Abstract
Background
The study was conducted to assess differences in overall survival (OS) in patients with non‐small cell lung cancer (NSCLC) receiving different treatment modalities of tyrosine kinase inhibitors (TKIs).
Methods
A total of 463 NSCLC patients receiving TKI treatment were included. OS was compared according to treatment timing in all patients, the elderly, and patients positive for EGFR mutations.
Results
One hundred and seventy two patients received TKIs as first‐line treatment, 220 as second‐line, and 67 as third‐line. The results between the three groups were not statistically significant: the one, two, and three‐year OS rates were: 55.3%, 22.3%, and 11.3% (first‐line); 59.6%, 27.8%, and 14.9% (second‐line); and 53.8%, 41.3%, and 29.5% (third‐line), respectively (P = 0.095). Results between the three groups of elderly patients were also not statistically significant (P = 0.469). The one and two‐year OS rates in EGFR mutation‐positive patients receiving first‐line treatment were 48% and 17.5%, respectively. The one, two, and three‐year OS rates of patients receiving second‐line treatment were: 54.2%, 30.3%, and 20.2%, respectively. There were no statistically significant differences between the groups with EGFR mutations receiving first‐line or second‐line treatment. Thirteen EGFR mutation‐positive patients received third‐line TKI treatment for a median duration of 7 months. Their one and two‐year OS rates were 69.8% and 58.2%, respectively, which were higher than in the other two groups (P = 0.015).
Conclusion
Three lines of TKI therapy can prolong survival in NSCLC patients. Elderly patients can benefit from TKI therapy. EGFR mutation‐positive patients can benefit from second‐line or third‐line TKI therapy.
Keywords: EGFR‐TKIs, elderly patients, survival analysis
Introduction
The era of molecular‐targeted therapy for non‐small cell lung cancer (NSCLC) is emerging. Previously, platinum‐based doublet chemotherapy was the standard of care, but resulted in poor prognosis with median overall survival (OS) of 8–10 months and a one‐year survival rate of 30–35%.1 The adoption of EGFR‐ tyrosine kinase inhibitors TKIs, such as erlotinib and gefitinib, has changed the potential outcome for EGFR mutation‐positive metastatic NSCLC patients dramatically.2, 3, 4, 5, 6, 7, 8 A series of studies have focused on comparing TKIs to chemotherapy. The IPASS study found that chemotherapy and gefitinib could significantly improve efficiency (response rate, RR) and progression‐free survival (PFS), particularly in patients with specific characteristics (i.e. Asian, female, non‐smoker) for whom gefitinib showed superiority over chemotherapy.4 Other phase III studies have achieved similar results.9, 10, 11 The TORCH study, which included patients with no specific molecular biology requirements, showed that chemotherapy as first‐line and erlotinib as second‐line treatment confers better survival rates than erlotinib as first‐line and chemotherapy as second‐line treatment.12 However, the optimal treatment regimen has not yet been discovered. We conducted this retrospective study to determine the kinds of treatments that NSCLC patients in China receive in real world clinical practice that might contribute to improved OS in patients treated with EGFR‐TKIs. With the current aging of the world's population and the popularity of physical examinations, an increasing number of elderly patients are being diagnosed. Very few studies have been specifically designed to definitively identify whether TKIs should be administered to elderly patients.13 Thus, this study also sheds light on whether elderly patients may benefit from TKI treatment.
Methods
Patients
Patients with previous histologic or cytologic confirmation of local; advanced stage (IIIA or IIIB) or stage IV; or recurrent, metastatic NSCLC were eligible for the study. The patients were required to be aged ≥18, with at least one measurable focus fitting the Response Evaluation Criteria in Solid Tumors, and had been diagnosed using computed tomography or nuclear magnetic resonance imaging. Neoplasms at unique foci must have been histologically or cytologically confirmed. This study was conducted in accordance with the Declaration of Helsinki and received approval from the Ethics Committee of Anhui Chest Hospital. Written informed consent was obtained from all participants.
Toxicity evaluation
Drug‐induced toxicity was classified in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
Related medical definition of the concept
Median survival time (MST) is the time by which death occurs in 50% of cases. OS refers to the duration from initial treatment to death from any cause.
Statistical analysis
EpiData bidirectional verification was used for data entry (EpiData Association, Odense, Denmark). A Chi square test was used to compare differences based on stratification: gender, performance status, age, histology, EGFR status, staging, and prior chemotherapy regimens. Median OS was calculated using the Kaplan–Meier method and differences between the levels of possible prognostic factors were compared using the log rank test in univariate analyses. Multivariate analysis with covariate adjusted Cox regression was then performed to identify prognostic factors. A value of P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
Data of 463 patients (212 men, 251 women) was collected from 16 hospitals. Patient age ranged from 22 to 93, with a median of 62 years. Pathological types included adenocarcinoma (340 cases), squamous cell carcinoma (48 cases), large cell carcinoma (4 cases), undifferentiated (35 cases), and other (36 cases). EGFR was detected in 130 cases (28.1%), of which 11 harbored wild type and 119 harbored EGFR mutations. The classified stages were distributed as follows: IIIa, 9 cases; IIIb, 48 cases; IVa, 152 cases; and IVb, 243 cases.
Tyrosine kinase inhibitors were administered as first‐line treatment in 172 cases (37.1%), as second‐line in 220 (47.5%), and as third‐line in 67 (14.4%). Four patients received TKIs beyond third‐line treatment, four patients received both gefitinib and icotinib as second‐line treatment, and three patients received both gefitinib and erlotinib as third‐line treatment. A comparison of the baseline characteristics of patients according to the timing of EGFR‐TKI treatment is summarized in Table 1.
Table 1.
Baseline characteristics of the three groups
| Characteristic | First‐line | Second‐line | Third‐line |
|---|---|---|---|
| Age | |||
| < 65 | 74 | 149 | 41 |
| ≥ 65 | 96 | 71 | 26 |
| Gender | |||
| Male | 71 | 94 | 44 |
| Female | 99 | 126 | 23 |
| Adenocarcinoma | |||
| 116 | 181 | 41 | |
| Non‐adenocarcinoma | |||
| 54 | 39 | 26 | |
| Stage | |||
| IIIa | 5 | 2 | 2 |
| IIIb | 18 | 24 | 4 |
| IVa | 75 | 65 | 12 |
| IVb | 70 | 124 | 49 |
| KPS | 70 (30–100) | 70 (30–100) | 70 (10–100) |
KPS, Karnofsky performance status.
The 220 patients in the second‐line treatment group tended to be younger than those who received EGFR‐TKIs as first‐line or third‐line therapy (<65 years 67.7% vs. 43.5% and 61.2%, respectively; P = 0.000), women (42.7% vs. 41.8% and 65.7%, respectively; P = 0.000), and with adenocarcinoma (81.8% vs. 68.2% and 61.2%, respectively; P = 0.001). There was no significant difference in stage or Karnofsky performance score (KPS) between the three groups.
Survival analysis
The median OS in the first‐line treatment group was 15 months (95% confidence interval [CI] 12.3–17.6), in the second‐line group 16 months (95% CI 13.7–18.2), and in the third‐line group 16 months (95% CI 5.4–26.6); these results were relatively insignificant (P = 0.146) (Fig 1a).
Figure 1.
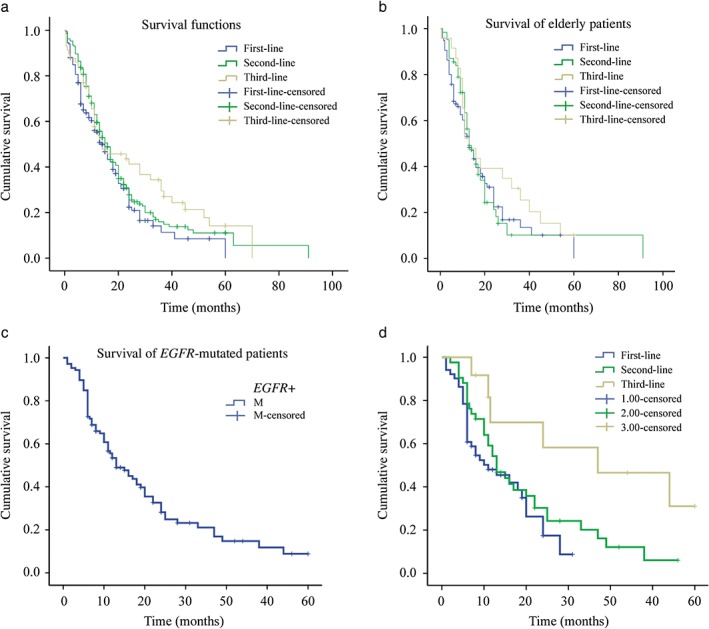
Overall survival comparison of: (a) the three groups, (b) elderly, (c) EGFR‐mutation positive, and (d) the three EGFR‐mutation positive groups of patients. Blue, first‐line; green, second‐line; yellow, third‐line; blue line with dash, first‐line censored; green line with dash, second‐line censored; yellow line with dash, third‐line censored.
The one, two, and three‐year survival rates were: 55.3%, 22.3%, and 11.3% in the first‐line treatment group; 59.6%, 27.8%, and 14.9% in the second‐line; and 53.8%, 41.3%, and 29.5% in the third‐line treatment group, respectively. The survival rates between the three groups were relatively insignificant (P = 0.095).
Survival analysis of elderly patients
A total of 194 patients were aged >65 years. Ninety‐seven patients received TKIs as first‐line treatment. The MST was 13 months (95% CI 9.9–16.1), and the one, two, and three‐year survival rates were 53.1%, 22.4%, and 13.4%, respectively. Seventy‐one patients received TKIs as second‐line treatment. The MST was 13 months (95% CI 10.1–15.9), and the one, two, and three‐year survival rates were 56.3%, 21.3%, and 10.1%, respectively. Twenty‐six patients received TKIs as third‐line treatment. The MST was 13 months (95% CI 7.4–18.6), and the one, two, and three‐year survival rates were 52.3%, 39.2%, and 25.4%, respectively. The survival differences between the three groups of patients were relatively insignificant (P = 0.469) (Fig 1b).
Survival analysis of EGFR mutation‐positive patients
Comparisons of the baseline characteristics of EGFR mutation‐positive patients according to the timing of EGFR‐TKI treatment are summarized in Table 2.
Table 2.
Baseline characteristics of EGFR mutation‐positive patients
| Characteristic | First‐line | Second‐line | Third‐line |
|---|---|---|---|
| Age | |||
| < 65 | 28 | 9 | 3 |
| ≥ 65 | 29 | 40 | 10 |
| Gender | |||
| Male | 22 | 25 | 7 |
| Female | 35 | 24 | 6 |
| Adenocarcinoma | |||
| 46 | 42 | 8 | |
| Non‐adenocarcinoma | |||
| 11 | 7 | 5 | |
| Stage | |||
| IIIa | 2 | 0 | 1 |
| IIIb | 5 | 10 | 0 |
| IVa | 45 | 9 | 2 |
| IVb | 15 | 30 | 10 |
| KPS | 60 (30–100) | 70 (50–90) | 70 (30–80) |
KPS, Karnofsky performance status.
EGFR mutation‐positive patients who received EGFR‐TKIs as first‐line treatment were younger than those who received EGFR‐TKIs as second‐line treatment (<65 years, 49% vs. 18.4%; P = 0.001). The KPS was higher in the second‐line than in the third‐line treatment group (P = 0.03). There were no significant differences in gender, pathology, or stage between the three groups.
The one, two, and three‐year OS rates of EGFR mutation‐positive patients were 53.4%, 28.2%, and 21.1%, respectively (Fig 1c).
Fifty‐seven EGFR mutation‐positive patients received TKIs as first‐line therapy. The one and two‐year survival rates were 48% and 17.5%, respectively. Forty‐nine patients received second‐line treatment and the one, two, and three‐year survival rates were 54.2%, 30.3%, and 20.2%, respectively. Thirteen patients received third‐line TKIs. The one and two‐year survival rates were 69.8% and 58.2%, respectively, which were higher than in the other two groups (P = 0.015) (Fig 1d).
Prognostic factors
Clinical variables associated with the OS of all patients were analyzed (Table 3). Higher KPS correlated with improved OS (P = 0.059).
Table 3.
Prognostic factors for overall survival
| Factors | N (%) | MST (months) | 95% CI (months) | x2 | P |
|---|---|---|---|---|---|
| All | 463 | 15 | 13.106–16.894 | ||
| Gender | |||||
| Male | 212 | 11 | 8.798–13.202 | 2.673 | 0.102 |
| Female | 251 | 17 | 15.219–18.781 | ||
| Age | |||||
| < 65 | 263 | 17 | 13.786–20.214 | 0.406 | 0.524 |
| ≥ 65 | 198 | 13 | 10.323–15.677 | ||
| Histology | |||||
| Adenocarcinoma | 340 | 16 | 13.782–18.218 | 1.147 | 0.284 |
| Non‐adenocarcinoma | 113 | 11 | 8.443–13.557 | ||
| KPS | |||||
| < 70 | 174 | 14 | 11.417–16.583 | 3.564 | 0.059 |
| ≥ 70 | 257 | 17 | 13.475–20.525 | ||
| Metastasis before treatment | |||||
| Yes | 281 | 16 | 13.161–18.839 | 0.12 | 0.729 |
| No | 156 | 14 | 11.120–16.880 | ||
| Disease progress | |||||
| Pulmonary | 136 | 12 | 9.765–14.235 | 0.149 | 0.7 |
| Extra‐pulmonary | 221 | 16 | 13.226–18.774 |
CI, confidence interval; KPS, Karnofsky performance status; MST, median survival time.
Discussion
In the last 10 years, the advent of targeted drugs has offered new treatment options for patients with NSCLC. EGFR is an important mature research target as it can activate multiple downstream signaling pathways, such as the Ras‐Raf‐MAPK, JAK‐STAT, and P13K‐Akt pathways, which contribute to cell signaling, promotion of cell proliferation, metastasis, and inhibition of apoptosis. EGFR‐TKIs, by binding to the EGFR adenosine triphosphate (ATP)‐competitive inhibitory site of the EGFR intracellular tyrosine kinase moiety, directly reduce the autophosphorylation of EGFR, leading to cell growth arrest and the promotion of apoptosis.14
This multi‐center study involved retrospective analysis. All patients were diagnosed with advanced NSCLC, 73.4% of which were adenocarcinomas. The detection rate of EGFR gene mutations was 28.1%.
In our study, the majority (47.5%) of patients received TKIs as second‐line treatment. Their one, two, and three‐year survival rates (59.6%, 27.8%, and 14.9%, respectively) were slightly higher than those of the first‐line treatment group (55.3%, 22.3%, and 11.3%, respectively). This may be explained by the fact that patients receiving second‐line treatment were younger, women, and had adenocarcinomas. However, this finding was statistically insignificant.
In this study, 47.9% of elderly patients received TKIs as first‐line treatment. The IPASS study found that the EGFR mutation‐positive rate was 68.5% in patients aged >65 and 56.7% in patients aged <65.4 The EGFR mutation rates in elderly patients with advantageous characteristics (i.e. Asian, female, non‐smoker) may be even higher. The TORCH study found that after first‐line chemotherapy, 28.5% patients died as a result of deteriorating health conditions that prevented them from receiving erlotinib as second‐line treatment.12 A study in Korea showed that the efficacy rate of octogenarians receiving first‐line TKI therapy was 80%, and the median OS of patients receiving TKIs was 24.1 months.15 EGFR‐TKI therapy is more suitable for older patients because of its lower cytotoxicity, allowing patients to lead a better quality of life.16, 17
Some phase III studies involving patients with EGFR‐positive NSCLC did not demonstrate improved OS, despite an improvement in PFS with first‐line EGFR‐TKI therapy.12 According to our analysis, the OS of EGFR mutation‐positive patients was not superior to other patients, and there were no significant survival differences between the patients that received first‐line EGFR‐TKI therapy and those that underwent one or two courses of prior chemotherapy. This finding is similar to the results demonstrated by previous Asian studies.18, 19, 20 Survival rates in the third‐line treatment group were higher than those in the other two groups because there were only 13 patients in this group.
This study demonstrates that TKIs confer survival benefits to patients; however no statistically significant survival difference was observed between different treatment timings.
Disclosure
No authors report any conflict of interest.
References
- 1. Inoue A, Yoshida K, Morita S et al Characteristics and overall survival of EGFR mutation‐positive non‐small cell lung cancer treated with EGFR tyrosine kinase inhibitors: A retrospective analysis for 1660 Japanese patients. Jpn J Clin Oncol 2016; 46: 462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maemondo M, Inoue A, Kobayashi K et al Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR . N Engl J Med 2010; 362: 2380–8. [DOI] [PubMed] [Google Scholar]
- 3. Mitsudomi T, Morita S, Yatabe Y et al Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–8. [DOI] [PubMed] [Google Scholar]
- 4. Fukuoka M, Wu YL, Thongprasert S et al Biomarker analyses and final overall survival results from a phase III, randomized, open‐label, first‐line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non‐small‐cell lung cancer in Asia (IPASS). J Clin Oncol 2011; 29: 2866–74. [DOI] [PubMed] [Google Scholar]
- 5. Han JY, Park K, Kim SW et al First‐SIGNAL: First‐line single‐agent iressa versus gemcitabine and cisplatin trial in never‐smokers with adenocarcinoma of the lung. J Clin Oncol 2012; 30: 1122–8. [DOI] [PubMed] [Google Scholar]
- 6. Sequist LV, Yang JC, Yamamoto N et al Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–34. [DOI] [PubMed] [Google Scholar]
- 7. Wu YL, Zhou C, Liam CK et al First‐line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer: Analyses from the phase III, randomized, open‐label, ENSURE study. Ann Oncol 2015; 26: 1883–9. [DOI] [PubMed] [Google Scholar]
- 8. Zhou C, Wu YL, Chen G et al Erlotinib versus chemotherapy as first line treatment for patients with advanced EGFR mutation positive non‐small‐cell lung cancer (OPTIMAL, CTONG 0802): A multicentre, open label, randomised, phase 3 study. Lancet Oncol 2011; 12: 735–42. [DOI] [PubMed] [Google Scholar]
- 9. Kim ES, Hirsh V, Mok T et al Gefitinib versus docetaxel in previously treated non‐small‐cell lung cancer (INTEREST): A randomised phase III trial. Lancet 2008; 372: 1809–18. [DOI] [PubMed] [Google Scholar]
- 10. Rosell R, Carcereny E, Gervais R et al Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–46. [DOI] [PubMed] [Google Scholar]
- 11. Schuler M, Yang JC, Park K et al Afatinib beyond progression in patients with non‐small‐cell lung cancer following chemotherapy, erlotinib/gefitinib and afatinib: Phase III randomized LUX‐Lung 5 trial. Ann Oncol 2016; 27: 417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gridelli C, Ciardiello F, Gallo C et al First‐line erlotinib followed by second‐line cisplatin‐gemcitabine chemotherapy in advanced non‐small‐cell lung‐cancer: The TORCH randomized trial. J Clin Oncol 2012; 30: 3002–11. [DOI] [PubMed] [Google Scholar]
- 13. Hohenforst‐Schmidt W, Zarogoulidis P, Steinheimer M et al Tyrosine kinase inhibitors for the elderly. J Cancer 2016; 7: 687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berger MS, Gullick WJ, Greenfield C, Evans S, Addis BJ, Waterfield MD. Epidermal growth factor receptors in lung tumours. J Pathol 1987; 152: 297–307. [DOI] [PubMed] [Google Scholar]
- 15. Sim SH, Kim YJ, Kim SH et al Current status of chemotherapy use and clinical outcome in octogenarians with advanced non‐small cell lung cancer. J Cancer Res Clin Oncol 2015; 141: 1073–81. [DOI] [PubMed] [Google Scholar]
- 16. Oizumi S, Kobayashi K, Inoue A et al Quality of life with gefitinib in patients with EGFR‐mutated non‐small cell lung cancer: Quality of life analysis of North East Japan Study Group 002 Trial. Oncologist 2012; 17: 863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morikawa N, Minegishi Y, Inoue A et al First‐line gefitinib for elderly patients with advanced NSCLC harboring EGFR mutations. A combined analysis of North‐East Japan Study Group studies. Expert Opin Pharmacother 2015; 16: 465–72. [DOI] [PubMed] [Google Scholar]
- 18. Miyauchi E, Inoue A, Kobayashi K et al Efficacy of chemotherapy after first‐line gefitinib therapy in EGFR mutation‐positive advanced non‐small cell lung cancer‐data from a randomized Phase III study comparing gefitinib with carboplatin plus paclitaxel (NEJ002). Jpn J Clin Oncol 2015; 45: 670–6. [DOI] [PubMed] [Google Scholar]
- 19. Koo DH, Kim KP, Choi CM et al EGFR‐TKI is effective regardless of treatment timing in pulmonary adenocarcinoma with EGFR mutation. Cancer Chemother Pharmacol 2015; 75: 197–206. [DOI] [PubMed] [Google Scholar]
- 20. Wu JY, Yu CJ, Yang CH et al First‐ or second‐line therapy with gefitinib produces equal survival in non‐small cell lung cancer. Am J Respir Crit Care Med 2008; 178: 847–53. [DOI] [PubMed] [Google Scholar]


