Abstract
Background
The major challenge for treating non‐squamous (non‐Sq) non‐small cell lung cancer (NSCLC) patients without actionable biomarkers is the actual selection of proper treatment, weighing expected clinical outcomes and safety profile.
Methods
Consecutive non‐Sq NSCLC patients were treated with platinum‐pemetrexed (PP) doublets in clinical practice. Subgroup analyses were conducted in patients treated with standard (s)PP and modified (m)PP doublets (because of age, performance status, and/or comorbidities) and in patients treated with cisplatin‐based and carboplatin‐based PP doublets. Activity, efficacy, safety, and toxicities were evaluated.
Results
From November 2009 to April 2017, 111 patients were treated: 87 (78.4%) with sPP and 24 (21.6%) with mPP; 76 (68.5%) with cisplatin‐based and 35 (31.5%) with carboplatin‐based regimens. The objective response rate (ORR), median progression‐free survival (PFS), and median overall survival (OS) were 49.0%, 7, and 13 months in the entire patient population, respectively. We found no significant differences in ORR, median PFS, and median OS between sPP and mPP. Cisplatin‐based PP showed higher ORR (53.7%) versus carboplatin‐based PP (38.7%) and longer PFS (7 vs. 6 months; P = 0.028) and OS (18 vs. 11 months; P = 0.006). We confirm that carboplatin has a better toxicity profile than cisplatin. The received dose‐intensities were ~80% of standard full doses.
Conclusions
Accurate management allowed us to treat the majority of advanced non‐Sq NSCLC patients with PP combination therapy without significant differences in ORR, median PFS, and median OS. Even considering the selection bias, our data seems to confirm the greater effectiveness of cisplatin‐based over carboplatin‐based regimens.
Keywords: Clinical practice, community oncology, non‐squamous non‐small cell lung cancer, pemetrexed
Introduction
Lung cancer (LC) is the most common cancer worldwide and remains the major cause of cancer‐related death.1, 2 Non‐squamous (non‐Sq) non‐small cell lung cancer (NSCLC) (adenocarcinoma, large cell, and undifferentiated carcinoma) constitutes approximately 70% of LCs.3 Despite the advantages in molecular characterization of non‐Sq NSCLC, first‐line treatment of metastatic patients without EGFR activating mutations or ALK translocation, and with negative PD‐L1 immunohistochemistry assay (or positive but < 50%), still represents the greatest challenge in “real‐life” thoracic oncology. In these patients, guidelines recommend first‐line induction with platinum‐based doublet chemotherapy using either carboplatin or cisplatin for four or six cycles, followed by maintenance monotherapy in cases of stable disease or partial response.4, 5
Advanced NSCLC patients encountered in common clinical practice are often elderly and/or unfit as a result of age, performance status (PS), and/or comorbidities. These patients are under‐represented in randomized trials, thus there is a lack of data regarding their management.6 The efficacy of a platinum‐based combination in elderly advanced NSCLC patients, compared to single‐agent chemotherapy, has not been specifically evaluated, but it seems to be a feasible option for elderly patients eligible for such therapy.7 In recent years, some experiences in frail patients have shown that carboplatin plus nab‐paclitaxel combination therapy were well tolerated, with significant advantages in efficacy.8, 9, 10
Pemetrexed in non‐Sq NSCLC patients is actually the preferred combination for first‐line platinum‐based chemotherapy in clinical practice,11 as a result of its favorable toxicity profile and robust data about maintenance monotherapy.12, 13 In a retrospective subgroup analysis of a randomized phase III trial evaluating first‐line pemetrexed plus carboplatin versus docetaxel plus carboplatin in elderly non‐Sq NSCLC patients, pemetrexed showed a favorable safety profile and risk‐benefit ratio, with significantly higher overall survival (OS), without limiting toxicities.14 In a randomized phase III trial, a first‐line standard dose carboplatin and pemetrexed combination was compared to single‐agent pemetrexed in Eastern Cooperative Oncology Group (ECOG) PS 2 NSCLC patients, and showed a significant improvement in progression‐free survival (PFS) (5.8 vs. 2.8 months, respectively; P = 0.001) and OS (9.3 vs. 5.3 months, respectively; P = 0.001), but with increased hematological toxicity.15 In subgroup analysis of another phase III trial comparing first‐line cisplatin/pemetrexed to cisplatin/gemcitabine in all histological subtypes of NSCLC patients, a survival benefit in favor of the experimental arm was maintained in patients aged < 65 and ≥ 65, and ECOG PS ≤ 1.16
Cisplatin and carboplatin share the same mechanism of action, binding to cellular DNA to form cross‐links; the equivalent tumor cytotoxicity depends on the equivalent level of DNA binding.17 Carboplatin is more stable than cisplatin, so a higher absolute dose is necessary to obtain comparable cytotoxic effects in vitro; the ratio of therapeutic doses of carboplatin over cisplatin is approximately 4:1 (400–500 mg/m2 vs. 100 mg/m2). Cisplatin has a poorer toxicity profile, which is more nephrotoxic and emetogenic than carboplatin, and has greater cumulative neurotoxicity and ototoxicity; myelosuppression is the major toxic effect of carboplatin with a pattern typically delayed to a nadir at days 21–28.18 Few randomized trials have compared various cisplatin and carboplatin‐based doublets, showing a trend of a greater activity of cisplatin at the cost of greater toxicity, but without significant advantages of one over the other19, 20, 21, 22 Some meta‐analyses have attempted to answer this missing point, with mixed results.23, 24 While in Europe, cisplatin‐based regimens remain the preferred choice in clinical practice, in the United States most patients are treated with carboplatin‐based regimens.25 A retrospective analysis of real life data showed no survival benefit in using cisplatin rather carboplatin combination therapy.26 Even if the use of cisplatin seems to be less prudent in “unfit” patients, a randomized phase III trial evaluated a modified cisplatin and gemcitabine schedule compared to gemcitabine alone in PS 2 patients and showed significantly longer median PFS and higher objective response rate (ORR) in favor of the doublet, without significantly different toxicity.27 Two ongoing Italian randomized trials were designed to compare cisplatin‐based combinations to the relative single‐agent using groups aged > 70 years and patients with PS 0–1.28
Herein, we report a “real‐life” retrospective multicentric analysis of consecutive non‐Sq NSCLC EGFR wild type patients, treated with tailored first‐line platinum‐pemetrexed (PP) doublets based on patient fitness.
Methods
Patient eligibility
This retrospective multicentric study evaluated consecutive non‐Sq NSCLC patients treated with first‐line PP doublets from November 2009 to April 2017 at Medical Oncology Units at St. Salvatore University Hospital in L'Aquila and S.S. Annunziata University Hospital in Chieti, Italy. Patients were eligible if they had: a histologically confirmed diagnosis of metastatic measurable non‐Sq NSCLC, EGFR wild‐type disease, or mutant unsuitable for anti‐EGFR treatments at the time of first‐line treatment; and ECOG PS ≤ 2, adequate hematological, renal, and hepatic functions, and a life expectancy of > 3 months. Because crizotinib was only approved in Italy in March 2017 for the use of first‐line treatment in ALK positive non‐Sq NSCLC patients, the treatment options available were those used for ALK negative patients. As a retrospective collection, there were no defined parameters for the choice of treatment schedules, and clinicians tailored PP doublets in keeping with patient fitness, which was defined according to age, “non‐elderly” (< 65 years), “young‐elderly” (≥ 65, < 75 years), “old‐elderly” (≥ 75), ECOG PS, and comorbidities. Comorbidities were evaluated using the Cumulative Index Rating Scale (CIRS)29 The primary CIRS stage consisted of independent Instrumental Activity of Daily Living (IADL), and absent or mild grade comorbidities; intermediate CIRS consisted of dependent or independent IADL, and < 3 mild or moderate grade comorbidities; and secondary CIRS stage consisted of > 3 comorbidities or a severe comorbidity, with or without dependent IADL. Patients with PS 3 were not treated with PP doublets. All patients provided written informed consent for the proposed treatment. The procedures followed were in accordance with the ethical standards of the responsible local committees on human experimentation (Bioethics Committees).
Schedule
First‐line standard (s)PP doublets consisted of cisplatin 75 mg/m2 or carboplatin area under the curve (AUC) 5, day 1, and pemetrexed 500 mg/m2 (Alimta, Eli‐Lilly, Houten, Netherlands), day 1 cycles every three weeks. Modified (m)PP doublet regimens were defined by any projected dose reduction or schedule modification compared to sPP. The mPP schedules are summarized in Table 1. Prophylactic pegylated granulocyte‐colony stimulating factor was administered based on the investigators’ choice.
Table 1.
mPP schedules according to cisplatin/carboplatin‐based doublets
| 24 mPP doublets | |
|---|---|
| Cisplatin‐based mPP doublets | 2 Cisplatin 75 mg/m2 day 1 ‐ Pemetrexed 400 mg/m2 day 1 q 21 |
| 2 Cisplatin 70 mg/m2 day 1 ‐ Pemetrexed 500 mg/m2 day 1 q 21 | |
| 1 Cisplatin 70 mg/m2 day 1 ‐ Pemetrexed 400 mg/m2 day 1 q 21 | |
| 2 Cisplatin 60 mg/m2 day 1 ‐ Pemetrexed 500 mg/m2 day 1 q 21 | |
| 1 Cisplatin 60 mg/m2 day 1 ‐ Pemetrexed 375 mg/m2 day 1 q 21 | |
| Carboplatin‐based mPP doublets | 5 Carboplatin (AUC 5) day 1 ‐ Pemetrexed 400 mg/m2 day 1 q 21 |
| 1 Carboplatin (AUC 2.5) days 1, 8 ‐ Pemetrexed 500 mg/m2 day 1 q 21 | |
| 1 Carboplatin (AUC 4) day 1 ‐ Pemetrexed 400 mg/m2 day 1 q 21 | |
| 2 Carboplatin (AUC 2.5) days 1, 8 ‐ Pemetrexed 400 mg/m2 day 1 q 21 | |
| 3 Carboplatin (AUC 2) days 1, 8 ‐ Pemetrexed 400 mg/m2 day 1 q 21 | |
| 3 Carboplatin (AUC 2) days 1, 8 ‐ Pemetrexed 375 mg/m2 day 1 q 21 | |
| 1 Carboplatin 200 mg days 1, 8 ‐ Pemetrexed 300 mg/m2 day 1 q 21 |
AUC, area under the curve; mPP, modified platinum‐pemetrexed.
Molecular analysis
EGFR (exons 18, 19, 20, 21) genetic analysis was performed on paraffin‐embedded tissue blocks from the primary tumor and/or metastases using direct sequencing, pyrosequencing, and real‐time PCR (Cobas Z480 analyzer, Roche Molecular Diagnostics Inc., South Branchburg, NJ, USA) in clinical practice. Moreover, ALK rearrangement analysis was performed when sample tissues were available using immunohistochemistry (ALK [D5F3] CDx Assay, Ventana Medical Systems, Inc. Tucson, AZ, USA; Omnis CD246, ALK Protein, Dako Denmark A/S, Glostrup, Denmark) and fluorescence in situ hybridization (FISH) (Vysis ALK Break Apart FISH Probe Kit, Abbott Molecular Inc., Des Plaines, IL, USA).
Study design
A multicentric retrospective analysis of consecutive non‐Sq NSCLC patients treated with first‐line PP doublets was conducted to evaluate activity, safety, and efficacy of the schedules in clinical practice. Subgroup analysis was conducted in patients treated with sPP and mPP doublets and in patients treated with cisplatin‐based and carboplatin‐based PP doublets. Clinical evaluation of response was evaluated by computed tomography (CT) scan; positron emission tomography (PET) was added based on the investigators’ assessment. Follow‐up was scheduled every three months until progression or death. ORR was defined as the portion of patients experiencing an objective response (complete response [CR] or partial response [PR]) as the best response; disease‐control rate (DCR) was defined as the portion of patients that experienced an objective response or demonstrated stable disease (SD) as the best response. PFS was defined as the length of time from the beginning of treatment to disease progression or death (resulting from any cause) or to the last contact; OS as the length of time between the beginning of treatment to death or last contact. ORR and DCR were evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST version 1.1).30 Median PFS and median OS were evaluated using the Kaplan–Meier method.31 Median periods of follow‐up were calculated according to the reverse Kaplan–Meier method.32 In subgroup analysis, the chi‐square test was used to compare ORR, and the log‐rank test to compare median PFS and OS, according to first‐line regimens.33, 34 A Cox proportional hazards model was used to evaluate prognostic factors.35 Toxicity was registered according to National Cancer Institute Common Toxicity Criteria (version 4.0). Limiting toxicity (LT) was defined as grade 3–4 non‐hematologic toxicity, grade 4 hematologic toxicity, febrile neutropenia, or any toxicity causing a > 2 week treatment delay and/or a modification in the schedule, such as dose reduction. Median received dose intensities (rDI) were calculated “per cycle” as mg/m2/week for cisplatin and pemetrexed and AUC/week for carboplatin; percentage values referred to standard regimens for each drugs. The data cut‐off period was May 2017.
Results
Patient features
From November 2009 to April 2017, 111 consecutive non‐sq NSCLC patients were treated with first‐line PP doublets in our institutions: 87 (78.4%) patients were treated with sPP doublets and 24 (21.6%) with mPP regimens because of age, PS, and/or comorbidities. The clinical features of the 111 patients are shown in Table 2: male: female ratio, 75:36; median age, 66.0 years; PS 0, 61 (55%), PS 1, 44 (39.6%), and PS 2, 6 (5.4%). Patient distribution according to age and comorbidity stage was: non‐elderly 50 (45.0%), young‐elderly (65–74 years) 46 (41.5%), and old‐elderly (≥ 75 years old) 15 (13.5%). The CIRS stages were: primary 41 (36.9%), intermediate 45 (40.6%), and secondary 25 (22.5%). The metastatic sites were: lung 84 (75.7%), liver 14 (12.6%), lymph nodes 88 (79.3%), bone 37 (33.3%), others 29 (26.1%), and central nervous system (CNS) 22 (19.8%). In patients treated with sPP, the median age, PS, and CIRS were: 64 years; PS 0, 53 (60.9%), PS 1, 33 (37.9%), PS 2, 1 (1.2%); CIRS primary stage 40 (46.0%), intermediate 35 (40.2%), and secondary 12 (13.8%). In patients treated with sPP, the median age, PS, and CIRS were: 73 years; PS 0, 8 (33.3%), PS 1, 11 (45.8%), PS 2, 5 (20.9%); CIRS primary stage 1 (4.2%), intermediate 10 (41.7%), and secondary 13 (54.1%). There were 68 (89.5%) patients treated with cisplatin‐based PP doublets in the sPP and 8 (10.5%) in the mPP group. Their median age, PS, and CIRS were: 64.5 years; PS 0, 55 (72.3%), PS 1, 19 (25.0%), PS 2, 2 (2.7%); and CIRS primary stage 37 (48.7%), intermediate 30 (38.5%), and secondary 9 (11.8%). There were 16 (45.7%) patients treated with carboplatin‐based PP doublets in the sPP group and 19 (54.3%) in the mPP group. Their median age, PS, and CIRS were: 67 years; PS 0, 6 (17.2%), PS 1, 25 (71.4.0%), PS 2, 4 (11.4%); and CIRS primary stage 4 (11.4%), intermediate 15 (42.9%), and secondary 16 (45.7%).
Table 2.
Patient characteristics
| Overall | sPP | mPP | Cisplatin‐based | Carboplatin‐based | |
|---|---|---|---|---|---|
| N° (%) | N° (%) | N° (%) | N° (%) | N° (%) | |
| Patients (N°) | 111 | 87 | 24 | 76 | 35 |
| Age, years | |||||
| Range | 24–82 | 24–76 | 55–82 | 33–79 | 24–82 |
| Mean | 63.0 | 60.7 | 71.6 | 61.4 | 66.6 |
| Median | 66.0 | 64.0 | 73.0 | 64.5 | 67.0 |
| Gender | |||||
| Male | 75 (67.6) | 58 (66.7) | 17 (70.8) | 52 (68.4) | 23 (65.7) |
| Female | 36 (32.4) | 29 (33.3) | 7 (29.2) | 24 (31.6) | 12 (34.3) |
| Age | |||||
| Non‐elderly | 50 (45.0) | 47 (54.0) | 3 (12.5) | 38 (50) | 12 (34.3) |
| Young‐elderly | 46 (41.5) | 34 (39.1) | 12 (50) | 35 (46.1) | 11 (31.4) |
| Old‐elderly | 15 (13.5) | 6 (6.9) | 9 (37.5) | 3 (3.9) | 12 (34.3) |
| ECOG PS | |||||
| 0 | 61(55.0) | 53 (60.9) | 8 (33.3) | 55 (72.3) | 6 (17.2) |
| 1 | 44 (39.6) | 33 (37.9) | 11 (45.8) | 19 (25.0) | 25 (71.4) |
| 2 | 6 (5.4) | 1 (1.2) | 5 (20.9) | 2 (2.7) | 4 (11.4) |
| CIRS | |||||
| Primary | 41 (36.9) | 40 (46.0) | 1 (4.2) | 37 (48.7) | 4 (11.4) |
| Intermediate | 45 (40.6) | 35 (40.2) | 10 (41.7) | 30 (39.5) | 15 (42.9) |
| Secondary | 25 (22.5) | 12 (13.8) | 13 (54.1) | 9 (11.8) | 16 (45.7) |
| EGFR mutation status | |||||
| Wild‐type | 102 (91.9) | 80 (92.0) | 22 (91.7) | 71 (93.4) | 28 (88.6) |
| Mutant | 2 (1.8) | 2 (2.3) | — | 2 (2.7) | — |
| Not tested | 7 (6.3) | 5 (5.7) | 2 (8.3) | 3 (3.9) | 4 (11.4) |
| ALK rearrangement | |||||
| Positive | 7 (6.3) | 6 (6.9) | 1 (4.2) | 5 (6.6) | 2 (5.7) |
| Negative | 40 (36.0) | 33 (37.9) | 7 (29.2) | 26 (34.2) | 4 (11.4) |
| Not tested | 64 (57.7) | 48 (55.2) | 16 (66.6) | 45 (59.2) | 19 (82.9) |
| Previous adjuvant therapy | 5 (4.5) | 4 (4.6) | 1 (4.2) | 4 (5.3) | 1 (2.9) |
| Sites of metastasis | |||||
| Lung | 84 (75.7) | 65 (74.7) | 19 (79.1) | 56 (73.7) | 25 (71.4) |
| Liver | 14 (12.6) | 10 (11.5) | 4 (16.7) | 8 (10.5) | 6 (17.2) |
| Lymph nodes | 88 (79.3) | 72 (82.8) | 16 (66.6) | 55 (72.3) | 33 (94.3) |
| Bone | 37 (33.3) | 29 (33.3) | 8 (33.3) | 20 (26.3) | 17 (48.6) |
| Other | 29 (26.1) | 22 (25.3) | 7 (29.2) | 20 (26.3) | 9 (25.7) |
| Central nervous system metastasis | 22 (19.8) | 18 (20.7) | 4 (16.7) | 13 (17.1) | 9 (25.7) |
| Single | 5 (4.5) | 5 (5.7) | — | 3 (3.9) | 2 (5.7) |
| Multiple | 17 (15.3) | 13 (14.9) | 4 (16.7) | 10 (13.2) | 7 (20) |
CRIS, Cumulative Index Rating Scale; ECOG, Eastern Cooperative Oncology Group; mPP, modified platinum‐pemetrexed; sPP, standard PP.
Activity and efficacy
Thirteen (11.7%) of the 111 patients treated with first‐line PP doublets were excluded from activity data (ORR) because they did not receive at least two cycles of treatment, and thus their disease had not been evaluated (2 patients were still on treatment at the date of data analysis, 7 were lost to follow‐up, 3 died during the first two cycles of no established cause, and 1 died after the first cycle as a result of adverse events). The activity and efficacy details are listed in Table 3. In the intent‐to‐treat analysis, the ORR was 49.0% (95% confidence interval [CI] 36–65) and the DCR was 77.6% (95% CI 61–97) (4 CR, 44 PR, and 28 SD). After a median follow‐up of 25 months, the median PFS was 7 months (range 1–52+): 89 events occurred; and the median OS was 13 months (range 1–67+): 66 events occurred (Fig 1). Ten (11.5%) of the 87 patients who underwent first‐line sPP doublets were excluded from activity analysis. The ORR was 48.1% (95% CI 34–66), and the DCR was 76.6% (95% CI 58–98) (4 CR, 33 PR, and 22 SD). After a median follow‐up of 25 months, the median PFS was 7 months (range 1–52+): 68 events occurred; and the median OS was 14 months (range 1–67+): 49 events occurred (Fig 2). Three (12.5%) of the 24 patients who underwent first‐line mPP doublets were excluded from activity analysis. The ORR was 52.4% (95% CI 26–94) and the DCR was 80.9% (95% CI 47–129) (11 PR, 6 SD). After a median follow‐up of 36 months, the median PFS was 7 months (range 1–18): 21 events occurred; and the median OS was 12 months (range 1–48+): 17 events occurred (Fig 2). Nine (11.8%) of the 76 patients who underwent first‐line cisplatin‐based PP doublets were excluded from activity analysis. The ORR was 53.7% (95% CI 38–74) and the DCR was 83.6% (95% CI 63–108) (4 CR, 32 PR, and 20 SD). After a median follow‐up of 22 months, the median PFS was 7 months (range 1–52+): 57 events occurred; and the median OS was 18 months (range 1–67+): 38 events occurred (Fig 3). Four (11.4%) of the 35 patients who underwent first‐line carboplatin‐based PP doublets were excluded from activity analysis. The ORR was 38.7% (95% CI 20–68) and the DCR was 64.5% (95% CI 39–99) (12 PR, 8 SD). After a median follow‐up of 25 months, the median PFS was 6 months (range 1–18): 32 events occurred; and the median OS was 11 months (range: 1–48+): 28 events occurred (Fig 3). The ORR, median PFS, and median OS of patients treated with sPP doublets compared to those treated with mPP doublets were not significantly different: P = 0.726, P = 0.631, and P = 0.194, respectively (Fig 2). The ORR of patients treated with cisplatin‐based PP doublets compared to those treated with carboplatin‐based PP doublets were not significantly different (P = 0.214), while the median PFS and median OS were significantly different: P = 0.028 and P = 0.006, respectively (Fig 3). In univariate analysis, none of the factors commonly associated with clinical outcomes were significant predictors for median PFS or median OS (Table 4). Notably, age (non‐elderly vs. young/old elderly), ECOG PS (0 vs. ≥ 1), CIRS (primary vs. intermediate/secondary), and the presence of CNS metastases (no vs. yes) were evaluated in this study, thus multivariate analysis did not reveal independent factors associated with median PFS and median OS.
Table 3.
Activity and efficacy of PP doublets according to standard/modified schedules
| Overall | sPP | mPP | Cisplatin‐based | Carboplatin‐based | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Criteria | N° | % | N° | % | N° | % | N° | % | N° | % |
| Enrolled patients | 111 | 100 | 87 | 100 | 24 | 100 | 76 | 100 | 35 | 100 |
| Evaluable patients | 98 | 88.3 | 77 | 88.5 | 21 | 87.5 | 67 | 88.2 | 31 | 88.6 |
| Objective response rate | 49.0 (95% CI 36–65) | 48.1 (95% CI 34–66) | 52.4 (95% CI 26–94) | 53.7 (95% CI 38–74) | 38.7 (95% CI 20–68) | |||||
| Partial response | 44 | 44.9 | 33 | 42.9 | 11 | 52.4 | 32 | 47.8 | 12 | 38.7 |
| Complete response | 4 | 4.1 | 4 | 5.2 | — | — | 4 | 5.9 | — | — |
| Disease control rate | 77.6 (95% CI 61–97) | 76.6 (95% CI 58–98) | 80.9 (95% CI 47–129) | 83.6 (95% CI 63–108) | 64.5 (95% CI 39–99) | |||||
| Stable disease | 28 | 28.6 | 22 | 28.6 | 6 | 28.6 | 20 | 29.8 | 8 | 25.8 |
| Progressive disease | 22 | 22.4 | 4 | 5.2 | 4 | 19 | 11 | 16.4 | 11 | 35.5 |
| Median PFS (months) | 7.0 | 7.0 | 7.0 | 7.0 | 6.0 | |||||
| Range | 1–52+ | 1–52+ | 1–18 | 1–52+ | 1–18 | |||||
| Progression events | 89 | 68 | 21 | 57 | 32 | |||||
| Median OS (months) | 13.0 | 14.0 | 12.0 | 18.0 | 11.0 | |||||
| Range | 1–67+ | 1–67+ | 1–48+ | 1–67+ | 1–48+ | |||||
| Deaths | 66 | 49 | 17 | 38 | 28 | |||||
CI, confidence interval; OS, overall survival; mPP, modified platinum‐pemetrexed; PFS, progression‐free survival; sPP, standard PP.
Figure 1.
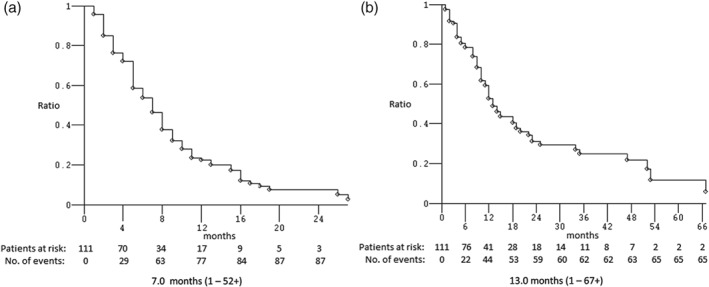
Kaplan–Meier survival estimate of treated patients: (a) progression‐free and (b) overall survival.
Figure 2.
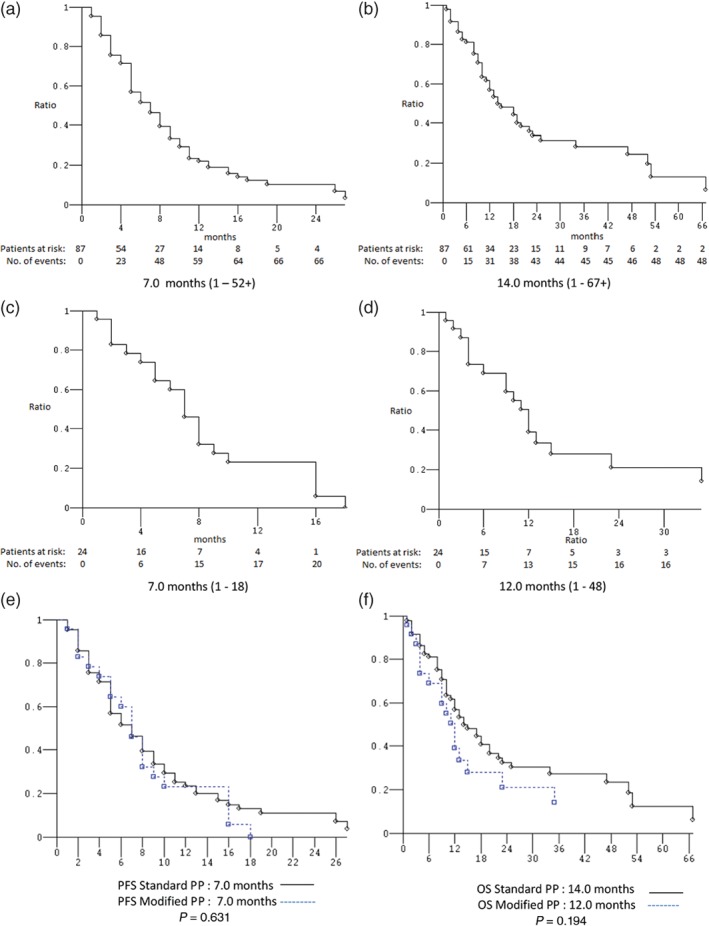
Kaplan–Meier survival estimates. Standard platinum‐pemetrexed (PP) doublets: (a) progression‐free survival (PFS) and (b) overall survival (OS). Modified PP doublets: (c) PFS and (d) OS. Standard versus modified PP doublets: (e) PFS and (f) OS.
Figure 3.
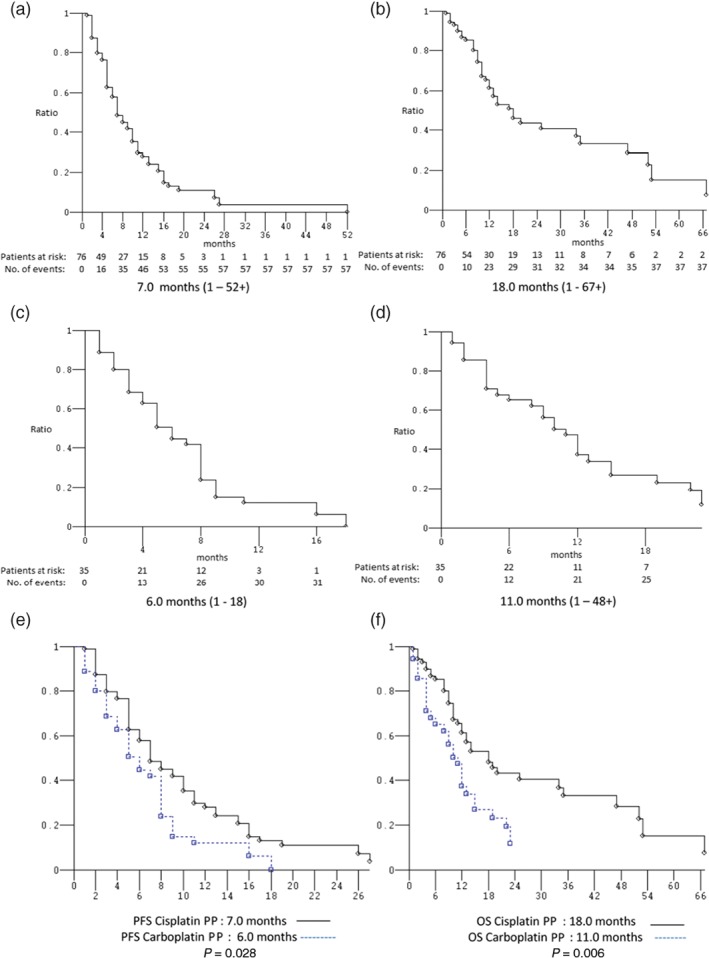
Kaplan–Meier survival estimate. Cisplatin‐based platinum‐pemetrexed (PP) doublets: (a) progression‐free survival (PFS) and (b) overall survival. Carboplatin‐based PP doublets: (c) PFS and (d) OS. Cisplatin‐based versus carboplatin‐based PP doublets: (e) PFS and (f) OS.
Table 4.
Univariate and multivariate analysis
| Variable | N° | PFS | OS | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Univariate analysis Age | |||||
| Non‐elderly | 50 | 1.01 (0.66–1.5) | 0.963 | 1.19 (0.68–1.84) | 0.660 |
| Elderly | 61 | ||||
| ECOG PS | |||||
| 0 | 61 | 1.24 (0.76–2.02) | 0.385 | 1.13 (0.70–1.83) | 0.611 |
| ≥ 1 | 50 | ||||
| CIRS | |||||
| Primary | 41 | 1.67 (0.75–1.81) | 0.495 | 1.39 (0.82–2.35) | 0.221 |
| Intermediate/secondary | 70 | ||||
| CNS* metastases | |||||
| No | 89 | 1.07 (0.64–1.79) | 0.791 | 0.91 (0.50–1.67) | 0.760 |
| Yes | 22 | ||||
| Multivariate analysis | |||||
| Age | |||||
| Non‐elderly/elderly | 0.94 (0.57–1.53) | 0.799 | 0.93 (0.52–1.66) | 0.811 | |
| ECOG PS | |||||
| 0/≥ 1 | 1.10 (0.69–1.75) | 0.698 | 1.03 (0.61–1.72) | 0.917 | |
| CIRS | |||||
| Primary/> primary | 1.15 (0.67–1.97) | 0.613 | 1.41 (0.75–2.66) | 0.279 | |
| CNS metastases | |||||
| No/Yes | 1.05 (0.62–1.77) | 0.854 | 0.92 (0.49–1.73) | 0.806 | |
CNS, central nervous system; CRIS, Cumulative Index Rating Scale; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; OS, overall survival; PFS, progression‐free survival.
Dose‐intensity
In the entire patient sample, the median number of administered cycles was 4 (range: 1–10): sPP doublets (range: 1–10), mPP doublets (range: 1–8), cisplatin‐based PP doublets (range: 1–9) and carboplatin‐based PP doublets (range: 1–10). Of the 465 administered cycles, 460 were evaluable for dose intensities. The median rDIs in the sPP doublet population were: cisplatin 25 (range: 0.25–25) mg/m2/week (100%), carboplatin 1.5 (range: 1–1.6) AUC/week (89.8% of standard full dose), and pemetrexed 166.6 (range: 12.5–166.6) mg/m2/week (100%). The median rDIs in the mPP doublet population were: cisplatin 20.6 (range: 14–25) mg/m2/week (82.4% of standard full dose), carboplatin 1.3 (range: 0.5–1.6) AUC/week (79.6% of standard full dose), and pemetrexed 133.3 (range: 36.1–166.6) mg/m2/week (80% of standard full dose). The median rDIs in the cisplatin‐based PP doublet population were: cisplatin 25 (range: 0.25–25) mg/m2/week (100%) and pemetrexed 166.6 (range: 12.5–166.6) mg/m2/week (100%). The median rDIs in the carboplatin‐based PP doublet population were: carboplatin 1.3 (range: 0.5–1.6) AUC/week (79.6% of standard full dose) and pemetrexed 136.1 (range: 36.1–166.6) mg/m2/w (81.7% of standard full dose).
Toxicity
Two patients in the sPP doublet subgroup (1 in the cisplatin‐based and 1 in the carboplatin‐based) were not evaluable for toxicity because of incomplete information in their clinical records. Nineteen patients were treated with prophylactic pegylated granulocyte‐colony stimulating factor: 12 in the sPP, 7 in the mPP doublet, 17 in the cisplatin‐based, and 2 in the carboplatin‐based subgroup. The treatment‐related grade 3 or 4 adverse events are summarized in Table 5. The most common adverse events were: leukopenia (6.4%) neutropenia (9.2%), anemia (10.1%), thrombocytopenia (11%), hypertransaminasemia (6.4%), nausea (3.7%), vomiting (3.7%), asthenia (4.6%), alopecia (4.6%), cardiac toxicity (4.6%), stomatitis/mucositis (3.7%), skin toxicity (3.7%), conjunctivitis (3.7), and creatinine increase (3.7). Febrile neutropenia was observed in one (0.9%) patient who died as result of adverse events.
Table 5.
G3/G4 toxicity of PP doublets according to standard/modified schedules, and cisplatin/carboplatin‐based doublets
| Overall patients | sPP doublets | mPP doublets | Cisplatin‐based PP | Carboplatin‐based PP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | 109 | 85 | 24 | 75 | 34 | |||||
| NCI‐CTC Grade | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 |
| Nausea (%) | 4 (3.7) | — | 4 (4.7) | — | 4 (16.6) | — | 4 (5.3) | — | — | — |
| Vomiting (%) | 4 (3.7) | — | 4 (4.7) | — | — | — | 4 (5.3) | — | — | — |
| Hiccup (%) | 1 (0.9) | — | 1 (1.8) | — | — | — | 1 (1.3) | — | — | — |
| Anorexia (%) | 1 (0.9) | — | 1 (1.8) | — | — | — | 1 (1.3) | — | — | — |
| Cardiac toxicity (%) | 5 (4.6) | — | 3 (3.5) | — | 2 (8.3) | — | 3 (4) | — | 2 (5.9) | — |
| Stomatitis/mucositis (%) | 3 (2.7) | 1 (0.9) | 3 (3.5) | 1 (1.8) | — | — | 3 (4) | 1 (1.3) | — | — |
| Asthenia (%) | 5 (4.6) | — | 4 (4.7) | — | 1 (4.1) | — | 3 (4) | — | 2 (5.9) | — |
| Hypertransaminasemia (%) | 5 (4.6) | 2 (1.8) | 4 (4.7) | 1 (1.8) | 1 (4.1) | 1 (4.1) | 2 (2.7) | — | 3 (8.9) | 2 (5.9) |
| Cholestasis (%) | 1 (0.9) | 2 (1.8) | 1 (1.8) | 1 (1.8) | — | 1 (4.1) | — | — | 1 (2.9) | 2 (5.9) |
| Creatinine increase (%) | 4 (3.7) | — | 4 (4.7) | — | — | — | 4 (5.3) | — | — | — |
| Hyperkalemia (%) | 2 (1.8) | — | 2 (2.3) | — | — | — | 2 (2.7) | — | — | — |
| Skin toxicity (%) | 4 (3.7) | — | 4 (4.7) | — | — | — | 4 (5.3) | — | — | — |
| Conjunctivitis (%) | 4 (3.7) | — | 4 (4.7) | — | — | — | 4 (5.3) | — | — | — |
| Hyperpyrexia (%) | 1 (0.9) | — | 1 (1.8) | — | — | — | 1 (1.3) | — | — | — |
| Alopecia (%) | 5 (4.6) | — | 4 (4.7) | — | 1 (4.1) | — | 5 (6.7) | — | — | — |
| Hypoalbuminemia (%) | 1 (0.9) | — | 1 (1.8) | — | — | — | 11(1.3) | — | — | — |
| Leukopenia (%) | 6 (5.5) | 1 (0.9) | 4 (4.7) | — | 2 (8.3) | 1 (4.1) | 4 (5.3) | — | 2 (5.9) | 1 (2.9) |
| Neutropenia (%) | 9 (8.2) | 1 (0.9) | 6 (7) | — | 3 (12.5) | 1 (4.1) | 6 (8) | — | 3 (8.9) | 1 (2.9) |
| Febrile neutropenia (%) | — | 1 (0.9) | — | 1 (1.8) | — | — | — | 1 (1.3) | — | — |
| Anemia (%) | 10 (9.2) | 1 (0.9) | 8 (9.4) | — | 2 (8.3) | 1 (4.1) | 7 (9.3) | — | 3 (8.9) | 1 (2.9) |
| Thrombocytopenia (%) | 10 (9.2) | 2 (1.8) | 7 (8.2) | — | 3 (12.5) | 2 (8.3) | 7 (9.3) | — | 3 (8.9) | 2 (5.9) |
mPP, modified platinum‐pemetrexed; NCI‐CTC, National Cancer Institute Common Terminology Criteria; sPP, standard PP.
Concomitant and subsequent treatments
Ten out of 22 patients with brain metastases underwent radiation therapy (RT) (8 whole brain RT and 2 stereotactic radiotherapy) before starting first‐line chemotherapy; 19 out of 37 patients with bone metastases underwent concomitant bone‐targeted therapy (17 zoledronic acid and 2 denosumab). Fifty‐four patients (48.6%) received pemetrexed maintenance therapy: 53 were administered second‐line systemic therapy and 22 third‐line. All subsequent treatments are itemized and summarized in Table 6.
Table 6.
Subsequent treatments according to standard/modified and cisplatin/carboplatin‐based first line PP doublets
| Treatment | Overall | sPP doublet | mPP doublet | Cisplatin‐based | Carboplatin‐based |
|---|---|---|---|---|---|
| (111) | (87) | (24) | (76) | (35) | |
| Maintenance therapy (pemetrexed), n (%) | 54 (48.6) | 45 (51.7) | 9 (37.5) | 37 (48.7) | 17 (48.6) |
| Progression events, n | 89 | 68 | 21 | 57 | 32 |
| Second‐line systemic therapy, n (%) | 53 (59.5) | 39 (57.3) | 14 (66.6) | 35 (61.4) | 18 (25) |
| Chemotherapy, n (%) | 18 (20.2) | 13 (19.1) | 5 (23.8) | 11 (19.3) | 7 (21.9) |
| Oral kinase inhibitor therapy, n (%) | |||||
| Erlotinib | 23 (25.8) | 18 (26.4) | 5 (23.8) | 18 (31.6) | 5 (15.6) |
| Gefitinib | 2 (2.2) | 1 (1.5) | 1 (4.8) | 1 (1.7) | 1 (3.1) |
| Crizotinib | 4 (4.5) | 4 (5.9) | — | 3 (5.3) | 1 (3.1) |
| Nintedanib–Docetaxel | 2 (2.2) | 2 (2.9) | — | 1 (1.7) | 1 (3.1) |
| Nivolumab | 4 (4.5) | 1 (1.5) | 3 (14.3) | 1 (1.7) | 3 (9.4) |
| Third‐line systemic therapy, n (%) | 22 (24.7) | 19 (27.9) | 3 (14.3) | 20 (35.1) | 2 (6.2) |
| Nivolumab | 3 (3.4) | 3 (4.4) | — | 3 (5.3) | — |
mPP, modified platinum‐pemetrexed; sPP, standard PP.
Discussion
The management of non‐Sq NSCLC patients without actionable biomarkers at the commencement of first‐line treatment remains a challenge for clinicians in a real life setting, because many patients have poor clinical conditions and are often unfit for standard chemotherapies. In the era before the introduction of immune‐checkpoint inhibitors, there was a lack of designed clinical trials for this patient population. The selection between mono or doublet chemotherapy is key, properly weighing expected clinical outcome and safety profile. In our study, the median patient age was 66 years, with 61 (54%) elderly patients (young/old elderly), 50 (45%) ECOG PS ≥ 1 patients, and 63 (56.7%) patients with ≥ 1 significant comorbidities. The ORR was 49.0%, median PFS 7 months, and median OS 13 months, with a good toxicity profile. As a real life retrospective collection, clinicians selected modified regimens taking into account patient fitness; therefore, several modified regimens were collected in the same groups and were not evaluated as stratified selected regimens, therefore creating selection bias. We hypothesized that the selection of proper treatment strategy may explain why the clinical outcomes in subgroup analysis comparing patients treated with sPP and mPP are not significantly different. Our data appeared to confirm the different toxicity profile and better manageability of carboplatin over cisplatin, with lower incidence of grade 3 and 4 non‐hematological toxicity (except for hypertransaminasemia), but with a mild tendency of greater myelosuppression. This perception probably influenced the clinicians, who chose carboplatin more often for frail patients. The prevalence of elderly patients treated with carboplatin was 65.7%, the median age 67 years, ECOG PS ≥ 1 82.8%, and patients with ≥ 1 significant comorbidity was 88.5%, compared to 50%, 64.5 years, 27.6%, and 51.3% in the cisplatin treated subgroup, respectively. A greater proportion of the patient sample was treated with sPP cisplatin doublets (68, 89.5%) compared to carboplatin (16, 45%). The comparison of clinical outcomes in favor of cisplatin treated patients, with statistically significant differences in median PFS and median OS, was probably influenced by this unbalanced distribution of patients. Our results cannot definitively conclude any advantage of cisplatin over carboplatin‐based doublets with pemetrexed, which is consistent with the results of previous studies.19, 20, 21, 22, 23, 24
Univariate analysis of factors commonly associated with clinical outcomes (age, performance status, comorbidities, and CNS metastases) did not confirm their role as significant predictors for median PFS and median OS, thus subsequent multivariate analysis also did not demonstrate independent factors. The PFS results could be attributed to the effectiveness of proper treatment, while the OS results could be attributed to the selection of candidates for doublet chemotherapy. Only six patients (5.4%) had an ECOG PS of 2, while significant differences in survival have historically been observed between ECOG PS 0/1 and ≥ 221 Our patient sample was fairly homogenous, with only 15 (13.5%) “old‐elderly” patients, a median age of 66, and only 25 (22.5%) patient with severe comorbidities (secondary CIRS stage). The sample size and the retrospective nature should be considered. Furthermore, as the factors that influence survival during follow‐up are variable (compared to constant factors during treatment) this could impair our results. In our experience, the proper selection of tailored first‐line treatment based on age, PS, and comorbidities, using schedule modifications and/or dosing reduction allowed us to reach an adequate rDI (~80% of standard full dose for each regimen), with better or equal safety profiles when compared to the literature in all subgroups. Good clinical management allowed a greater proportion of our patient sample to undergo second‐line (59.5%) and third‐line (24.7%) therapy. Our retrospective subgroup data of cisplatin‐based and carboplatin‐based PP doublets were slightly better compared to the results of previous studies. Subgroup analysis of non‐Sq NSCLC patients treated with cisplatin/pemetrexed in the first phase III trial using pemetrexed showed a median PFS of 5.3 months and a median OS of 11.8 months.16 In the combination arm of a randomized phase III trial comparing first‐line standard dose carboplatin/pemetrexed with single‐agent pemetrexed in selected ECOG PS 2 NSCLC patients, the ORR was 24%, the median PFS 5.8 months, and the median OS 9.3 months.14
This retrospective study shows that careful pretreatment evaluation and accurate clinical management allows the majority of advanced non‐Sq NSCLC patients to be treated with PP combination therapy with a good safety profile. The selection of proper treatment with modified schedules allowed us to treat even more frail patients without significant differences in response rates, median PFS, and median OS. Our data seem to confirm a greater effectiveness of cisplatin‐based over carboplatin‐based regimens, at the expense of a worse toxicity profile and manageability. Although these results do not identify new decision‐making paradigms, they do offer an opportunity to reflect on therapeutic strategies for advanced non‐Sq NSCLC patients in a real life setting, particularly those without actionable biomarkers. Further research needs to be performed, particularly regarding immune checkpoint inhibitors, in order to offer more first‐line treatment options for these patients.
Disclosure
No authors report any conflict of interest.
References
- 1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893–917. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J et al Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 2013; 49: 1374–403. [DOI] [PubMed] [Google Scholar]
- 3. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008; 359: 1367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ettinger DS, Wood DE, Aisner DL et al Non‐Small Cell Lung Cancer, Version 5. 2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017; 15: 504–35. [DOI] [PubMed] [Google Scholar]
- 5. Novello S, Barlesi F, Califano R et al Metastatic non‐small‐cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2016; 27 (Suppl 5): v1–v27. [DOI] [PubMed] [Google Scholar]
- 6. Meoni G, Cecere FL, Lucherini E, Di Costanzo F. Medical treatment of advanced non‐small cell lung cancer in elderly patients: A review of the role of chemotherapy and targeted agents. J Geriatr Oncol 2013; 4: 282–90. [DOI] [PubMed] [Google Scholar]
- 7. Weiss J, Langer C. Treatment of lung cancer in the elderly patient. Semin Respir Crit Care Med 2013; 34: 802–9. [DOI] [PubMed] [Google Scholar]
- 8. Socinski MA, Langer CJ, Okamoto I et al Safety and efficacy of weekly nab‐paclitaxel in combination with carboplatin as first‐line therapy in elderly patients with advanced non‐small‐cell lung cancer. Ann Oncol 2013; 24:314–21. [DOI] [PubMed] [Google Scholar]
- 9. Shiroyama T, Tamiya M, Minami S et al Carboplatin plus weekly nanoparticle albumin‐bound paclitaxel in elderly patients with previously untreated advanced squamous non‐small‐cell lung cancer selected based on Mini Nutritional Assessment short‐form scores: A multicenter phase 2 study. Cancer Chemother Pharmacol 2017; 80: 461– 7. [DOI] [PubMed] [Google Scholar]
- 10. Miyauchi E, Inoue A, Usui K et al Phase II study of modified carboplatin plus weekly nab‐paclitaxel in elderly patients with non‐small cell lung cancer: North Japan Lung Cancer Study Group Trial 1301. Oncologist 2017;22:640–e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abernethy AP, Arunachalam A, Burke T et al Real‐world first‐line treatment and overall survival in non‐small cell lung cancer without known EGFR mutations or ALK rearrangements in US community oncology setting. PLoS One 2017;12:e0178420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paz‐Ares L, de Marinis F, Dediu M et al Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non‐squamous non‐small‐cell lung cancer (PARAMOUNT): A double‐blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247–55. [DOI] [PubMed] [Google Scholar]
- 13. Paz‐Ares LG, de Marinis F, Dediu M et al PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non‐small‐cell lung cancer. J Clin Oncol 2013; 31: 2895–902. [DOI] [PubMed] [Google Scholar]
- 14. Pereira JR, Cheng R, Orlando M, Kim JH, Barraclough H. Elderly subset analysis of randomized phase III study comparing pemetrexed plus carboplatin with docetaxel plus carboplatin as first‐line treatment for patients with locally advanced or metastatic non‐small cell lung cancer. Drugs R D 2013; 13: 289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zukin M, Barrios CH, Pereira JR et al Randomized phase III trial of single‐agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non small‐cell lung cancer and eastern cooperative oncology group performance status of 2. J Clin Oncol 2013; 31: 2849–53. [DOI] [PubMed] [Google Scholar]
- 16. Scagliotti GV, Parikh P, von Pawel J et al Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naïve patients with advanced‐stage non‐small‐cell lung cancer. J Clin Oncol 2008;26: 3543–51. [DOI] [PubMed] [Google Scholar]
- 17. Weed DL. Methodologic guidelines for review papers. J Natl Cancer Inst 1997; 89: 6–7. [Google Scholar]
- 18. de Castria TB, da Silva EM, Gois AF, Riera R. Cisplatin versus carboplatin in combination with third‐generation drugs for advanced non‐small cell lung cancer. Cochrane Database Syst Rev 2013; 8: CD009256. [DOI] [PubMed] [Google Scholar]
- 19. Skarlos DV, Samantas E, Kosmidis P et al Randomized comparision of etoposide‐cisplatin vs. etoposide‐carboplatin and irradiation in small‐cell lung cancer. A Hellenic Co‐operative Oncology Group Study. Ann Oncol 1994; 5: 601–7. [DOI] [PubMed] [Google Scholar]
- 20. Fossella F, Pereira JR, von Pawel J et al Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non‐small‐cell lung cancer: The TAX 326 study group. J Clin Oncol 2003; 21: 3016–24. [DOI] [PubMed] [Google Scholar]
- 21. Schiller JH, Harrington D, Belani CP et al Comparison of four chemotherapy regimens for advanced non‐small‐cell lung cancer. N Engl J Med 2002; 346: 92–8. [DOI] [PubMed] [Google Scholar]
- 22. Rosell R, Gatzemeier U, Betticher DC et al Phase III randomised trial comparing paclitaxel/carboplatin with paclitaxel/cisplatin in patients with advanced non‐small‐cell lung cancer: A cooperative multinational trial. Ann Oncol 2002; 13: 1539–49. [DOI] [PubMed] [Google Scholar]
- 23. Ardizzoni A, Boni L, Tiseo M et al Cisplatin‐ versus carboplatin‐based chemotherapy in first‐line treatment of advanced non‐small‐cell lung cancer: An individual patient data meta‐analysis. J Natl Cancer Inst 2007; 99: 847–57. [DOI] [PubMed] [Google Scholar]
- 24. Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Meta‐analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non‐small‐cell lung cancer. J Clin Oncol 2004; 22: 3852–9. [DOI] [PubMed] [Google Scholar]
- 25. Zhu J, Sharma DB, Chen AB, Johnson BE, Weeks JC, Schrag D. Comparative effectiveness of three platinum‐doublet chemotherapy regimens in elderly patients with advanced non‐small cell lung cancer. Cancer 2013; 119: 2048–60. [DOI] [PubMed] [Google Scholar]
- 26. Santana‐Davila R, Szabo A, Arce‐Lara C, Williams CD, Kelley MJ, Whittle J. Cisplatin versus carboplatin based regimens for the treatment of patients with metastatic lung cancer. An analysis of Veterans Health Administration data. J Thorac Oncol 2014; 9: 702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morabito A, Gebbia V, Di Maio M et al Randomized phase III trial of gemcitabine and cisplatin vs. gemcitabine alone in patients with advanced non‐small cell lung cancer and a performance status of 2: The CAPPA‐2 study. Lung Cancer 2013; 81: 77–83. [DOI] [PubMed] [Google Scholar]
- 28. Gridelli C, Rossi A, Di Maio M et al Rationale and design of MILES‐3 and MILES‐4 studies: Two randomized phase 3 trials comparing single‐agent chemotherapy versus cisplatin‐based doublets in elderly patients with advanced non‐small‐cell lung cancer. Clin Lung Cancer 2014; 15: 166–70. [DOI] [PubMed] [Google Scholar]
- 29. Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol 1998; 16: 1582–7. [DOI] [PubMed] [Google Scholar]
- 30. Therasse P, Arbuck SG, Eisenhauer EA et al New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 31. Kaplan EL, Meier P. Nonparametric estimation of incomplete observations. J Am Stat Assoc 1958; 53: 457–81. [Google Scholar]
- 32. Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Control Clin Trials 1997; 17: 343–6. [DOI] [PubMed] [Google Scholar]
- 33. Mantel N. Chi‐square tests with one degree of freedom: Extensions of the Mendel‐Haenszel procedure. J Am Stat Assoc 1963; 58: 690–700. [Google Scholar]
- 34. Peto R, Peto J. Asymptomatically efficient rank invariant test procedures. J R Stat Soc Ser A 1972; 135: 185–206. [Google Scholar]
- 35. Cox DR. Regression models and life tables. J R Stat Soc Series B 1972; 34: 187–200. [Google Scholar]


