Abstract
This paper analyzed the results of a modified and simpler technique for distinguishing the intersegmental border during lung segmentectomy surgery. From January 2013 to December 2015, 539 patients with screening‐detected lung nodules <2 cm in maximum diameter underwent anatomic segmentectomy. With the guidance of preoperative three‐dimensional computed tomography bronchography and angiography, the bronchus, artery, and intrasegmental vein of the targeted segment could be precisely dissected under unilateral differential ventilation, and then intersegmental demarcation was confirmed by the modified inflation‐deflation method. The demarcation presented by this method was highly coincident with the real intersegmental border. Dissection along the border between the collapsed and inflated segments using either electrocautery or staples was safe, with almost no air leak or bleeding. This technique is a simple and effective alternative to previously described intersegmental border marking methods.
Keywords: Lung cancer, segmentectomy, surgery
Introduction
With the guidance of three‐dimensional computed tomography bronchography and angiography (3D‐CTBA), preoperative anatomical identification of the lung structure is becoming more and more accurate, and precise segmentectomy has gradually turned into the standard approach for small, early‐stage lung cancer.1 However, because of individual variation, identification of the intersegmental border still presents difficulties during surgery. Several methods for identifying the intersegmental border have been reported, most of which fall into two categories aiming to create a demarcation of the inflation‐deflation line or dyeing the border.2, 3 Herein, we report a modified and simpler technique of distinguishing the intersegmental border by combining the guidance of preoperative 3D‐CTBA and intraoperative discernment without any additional auxiliary materials.
Methods
This retrospective study was reviewed and approved by The First Affiliated Hospital of Nanjing Medical University Review Board, and individual patient consent was waived according to institutional guidelines.
From January 2013 to December 2015, 539 patients with screening‐detected lung nodules <2 cm in maximum diameter underwent anatomic segmentectomy (Table 1). The inclusion criteria for all surgical patients were adjusted according to the revision of National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Non‐Small Cell Lung Cancer. 3D–CTBA was applied to identify targeted segment bronchus and intrasegmental and intersegmental vein and artery, and the simulated surgical path and resection range were confirmed before surgery (Fig 1a,b).
Table 1.
Location of the resected segments (539 cases)
| Right | 281 Cases | Left | 258 Cases |
|---|---|---|---|
| S1 | 49 | S3 | 13 |
| S2 | 61 | S1+2 | 21 |
| S3 | 38 | S1+2+3 | 65 |
| S1+2/S1+3 | 8/2 | S3 + S4+5 | 2 |
| S4/S5 | 10/2 | S4+5 | 41 |
| S6 | 59 | S6 | 72 |
| S7/S8/S9 | 1/10/2 | S8 | 22 |
| S7+8/S8+9 | 7/2 | S9+10 | 3 |
| S9+10 | 6 | S8–10 | 15 |
| S6+9+10 | 9 | S4 | 1 |
| S7–10 | 14 | S8+9 | 1 |
| S6+9 | 1 | S9 | 1 |
| S* + S10 | 1 |
Right: S1, apical segment; S2, posterior segment; S3, anterior segment; S4, lateral segment; S5, medial segment; S6, superior segment; S7, medial‐basal segment; S8, anterior‐basal segment; S9, lateral‐basal segment; S10, posterior‐basal segment.
Left: S*, subsuperior segment; S1+2, apico‐posterior segment, S3, anterior segment; S4, superior lingular segment; S5, inferior lingular segment; S6, superior segment; S8, anterior‐basal segment; S9, lateral‐basal segment; S10, posterior‐basal segment.
Figure 1.
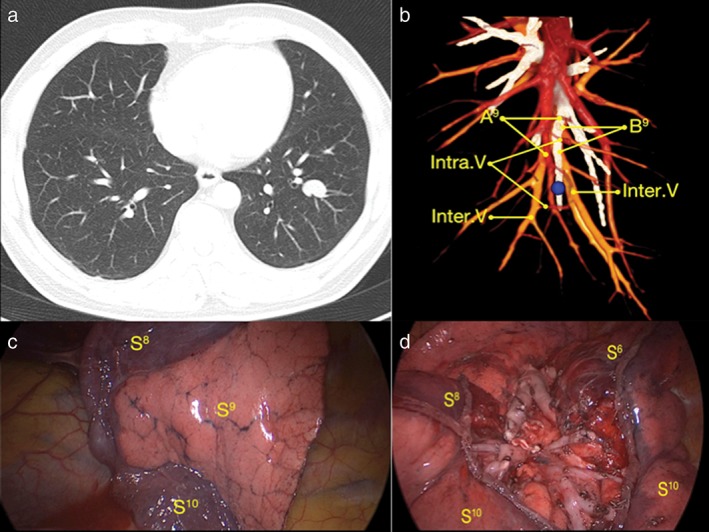
Example of a case of laterobasale segmentectomy. (a) The tumor was located in the left lower lobe. (b) Three‐dimensional computed tomography bronchography and angiography identified targeted the segment bronchus, artery, and intrasegmental and intersegmental veins before surgery. The tumor (blue ball) was located in the left segment laterobasale. (c) Demarcation created by the modified inflation‐deflation method. The inflated segment was the targeted segment (S9) and S8 and S10 were the collapsed segments. (d) The remaining segments (S6, S8, S10) and stumps after segmentectomy. A9, laterobasale segment artery; B9, laterobasale segment bronchus; Intra.V, intrasegmental vein; Inter.V, intersegmental vein; S6, segment superius; S8, segment ventrobasale; S9, laterobasale segment; S10, segment dorsobasale.
After endotracheal intubation, in a state of unilateral differential ventilation, the targeted segment bronchus, artery, and intrasegmental vein were identified and dissected by ligation or stapler cutting, and then the collapsed lung was re‐expanded completely with controlled airway pressure under 20 cmH2O, with the bronchus of the operation side open to atmosphere while continuing ventilation of the contralateral lung. Five to 12 minutes later, an irregular demarcation developed naturally between the inflated targeted segment and the deflated surrounding segments, which represented the intersegmental border to be operated on (Fig 1c). Combining the anatomical intersegmental vein orientation and the demarcation between the collapsed and inflated segments, a cone‐shaped dissection using either electrocautery or staples was performed safely to complete the anatomical segmentectomy (Fig 1d).4
Results
In 532 cases, there was a distinct demarcation between the targeted and surrounding segments, and the time required for this demarcation to develop ranged from 5 to 12 minutes (median 8 minutes). In the other seven cases, a clear line at the first inflation‐deflation process did not develop as the involved intrasegmental veins, which had not been reconstructed in the 3D‐CTBA models, were not adequately preserved. After cutting these intrasegmental veins and re‐attempting the inflation‐deflation process, satisfactory demarcation was observed in all seven cases. The cutting surface provided by this method was highly coincident with the real intersegmental border. Dissection along the border between the collapsed and inflated segments using either electrocautery or staples is safe, with almost no air leak or bleeding.
Discussion
The method developed in our center has a prominent advantage over other methods and does not require additional auxiliary materials. Tsubota reported a similar technique, but with his method, accurate identification and dissection of the targeted bronchus, artery, and intrasegmental venous under guidance of preoperative 3D‐CTBA was impossible, and the incorrect division of the main intersegmental vein may cause poor border development.5 Oizumi et al. also reported a modified Roeder knot technique for bronchial ligation and to visualize the anatomic plane during lung segmentectomy.6 However, we optimized the operational process by dissecting the bronchus, artery, and intrasegmental vein of the targeted segment under unilateral differential ventilation, which saved the steps of making an additional knot and tying this knot to dissect the targeted segmental bronchus after re‐expansion of the whole lung. Furthermore, in the above methods, the pressure to reflate the lung after dissection of the targeted bronchus, artery, and intrasegmental vein and the time required for the inflation‐deflation line to developed were not clearly defined.
The whole lung including the bronchus‐dissected segment should be confirmed with thorough re‐expansion, and we suggest 20 cmH2O as a moderate pressure, which can balance the need for re‐expansion and avoid possible pressure trauma. Only under given pressure can the bronchus‐dissected segment be inflated by the airstream passing through the pores of Kohn. After removal of the targeted segment, airway pressure under 20 cmH2O was applied to re‐expand the remnant lung to decrease the possibility of injury to the cutting edge. Waiting time was another critical factor for the emergence of ideal demarcation, although it varied from case to case. With the precise dissection of targeted tissue, the quality of demarcation was positively correlated to the waiting time to a certain extent.
As the prerequisite for obtaining a good demarcation between the targeted and surrounding segments, accurate identification and dissection of the targeted bronchus, arteries, and intrasegmental veins should be guaranteed by combining the guidance of 3D‐CTBA and intraoperative discernment. The seven cases that did not initially develop an ideal marking border at the first inflation‐deflation process demonstrate this necessity, because the preoperative 3D‐CTBA model is still not fully identical to intraoperative actual anatomy. The conditions of the lung during preoperative CT detection and intraoperatively are slightly different, thus experience is required for accurate identification. In addition, the reconstruction result is closely related to pulmonary parenchyma quality and the degree of bronchus inflation. When there is poor contrast, this has a detrimental effect on the reconstruction, which can cause a reduction in the quality of surgical guidance. In this case series, there were 21 patients with varying degrees of emphysema diagnosed via radiographic evidence but alack of clinical signs, and the waiting time for demarcation to appear was universally prolonged.
Development of the inflation‐collapse line was postulated to involve at least two mechanisms. First, without the segment bronchus as the gas outlet, the gaseous exchange between the targeted segment and the atmosphere was blocked. Second, the blood vessels were ligated simultaneously, so the gas transfer between the pulmonary alveoli and the blood vessels was blocked.
Another tip worth discussing is the method of cutting the intersegmental border. Because inflation‐deflation demarcation can wane from the hilum to the costal surface, caused by a decrease in the connective tissue of the septum lobulae, the hilum of the targeted segment should be dissected sharply, extending to the peripheral parenchyma as much as possible, while one third of the peripheral parenchyma with obscure intersegmental border can be tailored with a stapler. This method can eliminate the curling effect caused by linear cutting of the stapler on the convex of the lung.
In conclusion, this technique is a simple and effective alternative to previously described intersegmental border marking methods. However, the concrete physiological mechanisms and factors affecting inflation‐deflation development time still require further study.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This study was supported in part by the Jiangsu Province Natural Science Foundation (BK20151584), the Jiangsu Top Expert Program in Six Professions (WSW‐028) and the Jiangsu Province Key Research and Development Plan (BE2016789).
References
- 1. Oizumi H, Kanauchi N, Kato H et al Anatomic thoracoscopic pulmonary segmentectomy under 3‐dimensional multidetector computed tomography simulation: A report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011; 141: 678–82. [DOI] [PubMed] [Google Scholar]
- 2. Okada M, Mimura T, Ikegaki J, Katoh H, Itoh H, Tsubota N. A novel video‐assisted anatomic segmentectomy technique: Selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007; 133: 753–8. [DOI] [PubMed] [Google Scholar]
- 3. Sekine Y, Ko E, Oishi H, Miwa M. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012; 143: 1330–5. [DOI] [PubMed] [Google Scholar]
- 4. Wu WB, Xu XF, Wen W et al Three‐dimensional computed tomography bronchography and angiography in the preoperative evaluation of thoracoscopic segmentectomy and subsegmentectomy. J Thorac Dis 2016; 8 (Suppl 9): S710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsubota N. An improved method for distinguishing the intersegmental plane of the lung. Surg Today 2000; 30: 963–4. [DOI] [PubMed] [Google Scholar]
- 6. Oizumi H, Kato H, Endoh M, Inoue T, Watarai H, Sadahiro M. Slip knot bronchial ligation method for thoracoscopic lung segmentectomy. Ann Thorac Surg 2014; 97: 1456–8. [DOI] [PubMed] [Google Scholar]


