Abstract
Anorexia nervosa (AN) is a complex psychiatric disorder with high morbidity and mortality rates. While many individuals make full recoveries, up to a third of patients develop a chronic, treatment-resistant form of the illness that leads to a premature death in 15–20% of those affected. There have been few advances in treatment, both in terms of psychological or pharmacologic treatment over the last 30 years. Food aversion is commonly cited by patients with AN as a barrier to normalizing eating and weight. Our group has a keen interest in examining factors that might allow this to be addressed, thus improving treatment outcomes through personalized dietary plans or nutritional supplementation related to underlying genetic status.
We demonstrated that polyunsaturated fatty acids (PUFAs)-derived bioactive lipids (eicosanoids) are implicated in not only the risk of AN, but also with its comorbid psychopathology. Of interest, the differential postprandial omega 6-derived eicosanoid shift observed in AN highlights the possibility that the metabolism of PUFAs is an important mechanism underlying the profound food version, contributing to pathological food restriction in AN. A concise knowledge of the relationships among PUFAs, eicosanoids, and AN clinical course and psychopathology could be the key to developing personalized nutritional rehabilitative treatments for those suffering from AN.
This paper provides a comprehensive overview of the literature on PUFAs in AN. We also selectively reviewed the clinical benefits PUFA treatments exert in other psychiatric diseases, on weight and appetite regulation, and for resolution of inflammation, all of which are relevant in the disease course and outcome of AN. We propose that personalized PUFA formulation be developed and tested as a novel adjunctive treatment for patients with AN. We hypothesize that with personalized PUFA formulation, food aversion and anxiety about eating will decrease while mood, dietary behavior, and weight restoration will improve in AN, leading to improvements in the overall treatment outcome.
Keywords: Anorexia nervosa, Polyunsaturated fatty acid, Eicosanoid, Inflammation, Weight and appetite, Mental health
1. Introduction
Anorexia nervosa (AN) is a psychiatric disorder characterized by pathological restrictive eating patterns, an obsession of maintaining an unhealthy low weight, and body image disturbance. While some respond well to conventional treatments, many require multiple episodes of treatment over many years to get a good outcome, and one-third of patients go on to develop a chronic, treatment-resistant form of the illness that is associated with ongoing severe medical and psychiatric comorbidity, and high rates of premature death [1]. Research focused on understanding the etiology of AN, including core behaviors such as food avoidance, would be a major step in the direction of improving existing treatment outcomes and in developing new therapies.
Polyunsaturated fatty acids (PUFAs) include “essential fatty acids” linoleic (n-6) and alpha-linolenic (n-3(n-3) acids and long-chain fatty acids (LC n-PUFAs). Essential PUFAs are sourced from diet and serve as precursors to LC n-PUFAs such as n-3 eicosapentaenoic acid (EPA) and docosahexaenoic (DHA) acid, and n-6 arachidonic acid (ARA). The importance of PUFAs in weight restoration, a key clinical goal for patients with AN, was reported first in a 1930 rat study when Collin et al. stated that “Both linolenic acid and linoleic acid are effective in curing rats suffering from fat deficiency” [2]. Since then, PUFA treatments have shown to produce benefits in a small number of AN studies and in other psychiatric and neurobiological illnesses including schizophrenia, bipolar disease, major depressive disorder [3], and cognitive decline in Alzheimer’s disease [4]. Although the precise mechanism driving these clinical benefits is incompletely understood, PUFAs’ vital role in maintaining normal brain functions and inflammatory response regulation are likely the key factors to improve symptoms in patients with mental and neurodegenerative disease [5].
Although the health effects of individual PUFA have been established, the ratio of n-6 to n-3 PUFAs should also be considered as a significant determinant in health and disease outcomes given the complex, competitive relationship between n-3 and n-6 PUFAs and the resulting biological consequences [6–8]. The consideration of the ratio of n-6 to n-3 PUFAs is especially pertinent to today’s world given the dramatic impact of food industrialization which changed how foods are sourced, processed, packaged, regulated and distributed in the western societies. Such major food industry evolution significantly alters the nutritional composition of our meals, thereby profoundly affected human health without population awareness. For example, the n-6:n-3 ratio found in the Paleolithic diets were estimated to be 0.79, yet the ratio in today’s typical United States diet is estimated to be 16.74 [9,10]. While the effect of a high n-6 to n-3 ratio on human health has been determined as largely problematic [6,11], the increase in dietary n-6 also effects eicosanoids synthesis thereby disrupting resolution of inflammatory processes [12]. Eicosanoids are bioactive lipid mediators derived from the oxidation of several precursor PUFAs (including n-6 LA, and ARA, and n-3 ALA, EPA, and DHA) through enzymatic catalytic processing by cyclooxygenase (COX), lipoxygenase (LOX), or cytochrome P450 monooxygenases (CYP) pathways. Eicosanoids including prostaglandins, thromboxanes, leukotrienes, and epoxyeicosatrienoic acids form a complex regulatory network via competitive inhibition, displacement, or counteraction to regulate a number of important physiological processes including vascular permeability, platelet aggregation, and inflammation response and resolution (Fig. 1) [13,14].
Fig. 1. Families of polyunsaturated fatty acids and related eicosanoids.
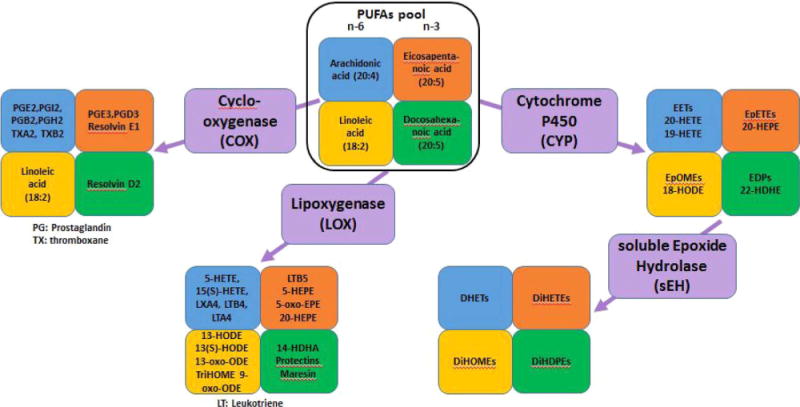
Selected major polyunsaturated fatty acids, families of enzymes, and bioactive lipid mediators (eicosanoids) that are hypothesized as contributors to the risk and progression of anorexia nervosa and disordered eating behavior.
Despite an abundance of studies demonstrating beneficial effects n-3 PUFAs have on various diseases and inflammation, there is a lack of comprehensive research on the role PUFAs play in AN risk and disease course. While a small number of studies suggest a potential for n-3 PUFAs to improve AN symptoms, the association between AN and specific PUFA is inconsistent, and the mechanisms by which PUFAs could benefit AN remain untested. We recently demonstrated that AN patients not only showed a significantly different pattern of PUFA concentration when compared with healthy controls [15], dysregulation in PUFA-derived eicosanoids were associated with AN risk [15] and comorbid psychopathology [16]. Of special interest is the finding that AN demonstrated a differential postprandial metabolism compared to healthy controls with n-6 ARA but not with n-3 PUFAs [16] (raw data in Table 1 of Ref 17)[17]. Our work to date highlights the possibility that the metabolism of PUFAs leading to increased production of proinflammatory bioactive lipid mediators may be a key mechanism underlying the profound food aversion in AN. This hypothesis is further supported by the finding that fat phobic-AN reported more episode and more severe gastrointestinal symptoms compared to nonfat phobic-AN [18].
Eating behavior and food selection of AN are characterized by an inadequate intake and a strong preference for low-fat foods [19]. Diet limited in total variety and specific food groups including “added fat” was associated with relapse risk in AN [20], highlighting the importance of dietary fat and adequate macronutrients for AN. Because correcting imbalanced nutritional state is a necessary step for weight restoration and healthy weight maintenance in AN, PUFA imbalance or deficiency is a critical missing link which should be researched and tested. The goal of this paper is to provide a comprehensive review on PUFAs and AN, and to present our conjectures through a selected review of PUFAs’ effectiveness in diseases and phenotypes related to AN. We hypothesize that nutritional rehabilitative plans utilizing personalized PUFA formulation will lead to significant improvements in the overall treatment outcome for AN.
2. Discussion
2.1. n-3 and n-6 polyunsaturated fatty acids (PUFAs) in anorexia nervosa
The first report of PUFA abnormality in AN was published in 1985 when Langan et al. investigated essential fatty acid levels using both total and phospholipid fractions of the plasma fatty acid levels of 17 patients hospitalized for anorexia nervosa and 11 healthy females control. In plasma phospholipid, anorexia nervosa patients showed a lower level of n-6 LA (19.35 ± 5.65 vs 24.96 ± 2.24, p < 0.01) but higher n-3 DHA (2.3 ± 0.72 vs 1.68 ± 0.36, p < 0.05) compared to controls. AN also had a lower level of total n-6 PUFA. The total lipid plasma PUFA showed that LA trended lower in AN, but the only fatty acid found to be statistically different between two groups was stearic acid (5.58 ± 1.06 vs 6.55 ± 0.73, p < 0.02) [21].
Holman et al. studied 8 patients with AN and 19 controls in 1995 and examined the fatty acid composition in phospholipids, triglycerides and cholesterol esters as well as non-esterified fatty acids. They identified in phospholipid normal levels of LA and ALA, but elevated GLA and a decrease in the other LC n-6 PUFAs and LC n-3 PUFAS. In cholesterol esters, total PUFA were suppressed due to LA, GLA, and DGLA. Most PUFAs were found to be within normal range with the exception that eicosadienoic acid was elevated 10-fold. In triglycerides, there was a suppression of PUFA especially of GLA, 22:5 n6 (osbond acid), ALA and DPA. Furthermore, non-esterified fatty acids in AN were shown to be significantly lower in DGLA and ARA [22].
Zak et al. examined a number of lipid classes in plasma in 16 AN women and 25 healthy control women and identified a decreased content of n-6 PUFA concentration in all lipid classes. No difference in n-3 PUFA concentration was observed between groups. Similar to Langan et al’s plasma phospholipid data, LA was decreased in plasma cholesteryl esters, triacylglycerols, and phosphatidylcholine in AN. Unlike Langan et al’s finding, DHA was decreased in cholesteryl esters [23].
In 2011 and 2012, Swenne et al. published 3 related papers [24–26] examining non-fasting plasma phospholipid and erythrocyte membranes (ERY) PUFAs in up to 220 girls with eating disorders and 39 healthy controls [24,25]. The authors also examined changes of ERY PUFAs in 24 ED who had PUFA measured at admission and the 1-year follow-up visit [26]. At the baseline assessment visit, 67% of ED subjects were demonstrating a clear weight loss course. In plasma phospholipid, ED subjects had a lower level of LA compared to controls (21.4 ± 3.00 vs 22.7 ± 2.59, p < 0.01) yet demonstrated a higher level of DPA (1.15 ± 0.26 vs 1.03 ± 0.20, p < 0.01) while showing lower total PUA and total n-6 PUFA levels. However, there was no significant difference in the levels of EPA and DHA. In erythrocyte membrane, ED patients also showed a lower level of ARA than that of the control (12.7 ± 1.54 vs 13.7 ± 1.54, p < 0.001), and the difference in levels of n-3 fatty acids were not significant [24]. When the ED subjects were stratified by the presence and absence of comorbid depression (n = 84 and 133), depression status was associated with lower EPA, DHA and DPA, decreased total LC n-3 PUFA, and higher total LC n-6⁄n-3 PUFA ratio and AA⁄DHA ratio [25]. Additionally, the authors observed that during nutritional rehabilitation, the FA profile was (partly) normalized following a normalization of eating without supplementation or weight gain. The increase of LC n-3 PUFA in ED patients, however, went above the level of the healthy controls. Studies by Swenne et al. show that aberrant PUFA is evident in individuals with ED, and restoration of healthy eating behavior and weight may lead to normalization of PUFA markers [26].
Caspar-Bauguil et al. [27] measured PUFA of phospholipids of red blood cell membranes (RBC phospholipid) in 22 AN and healthy controls. AN patients had lower level of total LC-PUFA (26.4 ± 2.1 vs 28.9 ± 1.9, p = 0.0004), ARA (14.7 ± 1.5 vs 15.8 ± 1.4, p = 0.016), EPA (0.74 ± 0.10 vs 0.96 ± 0.19, p = 0.02) and DHA (4.33 ± 1.3 vs 5.5 ± 0.92, p = 0.0012) yet a higher level of ALA (0.22 ± 0.13 vs 0.16 ± 0.04, p = 0.012). The AA/EPA ratio was elevated in AN patients (25.9 ± 16.3 vs 17.3 ± 4.6, p = 0.021) while the sum of all n-3 PUFA was lower compared to controls (7.7 ± 1.8 vs 9.0 ± 1.0, p = 0.007). One notable finding of this study was the inverse relationship between ALA levels and fat mass (r = −0.479, p = 0.032), which Caspar-Bauguil interprets as that diet rich in ALA was associated with more negative energy balance. However, it could also be a result of AN patients’ chronic replacement of ALA-rich foods to avoid consumption of unwanted fat-heavy diet.
Using a retrospective cross-sectional study design with 96 subjects, including 30 ill AN, 30 recovered AN and 36 health control, we reported plasma LA:ALA and ARA:EPA ratios in ill AN (126.7 and 7.9, respectively) to be lower than those found in remitted AN (153.2 and 14.9, respectively) and healthy controls (159.7 and 17.1, respectively) [15]. The pattern of lower n-6:n-3 ratio was largely driven by significantly higher levels of ALA (0.22 vs 0.11, p < 0.01) and EPA (0.15 vs 0.06, p < 0.001) in AN [15]. The EPA:DPA and EPA:DHA in ill AN and remitted AN were both significantly higher in comparison to healthy controls, suggesting an aberrant D6D desaturase and/or 3-keto acyl-CoA synthases (ELOVL2 and ELOVL5) efficiency in AN.
Our finding that plasma ALA was higher in both ill AN and recovered AN (compared to controls) is in agreement with Caspar-Bauguil’s 2012 RBC phospholipid PUFA data. Although our data on elevation of LC n-3 PUFAs (EPA and DHA) seems surprising initially, Swenne et al. found DPA to be higher in ED subjects [24] whereas Langan et al. also reported a higher DHA level in AN subjects [21]. For n-6 PUFAs, we did not identify any significant differences in LA or ARA, but DGLA and OBA were both elevated in AN compared to controls [15]. Langan et al. [21], Zak et al. [23], and Swenne et al. [24] found LA to be lower in AN, whereas Swenne et al. [26] and Caspar-Bauguil et al. [27] identified decreased ARA in erythrocyte membrane and RBC phospholipid of AN, respectively.
As summarized in Table 1, the semi-consistent, mixed results of PUFA presentation in subjects with AN is likely a result of the complexity of lipid metabolism combined with methodological factors in study design including different nationalities of subjects, treatment modalities including treatment meal used, varying nutritional composition in the diet specific to regional/national, culture, and industry processing influences, inconsistent fasting state during the biospecimen collection time, and technological advancement made in gas-liquid chromatography and enzymatic-colorimetric methods. The elevation of n-3 PUFAs has been most often explained by the elimination of n-6 rich diet due to AN’s chronic, profound fear of fat especially with animal-based fat [28]. Perhaps both acute and chronic effects from patients’ preferred diet (with little to no fat) have influenced the PUFA findings. Nonetheless, the differences in n-3 PUFA ratios [15] and DGLA⁄LA and ARA⁄DGLA (markers for D5- and D6- desaturases, respectively) [24] suggest the presence of additional factors, such as genetically moderated differential metabolism for PUFA, a consequence of chronic malnutrition, or changes in regulatory hormones [29,30]. Relating to the aberrant n-6:n3 PUFA ratios in AN, observation that fatty acid concentration in Escherichia coli bacteria cell membranes changes in response to temperature changes [31] and an increased prevalence of AN living in seasonal temperate climates (rather than tropical climates) [32] has also led to a “hibernation hypothesis” for AN [33].
Table 1.
Association reported for polyunsaturated fatty acids and anorexia nervosa.
| Authors, (Study location) | Ref | Subject and Study Design | PU FA examined | Key Findings |
|---|---|---|---|---|
| Langan et al, 1985 (USA) | [21] | 17 hospitalized AN patients (16 female, 1 male) and 11 healthy female controls. | Plasma and phospholipids PUFA | AN patients had lower plasma stearic acid, lower phospholipid LA and total n-6 PUFA, and higher phospholipid DHA compared to controls. |
| Holman et al, 1995 (USA) | [22] | 8 women with AN and 19 female controls | PUFA in plasma phospholipid, cholesteryl esters (CE), triglycerides (TG), and measurement of non-esterified fatty acids. | In AN: In phospholipids, LA was normal, GLA was elevated, the rest of n-6 PUFA were decreased. ALA was normal but the LC n-3 PUFA were subnormal. In CE, total PUFA, LA, and GLA were suppressed while EDA was elevated. In TG, AN had lower levels of GLA, osbond acid, ALA, DPA, free DGLA and ARA. |
| Zak et al, 2005 (Czech Republic) | [23] | 16 women with AN and 25 healthy controls | PUFA in plasma cholesteryl esters (CE), triglycerides (TG), and phosphatidylcholine (PC). | In AN, a decrease of LA and overall n-6 PUFA were found in CE, TG, and PC. DHA was decreased but no change was found in overall n-3 PUFA in CE. |
| Swenne et al, May 2011 Dec. 2011 Aug. 2012 (Sweden) | [24–26] | 220 patients with eating disorder (ED) and 39 healthy controls (May, 2011). 83 ED with depression and 133 ED without depression (Dec., 2011). 24 adolescents girls with ED with average > 10 kg weight loss at presentation (2012). |
Plasma phospholipid and erythrocyte membranes (ERY) PUFA | AN had lower levels of LA, DGLA, total PUFA, total n-6 PUFA, and a higher level of DPA. No differences in EPA or DHA (May 2011). ED patients with depression had lower EPA, DHA, DPA, total LC-n-3 PUFA, and higher total LC n-6:n-3 PUFA and AA:DHA ratios compared to patients without depression (Dec. 2011). N-3 PUFA improved after weight gain. During weight loss, saturated and monounsaturated FA increased while LA decreased (2012). |
| Caspar-Bauguil et al, 2012 (France) | [27] | 22 AN patients and 25 healthy controls. | RBC-phospholipids PUFA | RBC phospholipid LA was not different between the groups, but ALA was increased in AN and was inversely correlated with fat mass. EPA and DHA were reduced in AN. Total n-6 PUFA was positively correlated with fat mass. ALA was negatively correlated with fat mass. |
| Shih et al, 2016 (USA) | [15] | 30 ill AN (IAN), 30 recovered AN (RecAN), and 36 healthy controls. | n-6 and n-3 PUFA, PUFA ratios, PUFA-derived eicosanoids. | Ill AN had higher levels of n-3 PUFA and n-6:n-3 ratios compared to RecAN and controls. AN had higher EPA:DPA and EPA:DHA ratios. |
Adipocyte fatty acid binding proteins (FABP) is known to be critical for fatty acid uptake and intracellular transport and plays an important role in lipid metabolism and the integration of metabolic and inflammatory systems [34]. FABP has been shown to correlate with body weight and adiposity [35]. In a study of FABP and metabolic biomarkers in AN, lower levels of serum leptin and C-active protein and higher levels of .adiponectin and leptin receptors were found in AN compared to controls [36]. Surprisingly, there was no significant difference in FABP levels between AN and controls despite a significant difference in their BMI (15.9 vs 22.9 kg/m2 [mean BMI in AN and controls]) [36]. This finding suggests that decreased body fat has no direct influence on levels of FABP in AN patients. The disrupted relationship between FABP and metabolic parameters in AN may be a consequence of altered nutritional status [37], or a result of compensatory mechanism in response to the decreased body fat [36]. In fact, FABPs are known to regulate lipolysis by increasing fatty acid release. Bartak et al. had reported a higher rate of lipolysis in AN patients compared to healthy controls [38], supporting the possibility that aberrant FABP level may contribute to the heighten lipolysis in AN.
Ghrelin is a stomach-secreted hormone that stimulates appetite and impacts energy homeostasis through the regulation of central and peripheral lipid metabolism [39]. Several studies found plasma ghrelin to be elevated in AN [40], consistent with the pattern found in another “wasting” clinical population, cancer patients with cachexia [41]. Of interest, post chemotherapy ghrelin level was found to increase significantly only in cancer patients with decreased food intake, but not in those without decreased food intake [41]. Pathologic feeding behavior and nutritional status have been proposed for the elevation of ghrelin in AN although the exact mechanism remains elusive [40]. It is worth noting that in a study examining three types of high fat meal on physiological markers of hunger and satiety, a meal rich in PUFAs or monounsaturated fatty acids (MUFAs) reduced ghrelin significantly more than a meal rich in saturated fatty acids (SFAs) [42]. Determining the differential effects n-3 PUFAs and n-6 PUFAs exert on physiological markers of hunger (such as ghrelin) and eating behavior is an important next step to further our knowledge on therapeutic values of PUFAs in order to “personalize” such treatment for AN and other eating disorders.
2.2. Effectiveness of n-3 PUFA supplementation in AN
The patients with AN in the acute phase are defined as having a BMI less than 17, and those with severe weight loss (BMI < 16) almost always require hospitalization and sometimes forced feeding to restore weight and nutritional status. Weight gain is the number one clinical evidence of treatment response. Despite successful weight restoration in some AN patients, maintaining a healthy weight is the key clinical challenge as up to 40–60% patients relapse after completion of treatment programs. It is of high importance to develop improved therapies and preventions for this deadly neurobiological disorder. Polyunsaturated fatty acids exert important biological-, biochemical-, and neuronal mechanisms that are highly relevant to AN including increasing appetite [43], maintaining the integrity and restoring disturbed functions of blood bran barrier [5], stabilizing neuronal membrane fluidity index [44], and mediating cannabinoid signaling and type 1 cannabinoid receptor-mediated functions [45]. With these pivotal role of PUFA in mind, here, we summarized the findings from all studies to date that reported the effectiveness of PUFA supplementation in AN (Table 2).
Table 2.
Effectiveness of PUFA supplementation in anorexia nervosa.
| Authors, (Study location) | Ref | Subject and design | Intervention | Outcome variables | Key Findings |
|---|---|---|---|---|---|
| Ayton et al. (United Kingdom) | [46] | 1 patient with severe AN | 1 g/day EPA for 3 months | Changes in physical and psychological measures | After 3 months, weight (from 45.2 to 57) and food intake (from 1.6 to 8.2), and mood improved, and no relapse for the next 6 months. |
| Ayton et al. (United Kingdom) | [47] | 7 AN patients aged 13–22 were followed for at least 3 months | 1 g/day EPA versus standard treatment | BMI | All patients increased in body mass index |
| Mauler et al. (Germany) | [48] | 15 AN enrolled. 7 were on n-3 PUFA rich diet and 8 were on SFA diet (control diet) for 5 weeks. | n-3 PUFA rich diet versus saturated fatty acids rich control diet | Leptin, insulin, ghrelin, and weight gain. | No significant difference in leptin and insulin increase, ghrelin decrease, and efficiency of weight gain between two types of diet. |
The first report of n-3 supplementation having a beneficial effect on AN was a case report of one refractory patient [46]. The 15-year-old female patient had suffered from an eating disorder for 14 months and was admitted to the adolescent psychiatric clinic at the time of the study with a BMI of 16.71. Starting from month 4 of treatment, she was given 1 g/day EPA supplementation (together with Forceval) as an adjunct treatment for 3 months. There were significant improvements in both her weight and food intake. Her weight from months 5–7 went from 45.20 to 57 (average body weight [ABW] from 86.18 to 108.11) and the food intake (assessed by Morgal Russel outcome scale) improved from 1.6 to 8.2. In month 8, her mood and cognitions were significantly improved, and her weight and food intake increase continued (weight 62.2, ABW 117.29, and MR-A was 8.9). Moreover, there was no relapse in her eating disorder for the next 6 months [46].
To further investigate the potential beneficial effect of PUFA in AN, Ayton et al. [47] studied 7 AN patients aged 13–22 who completed the three-month treatment of 1 g EPA a day. While the outcome of each patient was reported on the individual case basis, a partial improvement was noted for 4 patients and full recovery for 3 patients. The primary endpoint, weight gain, was generally good as followed: Patient 1 had improvement in her appetite and mood with ongoing weight gain after two months of treatment. Patient 2 increased her BMI after 1 year, however, after she stopped the EPA for 6 months, significant weight loss occured. Patient 3 was able to consume high-calorie foods and maintain her weight after 1 year. Patient 4 saw improvements in her mood and weight but quickly lost the weight after stopping treatment. Patient 5 improved weight and eating habits, but her anxiety persisted. A reduction in panic attack was observed after EPA dose was increased to 4 g/day. For patient 6, his BMI increased after 3 months, and later had a complete resolution of his AN. Patient 7 received EPA treatment with oral re-feeding and individual and family therapy. Her BMI increased but suffered from emotional downturn because of family issues. This case series report shows some beneficial effects of n-3 PUFA, however, the lack of control group and small sample size limit its interpretation [47].
Mauler et al. tested their hypothesis that a hypercaloric diet rich in n-3 PUFA is associated with an attenuated increase in serum leptin and a higher efficiency of body weight gain in AN [48]. 7 AN patients received n-3 rich diet while 8 AN received a control diet high in saturated fatty acid for 5 weeks. There was a slower increase in energy intake necessary to achieve the goal of weekly weight gain (1.2 kg) in the n-3 PUFA group, and n-3 PUFA supplemented patients gained on average 2 kg more compared to control group despite the overall lower energy intake. Weight gained per 1000 kcal consumed did not change significantly between weeks 1 and 5 and was not significantly different between the two groups. The increase in serum leptin, insulin, and HDL-C and decrease in plasma ghrelin were similar in both groups. The hypothesis that n-3 PUFA decreases leptin concentration, thus supporting weight gain in AN patients was not observed in this study. The authors acknowledged the possibility that in patients with severe weight loss, the effect of increased dietary intake may dominate beneficial effect n-3 PUFA had on weight restoration [48].
Bulimia nervosa (BN) is another major eating disorder characterized by cycles of binge eating, followed by self-induced vomiting and other compensatory behaviors to undo the effects of binge eating. Biological consequences of excessive dieting and energy deprivation have been proposed to be relevant for the etiology of BN [49], this suggesting that an imbalance of n3:n6 PUFA ratio or PUFA deficiency may also play a role in BN. However, it is difficult to estimate how much energy and macronutrients are available for metabolism in BN because the contribution of food consumed during binge eating is generally neglected while purging frequency varies depending on the severity [50]. Currently, there are no studies of sufficient quality in BN to support the use of PUFA as an adjunctive treatment. It is of importance to also research PUFA’s role in treatment of other eating disorders including BN.
2.3. PUFAs and anorexia nervosa: the brain and body weight connections
Supplementation of EPA and DHA has been shown to improve appetite [43], restore weight [51], and inhibit inflammatory responses [52] in a number of health conditions and diseases. Although the studies in AN to date (Table 1 and Table 2) demonstrate weak evidence for the addition of n-3 PUFA. We contend that PUFA supplementation, if formulated based on empirical data obtained in properly-designed and well-powered studies, will lead to superior treatment outcome than the usual therapy for AN, via a mechanism involving down-regulation of the cellular inflammatory processes.
Although n-3 PUFAs have received significantly more recognition as the “good class” of PUFAs, several studies highlight the equally significant role n-6 PUFAs play in health, brain development and functions. Auestad et al. reported that infants who received n-3 DHA but without n-6 ARA scored lower on language vocabulary test than those breastfed or fed with un-supplemented formula [53], supporting the necessary role of ARA in cognitive development. While PUFAs serve a magnitude of important functions in human biology, the pertinent functions relevant to AN are their beneficial effects on the brain, body weight, and inflammation. Yehuda et al. reviewed the importance of PUFA in regulating the activity of blood brain barrier (BBB) and the consequences of PUFA deficiency [44]. Fatty acids occupy 50% of neuronal membrane and 70% of the myelin sheath, which protects and insulates axons to conduct electrical impulses [5]. The consequences of n-3 PUFA deficiency in myelination leads to impairment in learning, motor function, and vision in the postnatal period. n-3 PUFA deficiency is also associated with a decrease in tyrosine hydroxylase [54] and D2 receptors and dopaminergic presynaptic vesicles in the prefrontal cortex [55], which influences motivation-driven behaviors and response to punishment versus reward [5]. PUFAs also affect the membrane fluidity index, thus influence neuronal function by regulating n-6/n-3 PUFA ratios [56] and the subsequent outcomes of many chronic diseases.
AN often onsets in early adolescence, and neuroimaging studies have consistently shown significant differences in brain and neuro-circuitry of AN patients compared to healthy controls [57–60]. AN patients are known to have high comorbidity including developmental disorders, obsessive-compulsive disorder, and anxiety disorders [61]. Studies in children with neurodevelopmental disorders have demonstrated lower levels of n-3 PUFAs, a higher ratio of n-6/n-3 PUFAs, and high correlation between PUFA dysregulation and symptom severities [62,63]. In a randomized attention-deficit/hyperactivity disorder (ADHD) study, the n-3 PUFA supplemented group had a greater reduction in ADHD scores compared to the control group, suggesting that n-PUFA plays a major role in maintaining brain function and development in neurodevelopmental populations [64]. In an fMRI study, 33 healthy boys were randomly assigned either 400 mg/day or 1200 mg/day of DHA or corn oil for 8 weeks. Subjects on DHA showed significant differences in patterns of brain regional activation (e.g., right inferior frontal gyrus) during an attention task in comparison to subjects on corn oil, suggesting that the performance of attention tasks improved via DHA’s modulation of functional activity in cortical attention networks [65].
AN is one of the most visually striking illnesses due to the patients’ unrelenting efforts to lose weight by restricting food intake, excessively exercising, and self-inducing vomiting [61,66]. PUFA supplementation has shown benefits in improving body weight in several serious medical conditions including cancer [67,68]. Bayram et al. studied the effectiveness of EPA supplementation on weight loss in pediatric leukemia or solid tumor cancers. 33 patients who received a protein or energy dense EPA in addition to their normal diet had lost significantly less weight than the 19 patients received usual diet (control group) after 3 months. After 6 months, the percentage of patients with weight loss was lower (6.7%) in the treatment group as compared to the control group (50%), indicating a protective effect of EPA against weight loss [69]. Others have also demonstrated generally positive outcomes of EPA supplementation on weight gain in adult cancers during the cachexia phase, where rapid weight loss is accompanied by an uproar of inflammatory response [70,71]. In pancreatic cancer, Wigmore et al. provided oral fish oil supplementation (1 g/day, 18% EPA and 12% DHA) to 18 patients with unresectable patients and observed an arrest of cachexia and an unexpected average weight increase of 0.3 kg/month after 3 months. Moreover, changes in weight were associated with reduced inflammation and stabilization of resting energy expenditure [72]. Barber et al. reported that 20 stage III pancreatic cancer patients on EPA-containing supplementation significantly gained weight and increased dietary intake [51]. In another study, 40 lung cancer patients were assigned to either supplement containing 2 g/day EPA and 0.92 g/day DHA, or supplement with no EPA or DHA. Weight maintenance in the treatment group was significantly better than that of the control after 2 and 4 weeks with the difference of 1.3 kg and 1.7 kg (p < 0.05), respectively [73]. These findings suggest that the mechanism underlying clinical benefits of n-3 PUFAs in other disorders may also be relevant to AN treatment.
2.4. Resolving inflammation – utilize knowledge of PUFA and PUFA-associated eicosanoid network to improve weight and psychopathology
Although the etiological factors for the “anorexia” in cancer cachexia and anorexia nervosa are clearly different, molecular processes underpinning anorexia and weight loss, hallmarks of both illnesses, may overlap. Inflammation is thought to be a primary cause of loss of appetite (anorexia) in cancer cachexia, a syndrome characterized by systemic inflammation, negative protein and energy balance, and progressive loss of adipose tissue and skeletal muscle mass [68,74]. Various studies showed that n-3 PUFA supplementation improves anorexia via an anti-inflammatory effect, evident by inhibition of cytokine production and by promoting the release of hypothalamic orexigenic peptides and neurotransmitters to increase appetite [75]. Although patients with AN do not experience loss of appetite like cancer patients do, AN’s striking ability to resist hunger and restrict food results in a rapid weight loss accompanied by low-grade systemic inflammation [76].
Our multi-omics studies in AN [15,16] demonstrated for the first time that similar to cancer [77,78], PUFA and PUFA-associated eicosanoids play a role not only in AN risk but may also effect comorbid psychopathology [15] and postprandial metabolism dysregulation [79]. The increased activity of soluble epoxide hydrolase (sEH) observed in both ill and recovered AN patients compared to controls [15,16] implicates cellular inflammation as a key risk factor or disease modulator for AN. In effect, the sEH converts epoxyeicosatrienoic acids, which are anti-inflammatory, to corresponding diols that lessen anti-inflammation function and induce a shift to inflammation [80]. The interrelated associations of AN with plasma PUFA levels and with sEH-regulated PUFA eicosanoid are particularly intriguing because our earlier work revealed that the gene coding for sEH, EPHX2, is associated with AN risk [81]. Together, the data implies that biological effects of the interactions between EPHX2 and PUFA take place before the AN onset.
The aversion to high-fat food reported by AN may be partially psychological, however, the frequent complaint of pain and discomfort associated with eating suggests that certain nutrients in foods may adversely influence the risk of developing AN. Since sEH exerts catalytic functions on both n-3 PUFAs (e.g., ALA, EPA, DHA) and n-6 PUFAs (e.g., LA, ARA), we tested whether n-6 PUFAs differ from n-3 PUFAs in the context of postprandial metabolism by analyzing both the fasting and post-meal eicosanoids levels in subjects with AN and healthy controls. Having controlled for epoxyeicosatrienoic acids levels, an increase of postprandial proinflammatory diol fatty acid from n-6 ARA was found in AN whereas a decrease was present in healthy controls. Importantly, there was no difference in directional change of n-3 PUFA diols between AN and controls despite the assumption that intra-individual in vivo sEH catalyzes epoxy PUFAs from n-3 and n-6 PUFAs with equal efficiency. This opens the door to an important inflammatory dysregulation hypothesis in AN, indicating the specificity of dietary PUFA to be a significant determinant of inflammation regulation, AN risk and outcome. Indeed, PUFAs’ oxidative metabolites (Fig. 1) play an important role in the control of inflammatory response [82] in the context of weight loss. Mehra et al. reported that the heart failure patients who were randomized to n-3 PUFA not only had a larger weight gain (13% vs 5%), they also had a significant reduction in TNF-α (59%) and IL-1 (39%) compared to those on placebo. Moreover, the change in TNF-α level was inversely correlated with the change of body fat, suggesting fish oil can promote weight gain in patients with cardiac disease [83], and may also improve weight gain efficiency in AN.
The effects of n-3 and n-6 fatty acids on inflammation are thought to be mediated through conversion of short chain to long chain PUFAs, the competition between two PUFA classes, and resulting production and modulation of delta-5 and delta-6 desaturases, phospholipase A1, and eicosanoids and related compounds. The earlier literature strongly implicates long chain n-3 PUFA as having favorable effects on inflammatory processes marked by a decrease of cytokines [84] partly because of their inhibitory effect on ARA [85], a key precursor to a number of pro-inflammatory eicosanoids including leukotrienes and prostaglandins [86]. Newer data including ours, are now highlighting the importance of other contributory factors such as genotype × diet interaction effect [87] and n-6:n-3 ratios [6] on inflammatory processes and phenotype. For example, PUFA intake was positively associated with serum HDL cholesterol in carriers of the −238A of TNF-alpha gene but negatively associated in those with the −238GG genotype; PUFA was also inversely associated with HDL cholesterol in carriers of the −308A allele but not associated was found for those with the −308GG genotype [88]. ALOX12 and APOX 15 are genes that code lipoxygenases which synthesize the proinflammatory eicosanoid hydroperoxyeicosatetranoic acid (5-HPETE) from ARA (Fig. 1). Wild-type individuals homozygous for rs11568131 of ALOX15 had an increased colon cancer risk in the presence of high inflammation score. However, they had a lower risk for cancer when consuming high amounts of fish product. This protective effect was, however, absent in those carrying the variant allele [11]. This study shows that genetic polymorphism-induced lipoxygenase activity variation plays a key role in the phenotype impact dietary PUFA has in cancer [89]. Taken together, we also expect the genotypic difference in EPXH2 locus to have a synergistic effect with PUFA in AN risk, disease course, and outcome.
2.5. PUFA and other psychiatric illnesses
The studies of PUFA in several other major psychiatric illnesses suggest that there could be potential benefits of n-3 PUFA in AN. N-3 PUFAs suppress proinflammatory cytokine production (e.g., IL-2, IL-6, and TNF α), serve as important precursors for lipid-derived modulators of cell signaling (eicosanoids), effect gene expression and inflammatory processes, and protect the neurons [90]. In a study of major depressive disorder (MDD), 155 patients were randomized to either EPA, DHA, or placebo. The MDD symptom improvement did not differ significantly between groups after 8 weeks, however, subjects with high inflammation markers showed most improvement on EPA arm compared to the placebo or to DHA arms, suggesting specificity of EPA in resolving or improving inflammation and symptoms [91]. In another study with patients with persistent depression, Peet et al. showed that a higher proportion of patients on EPA improved compared to placebo group (59% vs 29%) [92]. In bipolar disorder studies, the effectiveness of EPA on decreasing depression symptoms has been replicated by several studies [93–95].
While the concept that manipulation of diet can modify inflammation responses has been around for some time [96], the recent advancement of biochemical technologies has enabled researchers to more comprehensively research the mechanisms by which dietary PUFAs modulate inflammation and effect phenotypes. Relating modulation of inflammatory eicosanoid to improve treatment outcome in psychiatric illnesses, Akhondzadeh et al. showed celecoxib to significantly enhance the effectiveness of risperidone (an antipsychotic medication) in improving symptoms and psychopathology in patients with chronic schizophrenia [97]. Muller et al. also identified similar clinical benefits of celecoxib as adjunctive treatment during the middle of the treatment course, and demonstrated the activation of the type-1 immune response coupling to down-regulation of the type-2 immune response mediated by celecoxib as a potential mechanism of action [98]. Our eicosanoid data which showed AN had a hyper postprandial responsiveness to ARA compared to healthy controls suggests that food-associated inflammatory processes may increase anxiety and pain, contributing to psychopathological eating behavior in AN [16]. In light of the aberrant PUFAs identified in AN (Table 1), a better understanding of how PUFAs interact with host factors (e.g., genetic variation) to modulate eicosanoid-induced inflammation in AN can provide much-needed knowledge to develop optimum PUFA formulation to improve AN symptoms and treatment outcomes.
3. Prospective and future directions
Treatment outcome of anorexia nervosa faces several challenges including insufficient weight restoration, limited psychopathology improvement, persistent temperament traits and unhealthy eating behavior that lead to high rates of relapse. Due in part to an incomplete understanding of pathophysiology, there is currently no sufficiently effective treatment for anorexia nervosa. The n-3 PUFAs have been found to be beneficial in the treatment of several psychiatric diseases, and in promoting weight gain in a number of chronic illnesses that lead to wasting. We agree that there is a potential for n-3 PUFA as an adjunct treatment to improve body conditions and psychological symptoms in anorexia nervosa. However, much more research focusing on specific role dietary PUFAs, including n-6 PUFAs, play in the network of eicosanoids synthesis and inflammatory modulation is needed. For clinical utility, a well-powered randomized clinical trial to test the effectiveness of specific PUFA formulation in AN is of urgent need. Such clinical trial should account for background dietary sources of PUFA and PUFA ratio in relation to the proposed therapeutic dose, the standardization of time of treatment and evaluation protocol, tight control of PUFA quality and composition, and careful consideration of the expected outcome differences that can be attributed to subtypes of AN. With the data derived from a tightly controlled clinical trial and related translational studies, we will be in a better position to further develop personalized PUFA formulation as a novel adjunctive treatment for patients with AN. We conject that with personalized PUFA formulation, food aversion and anxiety about eating will decrease while mood, dietary intake and weight restoration will improve in AN, leading to a speedier recovery and better long-term outcomes.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Health Grants: K01DK087813, NIH West Coast Metabolomics Center Pilot Award (U24DK097154), and R01MH106781.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.prostaglandins.2017.08.010.
Footnotes
Prepared for: 6th european workshop on lipid mediators special issue (SI: 6EWLM) prostaglandins and other lipid mediators.
References
- 1.Zipfel S, Giel KE, Bulik CM, Hay P, Schmidt U. Anorexia nervosa: aetiology, assessment, and treatment. Lancet Psychiatry. 2015;2(12):1099–1111. doi: 10.1016/S2215-0366(15)00356-9. [DOI] [PubMed] [Google Scholar]
- 2.Burr GO, Burr MM, Miller E. On the nature and role of the fatty acids essential in nutrition. J Biol Chem. 1930;86:587. [Google Scholar]
- 3.Perica MM, Delas I. Essential fatty acids and psychiatric disorders. Nutr Clin Pract. 2011;26(4):409–425. doi: 10.1177/0884533611411306. [DOI] [PubMed] [Google Scholar]
- 4.Thomas J, Thomas CJ, Radcliffe J, Itsiopoulos C. Omega-3 fatty acids in early prevention of inflammatory neurodegenerative disease: a focus on Alzheimer’s disease. BioMed Res Int (2015) 2015:172801. doi: 10.1155/2015/172801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yehuda S, Rabinovitz S, Mostofsky DI. Essential fatty acids and the brain: from infancy to aging. Neurobiol Aging. 2005;26(Suppl. 1):98–102. doi: 10.1016/j.neurobiolaging.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Simopoulos AP. An increase in the omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8(3):128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishizaki Y, Shimada K, Tani S, Ogawa T, Ando J, Takahashi M, Yamamoto M, Shinozaki T, Miyauchi K, Nagao K, et al. significance of imbalance in the ratio of serum n-3 to n-6 polyunsaturated fatty acids in patients with acute Coronary syndrome. Am J Cardiol. 2014;113(3):441–445. doi: 10.1016/j.amjcard.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Strandvik B. The omega-6/omega-3 ratio is of importance!, Prostaglandins Leukot. Essent Fatty Acids. 2011;85(6):405–406. doi: 10.1016/j.plefa.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Simopoulos AP. Importance of the omega-6/omega-3 balance in health and disease: evolutionary aspects of diet. World Rev Nutr Diet. 2011;102:10–21. doi: 10.1159/000327785. [DOI] [PubMed] [Google Scholar]
- 10.Eaton SB, Eaton SB, Sinclair AJ, Cordain L, Mann NJ. Dietary intake of long-chain polyunsaturated fatty acids during the paleolithic. World Rev Nutr Diet. 1998;83(3rd):12–23. doi: 10.1159/000059672. [DOI] [PubMed] [Google Scholar]
- 11.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med. 2008;233(6):674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 12.Kelley DS, Taylor PC, Nelson GJ, Mackey BE. Arachidonic acid supplementation enhances synthesis of eicosanoids without suppressing immune functions in young healthy men. Lipids. 1998;33(2):125–130. doi: 10.1007/s11745-998-0187-9. [DOI] [PubMed] [Google Scholar]
- 13.Tapiero H, Ba GN, Couvreur P, Tew KD. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed pharmacother = Biomed pharmacother. 2002;56(5):215–222. doi: 10.1016/s0753-3322(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 14.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shih PB, Yang J, Morisseau C, German JB, Zeeland AA, Armando AM, Quehenberger O, Bergen AW, Magistretti P, Berrettini W, et al. dysregulation of soluble epoxide hydrolase and lipidomic profiles in anorexia nervosa. Mol Psychiatry. 2016;21(4):537–546. doi: 10.1038/mp.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shih PB. Integrating multi-Omics biomarkers and postprandial metabolism to develop personalized treatment for anorexia nervosa. Prostaglandins Other Lipid Mediat. 2017 doi: 10.1016/j.prostaglandins.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Shih PA. Fasting and postprandial soluble epoxy hydrolase-associated eicosanoids in remitted patients with eating disorders. Data in Brief. 2017 doi: 10.1016/j.dib.2018.01.028. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S, Ng KL, Kwok KP, Thomas JJ, Becker AE. Gastrointestinal dysfunction in Chinese patients with fat-phobic and nonfat-phobic anorexia nervosa. Transcult Psychiatry. 2012;49(5):678–695. doi: 10.1177/1363461512459487. [DOI] [PubMed] [Google Scholar]
- 19.Steinglass J, Foerde K, Kostro K, Shohamy D, Walsh BT. Restrictive food intake as a choice–a paradigm for study. Int J Eat Disord. 2015;48(1):59–66. doi: 10.1002/eat.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schebendach JE, Mayer LE, Devlin MJ, Attia E, Contento IR, Wolf RL, Walsh BT. Food choice and diet variety in weight-restored patients with anorexia nervosa. J Am Diet Assoc. 2011;111(5):732–736. doi: 10.1016/j.jada.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langan SM, Farrell PM. Vitamin E, vitamin A and essential fatty acid status of patients hospitalized for anorexia nervosa. Am J Clin Nutr. 1985;41(5):1054–1060. doi: 10.1093/ajcn/41.5.1054. [DOI] [PubMed] [Google Scholar]
- 22.Holman RT, Adams CE, Nelson RA, Grater SJ, Jaskiewicz JA, Johnson SB, Erdman JW., Jr Patients with anorexia nervosa demonstrate deficiencies of selected essential fatty acids, compensatory changes in nonessential fatty acids and decreased fluidity of plasma lipids. J Nutr. 1995;125(4):901–907. doi: 10.1093/jn/125.4.901. [DOI] [PubMed] [Google Scholar]
- 23.Zak A, Vecka M, Tvrzicka E, Hruby M, Novak F, Papezova H, Lubanda H, Vesela L, Stankova B. Composition of plasma fatty acids and non-cholesterol sterols in anorexia nervosa. Physiol Res. 2005;54(4):443–451. [PubMed] [Google Scholar]
- 24.Swenne I, Rosling A, Tengblad S, Vessby B. Essential fatty acid status in teenage girls with eating disorders and weight loss. Acta Paediatr. 2011;100(5):762–767. doi: 10.1111/j.1651-2227.2011.02153.x. Oslo, Norway 1992. [DOI] [PubMed] [Google Scholar]
- 25.Swenne I, Rosling A, Tengblad S, Vessby B. Omega-3 polyunsaturated essential fatty acids are associated with depression in adolescents with eating disorders and weight loss. Acta Paediatr. 2011;100(12):1610–1615. doi: 10.1111/j.1651-2227.2011.02400.x. Oslo, Norway: 1992. [DOI] [PubMed] [Google Scholar]
- 26.Swenne I, Rosling A. Omega-3 essential fatty acid status is improved during nutritional rehabilitation of adolescent girls with eating disorders and weight loss. Acta Paediatr. 2012;101(8):858–861. doi: 10.1111/j.1651-2227.2012.02684.x. Oslo, Norway: 1992. [DOI] [PubMed] [Google Scholar]
- 27.Caspar-Bauguil S, Montastier E, Galinon F, Frisch-Benarous D, Salvayre R, Ritz P. Anorexia nervosa patients display a deficit in membrane long chain polyunsaturated fatty acids. Clin Nutr. 2012;31(3):386–390. doi: 10.1016/j.clnu.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Drewnowski A, Pierce B, Halmi KA. Fat aversion in eating disorders. Appetite. 1988;10(2):119–131. doi: 10.1016/0195-6663(88)90063-3. [DOI] [PubMed] [Google Scholar]
- 29.Warensjo E, Rosell M, Hellenius ML, Vessby B, De Faire U, Riserus U. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: links to obesity and insulin resistance. Lipids Health Dis. 2009;8:37. doi: 10.1186/1476-511X-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenner RR. Hormonal modulation of delta6 and delta5 desaturases: case of diabetes, Prostaglandins Leukot. Essent Fatty Acids. 2003;68(2):151–162. doi: 10.1016/s0952-3278(02)00265-x. [DOI] [PubMed] [Google Scholar]
- 31.Sinensky M. Homeoviscous adaptation–a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974;71(2):522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutierrez E, Carrera O, Vazquez R, Birmingham CL. Climate might be considered as a risk factor for anorexia nervosa? A hypothesis worth another look. Eating Bvehav. 2013;14(3):278–280. doi: 10.1016/j.eatbeh.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Scolnick B, Mostofsky DI. Anorexia nervosa: a rogue hibernation? Med Hypotheses. 2014;82(2):231–235. doi: 10.1016/j.mehy.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7(6):489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang J, Wat NM, Wong WK, Lam KS. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem. 2006;52(3):405–413. doi: 10.1373/clinchem.2005.062463. [DOI] [PubMed] [Google Scholar]
- 36.Haluzikova D, Dostalova I, Kavalkova P, Roubicek T, Mraz M, Papezova H, Haluzik M. Serum concentrations of adipocyte fatty acid binding protein in patients with anorexia nervosa. Physiol Res. 2009;58(4):577–581. doi: 10.33549/physiolres.931575. [DOI] [PubMed] [Google Scholar]
- 37.Engl J, Tschoner A, Willis M, Schuster I, Kaser S, Laimer M, Biebl W, Patsch JR, Mangweth B, Ebenbichler CF. Adipocyte fatty acid binding protein during refeeding of female patients with anorexia nervosa. Eur J Nutr. 2009;48(7):403–408. doi: 10.1007/s00394-009-0027-9. [DOI] [PubMed] [Google Scholar]
- 38.Bartak V, Vybiral S, Papezova H, Dostalova I, Pacak K, Nedvidkova J. Basal and exercise-induced sympathetic nervous activity and lipolysis in adipose tissue of patients with anorexia nervosa. Eur J Clin Invest. 2004;34(5):371–377. doi: 10.1111/j.1365-2362.2004.01344.x. [DOI] [PubMed] [Google Scholar]
- 39.Varela L, Vazquez MJ, Cordido F, Nogueiras R, Vidal-Puig A, Dieguez C, Lopez M. Ghrelin and lipid metabolism: key partners in energy balance. J Mol Endocrinol. 2011;46(2):R43–63. doi: 10.1677/JME-10-0068. [DOI] [PubMed] [Google Scholar]
- 40.Ogiso K, Asakawa A, Amitani H, Inui A. Ghrelin and anorexia nervosa: a psychosomatic perspective. Nutrition. 2011;27(10):988–993. doi: 10.1016/j.nut.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu Y, Nagaya N, Isobe T, Imazu M, Okumura H, Hosoda H, Kojima M, Kangawa K, Kohno N. Increased plasma ghrelin level in lung cancer cachexia. Clin Cancer Res. 2003;9(2):774–778. [PubMed] [Google Scholar]
- 42.Stevenson JL, Clevenger HC, Cooper JA. Hunger and satiety responses to high-fat meals of varying fatty acid composition in women with obesity. Obesity. 2015;23(10):1980–1986. doi: 10.1002/oby.21202. Silver Spring, Md. [DOI] [PubMed] [Google Scholar]
- 43.Damsbo-Svendsen S, Ronsholdt MD, Lauritzen L. Fish oil-supplementation increases appetite in healthy adults: a randomized controlled cross-over trial. Appetite. 2013;66:62–66. doi: 10.1016/j.appet.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Yehuda S, Rabinovitz S. The role of essential fatty acids in anorexia nervosa and obesity. Crit Rev Food Sci Nutr. 2016;56(12):2021–2035. doi: 10.1080/10408398.2013.809690. [DOI] [PubMed] [Google Scholar]
- 45.Lafourcade M, Larrieu T, Mato S, Duffaud A, Sepers M, Matias I, De Smedt-Peyrusse V, Labrousse VF, Bretillon L, Matute C, et al. nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat Neurosci. 2011;14(3):345–350. doi: 10.1038/nn.2736. [DOI] [PubMed] [Google Scholar]
- 46.Ayton AK, Azaz A, Horrobin DF. Rapid improvement of severe anorexia nervosa during treatment with ethyl-eicosapentaenoate and micronutrients. Euro Psychiatry: J Asso Euro Psychiatrists. 2004;19(5):317–319. doi: 10.1016/j.eurpsy.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Ayton AK, Azaz A, Horrobin DF. A pilot open case series of ethyl-EPA supplementation in the treatment of anorexia nervosa, Prostaglandins Leukot. Essent Fatty Acids. 2004;71(4):205–209. doi: 10.1016/j.plefa.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Mauler B, Dubben S, Pawelzik M, Pawelzik D, Weigle DS, Kratz M. Hypercaloric diets differing in fat composition have similar effects on serum leptin and weight gain in female subjects with anorexia nervosa. Nutr Res. 2009;29(1):1–7. doi: 10.1016/j.nutres.2008.12.001. New York, NY. [DOI] [PubMed] [Google Scholar]
- 49.Stice E. A prospective test of the dual-pathway model of bulimic pathology: mediating effects of dieting and negative affect. J Abnorm Psychol. 2001;110(1):124–135. doi: 10.1037//0021-843x.110.1.124. [DOI] [PubMed] [Google Scholar]
- 50.Alpers GW, Tuschen-Caffier B. Energy and macronutrient intake in bulimia nervosa. Eat Behav. 2004;5(3):241–249. doi: 10.1016/j.eatbeh.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 51.Barber MD, Ross JA, Voss AC, Tisdale MJ, Fearon KC. The effect of an oral nutritional supplement enriched with fish oil on weight-loss in patients with pancreatic cancer. Br J Cancer. 1999;81(1):80–86. doi: 10.1038/sj.bjc.6690654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68(5):280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 53.Auestad N, Scott DT, Janowsky JS, Jacobsen C, Carroll RE, Montalto MB, Halter R, Qiu W, Jacobs JR, Connor WE. Visual, cognitive, and language assessments at 39 months: a follow-up study of children fed formulas containing long-chain polyunsaturated fatty acids to 1 year of age. Pediatrics. 2003;112:e177–e183. doi: 10.1542/peds.112.3.e177. 3 Pt 1. [DOI] [PubMed] [Google Scholar]
- 54.Kuperstein F, Eilam R, Yavin E. Altered expression of key dopaminergic regulatory proteins in the postnatal brain following perinatal n-3 fatty acid dietary deficiency. J Neurochem. 2008;106(2):662–671. doi: 10.1111/j.1471-4159.2008.05418.x. [DOI] [PubMed] [Google Scholar]
- 55.Zimmer L, Delpal S, Guilloteau D, Aioun J, Durand G, Chalon S. Chronic n-3 polyunsaturated fatty acid deficiency alters dopamine vesicle density in the rat frontal cortex. Neurosci Lett. 2000;284(1–2):25–28. doi: 10.1016/s0304-3940(00)00950-2. [DOI] [PubMed] [Google Scholar]
- 56.Yehuda S, Rabinovitz S, Carasso RL, Mostofsky DI. The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol Aging. 2002;23(5):843–853. doi: 10.1016/s0197-4580(02)00074-x. [DOI] [PubMed] [Google Scholar]
- 57.Frank GK. Advances from neuroimaging studies in eating disorders. CNS Spectr. 2015;20(4):391–400. doi: 10.1017/S1092852915000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wagner A, Simmons AN, Oberndorfer TA, Frank GK, McCurdy-McKinnon D, Fudge JL, Yang TT, Paulus MP, Kaye WH. Altered sensitization patterns to sweet food stimuli in patients recovered from anorexia and bulimia nervosa. Psychiatry Res. 2015;234(3):305–313. doi: 10.1016/j.pscychresns.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bailer UF, Frank GK, Price JC, Meltzer CC, Becker C, Mathis CA, Wagner A, Barbarich-Marsteller NC, Bloss CS, Putnam K, et al. interaction between serotonin transporter and dopamine D2/D3 receptor radioligand measures is associated with harm avoidant symptoms in anorexia and bulimia nervosa. Psychiatry Res. 2013;211(2):160–168. doi: 10.1016/j.pscychresns.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC, Mathis CA, Wagner A, Hoge J, Ziolko S, et al. increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biol Psychiatry. 2005;58(11):908–912. doi: 10.1016/j.biopsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Treasure J, Claudino AM, Zucker N. Eating disorders. Lancet. 2010;375(9714):583–593. doi: 10.1016/S0140-6736(09)61748-7. [DOI] [PubMed] [Google Scholar]
- 62.Parletta N, Niyonsenga T. Duff J: omega-3 and omega-6 polyunsaturated fatty acid levels and correlations with symptoms in children with attention deficit hyperactivity disorder, autistic spectrum disorder and typically developing controls. PLoS One. 2016;11(5):e0156432. doi: 10.1371/journal.pone.0156432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sorgi PJ, Hallowell EM, Hutchins HL, Sears B. Effects of an open-label pilot study with high-dose EPA/DHA concentrates on plasma phospholipids and behavior in children with attention deficit hyperactivity disorder. Nutr J. 2007;6:16. doi: 10.1186/1475-2891-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anand P, Sachdeva A. Effect of poly unsaturated fatty acids administration on children with attention deficit hyperactivity disorder: a randomized controlled trial. J Clin Diagn Res: JCDR. 2016;10(9):OC01–OC05. doi: 10.7860/JCDR/2016/20423.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McNamara RK, Able J, Jandacek R, Rider T, Tso P, Eliassen JC, Alfieri D, Weber W, Jarvis K, DelBello MP, et al. docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: a placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am J Clin Nutr. 2010;91(4):1060–1067. doi: 10.3945/ajcn.2009.28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bewell-Weiss CV, Carter JC. Predictors of excessive exercise in anorexia nervosa. Compr Psychiatry. 2010;51(6):566–571. doi: 10.1016/j.comppsych.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Brown TT, Zelnik DL, Dobs AS. Fish oil supplementation in the treatment of cachexia in pancreatic cancer patients. Int J Gastrointest Cancer. 2003;34(2–3):143–150. doi: 10.1385/IJGC:34:2-3:143. [DOI] [PubMed] [Google Scholar]
- 68.Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83(6):1345–1350. doi: 10.1093/ajcn/83.6.1345. [DOI] [PubMed] [Google Scholar]
- 69.Bayram I, Erbey F, Celik N, Nelson JL, Tanyeli A. The use of a protein and energy dense eicosapentaenoic acid containing supplement for malignancy-related weight loss in children. Pediatr Blood Cancer. 2009;52(5):571–574. doi: 10.1002/pbc.21852. [DOI] [PubMed] [Google Scholar]
- 70.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, et al. definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 71.Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83(6):1345–1350. doi: 10.1093/ajcn/83.6.1345. [DOI] [PubMed] [Google Scholar]
- 72.Wigmore SJ, Ross JA, Falconer JS, Plester CE, Tisdale MJ, Carter DC, Fearon KC. The effect of polyunsaturated fatty acids on the progress of cachexia in patients with pancreatic cancer. Nutrition. 1996;12(1 Suppl):S27–S30. doi: 10.1016/0899-9007(96)90014-3. [DOI] [PubMed] [Google Scholar]
- 73.van der Meij BS, Langius JA, Smit EF, Spreeuwenberg MD, von Blomberg BM, Heijboer AC, Paul MA, van Leeuwen PA. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. J Nutr. 2010;140(10):1774–1780. doi: 10.3945/jn.110.121202. [DOI] [PubMed] [Google Scholar]
- 74.Ezeoke CC, Morley JE. Pathophysiology of anorexia in the cancer cachexia syndrome, Journal of Cachexia. Sarcopenia and Muscle. 2015;6(4):287–302. doi: 10.1002/jcsm.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goncalves CG, Ramos EJ, Suzuki S, Meguid MM. Omega-3 fatty acids and anorexia. Curr Opin Clin Nutr Metab Care. 2005;8(4):403–407. doi: 10.1097/01.mco.0000172580.02138.20. [DOI] [PubMed] [Google Scholar]
- 76.Agnello E, Malfi G, Costantino AM, Massarenti P, Pugliese M, Fortunati N, Catalano MG, Palmo A. Tumour necrosis factor alpha and oxidative stress as maintaining factors in the evolution of anorexia nervosa. Eat Weight Disord. 2012;17(3):e194–199. doi: 10.1007/BF03325347. [DOI] [PubMed] [Google Scholar]
- 77.Cathcart MC, Lysaght J, Pidgeon GP. Eicosanoid signalling pathways in the development and progression of colorectal cancer: novel approaches for prevention/intervention. Cancer Metastasis Rev. 2011;30(3–4):363–385. doi: 10.1007/s10555-011-9324-x. [DOI] [PubMed] [Google Scholar]
- 78.Panigrahy D, Kaipainen A, Greene ER, Huang S. Cytochrome P450-derived eicosanoids: the neglected pathway in cancer. Cancer Metastasis Rev. 2010;29(4):723–735. doi: 10.1007/s10555-010-9264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang J, Hammock BD, Halmi K, Woodside B, German B, Schork N, Bailer U, Kaye W, Morisseau C, Shih PB. Substrate-dependent postprandial oxylipin responses reveal the potential role of nutrient-Gene interaction in anorexia nervosa. Neuropsychopharmacology. 2015;40:S302. [Google Scholar]
- 80.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8(10):794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zeeland AA Scott-Van, Bloss CS, Tewhey R, Bansal V, Torkamani A, Libiger O, Duvvuri V, Wineinger N, Galvez L, Darst BF, et al. Evidence for the role of EPHX2 gene variants in anorexia nervosa. Mol Psychiatry. 2014;19(6):724–732. doi: 10.1038/mp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelley DS, Siegel D, Fedor DM, Adkins Y, Mackey BE. DHA supplementation decreases serum C-reactive protein and other markers of inflammation in hypertriglyceridemic men. J Nutr. 2009;139(3):495–501. doi: 10.3945/jn.108.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mehra MR, Lavie CJ, Ventura HO, Milani RV. Fish oils produce anti-inflammatory effects and improve body weight in severe heart failure. J Heart Lung Transplant. 2006;25(7):834–838. doi: 10.1016/j.healun.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 84.Galli C, Calder PC. Effects of fat and fatty acid intake on inflammatory and immune responses: a critical review. Ann Nutr Metab. 2009;55(1–3):123–139. doi: 10.1159/000228999. [DOI] [PubMed] [Google Scholar]
- 85.Calder PC. Long-chain fatty acids and inflammation. Proc Nutr Soc. 2012;71(2):284–289. doi: 10.1017/S0029665112000067. [DOI] [PubMed] [Google Scholar]
- 86.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15(8):511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lenihan-Geels G, Bishop KS. Ferguson LR: cancer risk and eicosanoid production interaction between the protective effect of long chain omega-3 polyunsaturated fatty acid intake and genotype. J Clin Med. 2016;5(2) doi: 10.3390/jcm5020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fontaine-Bisson B, Wolever TM, Chiasson JL, Rabasa-Lhoret R, Maheux P, Josse RG, Leiter LA, Rodger NW, Ryan EA, Connelly PW, et al. Genetic polymorphisms of tumor necrosis factor-alpha modify the association between dietary polyunsaturated fatty acids and fasting HDL-cholesterol and apo A-I concentrations. Am J Clin Nutr. 2007;86(3):768–774. doi: 10.1093/ajcn/86.3.768. [DOI] [PubMed] [Google Scholar]
- 89.Habermann N, Ulrich CM, Lundgreen A, Makar KW, Poole EM, Caan B, Kulmacz R, Whitton J, Galbraith R, Potter JD, et al. PTGS1, PTGS2, ALOX5, ALOX12, ALOX15, and FLAP SNPs: interaction with fatty acids in colon cancer and rectal cancer. Genes Nutr. 2013;8(1):115–126. doi: 10.1007/s12263-012-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Das UN. Can perinatal supplementation of long-chain polyunsaturated fatty acids prevents schizophrenia in adult life? Med Sci Monit. 2004;10(12):HY33–37. [PubMed] [Google Scholar]
- 91.Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, Mischoulon D. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry. 2016;21(1):71–79. doi: 10.1038/mp.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59(10):913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- 93.Osher Y, Bersudsky Y, Belmaker RH. Omega-3 eicosapentaenoic acid in bipolar depression: report of a small open-label study. J Clin Psychiatry. 2005;66(6):726–729. doi: 10.4088/jcp.v66n0608. [DOI] [PubMed] [Google Scholar]
- 94.Wozniak J, Biederman J, Mick E, Waxmonsky J, Hantsoo L, Best C, Cluette-Brown JE, Laposata M. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17(6–7):440–447. doi: 10.1016/j.euroneuro.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 95.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009;63(8):1037–1040. doi: 10.1038/ejcn.2008.81. [DOI] [PubMed] [Google Scholar]
- 96.Grimble RF. Dietary manipulation of the inflammatory response. Proc Nutr Soc. 1992;51(2):285–294. doi: 10.1079/pns19920039. [DOI] [PubMed] [Google Scholar]
- 97.Akhondzadeh S, Tabatabaee M, Amini H, Abbasi SA, Behnam SH. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr Res. 2007;90(1–3):179–185. doi: 10.1016/j.schres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 98.Muller N, Ulmschneider M, Scheppach C, Schwarz MJ, Ackenheil M, Moller HJ, Gruber R, Riedel M. COX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapy. Eur Arch Psychiatry Clin Neurosci. 2004;254(1):14–22. doi: 10.1007/s00406-004-0478-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


