Abstract
Background
Gemcitabine plus cisplatin (GP) is commonly used to treat lung squamous cell carcinoma (SCC); however, it is not clear which subgroup of lung SCC patients could benefit most from GP treatment. We explored the predictive factors in lung SCC patient cohorts.
Methods
Seventy‐eight lung SCC patients treated with a first‐line GP regimen were enrolled in this retrospective cohort study. Progression‐free survival (PFS) and overall survival (OS) were estimated using the Kaplan–Meier method. Classification tree models were used to explore the risk factors for PFS and OS in these patients.
Results
The median PFS and OS in SCC patients treated with a GP regimen were 6.0 and 13.6 months, respectively. Three terminal subgroups were formed for both PFS and OS. The subgroup with a body mass index (BMI) > 23.94 kg/m2 and aged ≤ 54.5 had the longest PFS (9.0 months); the subgroup with a BMI < 23.94 kg/m2 and aged ≤ 54.5 had the shortest PFS (4.05 months). Patients with an objective response (partial or complete response) to treatment had the longest OS (20.0 months), while patients with a BMI ≤ 26.92 kg/m2 and stable or progressive disease as the best response had the shortest OS (11.2 months).
Conclusions
BMI and age may be predictors of PFS in lung SCC patients who receive GP treatment. BMI and best response to GP treatment predicts OS in such patients. Patients’ clinical pathological characteristics may be used to predict the therapeutic efficacy of chemotherapy and survival.
Keywords: Body mass index (BMI), chemotherapy, non‐small cell lung cancer
Introduction
Squamous cell carcinomas (SCC) account for 30% of non‐small‐cell lung cancers worldwide.1 Unlike adenocarcinoma of the lung, SCC lacks effective target therapy. Although some potentially targetable markers have been identified,1, 2 including DDR2 mutation, and PIK3CA, FGFR1, and MET amplification, none has been validated in SCC for particular targeted drugs. The National Comprehensive Cancer Network guidelines recommend use of the PD‐1/PDL‐1 antibody and the human immunoglobulin G1 VEGFR‐2 antibody ramucirumab in combination with docetaxel. However, these treatments were only used for second or subsequent lines. As such, the available first‐line regimens have remained unchanged for nearly two decades. The platinum‐based doublet regimen is still standard chemotherapy for advanced squamous cell lung cancer. Gemcitabine plus cisplatin (GP) is the most commonly used regimen for squamous cell lung carcinoma.3 However, the effective response rate (RR) of the GP regimen is < 30% and the median progression‐free survival (PFS) is only three to five months.4, 5 Effective biomarkers are urgently needed.
Previous studies have suggested RRM1, ERCCl, hENT1, and BRCA1 as potential predictive biomarkers for a GP regimen. RRM1 is the binding site for chemotherapy drugs, particularly nucleoside analogues, such as gemcitabine.6 RRM1 expression is associated with gemcitabine resistance in non‐small cell lung cancer (NSCLC).7 ERCCl involved in DNA damage repair and excision caused by platinum. Studies have shown that ERCC1 expression level is critical for the evaluation of platinum resistance.8, 9 One recent study on pancreatic cancer patients showed that the hENT1 expression level correlated with higher survival rates with increased gemcitabine exposure.10, 11 BRCA1 is involved in mitosis and the repair of DNA damage. Studies have shown that NSCLC patients with high BRCA1 expression had low efficacy with cisplatin therapy.12, 13 Although all of these biomarkers for the GP regimen have been explored, none has been clinically implemented in routine daily practice and effective predictive biomarkers for GP cytotoxic chemotherapy are crucially lacking.
Body mass index (BMI), defined as weight in kilograms divided by the square of the height in meters, is positively associated with the risk of lung cancers.14 For early stage NSCLC, higher BMI is associated with improved OS after surgical resection.15 For advanced NSCLC, obese patients also had superior outcomes compared to normal/overweight patients.16
In this study, we analyze a group of SCC patients treated with a GP regimen to determine the predictive value of BMI and other clinical characteristics.
Methods
Patient population
Patients histologically or cytologically confirmed with stage IV SCC and administered GP as first‐line treatment at the Peking University Cancer Hospital, Beijing, China, between February 2008 and October 2016 were eligible for enrollment into this retrospective cohort study. Laboratory data was independently obtained and recorded and then analyzed by a biostatistician blinded to the clinical outcome. The study was reviewed and approved by the Institutional Ethic Committee. All patients routinely signed informed consent for the scientific use of their clinical data.
The study was reviewed and approved by the institutional ethic committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients routinely signed informed consent for the scientific use of their clinical data.
Evaluation of treatment response
Objective tumor response was determined using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The patients underwent computed tomography (CT) scans covering the target lesions every six weeks (two cycles) during the course of chemotherapy. Brain or bone metastases were evaluated every three or six months by magnetic resonance imaging or bone scintigraphy. According to the RECIST 1.1, the response to therapy was categorized into four groups: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Overall survival (OS) was calculated as the time from the beginning of therapy to death or the last follow‐up. Progression‐free survival (PFS) was calculated from the date of the beginning of chemotherapy to the date of tumor progression or death.
Statistical analysis
Statistical analysis was performed using R version 3.3.3 (http://www.r-project.org) and SPSS version 22.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as mean ± standard deviation, median (interquartile range), or absolute number and percentage, as appropriate. PFS and OS were estimated using the Kaplan–Meier method. A comparison of PFS and OS between groups was performed using the log‐rank test.
Classification tree analysis was used to classify PFS according to independent risk factors, including age, gender, BMI, Eastern Cooperative Oncology Group performance status, and location of cancer. For OS, we added response to GP regimen as an additional risk factor. The minimum number of patients for the parent node was set to 20, and the minimum for the child node was 5. The maximum classification tree depth was 3. We chose the chi‐square automatic interaction detection method, and its best model on the basis of the obtained predicted probabilities. The reported significance levels were all two‐sided, with statistical significance set at 0.05.
Results
Patient characteristics
A total of 78 SCC patients treated with a first‐line GP regimen were included in this retrospective study. Additional details are summarized in Table 1. At the last follow‐up date (1 October 2016), the median follow‐up duration was 16.8 months. The median age of the 78 patients was 59 years. The median BMI was 23.21. The location of cancer was well balanced (right‐sided 39 cases, left‐sided 39 cases) and most patients were male (67, 85.9%). All 78 patients were histologically confirmed with lung SCC.
Table 1.
Baseline characteristics
| No. of patients, n (%) | |
|---|---|
| Age | 59.0 (54.0–64.3) |
| Gender | |
| Male | 67 (85.9%) |
| Female | 11 (14.1%) |
| Location of cancer | |
| Right‐sided | 39 (50.0%) |
| Left‐sided | 39 (50.0%) |
| Body mass index | 23.21 (20.74–25.16) |
| ECOG PS | |
| 0 | 4 (5.1%) |
| 1 | 69 (88.5%) |
| 2 | 5 (6.4%) |
| Response rate | |
| Complete response | 1 (1.3%) |
| Partial response | 30 (38.5%) |
| Stable disease | 26 (33.3%) |
| Progressive disease | 21 (26.9%) |
Data are n (%) or median (interquartile range).
ECOG PS, Eastern Cooperative Oncology Group performance status.
Survival
Of the 78 patients included, 94.9% (74) had progressive disease; 87.2% (68) had died by the final follow‐up. The median PFS for this group of patients was 6.0 months (95% confidence interval 4.8–8.0) (Fig 1), and the median OS was 13.6 months (95% confidence interval 11.2–16.5) (Fig 2).
Figure 1.
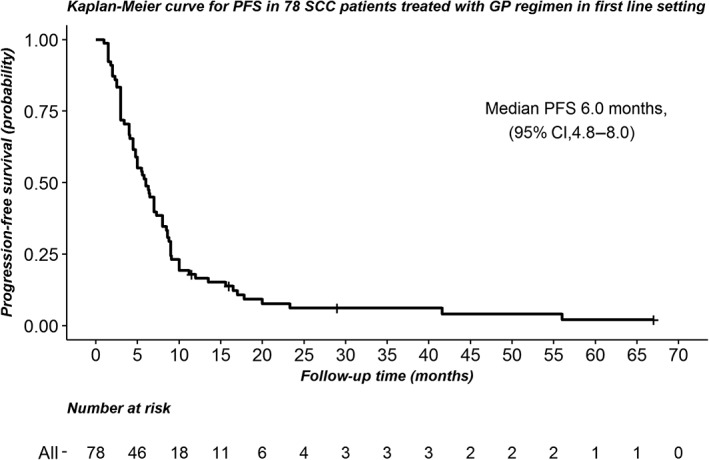
Kaplan‐Meier curve for progression‐free survival (PFS) in 78 squamous cell carcinoma patients treated with a gemcitabine and cisplatin regimen in a first‐line setting. CI, confidence interval.
Figure 2.
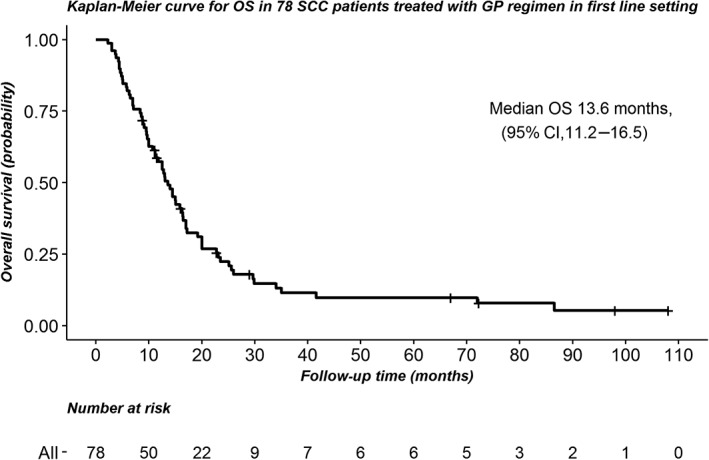
Kaplan‐Meier curve of overall survival (OS) in 78 squamous cell carcinoma patients treated a gemcitabine and cisplatin regimen in a first‐line setting. CI, confidence interval.
Classification tree model
Classification tree analysis was used to predict PFS according to independent risk factors, including age, gender, BMI, and location of cancer. The cut‐off point in classification tree analysis was obtained by chi‐square automatic interaction detection method. The first prognostic split was age, followed by BMI. Finally, the patients were divided into three groups (Fig 3): Group I, aged > 54.5 years; Group II, aged ≤ 54.5 years and BMI > 23.94 kg/m2; and Group III, aged ≤ 54.5 years and BMI ≤ 23.94 kg/m2. Among the three groups, Group II had the longest PFS (9.0 months), whereas Group III had the shortest PFS (4.05 months). The survival curves for the three groups are shown in Figure 4 (Log‐rank test, P = 0.008).
Figure 3.
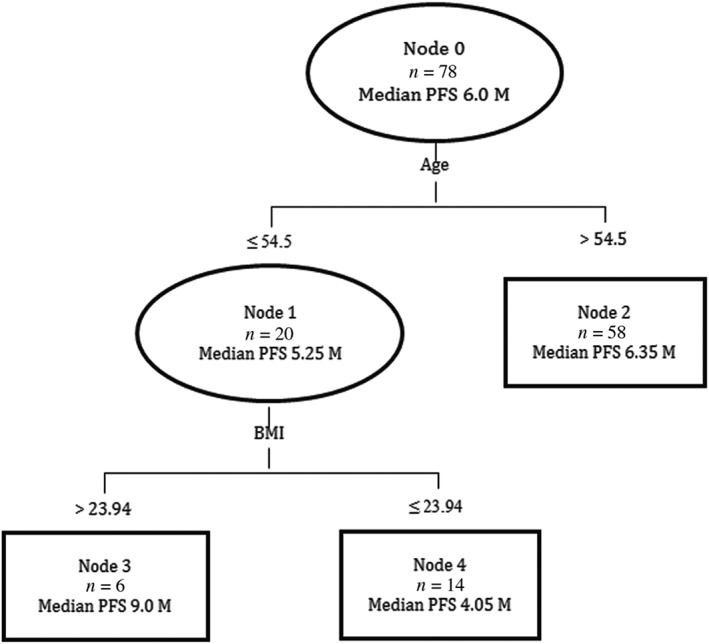
Classification tree model for progression‐free survival (PFS). BMI, body mass index; CI, confidence interval.
Figure 4.
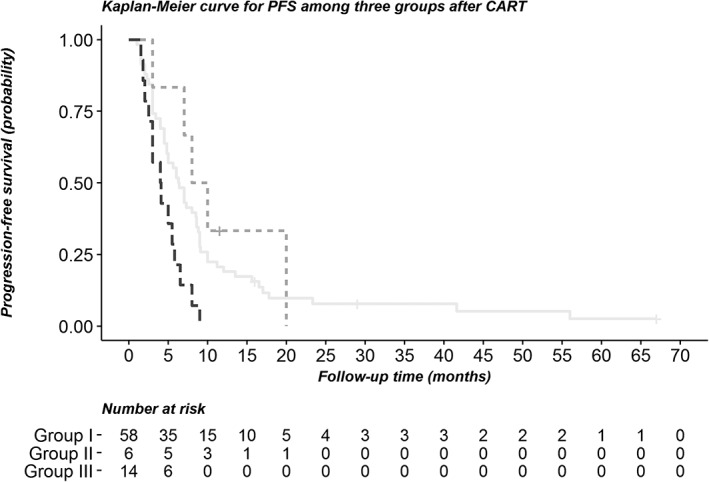
Kaplan‐Meier curve for progression‐free survival between the groups after classification regression tree analysis. ( ) Group I, (
) Group I, ( ) Group II, and (
) Group II, and ( ) Group III.
) Group III.
Another classification tree model was performed to analyze OS. Response to GP regimen was identified as the first split factor in the tree analysis for predicting OS, followed by BMI ≥ 26.92 kg/m2. The three groups were divided as follows: Group I: patients with CR or PR to GP regimen; Group II: patients with SD or PD response to GP regimen and BMI ≤ 26.92 kg/m2; and Group III: patients with SD or PD response to GP regimen and BMI > 26.92 kg/m2 (Fig 5). Patients who achieved PR + CR had the longest overall survival (20.0 months), compared to groups II and III. The OS curves are presented in Figure 6, and show significant differences between the groups (log‐rank test, P < 0.001).
Figure 5.
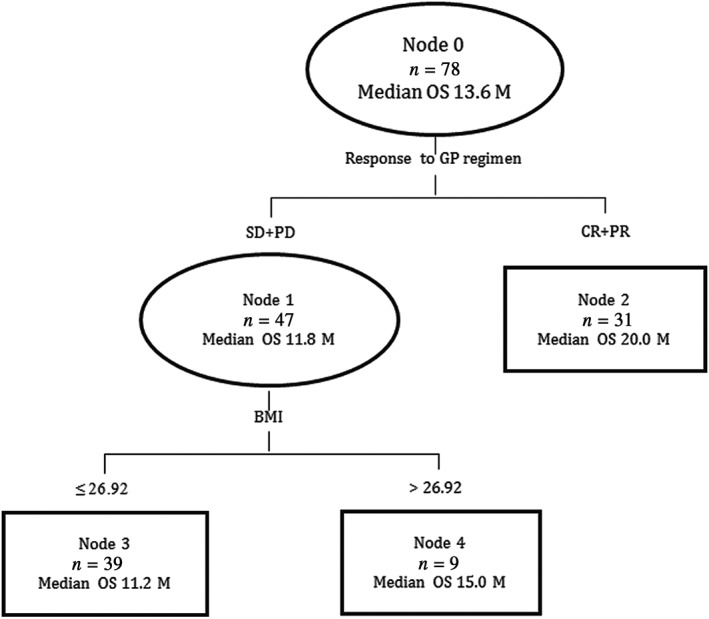
Classification tree model for overall survival. BMI, body mass index; CR, complete response; GP, gemcitabine and cisplatin; PR, partial response; PD, progressive disease; SD, stable disease.
Figure 6.
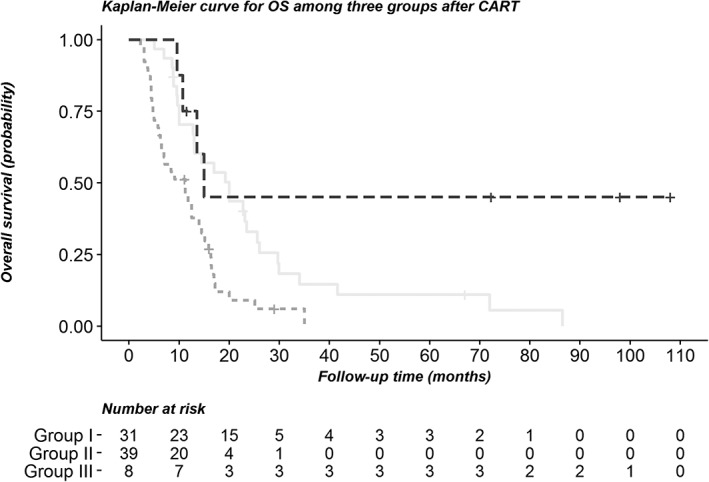
Kaplan‐Meier curve for overall survival between the groups after classification regression tree analysis. ( ) Group I, (
) Group I, ( ) Group II, and (
) Group II, and ( ) Group III.
) Group III.
Discussion
Our retrospective analysis of 78 SCC patients treated with a GP regimen demonstrates that high BMI and younger age are positive predictive factors for PFS. Sensitivity to initial treatment and high BMI predicts longer OS.
The World Health Organization defines BMI groups as: underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5 to < 25 kg/m2), overweight (BMI 25 to < 30 kg/m2), and obese (BMI ≥ 30 kg/m2).17 Our study showed that higher BMI predicts longer PFS and OS. These results are consistent with the previous studies that have evaluated the role of BMI in OS of advanced NSCLC16 as well as in surgically resected lung cancer.15, 18 However, our study also considers other clinical characteristics and we constructed a multivariable model using CART analysis.
The reason that high BMI is associated with a favorable outcome in our study may be because of the following reasons. First, previous studies have suggested that it is possible patients with a lower BMI have more aggressive disease and cachexia, meaning that they had a poor tolerance to chemotherapy and subsequent disease progression.19, 20 Secondly, differential pharmacokinetics may be affected by physical size.21 Won et al. found that clearance of doxorubicin was significantly reduced in individuals with a proportion of body fat exceeding 30%, even with a body surface area based dose adjustment.22 Similarly, a high BMI might also affect the clearance of gemcitabine and cisplatin. Thirdly, fatty acids (which are rich in patients with high BMI), can act as peroxisome proliferator‐activated receptor ligands, which have been shown to increase the efficacy of platinum‐based chemotherapy.23 Fourthly, patients with high BMI are more likely to receive metabolic drugs, such as metformin. These drugs induce apoptosis in LKB1‐deficient NSCLC.24 As inactivation of LKB1 has been found in 19% of lung SCC,25 and is also important for SCC transformation,26 patients with a high BMI have a reasonable likelihood of receiving metabolic drugs and to have an LKB1‐deficient tumor.
In our study, patients with a high BMI and aged < 54.5 experienced longer PFS, which indicates that advanced age might be a negative predictive factor of chemotherapy. A previous study reported that patients aged > 65 years were more likely to have poor performance status, multiple comorbidities, and survival rates became poorer with increasing age.27 The shorter PFS of older patients in our study might also be a result of poor performance status and a higher rate of comorbidities, although previous research has shown that age does not worsen efficacy or tolerance to NSCLC.28 A larger cohort and multivariate analysis of the relationship between age and treatment efficacy are required.
Patients in our study who achieved an objective response (PR + CR) to treatment had the longest OS, which has been previously been proven.29 The University of Texas M.D. Anderson Lung Cancer Collaborative Research Group found that CT RECIST is a reliable predictor of OS in NSCLC patients.30
Classification and regression tree programs effectively segregate patients into different groups with significantly different prognoses. Patients with a higher BMI and younger age benefit more from GP treatment. Patients who achieved a better response and had a higher BMI experienced longer OS.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
The authors wish to thank Dr. Mailin Chen and Ning Wang (Radiological Department, Peking University Cancer Hospital) for their assessments of response to treatment.
References
- 1. Perez‐Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: Molecular subtypes and therapeutic opportunities. Clin Cancer Res 2012; 18: 2443–51. [DOI] [PubMed] [Google Scholar]
- 2. Gandara DR, Hammerman PS, Sos ML, Lara PNJ, Hirsch FR. Squamous cell lung cancer: From tumor genomics to cancer therapeutics. Clin Cancer Res 2015; 21: 2236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Y, Wang LR, Chen J, Lou Y, Zhang GB. First‐line gemcitabine plus cisplatin in nonsmall cell lung cancer patients. Dis Markers 2014; 2014: 960458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scagliotti GV, Parikh P, von Pawel J et al Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J Clin Oncol 2008; 26: 3543–51. [DOI] [PubMed] [Google Scholar]
- 5. Paz‐Ares L, Socinski MA, Shahidi J et al Correlation of EGFR‐expression with safety and efficacy outcomes in SQUIRE: A randomized, multicenter, open‐label, phase III study of gemcitabine‐cisplatin plus necitumumab versus gemcitabine‐cisplatin alone in the first‐line treatment of patients with stage IV squamous non‐small‐cell lung cancer. Ann Oncol 2016; 27: 1573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosell R, Danenberg KD, Alberola V et al Ribonucleotide reductase messenger RNA expression and survival in gemcitabine/cisplatin‐treated advanced non‐small cell lung cancer patients. Clin Cancer Res 2004; 10: 1318–25. [DOI] [PubMed] [Google Scholar]
- 7. Zhao LP, Xue C, Zhang JW et al Expression of RRM1 and its association with resistancy to gemcitabine‐based chemotherapy in advanced nasopharyngeal carcinoma. Chin J Cancer 2012; 31: 476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olaussen KA, Dunant A, Fouret P et al DNA repair by ERCC1 in non‐small‐cell lung cancer and cisplatin‐based adjuvant chemotherapy. N Engl J Med 2006; 355: 983–91. [DOI] [PubMed] [Google Scholar]
- 9. Olaussen KA, Fouret P, Kroemer G. ERCC1‐specific immunostaining in non‐small‐cell lung cancer. N Engl J Med 2007; 357: 1559–61. [DOI] [PubMed] [Google Scholar]
- 10. Koay EJ, Truty MJ, Cristini V et al Transport properties of pancreatic cancer describe gemcitabine delivery and response. J Clin Invest 2014; 124: 1525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olaussen KA, Postel‐Vinay S. Predictors of chemotherapy efficacy in non‐small‐cell lung cancer: A challenging landscape. Ann Oncol 2016; 27: 2004–16. [DOI] [PubMed] [Google Scholar]
- 12. Taron M, Rosell R, Felip E et al BRCA1 mRNA expression levels as an indicator of chemoresistance in lung cancer. Hum Mol Genet 2004; 13: 2443–9. [DOI] [PubMed] [Google Scholar]
- 13. Rosell R, Skrzypski M, Jassem E et al BRCA1: A novel prognostic factor in resected non‐small‐cell lung cancer. PLoS One 2007; 2: e1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body‐mass index and incidence of cancer: A systematic review and meta‐analysis of prospective observational studies. Lancet 2008; 371: 569–78. [DOI] [PubMed] [Google Scholar]
- 15. Sepesi B, Gold KA, Correa AM et al The influence of body mass index on overall survival following surgical resection of non‐small cell lung cancer. J Thorac Oncol 2017; 12: 1280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dahlberg SE, Schiller JH, Bonomi PB et al Body mass index and its association with clinical outcomes for advanced non‐small‐cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J Thorac Oncol 2013; 8: 1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoon JL, Cho JJ, Park KM, Noh HM, Park YS. Diagnostic performance of body mass index using the Western Pacific Regional Office of World Health Organization reference standards for body fat percentage. J Korean Med Sci 2015; 30: 162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Attaran S, McShane J, Whittle I, Poullis M, Shackcloth M. A propensity‐matched comparison of survival after lung resection in patients with a high versus low body mass index. Eur J Cardiothorac Surg 2012; 42: 653–8. [DOI] [PubMed] [Google Scholar]
- 19. Pan C, He N, Zhao M et al Subdividing the M1 stage of liver metastasis for nasopharyngeal carcinoma to better predict metastatic survival. Med Oncol 2011; 28: 1349–55. [DOI] [PubMed] [Google Scholar]
- 20. Li W, Shen LJ, Chen T et al Overweight/obese status associates with favorable outcome in patients with metastatic nasopharyngeal carcinoma: A 10‐year retrospective study. Chin J Cancer 2016; 35 (1): 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bruno R, Vivier N, Vergniol JC, de Phillips SL, Montay G, Sheiner LB. A population pharmacokinetic model for docetaxel (Taxotere): Model building and validation. J Pharmacokinet Biopharm 1996; 24 (2): 153–72. [DOI] [PubMed] [Google Scholar]
- 22. Wong AL, Seng KY, Ong EM et al Body fat composition impacts the hematologic toxicities and pharmacokinetics of doxorubicin in Asian breast cancer patients. Breast Cancer Res Treat 2014; 144: 143–52. [DOI] [PubMed] [Google Scholar]
- 23. Girnun GD, Naseri E, Vafai SB et al Synergy between PPARgamma ligands and platinum‐based drugs in cancer. Cancer Cell 2007; 11: 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shackelford DB, Abt E, Gerken L et al LKB1 inactivation dictates therapeutic response of non‐small cell lung cancer to the metabolism drug phenformin. Cancer Cell 2013; 23: 143–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ji H, Ramsey MR, Hayes DN et al LKB1 modulates lung cancer differentiation and metastasis. Nature 2007; 448: 807–10. [DOI] [PubMed] [Google Scholar]
- 26. Han X, Li F, Fang Z et al Transdifferentiation of lung adenocarcinoma in mice with Lkb1 deficiency to squamous cell carcinoma. Nat Commun 2014; 5: 3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coate LE, Massey C, Hope A et al Treatment of the elderly when cure is the goal: The influence of age on treatment selection and efficacy for stage III non‐small cell lung cancer. J Thorac Oncol 2011; 6: 537–44. [DOI] [PubMed] [Google Scholar]
- 28. Marquez‐Medina D, Martin‐Marco A, Ojanguren‐Garranz A. Age does not worsen the efficacy nor tolerance to combined induction therapies in locally advanced non‐small cell lung cancer. Anticancer Res 2014; 34 (8): 4373–6. [PubMed] [Google Scholar]
- 29. Imai H, Mori K, Ono A et al Individual‐level data on the relationships of progression‐free survival and post‐progression survival with overall survival in patients with advanced non‐squamous non‐small cell lung cancer patients who received second‐line chemotherapy. Med Oncol 2014; 31 (8): 88. [DOI] [PubMed] [Google Scholar]
- 30. William WN Jr, Pataer A, Kalhor N et al Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non‐small‐cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2013; 8: 222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


