, infection costs, vertical transmission efficiency, and horizontal transmission rate (Hoffmann, Hercus, & Dagher, 1998; Werren, 1997). In this respect, empirical studies have revealed that facultative symbionts can spread quickly within the host population when they provide a large fitness benefit at no or relatively little cost (Himler et al., 2011; Jaenike et al., 2010; Oliver, Campos, Moran, & Hunter, 2008). In such cases, reproductive manipulations and/or providing fitness benefits of double infections would be predicted in T. truncatus.
Wolbachia‐induced CI phenotypes have been observed in some Tetranychus species (Gotoh, Noda, & Hong, 2003; Zhao, Chen, Ge, Gotoh, & Hong, 2013; Zhu et al., 2012). However, there was no evidence of Wolbachia‐mediated CI in T. truncatus, which is consistent with a previous finding (Zhao, Chen, et al., 2013). Singly Wolbachia‐infected females may suffer fitness costs, such as lower hatch percent, but they also have a higher female offspring proportion. Wolbachia strains that infect T. truncatus are phylogenetically diverse (Zhang et al., 2013), and non‐CI Wolbachia were present in this study. Some likely reasons have been explained for the lack of CI (Ros & Breeuwer, 2009). Although Spiroplasma carried by T. truncatus has a closer phylogenetic relationship with the male‐killing strain in small brown planthopper, Laodelphax striatellus (Sanada‐Morimura et al., 2013), it is not a male killer, because Spiroplasma did not cause significant differences in offspring sex ratio, and its incidence in male hosts was relatively high.
It is worth noting that doubly infected males were incompatible with both singly infected and uninfected females, which was inferred based on significant reduction in the egg hatchability. The female offspring proportions of uninfected females were significantly lower than those of other strains, and symbionts carried by females might have rescue effects on offspring sex ratio. These observations suggested that double infections of Wolbachia and Spiroplasma can induce a CI‐like phenotype in T. truncates, and this phenotype is only associated with double infections. By comparison, the CI phenotype is different from the reports of double infections of Wolbachia‐ and Cardinium‐induced CI in T. truncatus (Zhao, Chen, et al., 2013), T. piercei (Zhu et al., 2012), and Bryobia sarothamni (Ros & Breeuwer, 2009), in which double infections induced strong CI and single infections (Wolbachia or Cardinium) also induced CI. Together, we infer that interactions between Wolbachia and Spiroplasma resulted in CI induction, and the underlying mechanism merits further study.
Symbionts' complex effects on their hosts result from long‐term interactions and coevolution. Symbionts may manipulate their host physiological process and affect their fitness traits. wMelPop Wolbachia is well known for shortening the life of its Drosophila host (Min & Benzer, 1997). In Drosophila hydei, Spiroplasma showed enhanced survival when attacked by Leptopilina heterotoma wasps (Xie, Vilchez, & Mateos, 2010). Negative effects of symbionts on host survival were also observed (Costopoulos, Kovacs, Kamins, & Gerardo, 2014).
In this study, symbionts did not influence T. truncatus survival, but significantly regulated female fecundity. Relative to singly infected and uninfected females, doubly infected females produced more eggs. Fecundity advantage provided by symbionts is not surprising, and other studies report a similar phenomenon (Dobson et al., 2004; Himler et al., 2011; Zug & Hammerstein, 2015). These findings are concordant with a hypothesis that vertically transmitted symbionts cause their hosts to produce more infected daughters than are produced by uninfected females to facilitate their own spread (Werren & O'Neill, 1997). The infection density of symbionts is among the most important factors that influence their biological effects, such as intensity of reproductive phenotypes, level of fitness effects, and fidelity of vertical transmission. Oliver et al. (2006) proposed that over‐proliferation of symbionts may be harmful to the aphid host. In T. truncatus, the high density of symbionts may result in fecundity cost to singly infected females. Additionally, QPCR estimates also indicated that numbers of each of the two symbionts in singly infected females are significantly higher than those in doubly infected females.
In addition, double infections appeared to enhance female and male mites' developmental speed. Whatever the mechanism, it is evident that more generations will be produced within the doubly infected mite strains under certain circumstances. As a result, T. truncatus occurrence will directly benefit from these manipulations, and such manipulations will also maintain higher frequencies of double infection in nature. Similarly, Himler et al. (2011) found that Rickettsia‐infected whiteflies developed faster, and this observed increased performance can partly be explained by the rapid spread of Rickettsia across the southwestern United States within 8 years. Moreover, Jaenike et al. (2010) found that the frequent positive association between Wolbachia and Spiroplasma within D. neotestacea is a consequence of emerging symbiotic mutualism. Together, these observations strongly highlight symbionts' substantial influences on their hosts' ecology and population dynamics. Furthermore, these impacts are often influenced by symbionts (strains or infection patterns) and hosts (genetic background). For example, the fitness benefits to T. truncatus are mainly associated with double infections, which raises the possibility that Wolbachia and Spiroplasma coordinately regulate host biological traits.
When several symbionts are simultaneously present within the same host, symbiont–symbiont interactions can take place and substantially affect infection densities. The symbionts may compete for available resources and space in the host body or they may share the resources and habitats by regulating their own exploitation so as not to damage the whole symbiotic system. Alternatively, symbiont infection density is governed by symbiont genotype, host genotype, and environment. For example, Goto, Anbutsu, and Fukatsu (2006) revealed asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts that coexist in the same insect host, D. melanogaster. In this study, the QPCR result provided evidence of a competitive relationship between Wolbachia and Spiroplasma in doubly infected females of T. truncatus. The two symbionts are maternally transmitted and mostly located in the ovary; thus, their expansions are limited by resources and space in cases of co‐infection.
In summary, double infections of Wolbachia and Spiroplasma are beneficial to their mite host by inducing CI, and increasing fecundity and developmental rate. These benefits can help maintain stable double infections and also drive T. truncatus outbreaks in combination with other environmental factors.
AUTHOR CONTRIBUTIONS
YKZ and XYH conceived and designed experiments. YKZ, KY, YXZ performed the experiments. YKZ and KY analyzed the data. YKZ and XYH wrote the paper. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
This study was supported in part by a grant‐in‐aid for Scientific Research (31672035, 31371944) from the National Natural Science Foundation of China. We are grateful to Xiao‐Feng Xue and Jing‐Tao Sun of the Department of Entomology, Nanjing Agricultural University for their valuable comments on an earlier version of this manuscript.
Zhang Y‐K, Yang K, Zhu Y‐X, Hong X‐Y. Symbiont‐conferred reproduction and fitness benefits can favour their host occurrence. Ecol Evol. 2018;8:1626–1633. https://doi.org/10.1002/ece3.3784
REFERENCES
- Baldo, L. , Hotopp, J. C. D. , Jolley, K. A. , Bordenstein, S. R. , Biber, S. A. , Choudhury, R. R. , … Werren, J. H. (2006). Multilocus sequence typing system for the endosymbiont Wolbachia pipientis . Applied and Environmental Microbiology, 72(11), 7098–7110. https://doi.org/10.1128/AEM.00731-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann, P. (2005). Biology of bacteriocyte‐associated endosymbionts of plant sap‐sucking insects. Annual Review of Microbiology, 59, 155–189. https://doi.org/10.1146/annurev.micro.59.030804.121041 [DOI] [PubMed] [Google Scholar]
- Bolland, H. R. , Gutierrez, J. , & Flechtmann, C. H. W. (1998). World catalogue of the spider mite family (Acari: Tetranychidae). Leiden, The Netherlands: Brill Academic press. [Google Scholar]
- Cisak, E. , Wójcik‐Fatla, A. , Zając, V. , Sawczyn, A. , Sroka, J. , & Dutkiewicz, J. (2015). Spiroplasma‐an emerging arthropod‐borne pathogen? Annals of Agricultural and Environmental Medicine, 22(4), 589–593. https://doi.org/10.5604/12321966.1185758 [DOI] [PubMed] [Google Scholar]
- Costopoulos, K. , Kovacs, J. L. , Kamins, A. , & Gerardo, N. M. (2014). Aphid facultative symbionts reduce survival of the predator lady beetle Hippodamia convergens . BMC Ecology, 14(1), 5 https://doi.org/10.1186/1472-6785-14-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M. J. , Ying, Z. , Brunner, B. R. , Pantoja, A. , & Ferwerda, F. H. (1998). Rickettsial relative associated with papaya bunchy top disease. Current Microbiology, 26, 80–84. https://doi.org/10.1007/s002849900283 [DOI] [PubMed] [Google Scholar]
- Dobson, S. L. , Rattanadechakul, W. , & Marsland, E. J. (2004). Fitness advantage and cytoplasmic incompatibility in Wolbachia single‐ and superinfected Aedes albopictus . Heredity, 93(2), 135–142. https://doi.org/10.1038/sj.hdy.6800458 [DOI] [PubMed] [Google Scholar]
- Duron, O. , Bouchon, D. , Boutin, S. , Bellamy, L. , Zhou, L. , Engelstädter, J. , … Hurst, G. D. (2008). The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biology, 6(1), 27 https://doi.org/10.1186/1741-7007-6-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enigl, M. , & Schausberger, P. (2007). Incidence of the endosymbionts Wolbachia, Cardinium and Spiroplasma in phytoseiid mites and associated prey. Experimental and Applied Acarology, 42, 75–85. https://doi.org/10.1007/s10493-007-9080-3 [DOI] [PubMed] [Google Scholar]
- Goodacre, S. L. , Martin, O. Y. , Thomas, C. F. G. , & Hewitt, G. M. (2006). Wolbachia and other endosymbiont infections in spiders. Molecular Ecology, 15, 517–527. https://doi.org/10.1111/j.1365-294X.2005.02802.x [DOI] [PubMed] [Google Scholar]
- Goto, S. , Anbutsu, H. , & Fukatsu, T. (2006). Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Applied and Environmental Microbiology, 72(7), 4805–4810. https://doi.org/10.1128/AEM.00416-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh, T. , Noda, H. , & Hong, X. Y. (2003). Wolbachia distribution and cytoplasmic incompatibility based on a survey of 42 spider mite species (Acari: Tetranychidae) in Japan. Heredity, 91, 208–216. https://doi.org/10.1038/sj.hdy.6800329 [DOI] [PubMed] [Google Scholar]
- Gündüz, E. A. , & Douglas, A. E. (2009). Symbiotic bacteria enable insect to use a nutritionally inadequate diet. Proceedings of the Royal Society of London B: Biological Sciences, 276(1658), 987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges, L. M. , Brownlie, J. C. , O'Neill, S. L. , & Johnson, K. N. (2008). Wolbachia and virus protection in insects. Science, 322(5902), 702 https://doi.org/10.1126/science.1162418 [DOI] [PubMed] [Google Scholar]
- Himler, A. G. , Adachi‐Hagimori, T. , Bergen, J. E. , Kozuch, A. , Kelly, S. E. , Tabashnik, B. E. , … Hunter, M. S. (2011). Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science, 332(6026), 254–256. https://doi.org/10.1126/science.1199410 [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. A. , Hercus, M. , & Dagher, H. (1998). Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in Drosophila melanogaster . Genetics, 148, 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa, T. , Koga, R. , Kikuchi, Y. , Meng, X. Y. , & Fukatsu, T. (2010). Wolbachia as a bacteriocyte‐associated nutritional mutualist. Proceedings of the National Academy of Sciences of the United States of America, 107(2), 769–774. https://doi.org/10.1073/pnas.0911476107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst, G. D. , & Darby, A. C. (2009). The inherited microbiota of arthropods, and their importance in understanding resistance and immunity. Insect Infection and Immunity: Evolution, Ecology, and Mechanisms, 25, 119–136. https://doi.org/10.1093/acprof:oso/9780199551354.001.0001 [Google Scholar]
- Jaenike, J. , Polak, M. , Fiskin, A. , Helou, M. , & Minhas, M. (2007). Interspecific transmission of endosymbiotic Spiroplasma by mites. Biology Letter, 3, 23–25. https://doi.org/10.1098/rsbl.2006.0577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike, J. , Stahlhut, J. K. , Boelio, L. M. , & Unckless, R. L. (2010). Association between Wolbachia and Spiroplasma within Drosophila neotestacea: An emerging symbiotic mutualism? Molecular Ecology, 19, 414–425. https://doi.org/10.1111/j.1365-294X.2009.04448.x [DOI] [PubMed] [Google Scholar]
- Jiggins, F. M. , Hurst, G. D. D. , Jiggins, C. D. , VD Schulenburg, J. H. G. , & Majerus, M. E. N. (2000). The butterfly Danaus chrysippus is infected by a male‐killing Spiroplasma bacterium. Parasitology, 120(05), 439–446. https://doi.org/10.1017/S0031182099005867 [DOI] [PubMed] [Google Scholar]
- Ma, W. J. , Vavre, F. , & Beukeboom, L. W. (2014). Manipulation of arthropod sex determination by endosymbionts: Diversity and molecular mechanisms. Sexual Development, 8(1–3), 59–73. https://doi.org/10.1159/000357024 [DOI] [PubMed] [Google Scholar]
- Min, K. T. , & Benzer, S. (1997). Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proceedings of the National Academy of Sciences of the United States of America, 94(20), 10792–10796. https://doi.org/10.1073/pnas.94.20.10792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon, A. , & Dorkeld, F. (2006. –2017). Spider mites web: A comprehensive database for the Tetranychidae. Retrieved from http://www.montpellier.inra.fr/CBGP/spmweb
- Navajas, M. , Gutierrez, J. , Lagnel, J. , & Boursot, J. (1996). Mitochondrial cytochrome oxidase I in tetranychid mites: A comparison between molecular phylogeny and changes of morphological and life history traits. Bulletin of Entomology Research, 86, 407–417. https://doi.org/10.1017/S0007485300034994 [Google Scholar]
- Oliver, K. M. , Moran, N. A. , & Hunter, M. S. (2006). Costs and benefits of a superinfection of facultative symbionts in aphids. Proceedings of the Royal Society of London B: Biological Sciences, 273(1591), 1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, K. M. , Campos, J. , Moran, N. A. , & Hunter, M. S. (2008). Population dynamics of defensive symbionts in aphids. Proceedings of the Royal Society of London B: Biological Sciences, 275(1632), 293–299. https://doi.org/10.1098/rspb.2007.1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros, V. I. D. , & Breeuwer, J. A. J. (2009). The effects of, and interactions between, Cardinium and Wolbachia in the doubly infected spider mite Bryobia sarothamni . Heredity, 102, 413–422. https://doi.org/10.1038/hdy.2009.4 [DOI] [PubMed] [Google Scholar]
- Sanada‐Morimura, S. , Matsumura, M. , & Noda, H. (2013). Male killing caused by a Spiroplasma symbiont in the small brown planthopper, Laodelphax striatellus . Journal of Heredity, 104(6), 821–829. https://doi.org/10.1093/jhered/est052 [DOI] [PubMed] [Google Scholar]
- Shokal, U. , Yadav, S. , Atri, J. , Accetta, J. , Kenney, E. , Banks, K. , … Eleftherianos, I. (2016). Effects of co‐occurring Wolbachia and Spiroplasma endosymbionts on the Drosophila immune response against insect pathogenic and non‐pathogenic bacteria. BMC Microbiology, 16(1), 16 https://doi.org/10.1186/s12866-016-0634-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley, M. C. , & Majerus, M. E. N. (2006). A new male‐killing parasitism: Spiroplasma bacteria infect the ladybird beetle Anisosticta novemdecimpunctata (Coleoptera: Coccinellidae). Parasitology, 132(06), 757–765. https://doi.org/10.1017/S0031182005009789 [DOI] [PubMed] [Google Scholar]
- Vorburger, C. , Gehrer, L. , & Rodriguez, P. (2010). A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biology Letters, 6(1), 109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren, J. H. (1997). Biology of Wolbachia . Annual Review of Entomology, 42(1), 587–609. https://doi.org/10.1146/annurev.ento.42.1.587 [DOI] [PubMed] [Google Scholar]
- Werren, J. H. , Baldo, L. , & Clark, M. E. (2008). Wolbachia: Master manipulators of invertebrate biology. Nature Reviews Microbiology, 6(10), 741–751. https://doi.org/10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- Werren, J. H. , & O'Neill, S. L. (1997). The evolution of heritable symbionts In O'Neill S. L., Hoffmann A. A., & Werren J. H. (Eds.), Influential passengers: Inherited microorganisms and arthropod reproduction (pp. 1–41). Oxford: Oxford University Press. [Google Scholar]
- Williamson, D. L. , Sakaguchi, B. , Hackett, K. J. , Whitcomb, R. F. , Tully, J. G. , Carle, P. , & Henegar, R. B. (1999). Spiroplasma poulsonii sp. nov., a new species associated with male‐lethality in Drosophila willistoni, a neotropical species of fruit fly. International Journal of Systematic and Evolutionary Microbiology, 49(2), 611–618. [DOI] [PubMed] [Google Scholar]
- Xie, J. , Vilchez, I. , & Mateos, M. (2010). Spiroplasma, bacteria enhance survival of, Drosophila hydei, attacked by the parasitic wasp, Leptopilina heterotoma . PLoS ONE, 5(8), 3733–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zchori‐Fein, E. , & Perlman, S. J. (2004). Distribution of the bacterial symbiont Cardinium in arthropods. Molecular Ecology, 13, 2009–2016. https://doi.org/10.1111/j.1365-294X.2004.02203.x [DOI] [PubMed] [Google Scholar]
- Zhang, Y. K. , Chen, Y. T. , Yang, K. , Qiao, G. X. , & Hong, X. Y. (2016). Screening of spider mites (Acari: Tetranychidae) for reproductive endosymbionts reveals links between co‐infection and evolutionary history. Scientific Reports, 6, 27900 https://doi.org/10.1038/srep27900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. K. , Zhang, K. J. , Sun, J. T. , Yang, X. M. , Ge, C. , & Hong, X. Y. (2013). Diversity of Wolbachia in natural populations of spider mites (genus Tetranychus): Evidence for complex infection history and disequilibrium distribution. Microbial Ecology, 65, 731–739. https://doi.org/10.1007/s00248-013-0198-z [DOI] [PubMed] [Google Scholar]
- Zhao, D. X. , Chen, D. S. , Ge, C. , Gotoh, T. , & Hong, X. Y. (2013). Multiple infections with Cardinium and two strains of Wolbachia in the spider mite Tetranychus phaselus Ehara: Revealing new forces driving the spread of Wolbachia . PLoS ONE, 8, e54964 https://doi.org/10.1371/journal.pone.0054964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, D. X. , Zhang, X. F. , & Hong, X. Y. (2013). Host‐symbionts interactions in spider mite Tetranychus truncatus doubly infected with Wolbachia and Cardinium . Environmental Entomology, 42, 445–452. https://doi.org/10.1603/EN12354 [DOI] [PubMed] [Google Scholar]
- Zhu, L. Y. , Zhang, K. J. , Zhang, Y. K. , Ge, C. , Gotoh, T. , & Hong, X. Y. (2012). Wolbachia strengthens Cardinium‐induced cytoplasmic incompatibility in the spider mite Tetranychus piercei McGregor. Current Microbiology, 65, 516–523. https://doi.org/10.1007/s00284-012-0190-8 [DOI] [PubMed] [Google Scholar]
- Zug, R. , & Hammerstein, P. (2015). Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biological Reviews, 90(1), 89–111. https://doi.org/10.1111/brv.12098 [DOI] [PubMed] [Google Scholar]


