Abstract
Background
VEGF is critical in the pathogenesis of malignant pleural effusion (MPE). To understand the clinical benefits of antiangiogenic agents, the efficacy of chemotherapy containing bevacizmab was investigated in patients with lung adenocarcinoma‐induced MPE.
Methods
The data of lung adenocarcinoma patients with MPE treated with bevacizumab plus chemotherapy on day 1, every three weeks, for ≤ 6 cycles was retrospectively collected. Patients who achieved a response or stable disease were administered bevacizumab as maintenance therapy until progression. The primary outcomes of the study were MPE response rate (RR), MPE control rate, and pleural progression‐free survival (PPFS), while the secondary outcomes were PFS, overall survival (OS), changes to the lung volume and thoracic cage, and safety profiles.
Results
A total of 21 cases were collected, and all were evaluable for response, including 15 chemotherapy‐naïve patients and 6 who experienced relapse. The median cycle of treatments was 7 (1‐42) and 5 (2‐6) for bevacizumab and chemotherapy, respectively. The MPE RR reached 81.0%. The MPE control rate at 6, 12, 24, 48, and 96 weeks were 95.2%, 90.0%, 89.5%, 73.7%, and 43.8%, respectively. Median PPFS was significantly longer than PFS (22.2 vs. 7.8 months; P = 0.044), and median OS was 25.8 months. Nineteen (90.5%) patients experienced lung re‐expansion after treatment. Only one (4.8%) patient suffered thoracic volume decrease during treatment and the follow‐up period. No unexpected adverse events were observed.
Conclusions
Bevacizumab combined with chemotherapy demonstrated efficacious, persistence, and safety in controlling lung cancer‐induced MPE, indicating a potential superior therapeutic option.
Keywords: Bevacizumab, efficacy, lung adenocarcinoma, malignant pleural effusion
Introduction
Malignant pleural effusion (MPE) is a common and devastating complication of advanced tumors. Lung cancer is the leading cause, accounting for about 35%.1 MPE can cause significant symptoms, such as dyspnea, cough, and chest pain, and can result in a marked reduction in quality of life and a poor prognosis with survival of approximately six months.2
In the past few decades, intrapleural therapy has been widely used for the treatment of symptomatic MPE.3 Chest tube thoracostomy or pleuroscopy with subsequent chemical pleurodesis remain popular approaches for MPE management.3 However, the general effect is dissatisfactory, with a short MPE response duration. Side effects including fever, chest pain, dyspnea, and thoracic volume decrease are usually observed.4, 5
Studies on the mechanisms of MPE formation have made progress. VEGF is considered to be an essential intermediate in the development of MPE.6 Both basic research and clinical studies have shown that decreasing the level of VEGF can control pleural effusion.6, 7, 8 Bevacizumab, a humanized monoclonal antibody against VEGF, is reported to be effective in intrapleural therapy of malignant pleural fluid.9 In previous reports from Japan, an intravenous injection of bevacizumab plus chemotherapy was also shown to successfully block the accumulation of MPE in advanced NSCLC, with an MPE response rate of 71.4% and MPE control rate of approximately 92%.10, 11, 12 However, studies on long‐term outcomes of bevacizumab therapy for the control of MPE have not been intensive.
In this study, we retrospectively investigate the short and long‐term efficacy and safety of bevacizumab combined with chemotherapy in lung adenocarcinoma‐induced MPE in a Chinese population, to seek a more efficient and safe therapeutic approach for the clinical management of cancer‐associated pleural fluid.
Methods
Patient selection
The records of patients with advanced lung cancer with accompanying MPE who received bevacizumab combination chemotherapy between October 2007 and October 2016 at Beijing Chest Hospital, Capital Medical University, China were reviewed. Data were retrospectively collected from case records and radiographic imaging records. Inclusion criteria were: (i) histologically or cytologically documented advanced lung adenocarcinoma with symptomatic MPE; (ii) age ≥ 18 years, Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2, measurable lesion; (iii) adequate hematologic, hepatic, and renal function; (iv) normal or well‐controlled blood pressure; (v) no history of grade ≥ 2 hemoptysis, thrombotic, or hemorrhagic disorders; and (vi) the absence of therapeutic anticoagulation, active central nervous system metastases, or major blood vessel invasion.
The Ethics Committee of Beijing Chest Hospital, China approved the protocol. The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was provided by all patients prior to study commencement.
Treatment
Chest tube drainage was performed on all patients. At the same time, patients received bevacizumab administered intravenously (15 mg/kg or 7.5 mg/kg, d1) in combination with one of the following chemotherapy regimens every three weeks: paclitaxel 175 mg/m2 on d1 plus carboplatin AUC = 5 on d1 (TC regimen) or cisplatin 75 mg/m2 on d1 (TP regimen); gemcitabine 1250 mg/m2 on d1 plus cisplatin 75 mg/m2 on d1 (GP regimen); or pemetrexed single‐agent 500 mg/m2 on d1 (P regimen). If there was no evidence of disease progression following a maximum of six cycles of bevacizumab plus chemotherapy, patients continued to receive single‐agent bevacizumab until disease progression, unacceptable toxicity, or death.
Efficacy assessments
The volume of MPE and the size of measurable lesions were determined by computed tomography (CT) scan every six weeks. Three experienced oncologists independently performed efficacy evaluations. MPE response was determined according to previous studies, as follows. An MPE complete response (CR) was considered the complete disappearance of pleural fluid for four weeks; partial response (PR): a ≥ 50% decrease of accumulated fluid for four weeks, with improving symptoms; remission not obvious: (NC) a < 50% decrease of accumulated fluid; progressive disease (PD): an increase of accumulated fluid; response rate (RR): the percentage of CR + PR.9, 12 The MPE control rate was defined as the percentage of patients who did not experience re‐accumulation of pleural fluid for four weeks from the time of the catheter being removed, and lung re‐expansion > 70%.13 Pleural progression‐free survival (PPFS) was defined as the interval from the day of treatment to the date of an increase in pleural effusion or the final follow‐up date.14 Progression‐free survival (PFS) was defined as the period between the date of treatment and the date of progression or death. Overall survival (OS) was considered the period from therapy to death or loss to follow‐up. Tumor responses were assessed according to Response Evaluation Criteria in Solid Tumors Committee (RECIST) version 1.1. Tumor RR was defined as the percentage of CR + PR. Toxicity was graded based on the Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0.
Statistical analysis
Data analysis was performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Kaplan–Meier plots were used to evaluate PPFS, PFS, and OS. Median PPFS and PFS were compared by log‐rank test. The median values and 95% confidence intervals (CIs) are reported. Differences with a two‐sided P value of < 0.05 were considered statistically significant. All analyses were cutoff on 31 December 2016.
Results
Patient and treatment characteristics
A total of 21 patients were enrolled. The patient characteristics are summarized in Table 1. The median age was 58 years. Nineteen patients (90.5%) had a PS of 0 or 1. In patients whose gene status was known, six (6/12, 50%) harbored EGFR gene mutation and another three cases (3/9, 33.3%) ALK rearrangement. The median numbers of treatment cycles were 7 (range: 1–42) for bevacizumab and 5 (range: 2–6) for chemotherapy. A total of 15 patients (71.4%) received first‐line chemotherapy. In combination with bevacizumab, paclitaxel plus platinum chemotherapy was implemented in 16 patients (76.2%). Twelve patients (57.1%) underwent maintenance therapy.
Table 1.
Patient characteristics
| Characteristic | No. of patients | Percentage (%) |
|---|---|---|
| Gender | ||
| Male | 6 | 28.6 |
| Female | 15 | 71.4 |
| Age (years) | ||
| Median | 58 | |
| Range | 29–74 | |
| Smoking history | ||
| Yes | 4 | 19.0 |
| No | 17 | 81.0 |
| ECOG PS | ||
| 0–1 | 19 | 90.5 |
| 2 | 2 | 9.5 |
| Histology | ||
| Adenocarcinoma | 21 | 100 |
| Stage | ||
| IV | 21 | 100 |
| EGFR mutation | ||
| Positive | 6 | 28.6 |
| Negative | 6 | 28.6 |
| Unknown | 9 | 42.8 |
| ALK rearrangement | ||
| Positive | 3 | 14.3 |
| Negative | 6 | 28.6 |
| Unknown | 12 | 57.1 |
| Prior chemotherapy | ||
| No | 15 | 71.4 |
| Yes | 6 | 28.6 |
| Chemotherapy regimens | ||
| Paclitaxel + Platinum | 16 | 76.2 |
| Gemcitabine + Platinum | 3 | 14.3 |
| Pemetrexed | 2 | 9.5 |
ALK, anaplastic lymphoma kinase; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor.
Short‐term effect
The effect of the treatment on enrolled subjects was evaluated. The MPE and tumor responses are shown in Table 2. The MPE RR to therapy was 81.0% and the tumor RR was 42.9%.
Table 2.
Short‐term efficacy of bevacizumab‐containing treatment
| Observation items | Clinical outcomes | ||||
|---|---|---|---|---|---|
| MPE response | CR | PR | NC | PD | RR |
| No. of patients | 9 | 8 | 3 | 1 | 17 |
| Percentage (%) | 42.9 | 38.1 | 14.3 | 4.8 | 81.0 |
| Tumor response | CR | PR | SD | PD | RR |
| No. of patients | 0 | 9 | 11 | 1 | 9 |
| Percentage (%) | 0 | 42.9 | 52.4 | 4.8 | 42.9 |
CR, complete response; MPE, malignant pleural effusion; NC, Remission not obvious; PD, progressive disease; PR, partial response; RR, response rate; SD, stable disease.
Long‐term effect
The median follow‐up duration was 17.5 months. Kaplan–Meier curves showed a significant difference in PPFS and PFS (22.2 vs. 7.8 months; P = 0.044) (Fig 1). The median survival time was 25.8 months. The MPE control rates at 6, 12, 24, 48, and 96 weeks were 95.2%, 90.0%, 89.5%, 73.7%, and 43.8%, respectively (Table 3). By the last follow‐up, 16 patients (76.2%) had developed tumor PD. At the time of tumor PD, MPE was well controlled in most patients with 6 MPE CR, 7 MPE PR, and 3 MPE PD cases. Nineteen (90.5%) out of 21 patients experienced lung re‐expansion after treatment. Over the course of the treatment and follow‐up periods, only 1 (4.8%) patient suffered from thoracic volume decrease (Table 3). Loculated effusion was discovered in three patients before treatment commenced. By the end of the six cycles, two out of three patients exhibited effusion disappearance, while the other patient experienced obvious effusion decrease. None of the patients developed new fluid loculation during the treatment process. Long‐run radiological changes caused by bevacizumab plus chemotherapy are revealed in Figure 2.
Figure 1.
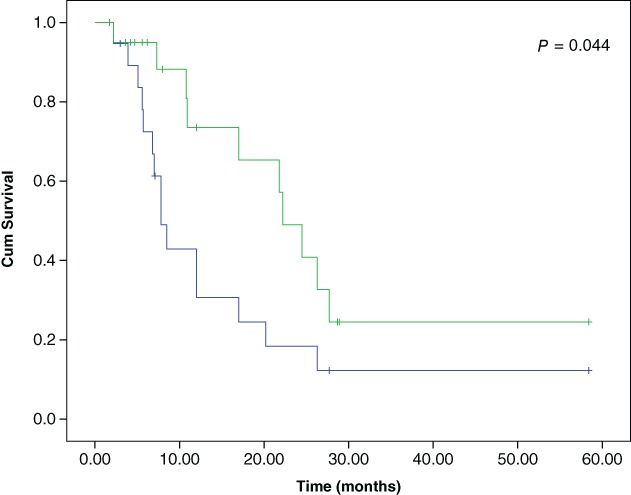
Kaplan–Meier curves for pleural progression‐free survival (PPFS) and progression‐free survival (PFS) of patients. ( ) PFS, (
) PFS, ( ) PPFS, (
) PPFS, ( ) PFS‐censored, and (
) PFS‐censored, and ( ) PPFS‐censored.
) PPFS‐censored.
Table 3.
Long‐term efficacy of bevacizumab‐containing treatment
| Observation items | No. of patients | Percentage (%) |
|---|---|---|
| MPE control rate | ||
| 6 weeks | 20/21 | 95.2 |
| 12 weeks | 18/20 | 90.0 |
| 24 weeks | 17/19 | 89.5 |
| 48 weeks | 14/19 | 73.7 |
| 96 weeks | 7/16 | 43.8 |
| MPE response at the time of tumor PD† | ||
| CR | 6/16 | 37.5 |
| PR | 7/16 | 43.8 |
| NC | 0/16 | 0 |
| PD | 3/16 | 18.8 |
| Lung re‐expansion ≥70% | 19/21 | 90.5 |
| Thoracic volume decrease | 1/21 | 4.8 |
| Outcome of loculated effusion | ||
| Disappearance | 2/3 | 66.7 |
| Decrease | 1/3 | 33.3 |
Sixteen patients experienced tumor PD at the last follow‐up.
CR, complete response; MPE, malignant pleural effusion; NC, remission not obvious; PD, progressive disease; PR, partial response.
Figure 2.
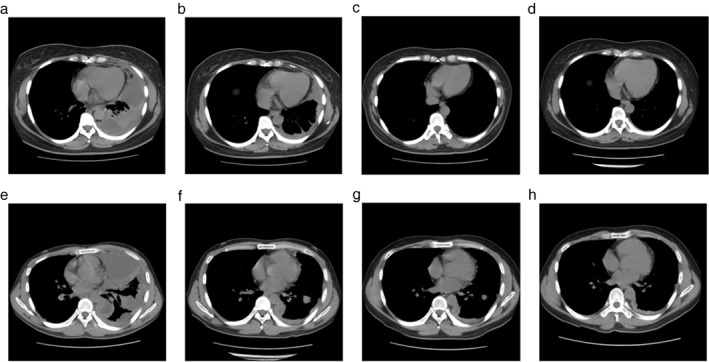
Chest computed tomography scans showed the outcomes of bevacizumab‐containing treatment. One patient who obtained malignant pleural effusion (MPE) complete response (a) at baseline, (b) after six weeks, (c) 12 weeks, and (d) 48 weeks of combined treatment. Another patient who obtained MPE partial response (e) at baseline, (f) after six weeks, (g) 12 weeks, and (h) 48 weeks of combined treatment.
Toxicity
Table 4 lists the adverse events experienced as a result of bevacizumab administration. Most adverse effects were < grade 3 and were reversible. Serious adverse events (SAE) were observed in two cases, with one cerebral infarction and one hemoptysis. No death related to toxic effects of the treatment occurred.
Table 4.
Adverse events relative to bevacizumab
| Adverse events | Grade < 3 | Grade ≥ 3 | All (%) |
|---|---|---|---|
| Epistaxis | 12 | 1 | 13 (61.9) |
| Hypertension | 8 | 2 | 10 (47.6) |
| Proteinuria | 8 | 1 | 9 (42.9) |
| Hemoptysis | 7 | 1 | 8 (38.1) |
| Dizziness | 5 | 1 | 6 (28.6) |
| Diarrhea | 4 | 0 | 4 (19.0) |
| Abnormal Liver Function | 4 | 0 | 4 (19.0) |
| Headache | 3 | 0 | 3 (14.3) |
| Phlebitis | 3 | 0 | 3 (14.3) |
| Pneumonia | 3 | 0 | 3 (14.3) |
| Arrhythmia | 2 | 0 | 2 (9.5) |
| Elevated Bilirubin | 1 | 1 | 2 (9.5) |
| Cerebral Infarction | 0 | 1 | 1 (4.8) |
| Lower Limb Deep Vein Thrombosis | 1 | 0 | 1 (4.8) |
| Myocardial Ischemia | 1 | 0 | 1 (4.8) |
| Hepatocirrhosis | 1 | 0 | 1 (4.8) |
| Splenauxe | 1 | 0 | 1 (4.8) |
Discussion
It is important to control MPE induced by lung cancer. Bevacizumab has been reported to reduce the accumulation of MPE in animal models.15 The use of bevacizumab with chemotherapy to treat MPE has been explored in small‐scale clinical trials.10, 11, 12, 16, 17 Although this therapy seemed effective, the long‐term influence of combined therapy has not received sufficient attention. In this study, we assessed the efficacy and safety of intravenous bevacizumab plus chemotherapy in patients with MPE resulting from lung adenocarcinoma in order to determine an optimized scheme for controlling MPE.
In a previous study, we preliminarily reported an MPE control rate of 100% in 13 patients, with 5 (38.5%) CR and 8 (61.5%) NC.18 The present study showed an MPE RR of 81.0% (n = 17), which suggests a good short‐term effect. With regard to long‐term influence, the data were inspiring. The MPE control rate was a high as 95.2% at six weeks. By the end of 48 weeks, it was still > 70%. Other studies have demonstrated the efficacy of chemotherapy plus bevacizumab in NSCLC‐related MPE. Masago et al. reported a 71.4% (15/21) response rate of pleural effusion,11 while Kitamura et al. revealed that 92.3% (12/13) of patients achieved MPE control lasting > 8 weeks after treatment with bevacizumab plus chemotherapy.12 A phase II study including 23 patients with NSCLC‐induced MPE administered bevacizumab with carboplatin‐paclitaxel therapy, yielding an MPE control rate of 91.3%.10 Another phase II study of bevacizumab with carboplatin‐pemetrexed reported an MPE control rate without pleurodesis at 8weeks of 92.8%, similar to our findings at 6 and 12 weeks.16
Previous research has reported median PFS without re‐accumulation of MPE of 312 days (10.4 months), which was markedly shorter than our finding (mPPFS 22.2 months).12 Median OS has also been reported as 11.7–18.6 months, which is also inferior to 25.8 months achieved in this study.10, 16, 19 The probable reasons for these differences include: (i) the small sample size of all studies; and (ii) the proportion of EGFR‐positive patients in our study (6/12) was larger than those in previous studies (1/15, 4/30, 2/13, and 4/19).
This study revealed promising antitumor synergy and a high MPE control rate after treatment with bevacizumab plus chemotherapy. One of the probable mechanisms of the synergistic effect of bevacizumab plus chemotherapy is based on the theory of bevacizumab‐induced tumor vascular normalization, which is considered to decrease tumoral interstitial hypertension, subsequently enhancing efficacious delivery and the uptake of drugs.20 A study from China showed that the use of paclitaxel and bevacizumab enhances the treatment effect in NSCLC patients with MPE.17 Qi et al. reported that T1/2a (hour) of paclitaxel in pleural fluid samples were 4.58 ± 0.45 and 0.83 ± 0.05 in paclitaxel and paclitaxel/bevacizumab‐treated patients, respectively (P < 0.01).17 The results of T1/2b (hour) of paclitaxel were 17.41 ± 1.12 and 51.35 ± 3.67 in the same groups, respectively (P < 0.01). Thus, pharmacokinetics for the combined treatment displayed a rapid distribution of the anticancer drug with an obvious increase in its elimination half‐life in the pleural fluid.17 Another possible mechanism lies in the efficacy of bevacizumab for inhibiting VEGF angiogenesis, which might suppress vascular permeability and cell proliferation.10 In one of the abovementioned phase II studies, patients received carboplatin/paclitaxel in the first cycle and carboplatin/paclitaxel with bevacizumab in 2–6 cycles.10 The median plasma VEGF levels significantly decreased after three chemotherapy cycles (baseline 513.6 ± 326.4 pg./mL; post‐chemotherapy 25.1 ± 14.1 pg./mL; P < 0.01).
The results in this study indicate that patients developed MPE progression much later than they developed tumor progression (mPPFS 22.2 vs. mPFS 7.8 months; P = 0.044). Therefore, it was concluded that bevacizumab therapy was beneficial and sustained activity to control MPE. In addition, anti‐VEGF therapy may be more effective for malignant effusion than for measurable tumors.11
To date there are no standard criteria to evaluate response in patients with MPE. In the past decades, successful pleurodesis, that is, no significant radiological effusion recurrence and no further ipsilateral pleural intervention, were regarded as evaluation criteria.1, 21 Recent trials have incorporated lung re‐expansion into their endpoints.13 Demmy et al., who conducted the CALGB 30102 study, considered that lung re‐expansion ≥ 70% would improve lung function.13 In addition, Davies and Lee proposed that “patient‐related outcome measures” should first be taken into account, which includes persistent symptom relief, elevation of quality of life, and reducing hospitalization.22 Therefore, in the present study, we defined lung re‐expansion ≥ 70% as one of the criterion of MPE control. In addition, we adopted several observation items, such as MPE control rate, MPE response at the time of tumor PD, and rate of thoracic volume decrease, in order to comprehensively evaluate the long‐term efficacy of bevacizumab combined with chemotherapy to control MPE and the effect of this kind of therapy on patients’ respiratory function.
In this study, lung re‐expansion ≥ 70% occurred in 19 cases (90.5%), and only one patient (4.8%) suffered from thoracic volume decrease. Fourteen out of of 19 (73.7%) patients achieved MPE control by 48 weeks without developing marked pleural thickening or thoracic alterations, which freed them from the symptoms of dyspnea and chest pain. In our study, combined therapy led to disappearance of loculated effusion in two out of three patients, the remaining patient exhibited effusion decrease. Loculated effusion commonly develops from NSCLC or traditional therapy, such as therapeutic thoracentesis or pleurodesis, which can make further thoracentesis or subsequent pleurodesis difficult.2 Thoracoscopy or surgery is theoretically feasible to exploring the mechanisms and treatment. However, as bevacizumab is associated with a higher risk of bleeding, thromboembolic events, wound healing delay, and so on, invasive procedures are not recommended during the course of bevacizumab administration. In addition, the management options for loculated effusion are restricted by symptoms, performance status, and expected survival, as well as the social and financial status of patients.2 Therefore, in the past, patients with loculated effusion suffered unalleviated symptoms and poor prognosis. On the one hand, it is difficult to deal with atelectasis, fluid loculation, and pleural adhesion caused by traditional intrapleural therapy in clinical practice. On the other hand, loculated effusion, thoracic volume decrease, and pleural thickening as a result of pleurodesis usually lead to the impairment of pulmonary function, which reduces patient quality of life.4, 5, 23 The results of our study show that intravenous bevacizumab plus chemotherapy not only had little effect on the thoracic cage, but also avoided the need for invasive procedures and facilitated lung recruitment, which were beneficial to pulmonary function and life quality.
This study had certain limitations. It was a retrospective study, with a small sample size, no control group, and no detection of biomarkers predicting efficacy and safety. Pleural VEGF level is reportedly significantly higher in lung cancer than in benign pleural effusion.7 Some reports have considered that VEGF levels in plasma or pleural effusion are associated with PFS and OS.19 However, there is a lack of definite effective predictors in clinical studies on bevacizumab. We plan further study to collect both blood and pleural effusion specimens and detect biomarkers, such as VEGF and PD‐L1, in order to determine potential predictive or prognostic factors and screen appropriate patients. With the widespread use of bevacizumab in NSCLC treatment, further prospective study with a larger number of cases and control groups is expected.
In conclusion, bevacizumab combined with chemotherapy provided efficacious, persistent, and safe therapy for patients with MPE caused by lung adenocarcinoma. With intravenous administration, patients not only avoided the invasive procedure of intrapleural local therapy and its side effects, but also received chemotherapy as scheduled. As a result, better responses to therapy and improved quality of life were achieved in most patients. Intravenously bevacizumab plus chemotherapy is an ideal options for patients with lung adenocarcinoma who develop MPE.
Disclosure
No authors report any conflict of interest.
Acknowledgments
We thank Huifang Tian and Xue Wang, Medical Department, Shanghai Roche Pharmaceuticals Ltd. for professional comments and references.
Contributor Information
Hong Tao, Email: taohong2000@126.com.
Zhe Liu, Email: lza@vip.163.com.
References
- 1. Sweatt AJ, Sung A. Interventional pulmonologist perspective: Treatment of malignant pleural effusion. Cur Treat Options Oncol 2014; 15: 625–43. [DOI] [PubMed] [Google Scholar]
- 2. Nam H. Malignant pleural effusion: Medical approaches for diagnosis and management. Tuberc Respir Dis (Seoul) 2014; 76: 211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uzbeck MH, Almeida FA, Sarkiss MG et al Management of malignant pleural effusions. Adv Ther 2010; 27: 334–47. [DOI] [PubMed] [Google Scholar]
- 4. Huggins JT, Doelkin P, Sahn SA. Intrapleural therapy. Respirology 2011; 16: 891–9. [DOI] [PubMed] [Google Scholar]
- 5. Becker G, Galandi D, Blum HE. Malignant ascites: Systematic review and guideline for treatment. Eur J Cancer 2006; 42: 589–97. [DOI] [PubMed] [Google Scholar]
- 6. Bradshaw M, Mansfield A, Peikert T. The role of vascular endothelial growth factor in the pathogenesis, diagnosis and treatment of malignant pleural effusion. Curr Oncol Rep 2013; 15: 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marquez‐Medina D, Popat S. Closing faucets: The role of anti‐angiogenic therapies in malignant pleural diseases. Clin Transl Oncol 2016; 18: 760–8. [DOI] [PubMed] [Google Scholar]
- 8. Economidou F, Margaritopoulos G, Antoniou KM, Siafakas NM. The angiogenetic pathway in malignant pleural effusions: Pathogenetic and therapeutic implications. Exp Ther Med 2010; 1: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Du N, Li X, Li F et al Intrapleural combination therapy with bevacizumab and cisplatin for non‐small cell lung cancermediated malignant pleural effusion. Oncol Rep 2013; 29: 2332–40. [DOI] [PubMed] [Google Scholar]
- 10. Tamiya M, Tamiya A, Yamadori T et al Phase2 study of bevacizumab with carboplatin – paclitaxel for non‐small cell lung cancer with malignant pleural effusion. Med Oncol 2013; 30: 676–81. [DOI] [PubMed] [Google Scholar]
- 11. Masago K, Fujimoto D, Fujita S et al Response to bevacizumab combination chemotherapy of malignant pleural effusions associated with non‐squamous non‐small‐cell lung cancer. Mol Clin Oncol 2015; 3: 415–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kitamura K, Kubota K, Ando M et al Bevacizumab plus chemotherapy for advanced non‐squamous non‐small‐cell lung cancer with malignant pleural effusion. Cancer Chemother Pharmacol 2013; 71: 457–61. [DOI] [PubMed] [Google Scholar]
- 13. Demmy TL, Gu L, Burkhalter JE et al Optimal management of malignant pleural effusions (results of CALGB 30102). J Natl Compr Canc Netw 2012; 10: 975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsubara N, Itoh K, Mukai H, Nagai S. Long‐term outcome of pleurodesis with OK‐432 in metastatic breast cancer: A new risk model for success from an analysis of 75 cases. Int J Clin Oncol 2012; 17: 470–6. [DOI] [PubMed] [Google Scholar]
- 15. Yano S, Herbst RS, Shinohara H et al Treatment for malignant pleural effusion of human lung adenocarcinoma by inhibition of vascular endothelial growth factor receptor tyrosine kinase phosphorylation. Clin Cancer Res 2000; 6: 957–65. [PubMed] [Google Scholar]
- 16. Usui K, Sugawara S, Nishitsuji M et al A phase II study of bevacizumab with carboplatin‐pemetrexed in non‐squamous non‐small cell lung carcinoma patients with malignant pleural effusions: North East Japan Study Group Trial NEJ013A. Lung Cancer 2016; 99: 131–6. [DOI] [PubMed] [Google Scholar]
- 17. Qi N, Li F, Li X, Kang H, Zhao H, Du N. Combination use of paclitaxel and avastin enhances treatment effect for the NSCLC patients with malignant pleural effusion. Medicine (Baltimore) 2016; 95: e5392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tao H, Tang JF, Zhu YZ et al Clinical observation of Bevacizumab combined with chemotherapy in advanced non‐squamous non‐small cell lung cancer patients. Prog Mod Biomed 2016; 16: 1289–94. [Google Scholar]
- 19. Tamiya M, Tamiya A, Yasue T et al Vascular endothelial growth factor in plasma and pleural effusion is a biomarker for outcome after Bevacizumab plus carboplatin‐paclitaxel treatment for non‐small cell lung cancer with malignant pleural effusion. Anticancer Res 2016; 36: 2939–44. [PubMed] [Google Scholar]
- 20. Assoun S, Brosseau S, Steinmetz C, Gounant V, Zalcman G. Bevacizumab in advanced lung cancer: State of the art. Future Oncol 2017; 13: 2515–35. [DOI] [PubMed] [Google Scholar]
- 21. Kheir F, Shawwa K, Alokla K, Omballi M, Alraiyes AH. Tunneled pleural catheter for the treatment of malignant pleural effusion: A systematic review and meta‐analysis. Am J Ther 2016; 23: e1300–6. [DOI] [PubMed] [Google Scholar]
- 22. Davies HE, Lee YC. Management of malignant pleural effusions: Questions that need answers. Curr Opin Pulm Med 2013; 19: 374–9. [DOI] [PubMed] [Google Scholar]
- 23. Thomas R, Francis R, Davies HE, Lee YC. Interventional therapies for malignant pleural effusions: The present and the future. Respirology 2014; 19: 809–22. [DOI] [PubMed] [Google Scholar]


