Abstract
The present study aimed to investigate the hepatoprotective effects of methyl ferulic acid (MFA) against oxidative stress and apoptosis in acute liver injury induced by carbon tetrachloride (CCl4) in rats, as well as the underlying mechanisms. Sprague Dawley rats were treated with CCl4 after oral administration of MFA (25, 50, and 100 mg/kg) or dimethyl diphenyl bicarboxylate (200 mg/kg) for 7 days. The hepatoprotective effects of MFA were determined by analyzing serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities as well as changes of oxidant parameters. Histopathological analysis was performed to determine the degree of hepatic injury. The mechanisms were investigated by detecting the levels of NADPH oxidase (NOX) trans-membrane subunit NOX4, its ligand p22phox, as well as caspase3, cleaved caspase3, B-cell lymphoma (Bcl)-2, Bcl-2-associated X protein (Bax), tumor necrosis factor (TNF)-α, interleukin (IL)-1, reactive oxygen species (ROS), thiobarbituric acid-reactive substances (TBARS), total anti-oxidant capacity (TAC), phosphorylated J-Jun N-terminal kinase (p-JNK) and p-p38 mitogen-activated protein kinase (MAPK) using semi-quantitative polymerase chain reaction, western blot analysis and colorimetric assays. MFA treatment significantly decreased serum enzymatic activities of ALT and AST. MFA markedly increased activities of liver superoxide dismutase, catalase and glutathione peroxidase, and reduced the malondialdehyde concentration. Histopathological examination demonstrated that MFA reduced lipid degeneration, cytoplasmic vacuolization, necrosis and inflammatory cell infiltration in the liversof CCl4-treated rats. MFA treatment markedly inhibited the expression of inflammatory factors TNF-α and IL-1β. Mechanistic study revealed that MFA decreased the TAC and the levels of ROS and TBARS. Furthermore, MFA treatment led to a reduction of the mRNA and protein expression of NOX4 and p22phox, as well as the protein levels of caspase3, cleaved caspase-3 and Bax, and an upregulation of p-JNK, p-p38 MAPK and Bcl-2 proteins in the liver. The present study demonstrated that MFA has hepatoprotective effects against CCl4-induced acute liver damage. MFA has anti-oxidant, anti-inflammatory and anti-apoptotic activities and was able to modulate the NOX4/p22phox/ROS-JNK/p38 MAPK signaling pathway.
Keywords: methyl ferulic acid, acute liver injury, oxidative stress, inflammatory response, apoptosis
Introduction
Liver injury or dysfunction is recognized as a serious worldwide health problem. Clinically available synthetic drugs for the treatment of liver diseases, such as interferon and corticosteroids, are expensive, particularly for patients in developing countries. These drugs may also cause adverse reactions and further damage (1). Therefore, traditional medicine is important in the treatment of liver diseases (2). Although traditional medicinal treatments have achieved good results, numerous problems remain due to the complexity of a single herbal component, the diversity of combination drugs, the uncertainty of drug formulation, randomness of oral administration and unknown mechanisms of action. Effective drugs with a clear mechanism and low incidence of side effects are urgently required.
Carbon tetrachloride (CCl4) has been widely used to induce chronic and acute liver damage in animal models (3). Liver damage caused by CCl4 is characterized by inflammation, formation of trichloromethyl radicals and overproduction of reactive oxygen species (ROS), which initiate lipid peroxidation and finally lead to hepatotoxicity (4). Anti-apoptotic, anti-oxidant and anti-inflammatory actions may be important in the protection against CCl4-induced liver damage.
Oxidative damage caused by ROS may lead to various human diseases, such as liver fibrosis, cancer and inflammation. It is well known that oxidative stress is involved in the pathogenesis of acute and chronic liver injury (5). Hepatic damage caused by viral infection, ethanol ingestion, iron overload and exposure to drugs or CCl4 is attributed to overproduction of ROS (6,7). NADPH oxidases (NOXs), which have a critical role in the inflammatory response, contribute to ROS production during liver injury (8).
The mitogen-activated protein kinase (MAPK) family is involved in the regulation of cell proliferation and death in response to various internal stresses. P38 MAPK and c-Jun N-terminal kinase (JNK), two members of the MAPK superfamily, are activated by cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β, or G protein-coupled receptors, and have an important role in inflammation and apoptosis in response to stress (9). JNKs have a vital role in the death receptor-initiated extrinsic and mitochondrial intrinsic apoptotic pathways (10). JNKs activate apoptotic signaling by upregulating pro-apoptotic genes via the transactivation of specific transcription factors or by modulating the activities of mitochondrial pro- and anti-apoptotic proteins through distinct phosphorylation (11). ROS may cause apoptosis by activating the JNK signaling pathway (12). CCl4 has been found to induce hepatic apoptosis via the mitochondrial intrinsic and extrinsic apoptotic pathways (13,14). p38 MAPK has an essential role in regulating numerous cellular processes, including inflammation and apoptosis. In turn, production of p38 MAPK may be induced by inflammatory factors and stress. CCl4 significantly increases the levels of p38 MAPK, and oxidative stress as well as certain cytokines activate p38 MAPK through phosphorylation (15). The activation of the p38 MAPK pathway accelerates cell apoptosis (16). Studies have confirmed that certain factors that activate JNKs also activate p38 MAPK (16,17). Furthermore, CCl4 was reported to induce apoptosis in the liver by modulating the JNK and p38 MAPK pathways. JNK regulates the expression of pro- and anti-apoptotic members of the Bcl-2 family such as B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X protein (Bax). p38 MAPK induces Bax translocation and enhances the expression of TNF-α to ultimately induce apoptosis (10,18). In response to extrinsic as well as intrinsic apoptotic stimuli, JNK and P38 MAPK have an important role by interacting and modulating the activities of caspase proteins (12,19). Caspase-3 is one of the critical executioners of apoptosis, capable of cleaving or degrading numerous key proteins such as nuclear lamins, fodrin and the nuclear enzyme poly (adenosine diphosphase ribose) polymerase (PARP) (12,20).
Methyl ferulic acid (MFA) is a monomer that is extracted and purified from Securidaca inappendiculata Hasskarl (21–23), which was traditionally used for the treatment of acute or chronic hepatitis and exhibited some inhibitory effects on hepatitis B surface antigen in T cell lines (24). However, only few studies have assessed the hepatoprotective effect of MFA (24). The present study investigated the effects of MFA on CCl4-induced acute liver injury in rats. Specifically, the inhibitory effect of MFA on inflammation, oxidative stress and apoptosis was assessed, as well as the involvement of p38 MAPK and JNK signaling.
Materials and methods
Animals
A total of 60 Sprague Dawley (SD) rats ((8–10 weeks; 30 males and 30 females) weighing 250–300 g were obtained from the Experimental Animal Center of Guilin Medical University (Guilin, China). The rats were kept in an environmentally controlled room with a temperature of 25±2°C, relative humidity of 55±10% and a 12-h light/dark cycle. The rats were allowed free access to food and water. The SD rats were randomly divided into six groups (n=10 in each). Rats in the control group and the CCl4-treated model group only received an equivalent of distilled water containing 0.1% Tween 80 by oral gavage once a day for one week. Rats in the dimethyl diphenyl bicarboxylate (DDB)-treated group (positive control group) received DDB in distilled water containing 0.1% Tween 80 at a dose of 200 mg/kg body weight by oral gavage once a day for one week. Low, medium and high MFA-treated groups received MFA in distilled water containing 0.1% Tween 80 at a dose of 25, 50 or 100 mg/kg body weight by oral gavage once a day for a week. One hour after the last treatment, all rats in the CCl4-treated model group, the DDB-treated group and the MFA-treated group received an intraperitoneal injection of CCl4 (1 ml/kg body weight), while the control group received an equivalent volume of 0.9% physiological saline solution instead. At 24 h after CCl4 treatment, all rats were sacrificed and a portion of liver tissues was immediately collected for analysis and placed in ice-cold 0.9% physiological saline solution to remove blood cells for ROS detection. The remaining liver tissues were immediately stored at −80°C for later use. The present study was performed in accordance with the Chinese legislation and the US National Institutes of Health guidelines for the use and care of experimental animals. All animal experiments were approved by the institutional ethical committee of Guilin Medical University (Guilin, China).
Measurement of serum aminotransferase activities
After blood collection, serum was separated by centrifugation at 3,200 × g for 20 min at room temperature. The activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum from rats were determined using commercially available diagnostic kits (Alanine aminotransferase assay kit; cat no. C009-2; Aspartate aminotransferase assay kit; cat no. C010-2; Nanjing Jiancheng Bio Co., Ltd., Nanjing, China) according to the manufacturer's instructions.
Assay of hepatic levels of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), malondialdehyde (MDA) and catalase (CAT)
Liver tissue samples were homogenized in nine volumes of ice-cold 50 mM phosphate buffer (pH 7.4) and centrifuged at 3,200 × g for 20 min at 4°C. Supernatants were used to determine SOD, GSH-Px, MDA, CAT and total protein concentrations by using commercially available diagnostic kits (SOD assay kit; cat no. A001-3; GSH-PX assay kit; cat no. A005; cat no. MDA assay kit; cat no. A003-1; CAT assay kit; cat no. A007-1; total protein assay kit; cat no. A045-3; Nanjing Jiancheng Bio Co., Ltd.). The levels of MDA, GSH-Px, SOD and CAT were normalized to the content of total protein.
Hematoxylin and eosin (H&E) staining
For histological examination, liver tissues were removed from a portion of the left lobe and fixed in 10% phosphate-buffered formalin. After being processed by routine histological procedures, the samples were cut into 5-µm slices. Sections were stained using hematoxylin for 5 min at 40°C and eosin solution for 1 min at room temperature, then the slides were observed for conventional morphological evaluation under a light microscope (BX41; Olympus, Tokyo, Japan) and images were captured at ×100 magnification. The degree of hepatic damage was evaluated. Histological changes were scored according to the following system: 0, no injury; 1, mild injury; 2, moderate injury; and 3, severe injury.
Semi-quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from liver tissues using a tissue total RNA isolation kit (cat no. B518651; Shanghai Sangong Pharmaceutical Co., Ltd., Shanghai, China) according to the manufacturer's protocol. Total RNA was reversibly transcribed into complementary DNA (cDNA) using a cDNA synthesis kit (TIANScript MMLV; cat no. ER104; Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer's protocol. An MJ PTC-200 PCR System (Bio-Rad, Hercules, CA, USA) and a qPCR kit (cat no. PC0902; 2× Taq PCR Master Mix; Aidlab Biotechnologies Co., Ltd., Beijing, China) were used based on the manufacturer's instructions for amplification of target genes. The primers used in the study are listed in Table I. The specific primers for target gene β-actin were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). As an internal standard control, the expression level of β-actin was simultaneously quantified. The PCR protocol was as follows: Initial denaturation for 3 min at 94°C; 30–40 cycles of denaturation for 30 sec at 94°C, annealing for 30 sec at 56–58°C, and extension for 1 min at 70°C; and a final extension for 5 min at 72°C. The PCR products were identified by electrophoresis using 1.5% agarose gel, and optical density of target gene bands was calculated in each sample using a Gel Doc XR+ automatic gel imaging analysis system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and with adjustment through β-actin correction to finally obtain the relative expression of target gene in each sample (25).
Table I.
Primer sequences used for the determination of NOX4, p22phox, TNF-α, IL-1β and β-actin gene expression.
| Genes | Oligonucleotide primer sequences (5′-3′) | Product length (bp) |
|---|---|---|
| NOX4 | Forward, TGTGCCGAACACTCTTGGC | 136 |
| Reverse, ATATGCACGCCTGAGAAAATA | ||
| p22phox | Forward, TATTGTTGCAGGAGTGCTCA | 103 |
| Reverse, CACAGCGGTCAGGTACTTCT | ||
| TNF-α | Forward, GGCAGGTCTACTTTGGAGTC | |
| Reverse, GCAGGCAGTATCACTCATTG | 233 | |
| IL-1β | Forward, GCAGGCAGTATCACTCATTG | |
| Reverse, CACACCAGCAGGTTATCATC | 165 | |
| β-actin | Forward, GACTCCTATGTGGGTGACGA | 199 |
| Reverse, ACGGTTGGCCTTAGGGTTCA |
NOX4, NADPH oxidase 4; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β.
Western blot analysis
Total protein was extracted from liver tissues with radio immunoprecipitation assay lysis buffer (cat no. P0013B, Beyotime Institutute of Biotechnology, Shanghai, China). The Mitochondrial protein was extracted from liver tissue using a Cytoplasmic and Mitochondrial Protein Extraction kit (cat no. C500051; Sangon Biotech Co., Ltd., Shanghai, China). Protein concentration was determined using a bicinchoninic acid assay kit (Beyotime Biotechnology, Inc.). A total of 50 µg/lane of sample proteins were separated by 12% SDS-PAGE (Bio-Rad Laboratories, Inc. USA). The separated proteins were then transferred to pure nitrocellulose blotting membranes. The membranes were then blocked for 1 h with 5% bovine serum albumin (cat no. B600036; Sangon Biotech Co., Ltd., Shanghai, China) in Tris-buffered saline containing 0.05% Tween 20 (TBST) at room temperature. The membranes were incubated with anti-NOX4 (1:500; cat no. D121050), anti-Bcl-2 (1:1,000; cat no. D151442), anti-caspase-3 (1:1,000; cat no. D220074), anti-cleaved caspase-3 (1:500; cat no. D260009), anti-Bax (1:1,000; cat no. D220073), anti-GAPDH (1:1,000; cat no. D110016; all from Sangon Biotech Co., Ltd., Shanghai, China), anti-p22phox (1:500; cat no. BS60290; Bioworld Technology, Inc., Nanjing, China), anti-phospho-p38 MAPK (1:1,000; cat no. 4511T), anti-p38 MAPK (1:1,000; cat no. 14451), anti-phospho-JNK (1:1,000; cat no. 4668T), anti-JNK (1:1,000; cat no. 9252T; all from Cell Signaling Technology, Inc., Danvers, MA, USA) or anti-IL-1β (1:1,000; cat no. sc-52012), anti-TNF-α (1:1,000; cat no. sc-33639; both from Santa Cruz Biotechnology, Inc., Dallas, TX, USA) primary antibodies at 4°C overnight. The samples were incubated with corresponding horseradish peroxidase-conjugated secondary antibodies (1:50,000; cat no. ZB2301; horseradish peroxidase (HRP) Affinipure Goat anti-rabbit immunoglobulin G or 1:50,000; ZB2305 HRP Affinipure Goat anti-Mouse immunoglobulin G; Zhongshang Goldenbridge Bio, Beijing, China) at room temperature for one hour and protein bands were visualized by enhanced chemiluminescence (cat no. E002-100; 7seapharmatech Co. Ltd, Shanghai, China). The imaging system Chemi Doc XRS+ (Bio-Rad Laboratories, Inc., USA) was used for imaging and quantitative analysis of the blots. VCDA1 or GAPDH protein was used as an internal control.
Fluorescent spectrophotometry
The level of ROS was determined by detecting the fluorescence intensity of the oxidant-sensitive probe 2,7-dichlorodihydrofluorescein diacetate (Molecular Probes; Thermo Fisher Scientific, Inc., Waltham, MA, USA) as described in a previous study (26). The amount of formed dichlorofluorescein in the clear supernatant was determined using a microplate reader (Infinite M200 PRO; Tecan, Zurich, Switzerland) at an excitation wavelength of 502 nm and an emission wavelength of 523 nm.
Thiobarbituric acid (TBA) reactive substances (TBARS) colorimetric assay
Tissue lipid peroxidation was measured using a TBARS colorimetric assay. Liver homogenate was incubated with 8.1% (w/v) SDS for 10 min, followed by addition of 20% acetic acid (pH 3.5). The reaction mixture was incubated with 0.6% TBA (w/v) for 1 h in a boiling water bath. Pink color chromogen was extracted in butanol-pyridine solution (15:1) and spectrophotometrically quantified at 532 nm.
Measurement of the total anti-oxidant capacity (TAC)
Based on the oxidation of intracellular anti-oxidants with iron (III) in acidic medium, the TAC in the liver was assayed with a commercially available assay kit (cat no. A015-1; Nanjing Jiancheng Bio Co., Nanjing, China). The TAC of the samples was measured according to the manufacturer's protocol. One unit of TAC was defined as the capability of increasing the optical density value at 520 nm by 0.01 per mg protein per min at 37°C.
Statistical analysis
All statistical analyses were performed using SPSS software (version 17.0; International Business Machines, Corp., Armonk, NY, USA). One-way analysis of variance was used to determine significant differences between groups. The Student-Newman-Keuls test was used for comparisons between groups. Values are expressed as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
MFA provides protection against CCl4-induced hepatic injury
To determine whether MFA attenuates liver damage in CCl4-treated rats, the activities of ALT and AST in serum were measured. Compared with those in the normal control, the activities of ALT and AST in serum from the model group were significantly increased (P<0.01). Of note, administration of MFA at all doses significantly inhibited the elevation of ALT levels, and MFA at 50 and 100 mg/kg significantly inhibited the elevation of AST levels in CCl4-treated rats in a dose-dependent manner (P<0.05; Table II). These results suggested that MFA provides protection against CCl4-induced liver injury.
Table II.
Effect of MFA administration on ALT and AST activities in serum of rats with liver damage induced by CCl4.
| Group | ALT (U/l) | AST (U/l) |
|---|---|---|
| Control | 23.85±9.50 | 61.14±17.35 |
| Model | 216.39±70.93a | 524.01±160.71a |
| DDB (200 mg/kg) | 130.69±41.33b | 360.28±102.76b |
| MFA (25 mg/kg) | 170.56±51.56c | 465.18±137.63 |
| MFA (50 mg/kg) | 150.72±36.99b | 380.04±111.66b |
| MFA (100 mg/kg) | 134.72±37.52b | 353.54±109.15b |
MFA, methyl ferulic acid; ALT, alanine aminotransaminase; AST, aspartate aminotransaminase; DDB, dimethyl diphenyl bicarboxylate.
P<0.01 compared with control group
P<0.01
P<0.05 compared with model group.
MFA suppresses CCl4-induced oxidative liver injury
To quantify oxidative liver injury, the hepatic levels of SOD, GSH-Px, MDA and CAT were assayed. The hepatic levels of MDA were assessed as an indicator of lipid peroxidation in oxidative liver damage, and CCl4 treatment obviously increased the hepatic MDA levels compared with those in the control group (P<0.01), which was significantly inhibited by pre-administration of MFA (P<0.05; Table III). Furthermore, the results demonstrated that the activities of SOD, GSH-Px and CAT in the model group were significantly decreased compared with those in the control group, but pre-treatment with DDB or MFA (50 or 100 mg/kg) significantly increased the activities of SOD, GSH-Px and CAT compared with those in the model group (P<0.01). In addition, CCl4 treatment significantly increased hepatic MDA levels compared with those in the control group (P<0.01), but pre-treatment with DDB or MFA significantly decreased MDA levels compared with those in the model group (P<0.05). Of note, the low dose of MFA had no significant effect on SOD or GSH-Px (Table III). These results indicated that MFA suppressed CCl4-induced oxidative liver injury.
Table III.
Effect of MFA administration on SOD, CAT and GSH-Px activities as well as the level of MDA in liver tissues of rats induced by CCl4.
| Group | SOD (U/mg prot) | CAT (U/mg prot) | GSH-Px (U/mg prot) | MDA (nmol/g prot) |
|---|---|---|---|---|
| Control | 5.14±1.36 | 66.70±6.16 | 515.36±133.47 | 22.78±7.63 |
| Model | 2.17±0.74a | 36.31±7.29a | 307.05±85.33a | 45.78±11.92a |
| DDB (200 mg/kg) | 4.30±0.79b | 59.79±6.21b | 492.07±127.63b | 32.55±9.50b |
| MFA (25 mg/kg) | 2.81±1.00 | 50.33±6.29b | 396.12±109.76 | 36.53±9.59c |
| MFA (50 mg/kg) | 3.34±0.72b | 60.37±5.49b | 456.87±131.95b | 32.43±6.52b |
| MFA (100 mg/kg) | 4.38±0.95b | 62.01±5.44b | 495.18±116.97b | 26.78±4.94b |
SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; CAT, catalase; MDA, malondialdehyde; MFA, methyl ferulic acid; DDB, dimethyl diphenyl bicarboxylate.
P<0.01 compared with control group
P<0.01
P<0.05 compared with model group.
MFA alleviates CCl4-induced histological changes in the liver
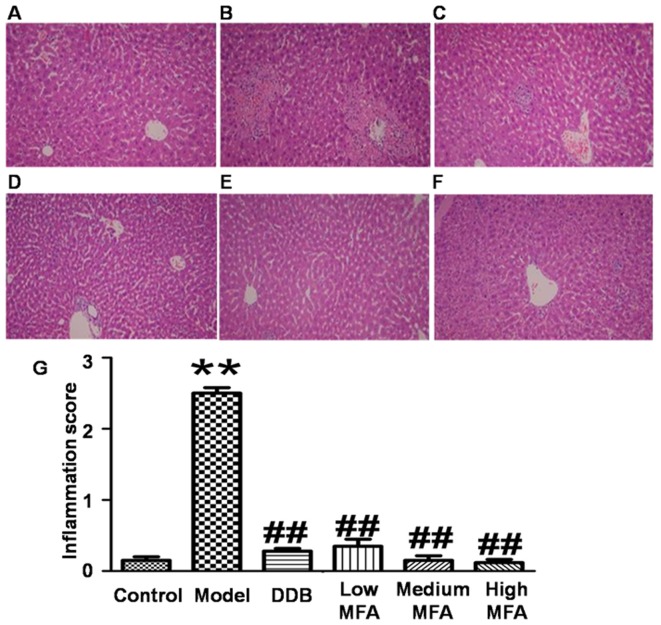
To detect histological changes in the liver, H&E staining was performed. Visual observation revealed that livers from the normal control group were reddish brown, soft and elastic, but livers from the CCl4 model group exhibited significantly increased liver volume, blood stasis, edge thickening and liver surface with petechial hemorrhage. In the MFA (25 mg/kg) group, the liver was slightly enlarged, and spot bleeding was significantly reduced. By contrast, the appearance of the liver in the medium and high MFA groups was close to normal. H&E staining of liver sections from the normal control group demonstrated an intact hepatic lobular structure, normal hepatic cells with well-preserved cytoplasm, prominent nuclei, hepatocytes that were radially arranged around the central vein, well-defined sinusoidal line, uniform size, no degeneration, no necrosis, and hepatic cords that were arranged in neat rows. In addition, no inflammatory cell infiltration was observed (Fig. 1A). In the model group treated with CCl4, typical pathological characteristics were observed, including destroyed hepatic lobule structure, liver sinus and central venous dilatation, hyperemia, a disorder in the arranged of hepatic cords, ballooning degeneration, broad infiltration of inflammatory cells, centrilobular fatty changes, apoptosis and widespread hepatocellular necrosis, particularly significant bridging necrosis, and inflammatory cell infiltration in hepatic lobules and portal area (Fig. 1B). By contrast, CCl4-intoxicated rats pre-treated with DDB had nearly normal liver tissues with no significant changes in hepatocytes (Fig. 1C). In the low MFA group, liver sections exhibited moderate hypertrophy of hepatocytes with a relatively intact central vein, spotty necrosis, a rare large area of necrosis, shrinking sinusoidal line and reduced number of inflammatory cells (Fig. 1D). Of note, hepatic lesions were markedly ameliorated in the medium and high MFA groups, with slight inflammatory cell infiltration (Fig. 1E and F). The inflammation score of CCl4-treated rats was significantly higher than that of normal control rats, while pre-treatment with MFA reduced the inflammation score (Fig. 1G). These results indicated that CCl4 treatment caused obvious histological changes in the liver, while pre-treatment with MFA prevented CCl4-induced damage.
Figure 1.
Effect of MFA on CCl4-induced liver damage. The rats were pre-treated with MFA (25, 50 or 100 mg/kg) once a day for seven consecutive days. One hour after the final treatment, the rats were treated with CCl4 (1.0 ml/kg, intraperitoneally). After 24 h, livers were histologically examined by hematoxylin and eosin staining (magnification, ×100). (A) Control group; (B) CCl4-treated model group; (C) CCl4+DDB group; (D) MFA (25 mg/kg) + CCl4 (1.0 ml/kg) group; (E) MFA (50 mg/kg) + CCl4 (1.0 ml/kg) group; (F) MFA (100 mg/kg) + CCl4 (1.0 ml/kg) group. (G) Tissue inflammation score, **P<0.01 compared with control group; ##P<0.01 compared with CCl4-treated model group. Values are expressed as the ± standard error of the mean. MFA, methyl ferulic acid; DDB, dimethyl diphenyl bicarboxylate.
MFA inhibits CCl4-induced oxidative stress in the liver
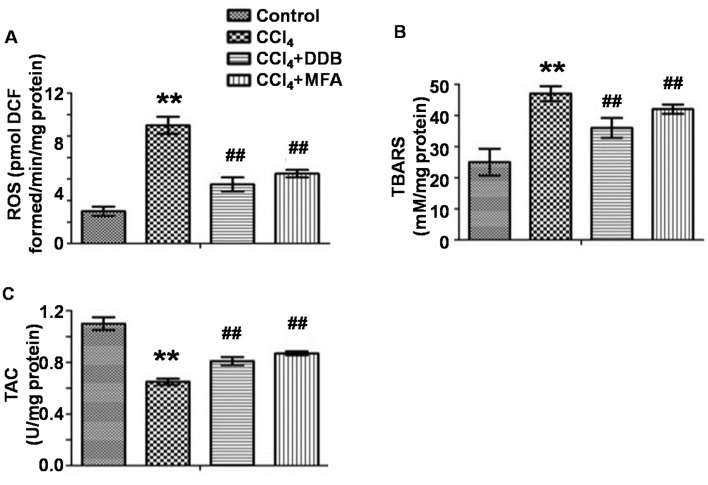
To evaluate oxidative stress in the liver, the levels of ROS and TBARS as well as the TAC were measured. The results demonstrated that CCl4 treatment markedly improved hepatic ROS and TBARS levels, while decreasing the TAC compared with those in the control group (P<0.01; Fig. 2A-C). Of note, pre-treatment with MFA significantly reduced CCl4-induced ROS and TBARS expression and significantly increased the TAC (P<0.01; Fig. 2A-C). These results indicated that MFA inhibited oxidative stress induced by CCl4 in rat livers.
Figure 2.
Effect of MFA on the oxidative stress in livers of CCl4-treated rats. (A) Levels of ROS; (B) Level of TBARS; (C) TAC. Values are expressed as the ± standard error of the mean (n=6). **P<0.01 compared with control group; ##P<0.05 compared with model group. MFA, methyl ferulic acid; DDB, dimethyl diphenyl bicarboxylate; ROS, reactive oxygen species; TBARS, thiobarbituric acid-reactive substances; TAC, total anti-oxidant capacity; DCF, dichlorofluorescein.
MFA inhibits NOX4 and p22phox mRNA and protein expression in the livers of rats treated with CCl4
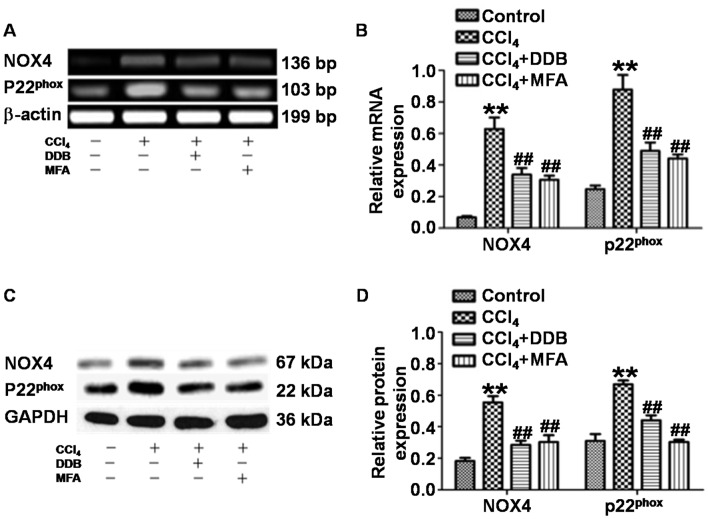
To assess whether MFA affects the generation of ROS by inhibiting NOX4 and p22phox in acute liver injury induced by CCl4, the present study determined the expression of NOX4 and p22phox in liver tissues. qPCR revealed that the levels of NOX4 and p22phox mRNA in the livers of CCl4-treated rats were significantly increased compared with those in the control group (P<0.01). By contrast, pre-treatment with DDB or MFA (100 mg/kg) decreased the expression of NOX4 and p22phox mRNA compared with that in rats treated with CCl4 only (P<0.01; Fig. 3A and B). Western blot analysis demonstrated significantly increased expression of NOX4 and p22phox protein in the liver of CCl4-treated rats compared with that in the control group (P<0.01). Of note, the protein expression of NOX4 and p22phox was significantly reduced by pre-treatment with DDB or MFA (100 mg/kg) compared with that in rats treated with CCl4 only (P<0.01; Fig. 3C and D). These results suggested that pre-treatment with MFA inhibited the mRNA and protein expression of NOX4 and p22phox in the livers of rats treated with CCl4.
Figure 3.
(A and B) mRNA expression and (C and D) protein expression of NOX4 and p22phox in liver tissues of rats. Semi-quantitative polymerase chain reaction and western blot analysis were used to measure mRNA and protein levels, respectively. **P<0.01 compared with control group; ##P<0.01 compared with CCl4-treated model group. MFA, methyl ferulic acid; DDB, dimethyl diphenyl bicarboxylate; NOX, NADPH oxidase.
MFA mitigates CCl4-induced pro-inflammatory responses by reducing the expression of TNF-α and IL-1β
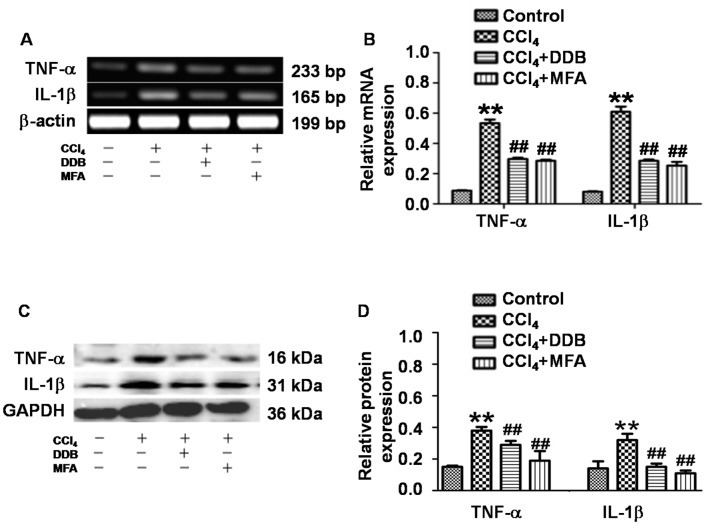
To determine the expression of TNF-α and IL-1β in liver tissues, qPCR and western blot analysis were employed. The results demonstrated that CCl4 treatment significantly increased the hepatic TNF-α and IL-1β mRNA and protein expression compared with that in the control group (P<0.01; Fig. 4A-D). Of note, pre-administration of DDB or MFA significantly suppressed the CCl4-induced mRNA and protein expression of hepatic TNF-α and IL-1β (P<0.01; Fig. 4A-D). These results indicated that pre-treatment with MFA prevented CCl4-induced pro-inflammatory responses by inhibiting the expression of TNF-α and IL-1β.
Figure 4.
Effect of MFA on CCl4-induced liver inflammation. (A and B) mRNA and (C and D) protein expression of TNF-α and IL-1β in liver tissues determined at 24 h after CCl4-induced acute liver injury. Semi-quantitative polymerase chain reaction and western blot analysis were used to measure mRNA and protein levels, respectively. **P<0.01 compared with control group; ##P<0.01 compared with CCl4-treated model group. MFA, methyl ferulic acid; DDB, dimethyl diphenyl bicarboxylate; TNF, tumor necrosis factor; IL, interleukin.
MFA inhibits CCl4-induced apoptosis in the livers of rats
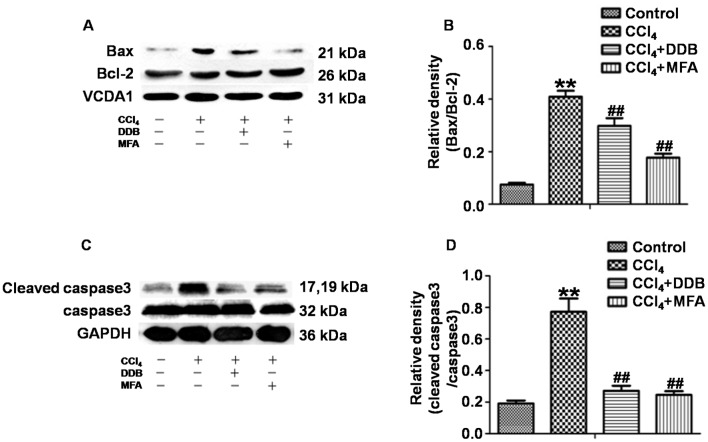
To investigate the effects of MFA on apoptosis induced by CCl4, western blot analysis was used to determine the ratio of Bax/Bcl-2 and the ratio of cleaved caspase3/caspase3. The results demonstrated that CCl4 treatment markedly increased the expression of Bax compared with that in the control group, while reducing the expression of Bcl-2, leading to a significantly increased Bax/Bcl-2 ratio. However, pre-treatment with MFA significantly decreased the CCl4-induced expression of the pro-apoptotic protein Bax and prominently decreased the Bax/Bcl-2 ratio as compared with that in the CCl4 treatment group (P<0.01; Fig. 5A and B). In addition, cleaved caspase3 levels in the livers of CCl4-treated rats were significantly elevated as compared with those in the controls (P<0.01). However, pre-treatment with MFA significantly inhibited this CCl4-induced elevation (P<0.01; Fig. 5C and D). These results suggested that MFA inhibits CCl4-induced apoptosis in the livers of rats.
Figure 5.
Effect of MFA on (A) Bcl-2, (B) Bax, (C) caspase3 and (D) cleaved caspase3 protein expression in rat livers following CCl4-induced acute injury. **P<0.01 compared with control group; ##P<0.01 compared with CCl4-treated model group. MFA, methyl ferulic acid; DDB, dimethyl diphenyl bicarboxylate; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein.
JNK and P38 MAPK activation is involved in the anti-apoptotic effect of MFA
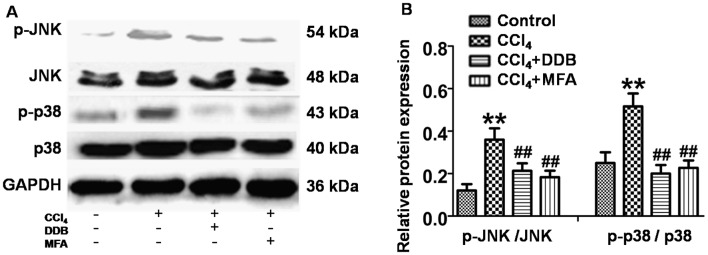
To investigate whether JNK and P38 MAPK signaling was involved in the mechanism of action of MFA, the present study investigated the effects of MFA on JNK and P38 MAPK in livers using western blot analysis. The results revealed that the levels of p-JNK and p-P38 MAPK were significantly increased in the livers of CCl4-treated rats compared with those in the controls (P<0.01; Fig. 6A and B). However, this upregulation of p-JNK and p-p38 MAPK was significantly suppressed by pre-treatment with MFA or DDP (P<0.01; Fig. 6A and B). These results indicated that JNK and P38 MAPK activation is involved in the anti-apoptotic effects of MFA.
Figure 6.
Involvement of JNK and p38 mitogen-activated protein kinase pathways in the anti-apoptotic effects of MFA. (A) The expression levels of JNK, p-JNK, p38 and p-p38 were analyzed by western blot analysis, using GAPDH as a reference. (B) The ratio of pJNK/JNK and p-p38/p38MAPK. **P<0.01 compared with control group; ##P<0.01 compared with CCl4-treated model group. MFA, methyl ferulic acid; DDB, dimethyl diphenyl bicarboxylate; p-JNK, phosphorylated c-Jun N-terminal kinase.
Discussion
During liver injury, liver cells exhibit varying degrees of swelling, degeneration, necrosis and apoptosis, which are the most basic pathological states of the development of various liver diseases. CCl4 has been widely used to generate models of hepatic injury for evaluating plant-based drugs for their hepatoprotective properties (3). It is well known that CCl4-induced liver damage involves the formation of free radicals (·CCl3) and the occurrence of lipid peroxidation in cellular and organelle membranes (13). After entering the body, CCl4 is metabolized by cytochrome P450 into free radicals (·CCl3), which are mainly associated with CCl4-induced hepatic damage. These free radicals react with oxygen to form trichloromethylperoxy radicals (CCl3OO·) and ROS, which trigger a chain reaction of lipid peroxidation, and attack and destroy polyunsaturated fatty acids, particularly those associated with phospholipids (27,28). All of this results in the breakdown of cell integrity and leakage of ALT and AST into the blood, leading to apoptosis and necrosis. Overall, oxidative stress, caused by the overproduction of ROS, is considered a vital risk factor in the development of liver disease. Numerous studies suggested that the levels of ROS and TBARS as well as the TAC may be indicators of oxidative stress (10,18). MFA is a monomer isolated from Securidaca inappendiculata Hasskarl with potent anti-viral activity (28). The present study investigated the hepatoprotective activity of MFA using a rat model of CCl4-induced acute liver damage, and DDB was used as a positive control drug (29). The results demonstrated that administration of MFA significantly inhibited CCl4-induced elevation of serum ALT and AST levels.
Oxidative stress has been postulated as an important molecular mechanism in acute liver injury induced by CCl4 (13,30). It was reported that the levels of MDA and GSH-Px are associated with CCl4-induced, oxidative stress-associated liver injury (29). MDA, the final product of lipid peroxidation, gradually accumulates during CCl4-induced liver injury and binds with biological macromolecules to form aldehydes, further destroying cell membrane structure and function (31). Increased MDA suggests enhanced peroxidation that results in tissue damage and failure of anti-oxidant defense mechanisms (32–34). The results of the present study indicated that increased MDA during CCl4-induced acute liver injury was prevented by pre-treatment with MFA.
GSH is a main intracellular anti-oxidant that exerts several main roles within a cell, including anti-oxidative effects, maintenance of the redox state, detoxification of xenobiotics and protection from damage by free radicals, toxins and peroxides (35–37). It is well known that the depletion of reduced GSH results in enhanced lipid peroxidation and superabundant lipid peroxidation may cause increased GSH consumption (38,39). Therefore, it is important to maintain sufficient GSH levels for the prevention of CCl4-induced damage. The results of the present study indicated that treatment with MFA markedly inhibited the formation of MDA and increased the level of GSH in the liver compared with that in the model group, suggesting that MFA increases the anti-oxidant capacity, clears free radicals and prevents cellular and organelle membranes from being damaged by free radicals. MFA contains phenolic hydroxyl and methoxy groups that directly or indirectly contribute to anti-oxidant action (32,39).
As an effective metalloenzyme, SOD catalyzes the dismutation of superoxide anions into hydrogen peroxide and O2 (35). GSH-Px catalyzes the reduction of toxic peroxide to a non-toxic hydroxyl compound as well as the reduction of H2O2 and hydroperoxides to water, removing lipid hydroperoxides from the cell membrane to thereby terminate the chain reaction of lipid peroxidation (32,35). The results of the present study suggested that CCl4 treatment lowers the activities of SOD and GSH-Px in the liver compared with those in the control group. In addition, administration of MFA led to significantly elevated activities of SOD and GSH-Px. Furthermore, MFA decreased ROS and TBARS production in CCl4-treated livers due to its powerful anti-oxidant and free radical scavenging activities. In CCl4-induced liver injury, GSH has an important role in detoxifying the toxic metabolites of CCl4, and once GSH is exhausted, hepatocelluar necrosis or apoptosis begin (40). In the present study, MFA exerted hepatoprotective effects by reducing CCl4-mediated oxidation of free radical species. In addition, MFA attenuated hepatic glutathione depletion after CCl4 treatment. In brief, the results of the present study confirmed that MFA effectively reduced oxidative stress and recovered anti-oxidant enzymes to their normal levels.
CCl4 has been reported to significantly elevate the concentration of hydrogen peroxide and the amount of lipid peroxidation in liver (41). Studies suggested that overproduction of ROS has an important role in the development and progression of CCl4-induced hepatic damage (42–45). In line with this, the present study also demonstrated the levels of ROS in CCl4-treated model group were significantly higher than those in the control group. Increased ROS generation stimulates pro-inflammatory cytokines and results in oxidative damage to macromolecules. In addition, pre-treatment with MFA significantly inhibited elevated CCl4-induced decreases of SOD activity and increased MDA levels in liver tissues of rats, suggesting that MFA exerts a hepatoprotective effect partly through efficiently eliminating excessive ROS in liver tissues. CCl4-induced ROS is generated mainly through NOX-mediated pathways and it was reported that NOX is a major source of ROS; furthermore, the NOX subunit NOX4 and its ligand p22phox are highly expressed in hepatocytes (30). NOX4 knock-out rats exhibited lower hepatic lipid peroxidation after CCl4 treatment compared with that in wild-type rats and NOX4 deficiency was effective in preventing liver injury in rats (46). The present study demonstrated that MFA treatment for seven days decreased the levels of ROS, NOX4 and its ligand p22phox. Overall, the protective effect of MFA against acute liver injury may be partly due to attenuating oxidative stress.
Apoptosis is a cell physiological self-extinction process controlled by multiple genes (47,48). The mitochondrial pathway is caused by a number of stress conditions, chemical agents and drugs, and controlled by numerous genes. Bax and Bcl-2 are important control factors (49), and caspase3 is the central effector of apoptosis. Cleaved caspase3 may be used as a reliable indicator to determine the severity of apoptosis (7,10,50). The present study demonstrated that MFA caused upregulation of Bcl-2 expression and downregulation of the expression of Bax and cleaved caspase3, leading to inhibition of apoptosis.
IL-1β and TNF-α are commonly considered as biomarkers of inflammatory conditions. Serum IL-1β is markedly increased during most inflammatory processes, and has been demonstrated to prevent hepatocyte proliferation (51). TNF-α, which has an important role in acute liver injury, is a mediator of hepatotoxicity (13). It activates intracellular pathways to regulate inflammation and proliferation, and has been identified as an attractive target for liver regeneration (13). TNF-α is also a pro-inflammatory mediator in hepatocyte apoptosis, which is tightly associated with cytotoxicity induced by CCl4 (13). In the present study, TNF-α and IL-1β levels in liver tissues were significantly increased by CCl4-induced hepatotoxicity, which was consistent with the findings of a previous study (32). By contrast, TNF-α and IL-1β levels in the MFA treatment group were lower than those in model group, suggesting an anti-inflammatory role of MFA to prevent acute liver injury.
The MAPK family is important for regulating cell proliferation and death in response to various internal stresses. JNKs are involved in stimulating apoptotic signaling. Oxidative stress may activate JNK to cause apoptosis by receptor-initiated extrinsic and mitochondrial intrinsic apoptotic pathways. JNKs also has an essential role in modulating the functions of pro- and anti-apoptotic proteins located in the mitochondria (12,52). JNK and ROS stimulate the activities of pro-apoptotic proteins such as Bax, and promote apoptosis by inhibiting anti-apoptotic proteins such as Bcl-2 to regulate the release of cytochrome C and apoptosis (10,52). The present study demonstrated that the levels of p-JNK, TNF-α and Bax were increased in the livers of CCl4-treated rats. Of note, pre-treatment with MFA significantly repressed the CCl4-induced increases of these proteins. Therefore, MFA exerted its protective effects on the liver by regulating JNK signaling.
CCl4 was previously reported to significantly increase the levels of p-p38 MAPK as a result of oxidative stress and certain cytokines, leading to the activation of p38 MAPK through its phosphorylation (15). The activation of the p38 MAPK pathway accelerates cell apoptosis (16,17). In the present study, the levels of p-p38/p38 MAPK ratio in the CCl4-treated model group were higher than those in the normal control group, which was consistent with the results of a previous study (53). The present study demonstrated that pre-treatment with MFA for seven days decreased the levels of p-p38/p38 MAPK ratio, as well as ROS levels. Therefore, the results demonstrated that during CCl4 challenge, ROS produced by CCl4 promoted the expression of p-p38 MAPK in the liver tissue, which in turn resulted in necrosis and liver cell apoptosis. However, MFA attenuated liver necrosis and cell apoptosis via reducing ROS production. The histopathological observations of the present study supported this notion.
p38 MAPK also causes mitochondria-dependent apoptosis. p38 MAPK activation promotes mitochondrial translocation of Bax and Bcl-2-like protein 11, while repressing the function of Bcl-2 by increasing the phosphorylation of p38 MAPK, and induces the activation of caspase3 (14). Therefore, it is concluded that p38 MAPK and Bcl-2/Bax signaling influence each other and cooperatively contribute to the protective effect of MFA on the acute liver injury induced by CCl4.
In summary, the present study demonstrated that MFA had strong protective effects against CCl4-induced acute oxidative liver injury and apoptosis by modulating JNK and p38 MAPK as well as Bcl-2/Bax signaling pathways in the liver. MFA alleviated CCl4-induced hepatic oxidative damage by inhibiting ROS generation and increasing liver TAC. It also effectively inhibited CCl4-induced inflammation and apoptosis in the liver by upregulating p-JNK, p-P38 MAPK, Bax, TNF-α and IL-1β, while downregulating Bcl-2 and cleaved caspase3. These results provided evidence that MFA may be used as a hepatoprotective agent for the treatment of liver diseases. However, further study is necessary to fully elucidate the molecular mechanisms that are responsible for the hepatoprotective effects of MFA.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant no. 81360497).
References
- 1.Stickel F, Schuppan D. Herbal medicine in the treatment of liver diseases. Dig Liver Dis. 2007;39:293–304. doi: 10.1016/j.dld.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Lal AA, Murthy PB, Pillai KS. Screening of hepatoprotective effect of a herbal mixture against CCl4 induced hepatotoxicity in Swiss albino mice. J Environ Biol. 2007;28:201–207. [PubMed] [Google Scholar]
- 3.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 4.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: New molecular links. Ann N Y Acad Sci. 2009;1155:206–221. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 6.Campo GM, Avenoso A, Campo S, Nastasi G, Traina P, D'Ascola A, Rugolo CA, Calatroni A. The antioxidant activity of chondroitin-4-sulphate, in carbon tetrachloride-induced acute hepatitis in mice, involves NF-kappaB and caspase activation. Br J Pharmacol. 2008;155:945–956. doi: 10.1038/bjp.2008.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang F, Wang X, Qiu X, Wang J, Fang H, Wang Z, Sun Y, Xia Z. The protective effect of Esculentoside A on experimental acute liver injury in mice. PLoS One. 2014;9:e113107. doi: 10.1371/journal.pone.0113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crosas-Molist E, Fabregat I. Role of NADPH oxidases in the redox biology of liver fibrosis. Redox Biol. 2015;6:106–111. doi: 10.1016/j.redox.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: An update. Arch Toxicol. 2015;89:867–882. doi: 10.1007/s00204-015-1472-2. [DOI] [PubMed] [Google Scholar]
- 10.Ma JQ, Ding J, Zhang L, Liu CM. Hepatoprotective properties of sesamin against CCl4 induced oxidative stress-mediated apoptosis in mice via JNK pathway. Food Chem Toxicol. 2014;64:41–48. doi: 10.1016/j.fct.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Xie J, Liu J, Chen TM, Lan Q, Zhang QY, Liu B, Dai D, Zhang WD, Hu LP, Zhu RZ. Dihydromyricetin alleviates carbon tetrachloride-induced acute liver injury via JNK-dependent mechanism in mice. World J Gastroenterol. 2015;21:5473–5481. doi: 10.3748/wjg.v21.i18.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013;87:1157–1180. doi: 10.1007/s00204-013-1034-4. [DOI] [PubMed] [Google Scholar]
- 13.Lu Y, Hu D, Ma S, Zhao X, Wang S, Wei G, Wang X, Wen A, Wang J. Protective effect of wedelolactone against CCl4-induced acute liver injury in mice. Int Immunopharmacol. 2016;34:44–52. doi: 10.1016/j.intimp.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Wang R, Wang Y, Peng R, Wu Y, Yuan Y. Ginkgo biloba extract mitigates liver fibrosis and apoptosis by regulating p38 MAPK, NF-κB/IκBα and Bcl-2/Bax signaling. Drug Des Devel Ther. 2015;9:6303–6317. doi: 10.2147/DDDT.S93732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bak J, Je NK, Chung HY, Yokozawa T, Yoon S, Moon JO. Oligonol ameliorates CCl4-induced liver injury in rats via the NF-Kappa B and MAPK signaling pathways. Oxid Med Cell Longev. 2016;2016:3935841. doi: 10.1155/2016/3935841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganai AA, Khan AA, Malik ZA, Farooqi H. Genistein modulates the expression of NF-κB and MAPK (P-38 and ERK1/2), thereby attenuating d-Galactosamine induced fulminant hepatic failure in Wistar rats. Toxicol Appl Pharmacol. 2015;283:139–146. doi: 10.1016/j.taap.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Xuan J, Wan L, Lin H, Couch L, Mei N, Dobrovolsky VN, Guo L. Sertraline, an antidepressant, induces apoptosis in hepatic cells through the mitogen-activated protein kinase pathway. Toxicol Sci. 2014;137:404–415. doi: 10.1093/toxsci/kft254. [DOI] [PubMed] [Google Scholar]
- 18.Ma JQ, Ding J, Zhang L, Liu CM. Ursolic acid protects mouse liver against CCl4-induced oxidative stress and inflammation by the MAPK/NF-κB pathway. Environ Toxicol Pharmacol. 2014;37:975–983. doi: 10.1016/j.etap.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu CM, Zheng GH, Ming QL, Chao C, Sun JM. Sesamin protects mouse liver against nickel-induced oxidative DNA damage and apoptosis by the PI3K-Akt pathway. J Agric Food Chem. 2013;61:1146–1154. doi: 10.1021/jf304562b. [DOI] [PubMed] [Google Scholar]
- 21.Zheng M. Inhibitory effect of 400 kinds of Chinese herbal medicine on HBsAg. Chin J Integr Trad Western Med Liver Dis. 1991;1:34. (In Chinese) [Google Scholar]
- 22.Qin Q, Yang X, Li Y, Li L, Li Y, Rong M. Isolation and identification of extracting methyl ferulic acid in cane peel onion. Asia Pac Trad Med. 2014;10:20–21. [Google Scholar]
- 23.Li L, Li Y, Tang A. The inhibitory effect of methyl ferulic acid on HBsAg and HBeAg in HepG2.2.15 cell. Pharmacol Clin Chin Mater Med. 2011;27:14–16. (In Chinese) [Google Scholar]
- 24.Li C, Li L, Yang CF, Zhong YJ, Wu D, Shi L, Chen L, LI YW. Hepatoprotective effects of Methyl ferulic acid on alcohol-induced liver oxidative injury in mice by inhibiting the NOX4/ROS-MAPK pathway. Biochem Biophys Res Commun. 2017;493:277–285. doi: 10.1016/j.bbrc.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Sun JJ, Chen GY, Wang WW, Xie ZT, Tang GF, Wei SD. Carnosic acid nanoparticles suppress liver ischemia/reperfusion injury by inhibition of ROS, Caspases and NF-κB signaling pathway in mice. Biomed Pharmacother. 2016;82:237–246. doi: 10.1016/j.biopha.2016.04.064. [DOI] [PubMed] [Google Scholar]
- 27.Jain A, Soni M, Deb L, Jain A, Rout SP, Gupta VB, Krishna KL. Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of Momordica dioica Roxb. leaves. J Ethnopharmacol. 2008;115:61–66. doi: 10.1016/j.jep.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Li Y, Tang A, Li M, Zhong Q. The anti-inflammatory and immunopotentiation effect of chloroform extracts isolated from Securidaca inappendiculata Hassk. Pharmacol Clin Chin Mater Med. 2011;27:62–64. (In Chinese) [Google Scholar]
- 29.Abdel-Hameid NA. Protective role of dimethyl diphenyl bicarboxylate (DDB) against erythromycin induced hepatotoxicity in male rats. Toxicol In Vitro. 2007;21:618–625. doi: 10.1016/j.tiv.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Roy S, Benz F, Alder J, Bantel H, Janssen J, Vucur M, Gautheron J, Schneider A, Schüller F, Loosen S, et al. Down-regulation of miR-192-5p protects from oxidative stress-induced acute liver injury. Clin Sci (Lond) 2016;130:1197–1207. doi: 10.1042/CS20160216. [DOI] [PubMed] [Google Scholar]
- 31.Ismail AF, Salem AA, Eassawy MM. Hepatoprotective effect of grape seed oil against carbon tetrachloride induced oxidative stress in liver of γ-irradiated rat. J Photochem Photobiol B. 2016;160:1–10. doi: 10.1016/j.jphotobiol.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 32.Cheng N, Ren N, Gao H, Lei X, Zheng J, Cao W. Antioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4-induced acute liver damage in mice. Food Chem Toxicol. 2013;55:234–240. doi: 10.1016/j.fct.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 33.NAIK SR, Panda VS. Antioxidants and their role in biological functions: An overview. Indian Drugs. 2007;27:393–399. [Google Scholar]
- 34.Pareek A, Godavarthi A, Issarani R, Nagori BP. Antioxidant and hepatoprotective activity of Fagonia schweinfurthii (Hadidi) Hadidi extract in carbon tetrachloride induced hepatotoxicity in HepG2 cell line and rats. J Ethnopharmacol. 2013;150:973–981. doi: 10.1016/j.jep.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 35.Ai G, Liu Q, Hua W, Huang Z, Wang D. Hepatoprotective evaluation of the total flavonoids extracted from flowers of Abelmoschus manihot (L.) Medic: In vitro and in vivo studies. J Ethnopharmacol. 2013;146:794–802. doi: 10.1016/j.jep.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Yuan L, Kaplowitz N. Glutathione in liver diseases and hepatotoxicity. Mol Aspects Med. 2009;30:29–41. doi: 10.1016/j.mam.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–155. doi: 10.1016/S0753-3322(03)00043-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong Y, Huang J, Lin X, Zhang S, Jiao Y, Liang T, Chen Z, Huang R. Hepatoprotective effects of Yulangsan polysaccharide against isoniazid and rifampicin-induced liver injury in mice. J Ethnopharmacol. 2014;152:201–206. doi: 10.1016/j.jep.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Onyema OO, Farombi EO, Emerole GO, Ukoha AI, Onyeze GO. Effect of vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats. Indian J Biochem Biophys. 2006;43:20–24. [PubMed] [Google Scholar]
- 40.Deng JS, Chang YC, Wen CL, Liao JC, Hou WC, Amagaya S, Huang SS, Huang GJ. Hepatoprotective effect of the ethanol extract of Vitis thunbergii on carbon tetrachloride-induced acute hepatotoxicity in rats through anti-oxidative activities. J Ethnopharmacol. 2012;142:795–803. doi: 10.1016/j.jep.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Xiang M, Wang J, Zhang Y, Ling J, Xu X. Attenuation of aortic injury by ursolic acid through RAGE-Nox-NFκB pathway in streptozocin-induced diabetic rats. Arch Pharm Res. 2012;35:877–886. doi: 10.1007/s12272-012-0513-0. [DOI] [PubMed] [Google Scholar]
- 42.Ranawat L, Bhatt J, Patel J. Hepatoprotective activity of ethanolic extracts of bark of Zanthoxylum armatum DC in CCl4 induced hepatic damage in rats. J Ethnopharmacol. 2010;127:777–780. doi: 10.1016/j.jep.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 43.Wu D, Zhai Q, Shi X. Alcohol-induced oxidative stress and cell responses. J Gastroenterol Hepatol. 2006;21(Suppl 3):S26–S29. doi: 10.1111/j.1440-1746.2006.04589.x. [DOI] [PubMed] [Google Scholar]
- 44.Stehbens WE. Oxidative stress, toxic hepatitis, and antioxidants with particular emphasis on zinc. Exp Mol Pathol. 2003;75:265–276. doi: 10.1016/S0014-4800(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Pan Y, Kan M, Xiao X, Wang Y, Guan F, Zhang X, Chen L. Hepatoprotective effects of berberine on liver fibrosis via activation of AMP-activated protein kinase. Life Sci. 2014;98:24–30. doi: 10.1016/j.lfs.2013.12.211. [DOI] [PubMed] [Google Scholar]
- 46.Lan T, Kisseleva T, Brenner DA. Deficiency of NOX1 or NOX4 prevents liver inflammation and fibrosis in mice through inhibition of hepatic stellate cell activation. PLoS One. 2015;10:e0129743. doi: 10.1371/journal.pone.0129743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Green DR, Fitzgerald P. Just So stories about the evolution of apoptosis. Curr Biol. 2016;26:R620–R627. doi: 10.1016/j.cub.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savitskaya MA, Onishchenko GE. Mechanisms of apoptosis. Biochemistry (Mosc) 2015;80:1393–1405. doi: 10.1134/S0006297915110012. [DOI] [PubMed] [Google Scholar]
- 49.Lindsay J, Esposti MD, Gilmore AP. Bcl-2 proteins and mitochondria-specificity in membrane targeting for death. Biochim Biophys Acta. 2011;1813:532–539. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W, Yin L, Tao X, Xu L, Zheng L, Han X, Xu Y, Wang C, Peng J. Dioscin alleviates dimethylnitrosamine-induced acute liver injury through regulating apoptosis, oxidative stress and inflammation. Environ Toxicol Pharmacol. 2016;45:193–201. doi: 10.1016/j.etap.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Tien YC, Liao JC, Chiu CS, Huang TH, Huang CY, Chang WT, Peng WH. Esculetin ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. Int J Mol Sci. 2011;12:4053–4067. doi: 10.3390/ijms12064053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim HY, Park J, Lee KH, Lee DU, Kwak JH, Kim YS, Lee SM. Ferulic acid protects against carbon tetrachloride-induced liver injury in mice. Toxicology. 2011;282:104–111. doi: 10.1016/j.tox.2011.01.017. [DOI] [PubMed] [Google Scholar]