Abstract
We report a case of concomitant EML4‐ALK and TPM3‐ROS1 fusion in non‐small cell lung cancer (NSCLC) in a 47‐year‐old Chinese man and review the clinical characteristics of this type double of fusion. The patient presented with a local tumor of the left upper lobe and underwent thoracoscopy. Postoperative surgical pathologic staging revealed T1aN0M0 stage IA. Histological examination of the tumor showed lung adenocarcinoma. Ventana ALK (D5F3) assay of the left lung tissue was ALK negative; however, immunohistochemical assay was positive for ROS1 protein. Using next generation sequencing, we found that the tumor had concomitant EML4‐ALK and TPM3‐ROS1 fusion. No recurrence was observed during seven months of follow‐up. Precise diagnostic techniques allow the detection of concomitant ROS1 fusion and other driver genes, including ALK or EGFR; therefore oncologists should consider this rare double mutation in NSCLC patients. Further exploration of treatment models is required to provide additional therapeutic options.
Keywords: Anaplastic lymphoma kinase, concomitant, lung cancer, ROS1
Introduction
Non‐small‐cell lung cancer (NSCLC) has the highest morbidity and mortality in the world. Incidence and mortality has risen in the female population and in developed countries.1, 2 Two to 7% of NSCLC patients are ALK positive.3 Previous studies have indicated that ALK gene rearrangement is mutually exclusive to EGFR gene mutation in lung cancers.4 However, the coexistence of EML4‐ALK fusion and EGFR mutations has occasionally been reported in a small proportion of patients at frequencies ranging from 0% to 8%.5, 6 In addition, genomic rearrangements involving chromosomal rearrangements of ROS1 occur in 1–2% of NSCLC patients.7 A total of 15 ROS1 fusion partner genes have been reported in NSCLC, including CD74, SLC34A2, GOPC, CCDC6, SDC4, TPM3, EZR, LRIG3, KDELR2, LIMA1, MSN, CLTC, TPD52L1, FIG, TMEM106B, FAM135B, and SLC6A17.8, 9, 10, 11 The clinicopathological features of ROS1 fusions or ALK rearrangements are associated with a history of never smoking, younger age, and adenocarcinoma histologic type.7, 12
The gold standard for the detection of ALK rearrangements is break‐apart fluorescence in situ hybridization.13 However, fluorescence in situ hybridization is usually only available in specialized institutions, while immunohistochemistry (IHC) is an easy, reliable, and cost‐effective technique that is available in almost all pathological institutions. IHC can be used as an initial screening approach to assess ALK or ROS1 rearrangement in NSCLC.14 As a result of the progression of precise detection assays, such as next generation sequencing (NGS), rare cases of double positive in lung cancer have been reported.
To our knowledge, only two cases harboring ROS1 and ALK concomitant rearrangements have been reported in the literature.15, 16 Herein, we report a third case of double TMP3‐ROS1 and EML4‐ALK rearranged NSCLC.
Case report
A 47‐year‐old Chinese male smoker (30 pack‐year) presented to our hospital with a local tumor of the left upper lobe. He underwent thoracoscopy and lymph node dissection. The size of tumor was 2.0 × 1.8 × 1.2 cm (Fig 1 ). The postoperative surgical pathologic diagnosis was papillary predominant with visceral pleural involvement (T1aN0M0 stage IA). Hematoxylin and eosin staining showed typical morphology of adenocarcinoma cells (Fig 2 ). IHC analysis was positive for TTF‐1, CK7, and Napsin A, and negative for CK 5/6 and P63. Ventana ALK (D5F3) assay (Ventana Medical Systems, Inc., Roche, Tucson, AZ, USA) of the left lung tissue was ALK negative. However, IHC assay was positive for ROS1 protein (3+, 90%). We performed next generation sequencing (NGS) assay (Geneplus, Beijing, China) on the surgical specimen and found that the tumor had concomitant EML4‐ALK fusion (abundance 0.92%) and TPM3‐ROS1 (abundance 25.53%) (Fig 3 ). NGS assay was conducted using HiSEquation 4000 (Illumina Inc., Madison, WI, USA). After seven months of follow‐up (to August 2017) no recurrence was observed.
Figure 1.
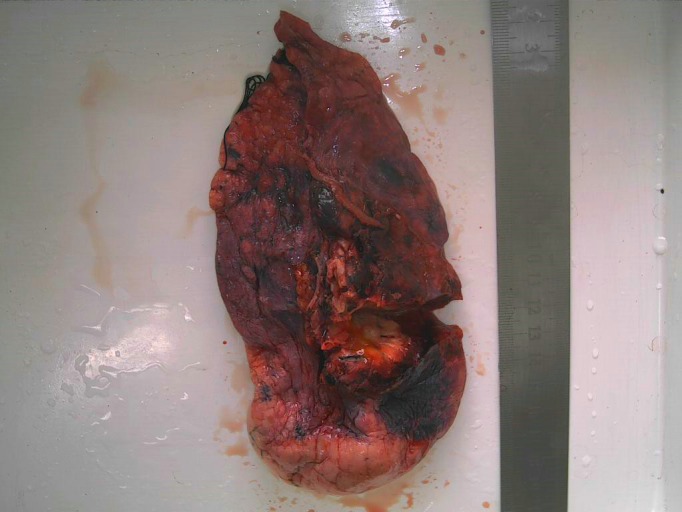
Gross pathologic findings; the mass size of the tumor was 2.0 cm × 1.8 cm × 1.2 cm.
Figure 2.
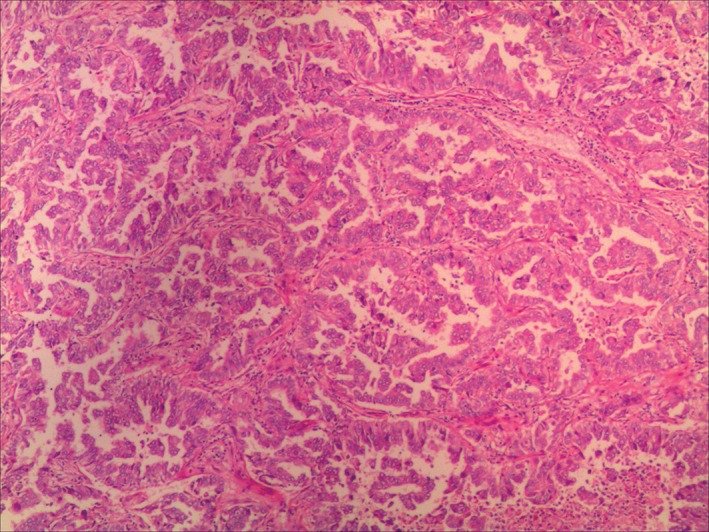
Hematoxylin and eosin staining of the left upper lobe revealed adenocarcinoma with a papillary growth pattern.
Figure 3.
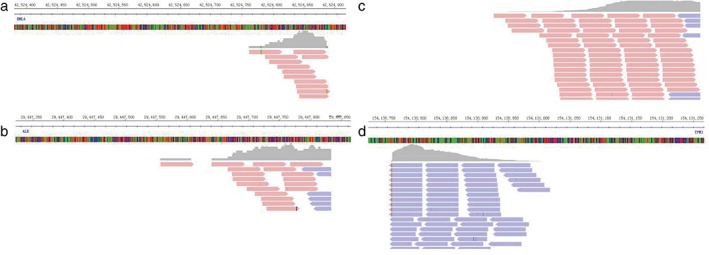
Paired‐end sequencing data indicated somatic intrachromosomal (a) EML4, (b) ALK, (c) TPM3, and (d) ROS1, as demonstrated by the Integrative Genomics Viewer program.
Discussion
The prevalence of a coexisting ROS1 gene rearrangement with other mutations is rare. Warth et al. screened 1478 completely resected NSCLCs with a ROS1‐specific antibody and 68 cases (4.6%) showed ROS1 immunoreactivity.17 They demonstrated that ROS1 translocations occurred in conjunction with other driver mutations (EGFR, KRAS, and BRAF) but none harbored concomitant ROS1 translocation and ALK fusion. Wiesweg et al. detected ROS1 status using IHC in 805 patients with metastatic lung adenocarcinoma.18 Twenty‐five lung cancer patients (4.8% of lung adenocarcinomas) showed ROS1‐positivity and 36% of ROS1‐IHC‐positive cases presented with concomitant oncogenic driver mutations involving EGFR (6 cases), KRAS (2 cases), PIK3CA, and BRAF; however, ALK and ROS1 coexistence was not observed. Recently, Lin et al. conducted a study of 228 NSCLC patients with ROS1 rearrangement.19 Sixty‐two cases were tested for ALK rearrangements, and EGFR and KRAS mutations, but none demonstrated concurrent ALK fusion or concurrent EGFR activating mutations. Lin et al. concluded that ROS1 rearrangements rarely overlap with alterations in other oncogenes. Therefore, concomitant ROS1 and ALK gene rearrangement is very rare. To date, only two cases harboring ROS1 and ALK concomitant rearrangements have been reported in the literature. Song et al. analyzed the data of 732 patients, 32 (4.4%) of which harbored ROS1 rearrangements.15 Of the 32 patients only one was identified with ALK/ROS1 coexistence. Uguen et al. reported a case of a 77‐year‐old female never‐smoker treated with crizotinib for three months who responded to this inhibitor; this was first case in a Chinese patient and only the second reported in the world.16
Crizotinib, an ALK/ROS1/MET inhibitor, was the first targeted agent approved by the United States Food and Drug Administration for the treatment of advanced ROS1‐rearraged NSCLC based on a phase I crizotinib trial. It demonstrated an objective response rate of 72% and median progression‐free survival of 19.2 months in advanced ROS1‐rearranged NSCLC patients.20 Therefore, we hypothesized that advanced NSCLC patients with ALK/ROS1 coexistence may benefit from anti‐ALK and anti‐ROS1 treatment. However, as our patient is at stage IA, follow‐up continues. Further reports of these rare cases are required to explore the best therapeutic strategy for advanced patients.
Immunohistochemistry has been demonstrated as a reliable prescreening test for detecting ALK expression in lung cancer in clinical practice.21 Currently, there are no approved companion assays for ROS1 fusion in NSCLC. Based on experience with ALK, commonly used methods to detect ROS1 fusion include FISH, IHC, reverse transcription‐PCR, and NGS.22 However, IHC results may exhibit false positive because of aneuploidy leading to aberrant expression, as in our case, which demonstrated ALK protein negative by IHC but EML4‐ALK fusion by NGS. Nowadays, in the era of personalized medicine, accurate multi‐gene diagnostics is crucial. Developments in NGS have created a new method for the simultaneous detection of a large number of gene fusions.23, 24 The incidence of double gene mutations in NSCLCs may be explained by different genetic alterations or multiple altered oncogenic pathways. In the future, NGS assay could be used to explore gene differential expression. Further studies are required to assess the performance of different NGS platforms.
In summary, cases of lung adenocarcinoma with concomitant ROS1 fusion and ALK gene rearrangement have been reported in advanced and early stage lung adenocarcinoma. IHC results may exhibit false positive because of aneuploidy, leading to aberrant expression. Currently, there is no consensus on standard therapy for tumors with double positive mutations or fusions. If concurrent driver mutations are identified, other testing assays should be considered to confirm the molecular diagnosis before proceeding with targeted therapy.
Disclosure
No authors report any conflict of interest.
Acknowledgments
The Science and Technology Planning project of Zhejiang Province (2015C33194), and the National Clinical Key Specialty Construction Program (2013) supported this study.
Contributor Information
Wen‐xian Wang, Email: helen-0407@163.com.
Chun‐wei Xu, Email: xuchunweibbb@163.com.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD et al Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 3. Lindeman NI, Cagle PT, Beasley MB et al Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. (Published erratum appears in J Thorac Oncol 2013;8:1343.) J Thorac Oncol 2013; 8: 823–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gainor JF, Varghese AM, SH O et al ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: An analysis of 1,683 patients with non‐small cell lung cancer. Clin Cancer Res 2013; 19: 4273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sahnane N, Frattini M, Bernasconi B et al EGFR and KRAS mutations in ALK‐positive lung adenocarcinomas: Biological and clinical effect. Clin Lung Cancer 2016; 17: 56–61. [DOI] [PubMed] [Google Scholar]
- 6. Kim TJ, Park CK, Yeo CD et al Simultaneous diagnostic platform of genotyping EGFR, KRAS, and ALK in 510 Korean patients with non‐small‐cell lung cancer highlights significantly higher ALK rearrangement rate in advanced stage. J Surg Oncol 2014; 110: 245–51. [DOI] [PubMed] [Google Scholar]
- 7. Bergethon K, Shaw AT, SH O et al ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012; 30: 863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cancer Genome Atlas Research Network . Comprehensive molecular profiling of lung adenocarcinoma. (Published erratum appears in Nature 2014;514:514.) Nature 2014; 511: 543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. SH O, Chalmers ZR, Azada MC et al Identification of a novel TMEM106B‐ROS1 fusion variant in lung adenocarcinoma by comprehensive genomic profiling. Lung Cancer 2015; 88: 352–4. [DOI] [PubMed] [Google Scholar]
- 10. Zhu VW, Upadhyay D, Schrock AB, Gowen K, Ali SM, Ou SH. TPD52L1‐ROS1, a new ROS1 fusion variant in lung adenosquamous cell carcinoma identified by comprehensive genomic profiling. Lung Cancer 2016; 97: 48–50. [DOI] [PubMed] [Google Scholar]
- 11. Zehir A, Benayed R, Shah RH et al Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. (Published erratum appears in Nat Med 2017;23:1004.) Nat Med 2017; 23: 703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shaw AT, Yeap BY, Solomon BJ et al Effect of crizotinib on overall survival in patients with advanced non‐small‐cell lung cancer harbouring ALK gene rearrangement: A retrospective analysis. Lancet Oncol 2011; 12: 1004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teixidó C, Karachaliou N, Peg V, Gimenez‐Capitan A, Rosell R. Concordance of IHC, FISH and RT‐PCR for EML4‐ALK rearrangements. Transl Lung Cancer Res 2014; 3: 70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bubendorf L, Büttner R, Al‐Dayel F et al Testing for ROS1 in non‐small cell lung cancer: A review with recommendations. Virchows Arch 2016; 469: 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song ZB, Zheng YH, Zhang YPALK. ROS1 rearrangements, coexistence and treatment in EGFR‐wild type lung adenocarcinoma: A multicenter study of 732 cases. J Thorac Oncol 2017; 12 (1 Suppl): s1160–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uguen A, Schick U, Quéré GA. rare case of ROS1 and ALK double rearranged non‐small cell lung cancer. J Thorac Oncol 2017; 12: e71–2. [DOI] [PubMed] [Google Scholar]
- 17. Warth A, Muley T, Dienemann H et al ROS1 expression and translocations in non‐small‐cell lung cancer: Clinicopathological analysis of 1478 cases. Histopathology 2014; 65: 187–94. [DOI] [PubMed] [Google Scholar]
- 18. Wiesweg M, Eberhardt WE, Reis H et al High prevalence of concomitant oncogene mutations in prospectively identified patients with ROS1‐positive metastatic lung cancer. J Thorac Oncol 2017; 12: 54–64. [DOI] [PubMed] [Google Scholar]
- 19. Lin JJ, Ritterhouse LL, Ali SM et al ROS1 fusions rarely overlap with other oncogenic drivers in non‐small cell lung cancer. J Thorac Oncol 2017; 12: 872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaw AT, Ou SH, Bang YJ et al Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med 2014; 371: 1963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wynes MW, Sholl LM, Dietel M et al An international interpretation study using the ALK IHC antibody D5F3 and a sensitive detection kit demonstrates high concordance between ALK IHC and ALK FISH and between evaluators. J Thorac Oncol 2014; 9: 631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin JJ, Shaw AT. Recent advances in targeting ROS1 in lung cancer. J Thorac Oncol 2017; 12: 1611–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pekar‐Zlotin M, Hirsch FR, Soussan‐Gutman L et al Fluorescence in situ hybridization, immunohistochemistry, and next‐generation sequencing for detection of EML4‐ALK rearrangement in lung cancer. Oncologist 2015; 20: 316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao X, Sholl LM, Nishino M, Heng JC, Jänne PA, Oxnard GR. Clinical implications of variant ALK FISH rearrangement patterns. J Thorac Oncol 2015; 10: 1648–52. [DOI] [PMC free article] [PubMed] [Google Scholar]


