Abstract
Tumor metastases are the basic biological characteristics of malignant tumors, and the lungs are the second most prominent metastatic organs in which these develop after the liver. Currently, with the rapid development of ablation technology, ablation therapy as a local treatment is playing an increasingly important role in the treatment of lung metastases. Whether alone or in combination with other treatments, ablation therapy has achieved good therapeutic effects for the treatment of partial lung metastases. This article briefly summarizes the results of current and previous ablation treatments for lung metastases, and focuses on the value of ablation therapy for different kinds of lung metastases.
Keywords: Ablation therapy, bone and soft tissue sarcoma, colorectal cancer, lung metastases, renal cancer
Introduction
Metastasis is the basic biological characteristic of malignant tumors and the main factor leading to treatment failure or death in most tumor patients. The lungs are the second most prominent metastatic organs, after the liver, in which malignant tumors develop. Lung metastasis often occurs through the following routes: hematogenous spread, lymphatic spread, direct infiltration or overspread, and airway transcoelomic spread.1 If patients do not receive timely and effective treatment, they may die as a result of respiratory failure.
Systemic chemotherapy is the main treatment for malignant tumors in patients with lung metastases. In recent years, with advances in chemotherapy drugs and the emergence of molecular targeted drugs, the efficacy of chemotherapy for lung metastases has significantly improved, but some patients still develop tumor progression during chemotherapy treatment.2, 3, 4, 5 Not all patients are tolerant to chemotherapy because of its side effects.
Surgical resection is the preferred topical treatment for lung metastases. Complete resection of lung metastases could help to prolong patient survival.1, 6 However, because of the severe trauma caused, the requirement of high patient lung function and other physical conditions, and the extent of lung metastases, many patients are not eligible for surgical treatment. Furthermore, the recurrence rate after surgical resection of lung metastases remains high, thus patients often require repeat surgical treatments.
For tumor patients with lung metastases ineligible for surgical treatment, radiotherapy and ablation therapy are currently the most commonly used local therapies. With the emergence and application of precise radiotherapy, the efficacy of radiotherapy for lung metastases has significantly improved.7, 8 However, because of radiation dose restrictions, it is not possible to apply the multiple radiation courses required to eliminate lung metastases and the patient would be intolerable to such doses. In addition, radiotherapy can cause radiation pneumonia and pulmonary fibrosis, which in serious cases can significantly impact quality of life. Ablation therapy is increasingly used for lung metastasis treatment because of its unique characteristics, including high efficacy, minimal invasion, and strong repeatability.9, 10, 11, 12, 13, 14, 15 The greatest advantage of ablation therapy is the ability to efficiently eliminate lung metastases and at the same time protect lung tissue. Ablation of pulmonary metastases should be performed to control the primary lesion, followed by surgery as soon as possible.
Currently, ablation therapy, whether performed alone or in combination with other treatments, has achieved good therapeutic effects for the treatment of partial lung metastases.
Ablation therapy of lung metastases from colorectal cancer
Colorectal cancer is one of the most common malignant tumors with 1.36 million new cases reported worldwide each year.16 The liver and lung are the main sites of these metastases.17 About 10–20% patients will develop lung metastases, with isolated lung metastases accounting for 7%.18, 19 Without treatment, the median survival duration of patients with lung metastases from colorectal cancer is eight months and the one‐year survival rate is only 30%.20
Chemotherapy is the most important treatment for patients who develop pulmonary metastasis from colorectal cancer. Initially, systemic chemotherapy based on 5‐fluorouracil increased the median survival duration to 12 months and the one‐year survival rate to 50%.21 With the continuous development of chemotherapy drugs, different combinations of oxaliplatin, irinotecan, fluorouracil, and leucovorin have effectively improved the median survival duration to 17 months.22, 23, 24 In recent years, molecular targeted drugs, such as bevacizumab, cetuximab and panitumumab, have further increased the median survival time to 20 months.25, 26, 27, 28 Although chemotherapy can improve patient survival, chemotherapy is not successful in all patients because of drug resistance, side effects, poor basic patient condition, and other factors.
For patients with pulmonary metastasis from colorectal cancer, surgical resection is a positive effective treatment. Accurate and complete resection of all lung metastases is the main surgical treatment principle. Several studies have shown that patients with pulmonary metastasis from colorectal cancer who have undergone surgical resection had median survival duration of 35–50 months and five‐year survival of 36–67.8%.11, 29, 30, 31, 32, 33, 34, 35 However, Inoue et al. reported a recurrence rate after the first surgical resection of 68% and that the lung was the most common site of recurrence.36 The five‐year survival rate after reoperation was 25–58%. However, not all patients are eligible for surgical treatment.
For patients with lung metastases from colorectal cancer that cannot be surgically resected, ablation therapy as a local treatment can effectively control and inactivate pulmonary lesions (Fig 1). The median survival duration of patients with pulmonary metastasis from colorectal cancer treated with radiofrequency ablation (RFA) is 33–67 months, the one, three, and five‐year survival rates are 83.9–95%, 46–76.1% and 35–56%, respectively, and the local recurrence rate is 13–38%.11, 29, 33, 34, 37 Therefore, ablation therapy can achieve similar efficacy as surgical resection. Meanwhile, RFA and surgery provide similar survival predictors, including the number of lung metastases, whether to perform R0 resection, preoperative ACE levels, and whether thoracic lymph node metastasis has developed.29, 33 However, it is noteworthy that pulmonary metastasis from colorectal carcinoma treated with RFA usually cannot be removed by surgery. Compared to surgery, RFA does less harm to the normal lung tissue, which does not cause changes to lung function and RFA can be repeatedly performed on the same or different lung metastases.
Figure 1.
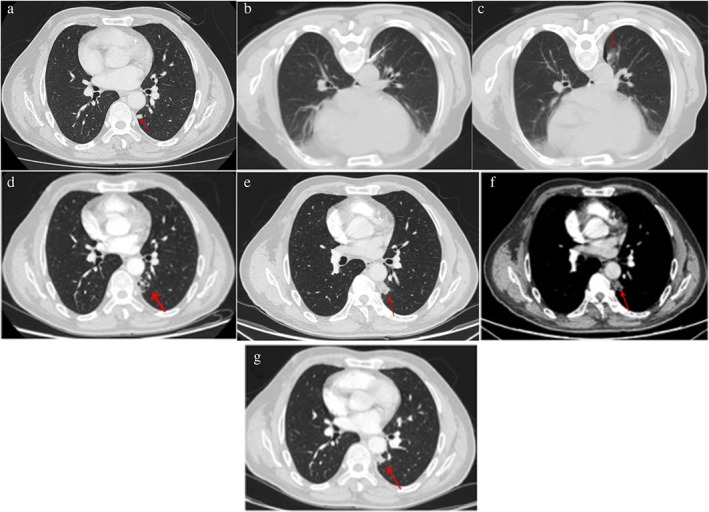
Ablation treatment of lung metastases from colorectal cancer. A 62‐year‐old man displayed left lung metastasis on computed tomography (CT) scans, after rectal adenocarcinoma surgery and seven‐course systemic chemotherapy. The patient received microwave ablation for the lung metastasis under CT‐guidance. No tumor recurrence was found during 11 months of follow‐up. (a) Chest CT scans: lung metastases (red arrows) were found in the superior segment of left lower lobe near the pleural. (b) CT‐guided microwave ablation of lung metastases. (c) Immediate chest CT scans after ablation: the periphery of the ablation lesion showed ground glass changes (red arrow). (d) Chest CT reviews one month after ablation: the ablation lesions showed mixed density changes (red arrow). (e,f) Chest CT reviews three months after ablation: ablation lesions showed substantial alteration without enhancement (red arrow; [e]: pulmonary window; [f]: mediastinal window). (g) Chest CT reviews 11 months after ablation: ablation lesions had significantly shrunk and no tumor recurrence was found.
Inoue et al. conducted a study of 17 patients with colorectal carcinoma with pulmonary metastasis treated with RFA combined with systemic chemotherapy (n = 10) and systemic chemotherapy alone (n = 7).38 The median survival duration of RFA combined with systemic chemotherapy versus systemic chemotherapy alone was 44.2 vs. 24.7, and the three‐year survival rates were 87.5% vs. 33.3% (P = 0.0041), respectively. Ablation therapy combined with systemic chemotherapy was superior to chemotherapy alone for the treatment of pulmonary metastasis in colorectal carcinoma. Ablation therapy can efficiently inactivate a tumor and reduce the tumor burden in a minimally invasive way; combined with chemotherapy, it can significantly stimulate the chemotherapy effect and help to extend patient survival.
Ablation treatment, whether alone or in combination with systemic chemotherapy, can effectively eliminate lung metastases and prolong patient survival.
Ablation therapy of lung metastases from bone and soft tissue sarcoma
Bone and soft tissue sarcoma in malignant tumors originate from mesenchymal and ectodermal nerve tissue, which mostly occur at the trunk, limbs, and retroperitoneal space. Morbidity and mortality account for 1% and 2% among adult malignancies, respectively.16
Ten to 15% of osteosarcoma and 20% of soft tissue sarcoma patients have developed distant metastases by the time they are diagnosed, and lung metastases account for 85%.39, 40, 41 Once metastasis occurs, treatment has a poor therapeutic effect. Lung metastasis is the main cause of death in bone and soft tissue sarcoma patients.42, 43
Despite improvements in chemotherapeutic drugs and schedules, chemotherapy continues to have poor efficacy (25%) in patients with bone or soft tissue sarcoma with metastases.44 Chemotherapy can effectively prolong survival in patients without local or distant metastasis, but fails to improve overall survival.45 Unfortunately, few developments have been made in systemic chemotherapy for bone and soft tissue sarcoma in recent years.
Bone and soft tissue tumors are not sensitive to conventional radiotherapy. Because of limitations to the radiation dose, radiotherapy is not effective for lung metastases in bone and soft tissue tumors. With developments in radiotherapy, stereotactic body radiotherapy (SBRT) is increasingly used in patients with bone and soft tissue tumors with lung metastasis and has achieved a promising effect.10, 46 Dhakal et al. found that in patients with soft tissue sarcoma with lung metastases treated with SBRT, the three‐year local control rate was 82% and the median survival duration was significantly longer than in patients not treated with SBRT (2.1 vs. 0.6, respectively; P = 0.006).8 Baumann et al. treated 39 lung metastases in 30 sarcoma patients with SBRT.47 The one and two‐year local control rates were 94% and 86% and the one and two‐year survival rates were 76% and 43%, respectively.
Surgical resection is the main treatment method for patients with lung metastases from bone and soft tissue tumors. The three and five‐year survival rates of surgical resection range from 25–54% and 14–25%, respectively, but only 25–30% of patients are operable47, 48, 49, 50 and 40–80% patients will develop recurrence.51 Although reoperation has been proven effective for controlling tumor recurrence, not all patients are able to tolerate reoperation.
For patients with lung metastases that cannot be surgically removed, ablation therapy is an effective supplemental treatment (Fig 2). Nakamura et al. performed RFA in 20 patients with lung metastases from osteosarcoma.52 The one and three‐year survival rates were 88.9% and 59%, respectively. Palussiere et al. conducted RFA in 29 patients with lung metastases from sarcoma.53 The one and three‐year survival rates were 92% and 63%, respectively. Koelblinger et al. also reported on 21 patients with lung metastases from sarcoma that underwent RFA.12 The two and three‐year survival rates were 94% and 85%, respectively. Thus, RFA can achieve a similar therapeutic effect to surgical resection for patients with lung metastases from bone and soft tissue sarcoma.
Figure 2.
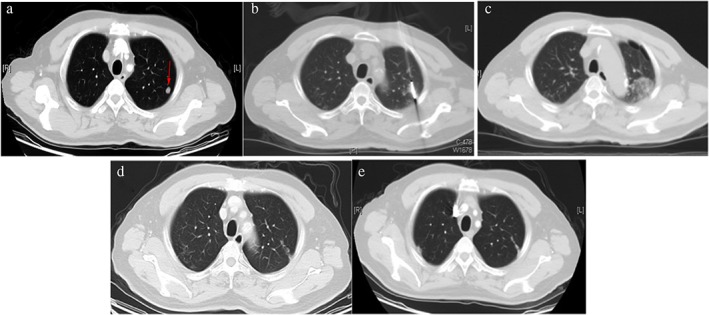
Ablation of lung metastases from sarcoma. A 56‐year‐old man displayed lung metastases on computed tomography (CT) scan four years after left nasal sarcoma resection. The patient received microwave ablation for the lung metastasis under CT‐guidance. No tumor recurrence was found during five months of follow‐up. (a). Chest CT scans shows lung metastases (red arrows) in left lung. (b) CT‐guided microwave ablation of lung metastases. (c) Immediate chest CT scans after first ablation: the ablation lesion showed patchy changes. (d) Chest CT reviews three months after ablation: the ablation lesion became a fibrotic streak. (e) Chest CT reviews five months after ablation: the fibrotic streak had significantly shrunk and no tumor recurrence was found.
The use of surgical resection for elderly patients with lung metastases from bone and soft tissue sarcoma has long been contentious. Ginsberg et al. and Deslauriers et al. reported high rates of perioperative mortality in elderly patients after surgery.54, 55 Because it is minimally invasive, ablation treatment is an effective method for patients with lung metastases from bone and soft tissue tumors. Nakamura et al. performed RFA in 12 elderly patients with lung metastases from osteosarcoma.52 The one and three‐year survival rates were 81.8% and 38.4%, respectively, and the median survival time was 19 months.
Ablation therapy of lung metastases from renal cancer
Renal cancer accounts for 2–3% of adult malignancies, and is the second most common urinary system tumor after bladder cancer.17 Twenty‐five to 30% of patients have distant metastases at the time of diagnosis, and lung is the most common site of metastasis.56
Lung metastases from renal cancer are not sensitive to traditional radiotherapy and chemotherapy. The median survival duration is only 8–12 months and the five‐year survival rate is only 2–3%.57 Treatment with IL‐2 and IFN‐a based immunotherapy is effective in < 20% of patients, the overall median survival duration is only 13.3 months, and such treatment is accompanied by significant adverse reactions.58
With the emergence of molecular targeted therapy, sorafenib has now become one of the preferred treatments for advanced renal cell carcinoma. In a phase III clinical study conducted by Escudier et al., 903 patients with advanced renal cell carcinoma who were treated with radical nephrectomy and/or cytokine but experienced disease progression were randomly assigned to sorafenib and placebo groups.59 Overall survival in the sorafenib group was significantly higher than in the placebo group (17.8 vs. 14.3 months, hazard ratio 0.78; P = 0.0287). Although targeted therapy is more tolerable than cytokine therapy, the side effects cannot be ignored, and most patients will experience disease progression or drug resistance after a period of medication.
Surgical resection is an effective method for the treatment of lung metastasis from renal carcinoma, with the five‐year survival rate ranging from 31% to 40%.60, 61, 62 Kanzaki et al. performed surgical resection in 48 patients with lung metastases from renal cancer, yielding three, five, and 10‐year survival rates of 60%, 47%, and 18%, respectively.63 Alt et al. also performed surgical resection in 224 patients with lung metastases from renal cancer.64 The five‐year tumor‐specific survival rates of the complete resection (n = 49) and palliative resection (n = 175) groups were 73.6% and 19%, respectively. However, only a small portion of patients is eligible for surgery as the extent of metastasis is too great in most patients.
In recent years, ablation therapy has been attempted as a method to treat lung metastasis from renal cancer (Fig 3). de Baère et al. reported a five‐year survival rate of 53.8% in 68 patients with lung metastases from renal cancer.33 Soga et al. used RFA to treat 39 patients with lung metastases from renal cancer.13 There were significant differences in the overall survival rates between the curative and palliative groups at one (100% vs. 90%), three (100% vs. 52%) and five (100% vs. 52%) years (P < 0.05). Whether the tumor was completely eliminated was an important prognostic factor.
Figure 3.
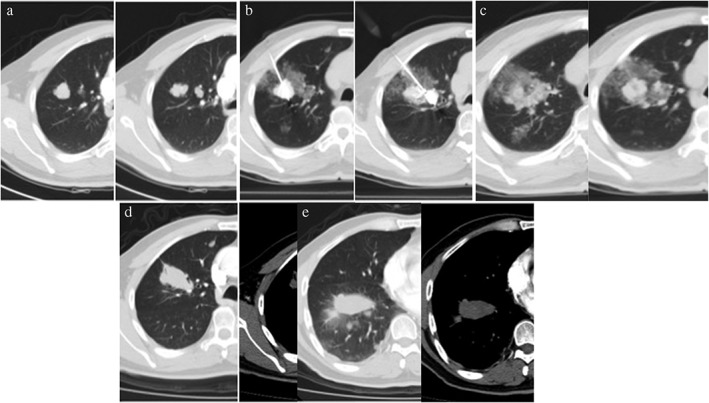
Ablation of lung metastases from renal cancer. A 51‐year‐old man with lung metastases from renal cancer received laparoscopic resection of left renal cell carcinoma in April 2010. The patient was administered sorafenib after surgery, but the lung metastases progressed. He received microwave ablation for the lung metastasis under CT‐guidance. No tumor recurrence was found during two months of follow‐up. (a) Chest computed tomography (CT) scans showed multiple metastases in the left superior lung. (b) CT‐guided microwave ablation of lung metastases. (c) Immediate chest CT scans after ablation: the periphery of the ablation lesion showed ground glass changes. (d,e) Chest CT reviews two months after ablation: ablation lesions showed substantial alteration without enhancement and no tumor recurrence was found.
Ablation therapy for lung metastasis from primary liver cancer
Primary liver cancer is one of the most common malignant tumors. The number of new cases reported annually in China accounts for 50% of global liver cancer incidence. The mortality rate of primary liver cancer is the second highest after lung cancer.17 Because there are usually no obvious symptoms in the early stage, most patients have already reached advanced stage when this disease is diagnosed, and thus, have an extremely poor prognosis. The incidence of lung metastases from primary liver cancer is as high as 20% or more, and reaches 40–73% in autopsy.65
Sorafenib is the preferred treatment for lung metastases from primary liver cancer.66 However, the low response rate, severe adverse reactions, and high cost restrict its application in patients with lung metastases from primary liver cancer.
Surgical resection of lung metastases from liver cancer can significantly improve patient survival. A study of 280 patients with lung metastases from liver cancer in Hong Kong indicated that resection of lung metastases can significantly improve patient survival.67 The median survival duration was 40.36 and 7.46 months, and the one, three, five, and 10‐year survival rates were 86.7%, 53.9%, 31.8%, and 26.9% and 34.1%, 8.1%, 3.5%, and 2.1% in resected and unresectable groups, respectively. However, because chronic hepatitis B cirrhosis is often associated with liver cancer, the patient's liver reserve function is so poor that they cannot tolerate surgical treatment for lung metastases.
If the primary lesion is well controlled, RFA could benefit patients with lung metastases from liver cancer (Fig 4). Baba et al. treated 83 lung metastases in 32 liver cancer patients with RFA, and the one, two, and three‐year survival rates were 83%, 57%, and 57%, respectively.14 Li et al. performed RFA for 68 lung metastases in 29 liver cancer patients, and the one, two, and three‐year survival rates were 73.1%, 41.1%, and 30%, respectively.68 Therefore, when good control of primary liver lesions is possible, ablation therapy is playing an increasingly important role in treatment for patients with lung metastases from primary liver cancer who cannot tolerate surgical treatment.
Figure 4.
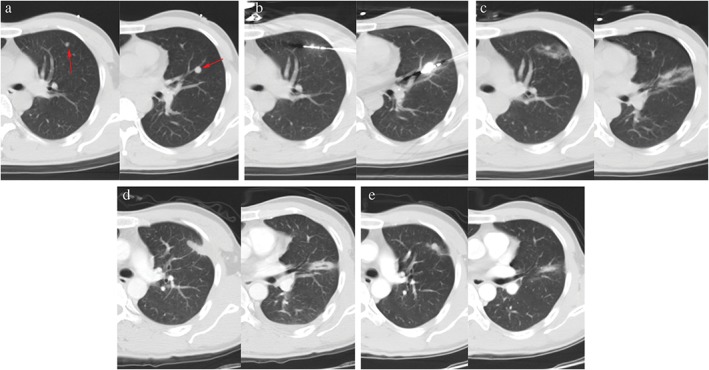
Ablation of lung metastasis from primary liver cancer. A 38‐year‐old man displayed two left lung metastases on chest computed tomography (CT) review one year after resection of primary liver cancer. The patient received microwave ablation for the lung metastasis under CT‐guidance. No tumor recurrence was found during five months of follow‐up. (a) Chest CT scans: lung metastases in the left superior and inferior lobes (red arrow). (b) CT‐guided microwave ablation of lung metastases. (c) Immediate CT scans after ablation: the periphery of the ablation lesion showed ground glass changes. (d) Chest CT reviews one month after ablation: the ablation lesion became fibrotic streaks. (e) Chest CT reviews five months after ablation: fibrotic streaks in the inferior lobe significantly shrank and the ablation lesion in superior lobe changed into a consolidation nodule. No tumor recurrence was found.
Ablation therapy for lung metastasis from nasopharyngeal cancer
Qi et al. performed microwave ablation of 29 lung metastases in 17 patients with nasopharyngeal cancer. The lung metastases were completely ablated in 27 patients and new lung metastases only occurred in five patients within the one‐year follow‐up period.69 A pairing study conducted by Pan et al. indicated that the median survival duration of patients with lung metastases from nasopharyngeal cancer (10 cases) treated with RFA combined with chemotherapy was significantly longer than those who received chemotherapy alone (77.1 vs. 32.4 months, respectively; P = 0.009).15 Although the number of cases is limited, ablation therapy has achieved good results for the treatment of lung metastasis from nasopharyngeal cancer.
Ablation therapy, as a local treatment method, is playing an increasingly important role in the comprehensive treatment of lung metastases. Particularly in patients with lung metastases from colorectal cancer, ablation therapy has been demonstrated to achieve the same effect as surgical resection. Evidence of the therapeutic effect of ablation treatment for lung metastases from other cancers continues to accumulate. However, ablation therapy should only be performed in cases where the primary tumor is well controlled. Individualized ablation is increasingly used but should be adapted according to patient characteristics, combined with different treatment methods, and in a multi‐disciplinary comprehensive treatment model in order to eliminate the tumor, protect the normal lung tissue, and avoid any occurrence of serious complications.
Disclosure
No authors report any conflict of interest.
Acknowledgments
The National Natural Science Foundation of China (Grant No. 8177070783) and the Science and Technology Program of Guangdong Province, China (Grant No. 2015A020214011) supported this study.
References
- 1. Pastorino U, Buyse M, Friedel G et al Long‐term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997; 113: 37–49. [DOI] [PubMed] [Google Scholar]
- 2. Cassidy J, Clarke S, Díaz‐Rubio E et al Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first‐line therapy for metastatic colorectal cancer. J Clin Oncol 2008; 26: 2006–12. [DOI] [PubMed] [Google Scholar]
- 3. Douillard JY, Cunningham D, Roth AD et al Irinotecan combined with fluorouracil compared with fluorouracil alone as first‐line treatment for metastatic colorectal cancer: A multicentre randomised trial. (Published erratum appears in Lancet 2000; 355: 1372.). Lancet 2000; 355: 1041–7. [DOI] [PubMed] [Google Scholar]
- 4. Golfinopoulos V, Salanti G, Pavlidis N, Ioannidis JPA. Survival and disease‐progression benefits with treatment regimens for advanced colorectal cancer: A meta‐analysis. Lancet Oncol 2007; 8: 898–911. [DOI] [PubMed] [Google Scholar]
- 5. Singer EA, Gupta GN, Srinivasan R. Targeted therapeutic strategies for the management of renal cell carcinoma. Curr Opin Oncol 2012; 24: 284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Colon Cancer version 3. 2014. [DOI] [PubMed]
- 7. Stragliotto CL, Karlsson K, Lax I et al A retrospective study of SBRT of metastases in patients with primary sarcoma. Med Oncol 2012; 29: 3431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dhakal S, Corbin KS, Milano MT et al Stereotactic body radiotherapy for pulmonary metastases from soft‐tissue sarcomas: Excellent local lesion control and improved patient survival. Int J Radiat Oncol Biol Phys 2012; 82: 940–5. [DOI] [PubMed] [Google Scholar]
- 9. Chua TC, Glenn D, Morris DL. Extending the survival of patients with melanoma lung metastases through radiofrequency ablation. Acta Oncol 2010; 49: 517–9. [DOI] [PubMed] [Google Scholar]
- 10. Smith SL, Jennings PE. Lung radiofrequency and microwave ablation: A review of indications, techniques and post‐procedural imaging appearances. Br J Radiol 2015; 88: 20140598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsui Y, Hiraki T, Gobara H et al Long‐term survival following percutaneous radiofrequency ablation of colorectal lung metastases. J Vasc Interv Radiol 2015; 26: 303–10. [DOI] [PubMed] [Google Scholar]
- 12. Koelblinger C, Strauss S, Gillams A. Outcome after radiofrequency ablation of sarcoma lung metastases. Cardiovasc Intervent Radiol 2014; 37: 147–53. [DOI] [PubMed] [Google Scholar]
- 13. Soga N, Yamakado K, Gohara H et al Percutaneous radiofrequency ablation for unresectable pulmonary metastases from renal cell carcinoma. BJU Int 2009; 104: 790–4. [DOI] [PubMed] [Google Scholar]
- 14. Baba Y, Watanabe M, Yoshida N, Kawanaka K, Yamashita Y, Baba H. Radiofrequency ablation for pulmonary metastases from gastrointestinal cancers. Ann Thorac Cardiovasc Surg 2014; 20: 99–105. [DOI] [PubMed] [Google Scholar]
- 15. Pan CC, Wu PH, Yu JR et al Comparative survival analysis in patients with pulmonary metastases from nasopharyngeal carcinoma treated with radiofrequency ablation. Eur J Radiol 2012; 81: e473–7. [DOI] [PubMed] [Google Scholar]
- 16. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 17. Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: Metastases to a single organ. World J Gastroenterol 2015; 21: 11767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moorcraft SY, Ladas G, Bowcock A, Chau I. Management of resectable colorectal lung metastases. Clin Exp Metastasis 2016; 33: 285–96. [DOI] [PubMed] [Google Scholar]
- 19. Tan KK, Lopes Gde L Jr, Sim R. How uncommon are isolated lung metastases in colorectal cancer? A review from database of 754 patients over 4 years. J Gastrointest Surg 2009; 13: 642–8. [DOI] [PubMed] [Google Scholar]
- 20. Mitry E, Guiu B, Cosconea S, Jooste V, Faivre J, Bouvier AM. Epidemiology, management and prognosis of colorectal cancer with lung metastases: A 30‐year population‐based study. Gut 2010; 59: 1383–8. [DOI] [PubMed] [Google Scholar]
- 21. Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: Systematic review and meta‐analysis. Colorectal Cancer Collaborative Group. BMJ 2000; 321: 531–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Gramont A, Figer A, Seymour M et al Leucovorin and fluorouracil with or without oxaliplatin as first‐line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938–47. [DOI] [PubMed] [Google Scholar]
- 23. Saltz LB, Cox JV, Blanke C et al Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000; 343: 905–14. [DOI] [PubMed] [Google Scholar]
- 24. Giacchetti S, Perpoint B, Zidani R et al Phase III multi‐center randomized trial of oxaliplatin added to chronomodulated fluorouracil‐leucovorin as first‐line treatment of metastatic colorectal cancer. J Clin Oncol 2000; 18: 136–47. [DOI] [PubMed] [Google Scholar]
- 25. Hurwitz H, Fehrenbacher L, Novotny W et al Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–42. [DOI] [PubMed] [Google Scholar]
- 26. Colucci G, Gebbia V, Paoletti G et al Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: A multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol 2005; 23: 4866–75. [DOI] [PubMed] [Google Scholar]
- 27. Porschen R, Arkenau HT, Kubicka S et al Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: A final report of the AIO Colorectal Study Group. J Clin Oncol 2007; 25: 4217–23. [DOI] [PubMed] [Google Scholar]
- 28. Geng F, Wang Z, Yin H, Yu J, Cao B. Molecular targeted drugs and treatment of colorectal cancer: Recent progress and future perspectives. Cancer Biother Radiopharm 2017; 32: 149–60. [DOI] [PubMed] [Google Scholar]
- 29. Petre EN, Jia X, Thornton RH et al Treatment of pulmonary colorectal metastases by radiofrequency ablation. Clin Colorectal Cancer 2013; 12: 37–44. [DOI] [PubMed] [Google Scholar]
- 30. Gillams A, Khan Z, Osborn P, Lees W. Survival after radiofrequency ablation in 122 patients with inoperable colorectal lung metastases. Cardiovasc Intervent Radiol 2013; 36: 724–30. [DOI] [PubMed] [Google Scholar]
- 31. Ferguson J, Alzahrani N, Zhao J et al Long term results of RFA to lung metastases from colorectal cancer in 157 patients. Eur J Surg Oncol 2015; 41: 690–5. [DOI] [PubMed] [Google Scholar]
- 32. Lencioni R, Crocetti L, Cioni R et al Response to radiofrequency ablation of pulmonary tumours: A prospective, intention‐to‐treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 2008; 9: 621–8. [DOI] [PubMed] [Google Scholar]
- 33. de Baère T, Aupérin A, Deschamps F et al Radiofrequency ablation is a valid treatment option for lung metastases: Experience in 566 patients with 1037 metastases. Ann Oncol 2015; 26: 987–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamakado K, Inoue Y, Takao M et al Long‐term results of radiofrequency ablation in colorectal lung metastases: Single center experience. Oncol Rep 2009; 22: 885–91. [DOI] [PubMed] [Google Scholar]
- 35. Yamakado K, Hase S, Matsuoka T et al Radiofrequency ablation for the treatment of unresectable lung metastases in patients with colorectal cancer: A multicenter study in Japan. J Vasc Interv Radiol 2007; 18: 393–8. [DOI] [PubMed] [Google Scholar]
- 36. Inoue M, Ohta M, Iuchi K et al Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg 2004; 78: 238–44. [DOI] [PubMed] [Google Scholar]
- 37. Saltz LB, Clarke S, Díaz‐Rubio E et al Bevacizumab in combination with oxaliplatin‐based chemotherapy as first‐line therapy in metastatic colorectal cancer: A randomized phase III study. (Published errata appear in J Clin Oncol 2009; 27: 653; J Clin Oncol 2008; 26: 3110.). J Clin Oncol 2008; 26: 2013–9. [DOI] [PubMed] [Google Scholar]
- 38. Inoue Y, Miki C, Hiro J et al Improved survival using multi‐modality therapy in patients with lung metastases from colorectal cancer: A preliminary study. Oncol Rep 2005; 14: 1571–6. [PubMed] [Google Scholar]
- 39. Chou AJ, Kleinerman ES, Krailo MD et al Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: A report from the Children's Oncology Group. Cancer 2009; 115: 5339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Billingsley KG, Burt ME, Jara E et al Pulmonary metastases from so tissue sarcoma: Analysis of patterns of diseases and postmetastasis survival. Ann Surg 1999; 229: 602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Daw NC, Chou AJ, Jaffe N et al Recurrent osteosarcoma with a single pulmonary metastasis: A multi‐institutional review. Br J Cancer 2015; 112: 278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kager L, Zoubek A, Pötschger U et al Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol 2003; 21: 2011–8. [DOI] [PubMed] [Google Scholar]
- 43. Kempf‐Bielack B, Bielack SS, Jürgens H et al Osteosarcoma relapse after combined modality therapy: An analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol 2005; 23: 559–68. [DOI] [PubMed] [Google Scholar]
- 44. García del Muro Solans X, Martín Broto J, Lianes Barragán P, Cubedo Cervera R, Spanish Society of Clinical Oncology . SEOM clinical guidelines for the management of adult soft tissue sarcomas. Clin Transl Oncol 2012; 14: 541–4. [DOI] [PubMed] [Google Scholar]
- 45. Tascilar M, Loos WJ, Seynaeve C, Verweij J, Sleijfer S. The pharmacologic basis of ifosfamide use in adult patients with advanced soft tissue sarcomas. Oncologist 2007; 12: 1351–60. [DOI] [PubMed] [Google Scholar]
- 46. Baumann BC, Nagda SN, Kolker JD et al Efficacy and safety of stereotactic body radiation therapy for the treatment of pulmonary metastases from sarcoma: A potential alternative to resection. J Surg Oncol 2016; 114: 65–9. [DOI] [PubMed] [Google Scholar]
- 47. Smith R, Pak Y, Kraybill W, Kane JM III. Factors associated with actual long‐term survival following soft tissue sarcoma pulmonary metastasectomy. Eur J Surg Oncol 2009; 35: 356–61. [DOI] [PubMed] [Google Scholar]
- 48. Briccoli A, Rocca M, Salone M, Guzzardella GA, Balladelli A, Bacci G. High grade osteosarcoma of the extremities metastatic to the lung: Long‐term results in 323 patients treated combining surgery and chemotherapy, 1985–2005. Surg Oncol 2010; 19: 193–9. [DOI] [PubMed] [Google Scholar]
- 49. Blackmon SH, Shah N, Roth JA et al Resection of pulmonary and extrapulmonary sarcomatous metastases is associated with long‐term survival. Ann Thorac Surg 2009; 88: 877–84. [DOI] [PubMed] [Google Scholar]
- 50. Harting MT, Blakely ML, Jaffe N et al Long‐term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg 2006; 41: 194–9. [DOI] [PubMed] [Google Scholar]
- 51. Olivier T, Pop D, Chouiter Djebaili A et al Treating metastatic sarcomas locally: A paradoxe, a rationale, an evidence? Crit Rev Oncol Hematol 2015; 95: 62–77. [DOI] [PubMed] [Google Scholar]
- 52. Nakamura T, Matsumine A, Yamakado K et al Lung radiofrequency ablation in patients with pulmonary metastases from musculoskeletal sarcoma. (Published erratum appears in Cancer 2009; 115: 4041.). Cancer 2009; 115: 3774–81. [DOI] [PubMed] [Google Scholar]
- 53. Palussière J, Italiano A, Descat E et al Sarcoma lung metastases treated with percutaneous radiofrequency ablation: Results from 29 patients. Ann Surg Oncol 2011; 18: 3771–7. [DOI] [PubMed] [Google Scholar]
- 54. Ginsberg RJ, Hill LD, Eagan RT et al Modern thirty‐day operative mortality for surgical resections in lung cancer. J Thorac Cardiovasc Surg 1983; 86: 654–8. [PubMed] [Google Scholar]
- 55. Deslauriers J, Ginsberg RJ, Piantadosi S, Fournier B. Prospective assessment of 30‐day operative morbidity for surgical resections in lung cancer. Chest 1994; 106(6 Suppl.): 329S–30S. [DOI] [PubMed] [Google Scholar]
- 56. Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastataic renal cell carcinoma (mRCC): A literature review. Cancer Treat Rev 2008; 34: 193–205. [DOI] [PubMed] [Google Scholar]
- 57. Bellmunt J, Montagut C, Albiol S, Carles J, Maroto P, Orsola A. Present strategies in the treatment of metastatic renal cell carcinoma: An update on molecular targeting agents. BJU Int 2007; 99: 274–80. [DOI] [PubMed] [Google Scholar]
- 58. Coppin C, Porzsolt F, Awa A, Kumpf J, Coldman A, Wilt T. Immunotherapy for advanced renal cell cancer. Cochrance Database Syst Rev 2005; (1); CD001425. [DOI] [PubMed] [Google Scholar]
- 59. Escudier B, Eisen T, Stadler WM et al Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 2009; 27: 3312–8. [DOI] [PubMed] [Google Scholar]
- 60. Hofmann HS, Neef H, Krohe K, Andreev P, Silber RE. Prognostic factors and survival after pulmonary resection of metastatic renal cell carcinoma. Eur Urol 2005; 48: 77–81. [DOI] [PubMed] [Google Scholar]
- 61. Swanson DA. Surgery for metastases of renal cell carcinoma. Scand J Surg 2004; 93: 150–5. [DOI] [PubMed] [Google Scholar]
- 62. Assouad J, Petkova B, Berna P, Dujon A, Foucault C, Riquet M. Renal cell carcinoma lung metastases surgery: Pathologic findings and prognostic factors. Ann Thorac Surg 2007; 84: 1114–20. [DOI] [PubMed] [Google Scholar]
- 63. Kanzaki R, Higashiyama M, Fujiwara A et al Long‐term results of surgical resection for pulmonary metastasis from renal cell carcinoma: A 25‐year single‐institution experience. Eur J Cardiothorac Surg 2011; 39: 167–72. [DOI] [PubMed] [Google Scholar]
- 64. Alt AL, Boorjian SA, Lohse CM, Costello BA, Leibovich BC, Blute ML. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer 2011; 117: 2873–82. [DOI] [PubMed] [Google Scholar]
- 65. Maida M, Orlando E, Cammà C, Cabibbo G. Staging systems of hepatocellular carcinoma: A review of literature. World J Gastroenterol 2014; 20: 4141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers version 2. 2015.
- 67. Chok KS, Yau TC, Cheung TT, Poon RT, Lo CM. Retrospective study of metachronous lung metastases from primary hepatocellular carcinoma. ANZ J Surg 2016; 86: 289–93. [DOI] [PubMed] [Google Scholar]
- 68. Li X, Wang J, Li W et al Percutaneous CT‐guided radiofrequency ablation for unresectable hepatocellular carcinoma pulmonary metastases. Int J Hyperthermia 2012; 28: 721–8. [DOI] [PubMed] [Google Scholar]
- 69. Qi H, Wan C, Li X, Zhang L, Song Z, Fan W. Computed tomography‐guided percutaneous microwave ablation treatment for lung metastases from nasopharyngeal carcinoma. Indian J Cancer 2015; 52 (Suppl. 2): e91–5. [DOI] [PubMed] [Google Scholar]


