Abstract
Background
Pulmonary large cell neuroendocrine carcinoma (LCNEC) is a relatively rare subtype of lung malignancy. According to revised 2015 World Health Organization (WHO) criteria for the pathological diagnosis of LCNEC, neuroendocrine markers must be examined by immunohistochemistry. In this study, we reevaluated endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA) samples of patients previously diagnosed with LCNEC using the revised WHO criteria.
Methods
Clinical tissue samples that had been obtained by EBUS‐TBNA between January 2004 and December 2011, and that had been pathologically diagnosed as LCNEC according to the previous criteria, were reevaluated according to the revised WHO criteria.
Results
The records of 471 lung cancer patients with mediastinal or hilar lymph node metastasis diagnosed by EBUS‐TBNA were analyzed. Thirteen patients were diagnosed with LCNEC; one of which was diagnosed based on cytology alone because the histological material was insufficient for a histological examination. Among the 12 cases in which a histological examination was performed, nine were diagnosed with possible LCNEC based on neuroendocrine marker positivity, while three were diagnosed with suspected LCNEC because they did not meet the immunostaining criteria. The patient who was cytologically diagnosed was found to have non‐small cell carcinoma with neuroendocrine morphology.
Conclusion
LCNEC could be pathologically diagnosed based on 2015 WHO criteria using EBUS‐TBNA samples.
Keywords: Endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA), large cell neuroendocrine carcinoma (LCNEC), needle biopsy, neuroendocrine carcinoma
Introduction
Large cell neuroendocrine carcinoma (LCNEC) is a rare subtype of neuroendocrine carcinoma that accounts for approximately 3% of lung cancers.1 The prognosis of patients with LCNEC is reported to be poor, with five‐year survival rates of 15–57%.2 According to the revised World Health Organization (WHO) criteria for the pathological diagnosis of LCNEC, neuroendocrine markers must be examined by immunohistochemistry (IHC). In the 2004 WHO criteria, LCNEC was included as a variant of large cell carcinoma in the lung neuroendocrine tumor group. However, in the 2015 WHO criteria, LCNEC was separated from the large cell carcinoma group and all lung neuroendocrine tumors are now included as a single entity. LCNEC may be pathologically diagnosed based on the results of an immunohistochemical examination and genetic analysis. Immunohistochemical neuroendocrine markers, such as CD56, synaptophysin, and chromogranin A, can be helpful in diagnosing pulmonary neuroendocrine tumors. The role of Ki‐67 is mainly to separate high‐grade small‐cell lung carcinoma (SCLC) and LCNEC from carcinoid tumors, especially in small biopsies with crushed and qualified tumor cells. The mitotic rate is the most important criteria for separating LCNEC and SCLC from carcinoids in the 2015 WHO criteria. The mitotic rate of LCNEC is high (>10 mitoses per 10 high‐power fields).3
Endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA) is an established modality for mediastinal staging in lung cancer patients with radiologically suspected lymph node metastasis.4 Definitive diagnosis of LCNEC (according to the new criteria) using EBUS‐TBNA samples would enable the selection of an appropriate therapeutic strategy for patients with this unique subtype of lung cancer. We therefore used the revised WHO criteria to reevaluate EBUS‐TBNA samples of patients who had previously been diagnosed with LCNEC.
Methods
Patients
Between January 2004 and December 2011, 1365 patients underwent EBUS‐TBNA for the diagnosis or staging of lung cancer by board certified bronchoscopists of The Japan Society for Respiratory Endoscopy (JSRN) or trainees under their supervision at Chiba University Hospital. Conventional TBNA was not performed in any cases in this study. Four hundred and seventy‐one lung cancer patients with mediastinal or hilar lymph node metastasis, diagnosed by EBUS‐TBNA, were evaluated. Hematoxylin and eosin staining was performed for 13 of the patients who were diagnosed with LCNEC according to the 2004 WHO criteria. The Ethics Committee at Chiba University approved this study (No.2192), and the need for consent was waived because of the retrospective nature of this chart review.
Endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA)
Endobronchial ultrasound‐guided transbronchial needle aspiration was performed under local anesthesia with conscious sedation. A convex probe EBUS (BF‐UC260FW) and ultrasound scanner (EU‐ME1), combined with a dedicated 22G needle (NA‐201SX‐4022), were used in this study (Olympus, Tokyo, Japan).
The aspirated “core” material was processed using a tissue coagulation clot method and the formalin‐fixed paraffin‐embedded (FFPE) sample was used for histology as well as IHC. The remaining aspirates inside of the needle were sprayed on glass slides for both air‐dried smears and immediately fixed with 95% ethanol smears. The air‐dried smears were stained by Diff‐Quik staining for rapid onsite cytology evaluation or Giemsa stain solution and the ethanol‐fixed smears were stained by Papanicolaou staining.
Pathological evaluation
During the clinical period, pathological diagnosis was routinely determined according to the 2004 WHO lung cancer classification, using hematoxylin and eosin‐stained formalin‐fixed paraffin‐embedded 4 μm sections.5 IHC was then performed using the universal immune‐peroxidase polymer method to detect chromogranin A (IR502, Agilent, Santa Clara, USA), synaptophysin (PA0299, Leica Biosystems, Wetzlar, Germany), and CD56 (MRQ‐42, Nichirei Biosciences, Tokyo, Japan). Each antibody was used and stained according to the manufacturer's instructions. The sections were reevaluated according to 2015 WHO criteria by independent board‐certified pathologists (Fig 1).5
Figure 1.
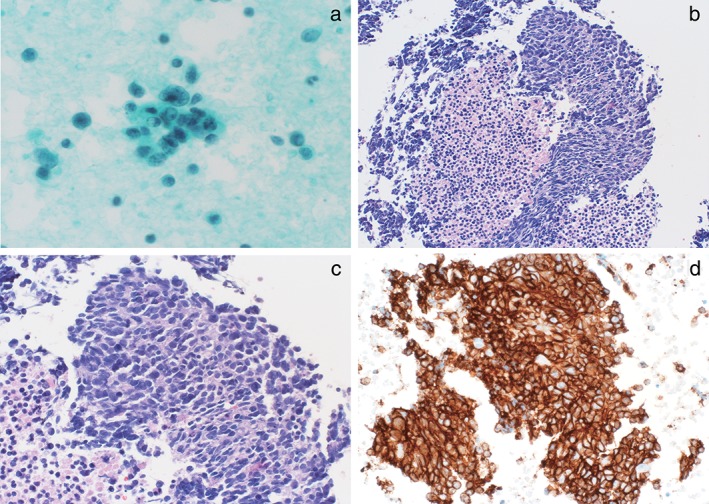
Cytological/histological diagnosis of large cell neuroendocrine carcinoma (LCNEC). (a) Cytological features of LCNEC. The tumor cells exhibit a rosette‐like pattern and abundant cytoplasm is arranged in a radial pattern. (b) Low‐power and (c) high‐power images of hematoxylin and eosin stained sections demonstrating LCNEC morphology, including neuroendocrine architecture with a granular pattern of chromatin in the nuclei. (d) Immunohistochemical staining for synaptophysin shows diffuse labeling.
Histological diagnosis was initially made with hematoxylin and eosin‐stained sections. The histological features of lung LCNEC are large cell size; low ratio of nuclear/cytoplasm; areas of necrosis; neuroendocrine differentiation growth patterns, such as rosette features, organoid nests, trabecular, a granular pattern of chromatin, and clear or atypical nucleoli; and high mitotic rate (> 10 mitoses per 10 high‐power fields). IHC was then performed for chromogranin A, synaptophysin, and CD56 (Figure S1).
Results
In the present study, 471 lung cancer patients with mediastinal or hilar lymph node metastasis diagnosed by EBUS‐TBNA were evaluated. Thirteen of these patients had been diagnosed with LCNEC according to the 2004 criteria (Table 1). The median age of patients was 70 years (range 36–77). In the 13 LCNEC patients, the location of the EBUS‐TBNA samples were: eight in the right lower paratracheal lymph node (#4R), three in subcarinal lymph nodes (#7), and one in the left lower paratracheal lymph nodes (#4L) (Table 1). Twelve of the 13 (92.3%) cases were histologically diagnosed; one was cytologically diagnosed because the sample was insufficient for histological diagnosis. Among the 12 histologically diagnosed cases, five were diagnosed based on morphology and seven by IHC.
Table 1.
Clinicopathological patient characteristics
| Case | Age | Gender | Lymph node | Previous treatment | Cytology† (Papanicolaou) | Histolology†,‡ (H&E) | IHC for neuroendocrine markers‡ | ||
|---|---|---|---|---|---|---|---|---|---|
| CD56 | Synaptophysin | Chromogranin A | |||||||
| 1 | 69 | M | 7 | Surgery | Carcinoma | Neuroendocrine | − | − | − |
| 2 | 75 | M | 4L | Surgery + adjuvant chemotherapy | Carcinoma | Neuroendocrine | − | − | − |
| 3 | 54 | M | 4R | None | LCNEC | Not determined | NA | NA | NA |
| 4 | 74 | M | 4R | None | No malignancy | Neuroendocrine | − | − | − |
| 5 | 76 | M | 4L | Surgery + adjuvant chemotherapy | LCNEC suspected | Neuroendocrine | + | − | + |
| 6 | 77 | M | 4R | None | Carcinoma | Neuroendocrine | + | + | + |
| 7 | 36 | M | 4R | None | LCNEC suspected | Neuroendocrine | − | + | − |
| 8 | 68 | M | 7 | Surgery | No malignancy | Neuroendocrine | + | + | + |
| 9 | 71 | M | 4R | None | Carcinoma | Neuroendocrine | + | + | − |
| 10 | 62 | M | 7 | None | LCNEC suspected | Neuroendocrine | + | − | + |
| 11 | 69 | M | 4R | None | LCNEC suspected | Neuroendocrine | + | + | + |
| 12 | 77 | M | 4R | None | LCNEC suspected | Neuroendocrine | + | + | + |
| 13 | 67 | M | 4R | None | Carcinoma | Neuroendocrine | + | + | + |
Diagnosis using 2004 World Health Organization (WHO) classification;
revised diagnosis using 2015 WHO classification. Neuroendocrine refers to neuroendocrine morphology: neuroendocrine architecture with a granular chromatin pattern in the nuclei and a rosette‐like pattern.
H&E, hematoxylin and eosin; IHC, immunohistochemistry; LNCEC, large cell neuroendocrine carcinoma; NA, not applicable.
The previous treatment regimens are shown in Table 1. Four of the 13 patients underwent surgical resection and two received adjuvant chemotherapy with regimens used for SCLC (platinum‐based combined chemotherapy including etoposide or irinotecan) (Table S2).
Pathological diagnoses were reevaluated according to the 2015 WHO lung cancer classification (Table 1). IHC with neuroendocrine markers (CD56, synaptophysin, chromogranin) was additionally performed for the five patients who were previously diagnosed based on morphology alone. Finally, of the 12 patients who had been histologically diagnosed according to the 2004 WHO criteria, nine were subsequently diagnosed with possible LCNEC based on neuroendocrine marker positivity and three with suspected LCNEC because they did not meet immunostaining criteria. The patient who was previously diagnosed based on cytological examination was diagnosed with non‐small cell carcinoma (NSCC) with neuroendocrine morphology (Fig 2).
Figure 2.
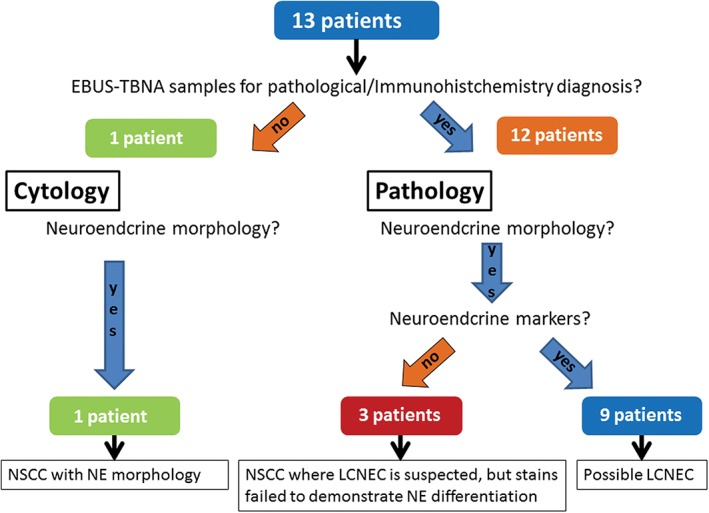
A diagnostic flowchart of the 13 patients included in the study. EBUS‐TBNA, endobronchial ultrasound‐guided transbronchial needle aspiration; LCNEC, large cell neuroendocrine carcinoma; NE, neuroendocrine; NSCC, non‐small cell carcinoma.
Discussion
In the most recent WHO classification of lung tumors, LCNEC has been re‐categorized as a neuroendocrine tumor subtype (Table S1).3 According to this new classification, LCNEC may be pathologically diagnosed using small biopsy specimens based on the results of IHC and genetic analysis.6 With this revision, the following diagnoses can be obtained based on the results of histological or cytological examination of small biopsy specimens: (i) possible NSCC with neuroendocrine morphology and neuroendocrine marker positivity; and (ii) suspected NSCC with neuroendocrine morphology (negative neuroendocrine markers) but without immunohistochemical evidence of neuroendocrine differentiation.7 We previously reported that samples obtained by EBUS‐TBNA can be used for histological examination, including IHC. IHC using EBUS‐TBNA samples can be used to support a diagnosis of lung cancer subtype.8 In this study, five cases (38.4%) were diagnosed as LCNEC based on morphology alone, while the other seven were diagnosed based on the results of IHC; 92.3% of LCNEC diagnoses made using specimens obtained by EBUS‐TBNA were based on the results of histological examinations, including IHC.
Accurate pretreatment diagnosis and differentiation of LCNEC from other NSCLC subtypes is important for selecting an appropriate treatment strategy. In this study, two cases of LCNEC with mediastinal lymph node metastasis were preoperatively diagnosed. The two patients received chemotherapy in an induction setting followed by complete surgical resection. However, because of the difficulty of making a preoperative pathological diagnosis of LCNEC, most previous reports have focused on the postoperative diagnosis of surgical specimens.2, 9 Even when complete surgical resection is achieved, recurrence is frequently observed in patients with LCNEC. The confirmation of LCNEC in tissue specimens from sites of metastasis is warranted for the selection of treatment. In this study, the examination of lymph node specimens obtained by EBUS‐TBNA revealed the recurrence of LCNEC in four patients who had previously undergone treatment. Thus, EBUS‐TBNA was also useful for the diagnosis of post‐treatment recurrence. According to the 2015 WHO criteria, histological examination that includes IHC is mandatory for a diagnosis of LCNEC; thus, LCNEC can be diagnosed using specimens obtained by EBUS‐TBNA as long as the sample is adequate for histological examination. Histological diagnosis of NSCLC using specimens obtained by EBUS‐TBNA will assist in determining the histological subtype and may help to improve the quality of lung cancer treatment.
Disclosure
Takahiro Nakajima and Taiki Fujiwara received honoraria and lecture fees from Olympus Corporation for EBUS‐TBNA training courses. No other authors report any conflict of interest.
Supporting information
Figure S1 The diagnostic algorithm includes control samples for Small biopsies and cytology specimens.
Table S1 2004 and 2015 WHO classification of malignant epithelial tumors.
Table S2 The details of clinicopathological patient characteristics.
Acknowledgments
The authors thank Japan Medical Communication for reviewing the English in this manuscript.
References
- 1. Iyoda A, Hiroshima K, Toyozaki T, Haga Y, Fujisawa T, Ohwada H. Clinical characterization of pulmonary large cell neuroendocrine carcinoma and large cell carcinoma with neuroendocrine morphology. Cancer 2001; 91: 1992–2000. [DOI] [PubMed] [Google Scholar]
- 2. Kozuki T, Fujimoto N, Ueoka H et al Complexity in the treatment of pulmonary large cell neuroendocrine carcinoma. J Cancer Res Clin Oncol 2005; 131: 147–51. [DOI] [PubMed] [Google Scholar]
- 3. Travis WD, Brambilla E, Nicholson AG et al The 2015 World Health Organization Classification of Lung Tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10: 1243–60. [DOI] [PubMed] [Google Scholar]
- 4. Silvestri GA, Gonzalez AV, Jantz MA et al Methods for staging non‐small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013; 143 (5 Suppl): e211S–50S. [DOI] [PubMed] [Google Scholar]
- 5. Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol 2005; 40: 90–7. [DOI] [PubMed] [Google Scholar]
- 6. Travis WD, Rekhtman N. Pathological diagnosis and classification of lung cancer in small biopsies and cytology: Strategic management of tissue for molecular testing. Semin Respir Crit Care Med 2011; 32: 22–31. [DOI] [PubMed] [Google Scholar]
- 7. Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: Relevance for clinical practice and clinical trials. J Clin Oncol 2013; 31: 992–1001. [DOI] [PubMed] [Google Scholar]
- 8. Nakajima T, Yasufuku K, Iyoda A et al The evaluation of lymph node metastasis by endobronchial ultrasound‐guided transbronchial needle aspiration: Crucial for selection of surgical candidates with metastatic lung tumors. J Thorac Cardiovasc Surg 2007; 134: 1485–90. [DOI] [PubMed] [Google Scholar]
- 9. Iyoda A, Makino T, Koezuka S, Otsuka H, Hata Y. Treatment options for patients with large cell neuroendocrine carcinoma of the lung. Gen Thorac Cardiovasc Surg 2014; 62: 351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The diagnostic algorithm includes control samples for Small biopsies and cytology specimens.
Table S1 2004 and 2015 WHO classification of malignant epithelial tumors.
Table S2 The details of clinicopathological patient characteristics.


