Abstract
Testicular cell apoptosis is associated with impaired spermatogenesis. It has been reported that Asiatic acid (AA) may suppress apoptosis. However, little is known about the effect of AA on high-fat diet (HFD)-induced impairment of spermatogenesis. The aim of the present study was to determine whether AA protects against HFD-induced impairment of spermatogenesis. Sprague-Dawley rats were randomly divided into three groups: Control group, HFD group and AA (50 mg/kg) + HFD group. Rats fed an HFD were orally administered with AA (50 mg/kg) daily for 12 weeks, and blood samples, testis and epididymis were harvested for further analysis. Sex hormones were detected and hematoxylin and eosin staining was performed to examine the morphological changes of the testis. Semen samples were collected to evaluate sperm quality and apoptosis was determined. The results indicate that AA treatment significantly increased testis weight, testis/body weight, spermatogonia, Leydig cells and Sertoli cells in the testis of obese mice (P<0.05). AA treatment also attenuated HFD-induced histological change. AA treatment prevented HFD-induced decrease of sex hormones and the quality of semen samples (P<0.05). Furthermore, HFD-induced apoptosis was significantly attenuated by AA treatment (P<0.05). In conclusion, the results suggest that AA is able to ameliorate HFD-induced impaired spermatogenesis via inhibiting apoptosis in Sprague-Dawley rats. AA may have therapeutic value in the treatment of obesity-related impairment of spermatogenesis.
Keywords: asiatic acid, high-fat diet, spermatogenesis, apoptosis, testis
Introduction
Infertility is defined as the inability to conceive following >1 year of regular unprotected sexual intercourse and now affects 10–15% of couples of reproductive age (1,2). Defective sperm function is a common contributing factor, accounting for 30–40% of couples attending infertility clinics (3). Obesity, which is the sixth most important risk factor contributing to the overall burden of infertility worldwide (4), has been reported to impair male infertility (5,6). Accumulating evidence suggests that male infertility is regulated by an orchestrated network comprising numerous pathological changes (7,8). The mechanisms underlying obesity-associated infertility are complex and remain unclear; however, previous studies have indicated that excessive cell apoptosis serves an important role (7,9–11).
Apoptosis is essential for cellular homeostasis and male germ cell development (12,13). Increased apoptosis has been observed in the spermatozoa of male patients with infertility, as well as in the sperm of infertile mice (14,15). High-fat diet (HFD) has been demonstrated to induce apoptosis in rodents, which in turn promotes the progression of infertility (16). Conversely, inhibiting excessive apoptosis attenuates HFD-induced impairment of spermatogenesis (17). Therefore, pharmacological agents that are able to inhibit testicular cell apoptosis are of great therapeutic interest.
Asiatic acid (AA), which is a pentacyclic triterpene isolated from Centella asiatica, has been demonstrated to possess a number of pharmacological activities (18). AA is able to protect against cardiac hypertrophy (19), reduce islet fibrosis in animal models of diabetes (20) and ameliorate hepatic lipid accumulation (21). Furthermore, previous studies have indicated that AA attenuates glutamate-induced apoptosis in SH-SY5Y cells (22) and inhibits apoptosis in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice (23). However, the effect of AA on obesity-induced impaired spermatogenesis has not yet been reported. The aim of the present study was to investigate whether AA is able to protect against HFD-induced defective spermatogenesis function.
Materials and methods
Reagents
AA (purity, 97%; cat. no. 546712) was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Rabbit anti-B-cell lymphoma (Bcl)-xl antibody (cat. no. ab32370), rabbit anti-Fas antibody (cat. no. ab82419, and anti-GAPDH antibody (cat. no. ab8245) were obtained from Abcam (Cambridge, UK). Anti-Bcl-2 antibody (cat. no. 2870) and anti-Bcl-2-associated X protein (Bax) antibody (cat. no. 2722) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). TUNEL kits (cat. no. 11684817910) were purchased from Roche Applied Science (Penzberg, Germany).
Animal treatment
All experiments in the present study were performed in compliance with National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committees of Puai Hospital of Huazhong University of Science and Technology (Wuhan, China). A total of 24 adult male Sprague-Dawley rats (180–200 g, 8–9 weeks) were obtained from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences (Beijing, China). All rats were housed with a 12-h light/dark cycle at 20–25°C and 50±5% humidity, with ad libitum access to food and water. Rats were randomly divided into three groups: Control group (n=8), HFD group (n=8) and the AA + HFD (n=8) group. Rats in the control group were fed with a normal diet, whereas the other rats were fed with an HFD (protein, 18.1%; fat, 61.6%; carbohydrates, 20.3%) for 12 weeks to induce obesity. AA was dissolved in 1% CMC-Na as a vehicle for in vivo experiments. Rats in the AA + HFD group were orally administered with 50 mg/kg AA once per day for 12 weeks, and the other two groups received the same volume of vehicle as control. At the end of the study period, rats were euthanized with an overdose of sodium pentobarbital (200 mg/kg, Sigma-Aldrich; Merck KGaA) and blood samples were harvested from the abdominal aorta for further analysis. Finally, rats were sacrificed via cervical dislocation and testes and adipose tissues were harvested.
Histological analysis
Testis samples were fixed in 4% paraformaldehyde at room temperature for 24 h, dehydrated and embedded in paraffin. Tissues were cut into 5-µm sections for further analysis. The sections were stained with hematoxylin and eosin at room temperature and observed under a light microscope (magnification, ×400; E100; Nikon Corporation, Tokyo, Japan). In each group, 30 fields in 6 rats were randomly selected and the number of spermatogonia, Leydig cells and Sertoli cells were calculated using Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Blood pressure
Prior to sacrifice, rats were anesthetized using 0.5% isoflurane (Sigma-Aldrich; Merck KGaA), and a microtip catheter transducer (SPR-839; Millar, Inc., Houston, TX) was inserted into the right carotid artery and left ventricle to detect the systolic blood pressure according to the manufacturers protocol.
Hormone detection
Serum was collected from the tail vein of animals and fasting insulin was determined using a rat insulin ELISA Kit (cat. no. EZRMI-13K, EMD Millipore, Billerica, MA, USA) 3 days prior to sacrifice. Sex hormones were detected using kits for estradiol (E2; cat. no. E-EL-R0065c), testosterone (T; cat. no. E-EL-R0072c), follicle stimulating hormone (FSH; cat. no. E-EL-R0391c) and luteinizing hormone (LH; cat. no. E-EL-R0026c) purchased from Elabscience Biotechnology Co., Ltd. (Wuhan, China) according to the manufacturers protocol. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as previously described (24).
Semen analysis
Isolated epididymides were immediately placed in Ringers solution (Wuhan Servicebio Technology Co., Ltd., Wuhan, China) and cut into pieces. The concentration, viability and motility of sperm were determined as previously described (25). The sperm gradually left the epididymis and semen samples were carefully collected. The number of sperm was counted using a hemocytometer (AMQAX1000, Thermo Fisher Scientific, Inc., Waltham, MA, USA) and the concentrations were calculated according to the manufacturers protocol. Eosin-nigrosin staining solution was used to determine sperm viability at room temperature for 5 min, and light microscopy (magnification, ×400) was used to observe the spermatozoa. Using this staining live spermatozoa are white in color, whereas dead spermatozoa are pink or red (26). Sperm motility was detected by computer-assisted sperm analysis (CASA). Sperm was incubated in Ringers solution at room temperature for 30 min and subsequently placed in CASA assay chambers (Hamilton Thorne Research, Beverly, MA, USA). Sperm tracks (1.5 sec, 30 frames) were captured (frequency, 60 Hz) and further analyzed by HTM-IVOS Sperm Analyzer software (version 12.2L; Hamilton Thorne Research) (27).
Immunohistochemistry and TUNEL staining
Immunohistochemistry was performed to detect the expression of Fas and Bcl-xl. Testis tissue sections were deparaffinized and boiled in sodium citrate buffer (pH=7.0, 5 min, MXB Biotechnologies, Fuzhou, China) for antigen retrieval after rehydration in a descending alcohol series. Sections were subsequently incubated with primary antibodies (anti-Fas, 1:1,000; anti-Bcl-xl, 1:500) at 4°C overnight after eliminating the internal peroxidase activity using 3% hydrogen peroxide incubation at room temperature for 20 min. Sections were subsequently incubated with the secondary antibody (EnVision™+/HRP reagent; 1:100; cat. no. GK500610A, Gene Technology Co., Ltd., Shanghai, China) at 37°C for 30 min. Sections were incubated with diaminobenzidine at room temperature for 2 min and observed under a light microscope (magnification, ×100 and ×400; E100; Nikon Corporation). A total of 30 fields were randomly selected in 6 rats from each group and the expression of Fas and Bcl-xl were identified using integrated optical density. Apoptosis was detected using the TUNEL kit according to the manufacturers protocol. A total of 100 cells were randomly selected in each group and the number of positive cells was calculated manually.
Western blot analysis and reverse transcription-quantitative polymerase chain analysis (RT-qPCR)
Total proteins from fresh testis tissues were isolated using radioimmunoprecipitation assay lysis buffer (Wuhan Servicebio Technology Co., Ltd.). Protein concentrations were determined using a bicinchoninic acid assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.). Proteins (50 µg) were separated by 10% SDS-PAGE and transferred to a polyvinylidene fluoride membrane. The membrane was blocked with 5% non-fat milk at room temperature for 1 h, and subsequently incubated overnight at 4°C with the following primary antibodies: Anti-GAPDH antibody, anti-Bax antibody and anti-Bcl-2 antibody (all 1:1,000). The membrane was subsequently incubated with IRDye 800CW-conjugated secondary antibody (1;10,000, cat. no. LI 926-32211; LI-COR Biosciences, Lincoln, NE, USA) at room temperature for 1 h. Finally, the membrane was scanned using a two-color infrared imaging system (Odyssey; LI-COR Biosciences) and protein expression levels were normalized to GAPDH.
RNA was isolated from tissues using an RNeasy mini kit (Qiagen AB, Sollentuna, Sweden) and RT-qPCR was performed with a Bio-Rad iCycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using PrimeScriptTM RT reagent kit with gDNA Eraser (RR047A, Takara Bio, Inc., Otsu, Japan). SYBR Premix Ex TaqTM II was obtained from Takara Bio, Inc. (DRR820A). The temperature protocol for reverse transcription was: 37°C for 15 min, 85°C for 5 sec. The thermocycling conditions for PCR were: Initial denaturation at 95°C for 30 sec; 40 cycles of 95°C for 5 sec and 60°C for 45 sec; dissociation at 95°C for 15 sec and 60°C for 30 sec. The primers used were as follows: Bax, forward 5′-ATC CAG GAT CGA GCA GGG AGG ATG G-3 and reverse, 5′-TGC CCG CCT ACT TCA ACG A-3; Bcl-2, forward 5′-CTT CCA GCC TGA GAG CAA CC-3 and reverse 5′-CAT CCC AGC CTC CGT TAT CC-3; GAP DH, forward 5′GAC ATG CCG CCT GGA GAA AC-3 and reverse 5′AGCC CAG GAT GCC CTT TAG T-3. Relative mRNA expression levels were analyzed using the 2−∆∆Cq method (28). The mRNA levels were normalized to GAPDH.
Data analysis
Results in each group are expressed as the mean + standard deviation. All statistical tests were conducted using SPSS 19.0 (IBM Corp., Armonk, NY, USA). Multiple group comparisons were made using one-way ANOVA followed by a post hoc Tukeys test. P<0.05 was considered to indicate a statistically significant difference.
Results
AA improves cardiometabolic profile in rats subjected to HFD
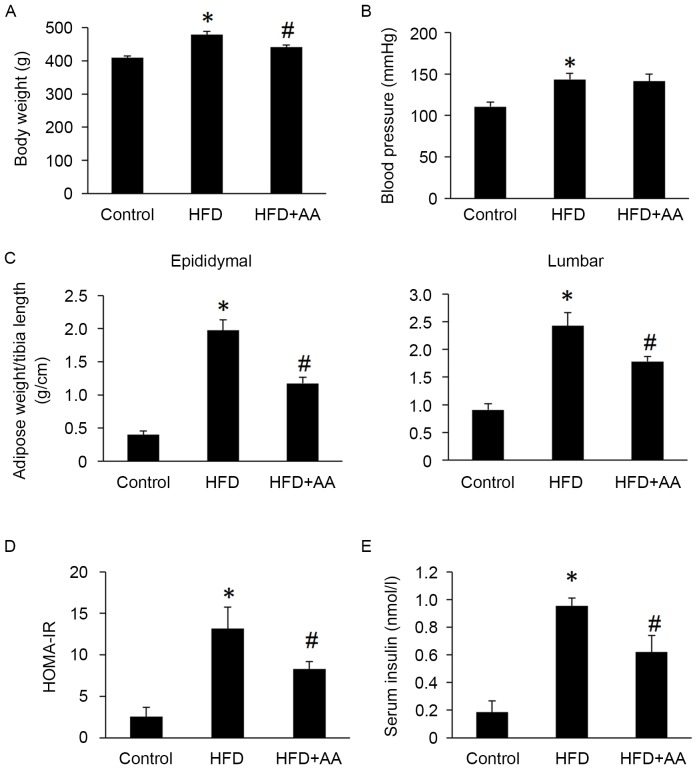
Body weight, HOMA-IR, serum insulin, epididymal and lumbar adipose tissues weights were all significantly increased in rats with HFD compared with control rats (P<0.05; Fig. 1). However, AA treatment significantly attenuated these HFD-induced increases (P<0.05; Fig. 1). Rats in the HFD also had significantly increased systolic blood pressure compared with the control group (P<0.05); however, no significant difference was observed between the HFD and AA + HFD group (Fig. 1).
Figure 1.
AA improves the cardiometabolic profile in rats fed with an HFD (n=8). (A) Body weight, (B) blood pressure, (C) epididymal and lumbar adipose weight/tibia length, (D) HOMA-IR and (E) serum insulin levels. *P<0.05 vs. control and #P<0.05 vs. HFD. AA, Asiatic acid; HFD, high fat diet; HOMA-IR, homeostasis model assessment of insulin resistance.
AA treatment improves pathological changes of testes induced by HFD
Following 12 weeks of HFD, rats in the HFD group had significantly decreased testis weight and testis weight/body weight compared with the control group (P<0.05; Table I), which was also confirmed by histological analysis (Fig. 2). Atrophic seminiferous tubules with smaller diameters were also observed in the testis of rats with HFD (Fig. 2). HFD also resulted in a significant reduction in spermatogonia, Leydig cells and Sertoli cells compared with the control group (P<0.05; Table I). Sperm concentration, sperm viability and motility were significantly decreased in rats subjected to an HFD diet compared with control rats (P<0.05; Table I). AA treatment significantly attenuated the decreased testis weight, testis/body weight, spermatogonia, Leydig cells and Sertoli cells (P<0.05; Table I), and markedly improved the HFD-induced atrophy of seminiferous tubules (Fig. 2).
Table I.
Effects of AA on testis weight, germ cell count and sperm quality.
| Parameter | Control | HFD | AA + HFD |
|---|---|---|---|
| Testis weight (g) | 3.32±0.13 | 2.64±0.09a | 2.86±0.09b |
| Testis weight/body weight (g/kg) | 8.11±0.39 | 5.52±0.14a | 6.54±0.21b |
| Spermatogonia (number/field) | 25.26±2.18 | 15.52±1.79a | 23.10±1.39b |
| Leydig cells (number/field) | 8.21±0.15 | 4.53±0.33a | 5.21±0.08b |
| Sertoli cells (number/field) | 9.21±0.14 | 5.34±0.24a | 7.16±0.13b |
| Sperm concentration (×106/ml) | 57.61±4.21 | 45.36±3.17a | 53.11±2.54b |
| Sperm viability (%) | 93.39±2.65 | 85.31±1.70a | 92.02±1.33b |
| Sperm motility (%) | 75.07±1.60 | 66.28±1.88a | 71.10±1.48b |
Data are expressed as the mean ± standard deviation.
P<0.05 vs. control
P<0.05 vs. HFD. AA, Asiatic acid; HFD, high fat diet.
Figure 2.
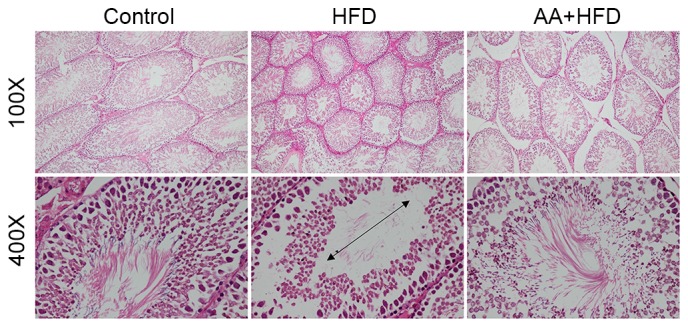
Hematoxylin and eosin staining of testis tissue in rats fed with an HFD (n=6). Arrowheads indicates the diameter of seminiferous tubules. HFD, high fat diet; AA, Asiatic acid.
AA treatment attenuates the HFD-induced abnormal serum sexual hormone levels
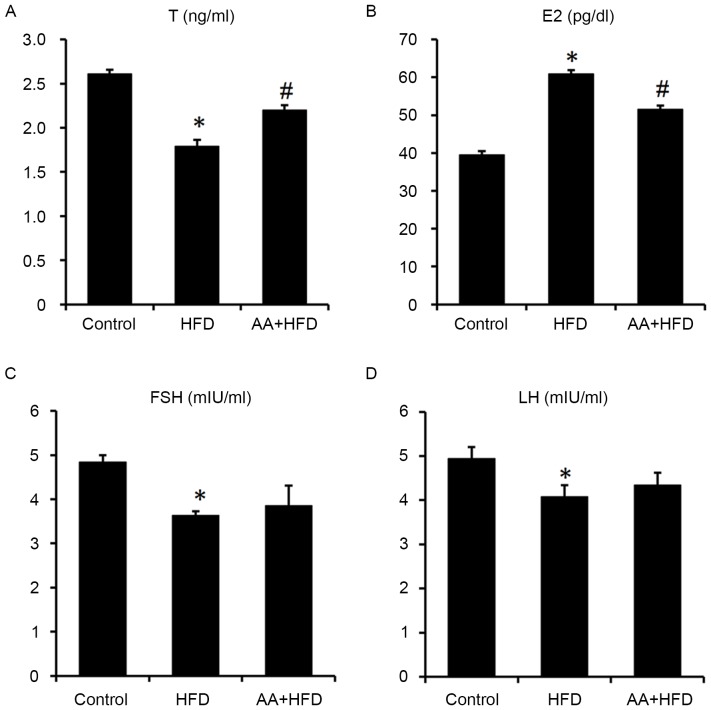
Serum E2 levels were significantly increased and T levels were significantly decreased in the HFD compared with the control group (P<0.05; Fig. 3A and B). However, AA treatment for 12 weeks significantly ameliorated the HFD-induced abnormal serum sexual hormone levels (Fig. 3A and B). Serum FSH and LH were both significantly reduced in HFD rats compared with the control group (P<0.05), whereas no significant difference was observed between the HFD and AA + HFD groups (Fig. 3C and D).
Figure 3.
AA treatment improves abnormal serum hormone levels of (A) T, (B) E2, (C) FSH and (D) LH induced by HFD (n=8). *P<0.05 vs. control and #P<0.05 vs. HFD. AA, Asiatic acid; T, testosterone; E2, estradiol; FSH, follicle stimulating hormone; LH, luteinizing hormone; HFD, high fat diet.
AA suppresses apoptosis in the testis of rats fed with HFD
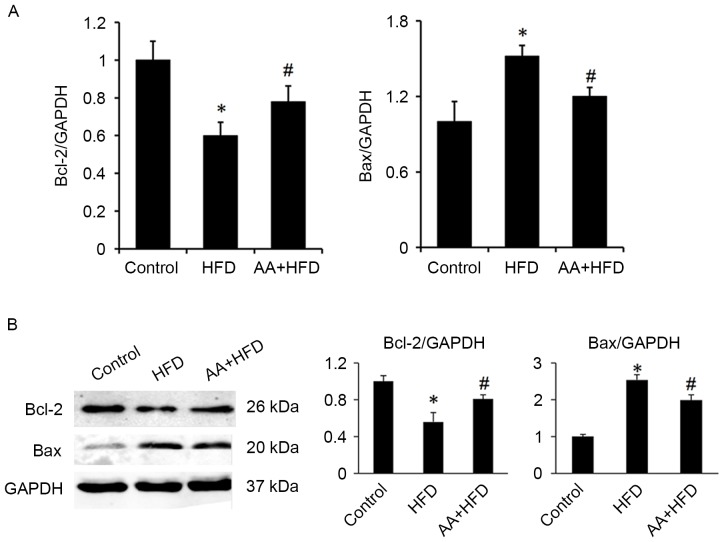
Immunohistological analysis revealed that the protein level of Bcl-xl was significantly decreased in the HFD rats compared with the control group (P<0.05) and that AA treatment significantly ameliorated this effect (P<0.05; Fig. 4). Fas was also significantly upregulated in the HFD group compared with the control rats (P<0.05) and this effect was significantly ameliorated with AA treatment (P<0.05; Fig. 4). TUNEL analysis was used to investigate the apoptotic rate of germ cells in the testis. The results revealed that rats in the HFD group had a significantly higher rate of apoptosis compared with the control rats (P<0.05), and that AA significantly inhibited HFD-induced testicular cell apoptosis (P<0.05; Fig. 5). These results were corroborated by subsequent analysis of mRNA and protein levels, which indicated that HFD induced a significant downregulation in Bcl-2 and a significant increase in Bax expression compared with the control group (P<0.05; Fig. 6). Treatment with AA, however, significantly ameliorated these effects, inducing a significant increase in Bcl-2 and decrease in Bax compared with the HFD group (P<0.05; Fig. 6).
Figure 4.
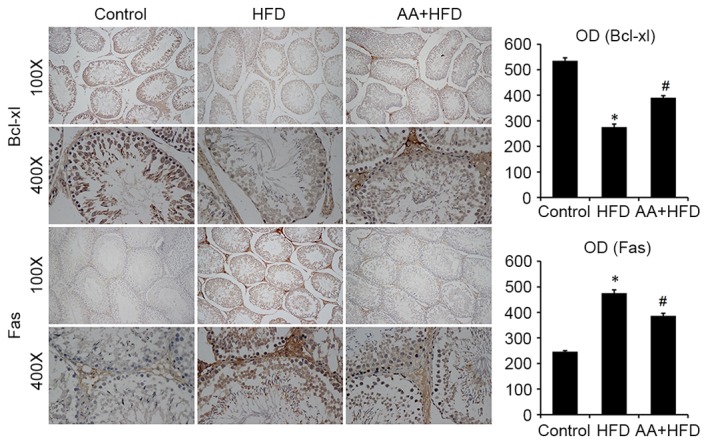
Immunohistochemistry revealed that AA increases the expression of Bcl-xl and decreases the expression of Fas compared with the HFD group (n=8). *P<0.05 vs. control and #P<0.05 vs. HFD. AA, Asiatic acid; Bcl-xl, B-cell lymphoma extra large; HFD, high fat diet; OD, optical density.
Figure 5.
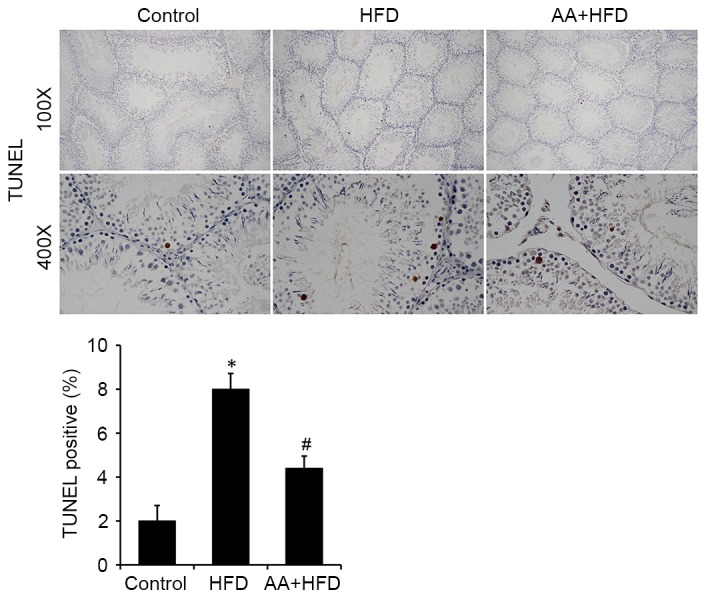
AA treatment reduces HFD-induced apoptosis as detected by TUNEL staining (n=8). *P<0.05 vs. control and #P<0.05 vs. HFD. AA, Asiatic acid; HFD, high fat diet.
Figure 6.
AA increases levels of Bcl-2 and decreases levels of Bax in rats fed with an HFD (n=6). (A) mRNA and (B) protein levels of Bax and Bcl-2. *P<0.05 vs. control and #P<0.05 vs. HFD. AA, Asiatic acid; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; HFD, high fat diet.
Discussion
The number of worldwide overweight individuals has grown rapidly, resulting in an escalation of obesity-associated health problems including infertility (29). As a result, there is a greater need to develop pharmacological agents for and to explore the novel and specific regulators of obesity-associated infertility. The results of the present study indicate that AA may attenuate HFD-induced impaired spermatogenesis. AA was also demonstrated to ameliorate endocrine disorders and suppress HFD-induced testicular cell apoptosis.
Endocrine disorders are key features of spermatogenesis dysfunction (30). T is able to promote spermatogenesis via intracellular signaling pathways (31). A recent study indicated that metformin-induced T level increases were able to improve reproductive function in obese male rats (17). Consistent with this, the present study demonstrated that AA upregulates the level of T and reduces the level of E2, which suggests that improved sex hormone levels may contribute to the protective effects of AA. AA had no significant effect on FSH and LH levels, which indicates that it does not affect pituitary hormones.
It is known that spermatogenesis is a complex process that relies on coordinated cell proliferation and apoptosis (32). Excessive cell apoptosis is reported to be a prevalent phenomenon in defective spermatogenesis (33); therefore, inhibiting excessive cell apoptosis and reconstructing the balance between cell proliferation and apoptosis may be an effective treatment for defective spermatogenesis. In view of the antiapoptotic properties of AA (22,23), it was hypothesized that AA may suppress HFD-induced apoptosis in the testes. The results of the present study revealed that AA significantly inhibits testicular cell apoptosis, which suggests that apoptosis may be one of the underlying mechanisms by which AA protects against HFD-induced defective spermatogenesis. Conversely, it has previously been reported that AA induces tumor cell apoptosis (34,35). The reason for these incompatible results may be that apoptosis serves different roles in different pathological processes.
The precise mechanisms that mediate the antiapoptotic effects of AA remain to be elucidated. A recent study indicated that AA is able to activate AMP-activated protein kinase α (19), which has been demonstrated to be a negative regulator of apoptosis (36,37). AA has also been reported to suppress inflammation and oxidative injury in human bronchial epithelial cells (38), which is associated with apoptosis. Further study is required to determine the precise mechanisms underlying the protective effects of AA.
In conclusion, the results of the present study demonstrated that AA is able to attenuate HFD-induced spermatogenesis dysfunction via inhibiting excessive apoptosis. These findings provide theoretical evidence for the use of AA as a treatment for obesity-associated infertility.
References
- 1.Botelho F, Figueiredo L, Leite R, Carvalho A, Tomada N, Vendeira P. Predictive factors of a successful testicular biopsy and subsequent clinical pregnancy. Andrologia. 2012;44:237–242. doi: 10.1111/j.1439-0272.2012.01276.x. [DOI] [PubMed] [Google Scholar]
- 2.Ferlin A, Arredi B, Foresta C. Genetic causes of male infertility. Reprod Toxicol. 2006;22:133–141. doi: 10.1016/j.reprotox.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Adamson GD, Baker VL. Subfertility: Causes, treatment and outcome. Best Pract Res Clin Obstet Gynaecol. 2003;17:169–185. doi: 10.1016/S1521-6934(02)00146-3. [DOI] [PubMed] [Google Scholar]
- 4.Barnett R. Obesity. Lancet. 2005;366:984. doi: 10.1016/S0140-6736(05)67376-X. [DOI] [PubMed] [Google Scholar]
- 5.Katib A. Mechanisms linking obesity to male infertility. Cent European J Urol. 2015;68:79–85. doi: 10.5173/ceju.2015.01.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammoud AO, Meikle AW, Reis LO, Gibson M, Peterson CM, Carrell DT. Obesity and male infertility: A practical approach. Semin Reprod Med. 2012;30:486–495. doi: 10.1055/s-0032-1328877. [DOI] [PubMed] [Google Scholar]
- 7.Bellver J, Melo MA, Bosch E, Serra V, Remohi J, Pellicer A. Obesity and poor reproductive outcome: The potential role of the endometrium. Fertil Steril. 2007;88:446–451. doi: 10.1016/j.fertnstert.2006.11.162. [DOI] [PubMed] [Google Scholar]
- 8.Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW. Male obesity and alteration in sperm parameters. Fertil Steril. 2008;90:2222–2225. doi: 10.1016/j.fertnstert.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology. 1997;138:2081–2088. doi: 10.1210/endo.138.5.5110. [DOI] [PubMed] [Google Scholar]
- 10.Sinha HA, Swerdloff RS. Hormonal and genetic control of germ cell apoptosis in the testis. Rev Reprod. 1999;4:38–47. doi: 10.1530/ror.0.0040038. [DOI] [PubMed] [Google Scholar]
- 11.Tapanainen JS, Tilly JL, Vihko KK, Hsueh AJ. Hormonal control of apoptotic cell death in the testis: Gonadotropins and androgens as testicular cell survival factors. Mol Endocrinol. 1993;7:643–650. doi: 10.1210/me.7.5.643. [DOI] [PubMed] [Google Scholar]
- 12.Aitken RJ, Baker MA. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int J Dev Biol. 2013;57:265–272. doi: 10.1387/ijdb.130146ja. [DOI] [PubMed] [Google Scholar]
- 13.MacFarlane M, Williams AC. Apoptosis and disease: A life or death decision. EMBO Rep. 2004;5:674–678. doi: 10.1038/sj.embor.7400191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barroso G, Morshedi M, Oehninger S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum Reprod. 2000;15:1338–1344. doi: 10.1093/humrep/15.6.1338. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Zhao R, Guo C, Jiang S, Yang J, Xu Y, Liu Y, Fan L, Xiong W, Ma J, et al. Knockout of BRD7 results in impaired spermatogenesis and male infertility. Sci Rep. 2016;6:21776. doi: 10.1038/srep21776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhat GK, Sea TL, Olatinwo MO, Simorangkir D, Ford GD, Ford BD, Mann DR. Influence of a leptin deficiency on testicular morphology, germ cell apoptosis and expression levels of apoptosis-related genes in the mouse. J Androl. 2006;27:302–310. doi: 10.2164/jandrol.05133. [DOI] [PubMed] [Google Scholar]
- 17.Yan WJ, Mu Y, Yu N, Yi TL, Zhang Y, Pang XL, Cheng D, Yang J. Protective effects of metformin on reproductive function in obese male rats induced by high-fat diet. J Assist Reprod Genet. 2015;32:1097–1104. doi: 10.1007/s10815-015-0506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonte F, Dumas M, Chaudagne C, Meybeck A. Influence of asiatic acid, madecassic acid and asiaticoside on human collagen I synthesis. Planta Med. 1994;60:133–135. doi: 10.1055/s-2006-959434. [DOI] [PubMed] [Google Scholar]
- 19.Ma ZG, Dai J, Wei WY, Zhang WB, Xu SC, Liao HH, Yang Z, Tang QZ. Asiatic acid protects against cardiac hypertrophy through activating AMPKα signalling pathway. Int J Biol Sci. 2016;12:861–871. doi: 10.7150/ijbs.14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Lu Q, Yu DS, Chen YP, Shang J, Zhang LY, Sun HB, Liu J. Asiatic acid mitigates hyperglycemia and reduces islet fibrosis in Goto-Kakizaki rat, a spontaneous type 2 diabetic animal model. Chin J Nat Med. 2015;13:529–534. doi: 10.1016/S1875-5364(15)30047-9. [DOI] [PubMed] [Google Scholar]
- 21.Yan SL, Yang HT, Lee YJ, Lin CC, Chang MH, Yin MC. Asiatic acid ameliorates hepatic lipid accumulation and insulin resistance in mice consuming a high-fat diet. J Agric Food Chem. 2014;62:4625–4631. doi: 10.1021/jf501165z. [DOI] [PubMed] [Google Scholar]
- 22.Xiong Y, Ding H, Xu M, Gao J. Protective effects of asiatic acid on rotenone- or H2O2-induced injury in SH-SY5Y cells. Neurochem Res. 2009;34:746–754. doi: 10.1007/s11064-008-9844-0. [DOI] [PubMed] [Google Scholar]
- 23.Chao PC, Lee HL, Yin MC. Asiatic acid attenuated apoptotic and inflammatory stress in the striatum of MPTP-treated mice. Food Funct. 2016;7:1999–2005. doi: 10.1039/C6FO00041J. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Ghanayem BI, Bai R, Kissling GE, Travlos G, Hoffler U. Diet-induced obesity in male mice is associated with reduced fertility and potentiation of acrylamide-induced reproductive toxicity. Biol Reprod. 2010;82:96–104. doi: 10.1095/biolreprod.109.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kokilavani P, Suriyakalaa U, Elumalai P, Abirami B, Ramachandran R, Sankarganesh A, Achiraman S. Antioxidant mediated ameliorative steroidogenesis by Commelina benghalensis L. and Cissus quadrangularis L. against quinalphos induced male reproductive toxicity. Pestic Biochem Physiol. 2014;109:18–33. doi: 10.1016/j.pestbp.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Wei ZT, Lu XL, Zhang G, Yu J, Li H, Jia GH, Li JT, Zhang JM. The long-term effects of superovulation on fertility and sexual behavior of male offspring in mice. J Assist Reprod Genet. 2014;31:555–560. doi: 10.1007/s10815-014-0191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-tie quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Pasquali R, Patton L, Gambineri A. Obesity and infertility. Curr Opin Endocrinol Diabetes Obes. 2007;14:482–487. doi: 10.1097/MED.0b013e3282f1d6cb. [DOI] [PubMed] [Google Scholar]
- 30.Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22:764–786. doi: 10.1210/edrv.22.6.0446. [DOI] [PubMed] [Google Scholar]
- 31.Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in sertoli cells; Proc Natl Acad Sci USA; 2004; pp. 6876–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakkas D, Seli E, Bizzaro D, Tarozzi N, Manicardi GC. Abnormal spermatozoa in the ejaculate: Abortive apoptosis and faulty nuclear remodelling during spermatogenesis. Reprod Biomed Online. 2003;7:428–432. doi: 10.1016/S1472-6483(10)61886-X. [DOI] [PubMed] [Google Scholar]
- 33.Sukhotnik I, Nativ O, Roitburt A, Bejar D, Coran AG, Mogilner JG, Nativ O. Methotrexate induces germ cell apoptosis and impairs spermatogenesis in a rat. Pediatr Surg Int. 2013;29:179–184. doi: 10.1007/s00383-012-3197-0. [DOI] [PubMed] [Google Scholar]
- 34.Gurfinkel DM, Chow S, Hurren R, Gronda M, Henderson C, Berube C, Hedley DW, Schimmer AD. Disruption of the endoplasmic reticulum and increases in cytoplasmic calcium are early events in cell death induced by the natural triterpenoid Asiatic acid. Apoptosis. 2006;11:1463–1471. doi: 10.1007/s10495-006-9086-z. [DOI] [PubMed] [Google Scholar]
- 35.Kavitha CV, Jain AK, Agarwal C, Pierce A, Keating A, Huber KM, Serkova NJ, Wempe MF, Agarwal R, Deep G. Asiatic acid induces endoplasmic reticulum stress and apoptotic death in glioblastoma multiforme cells both in vitro and in vivo. Mol Carcinog. 2015;54:1417–1429. doi: 10.1002/mc.22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi D, Young LH. AMPK: Energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol Metab. 2015;26:422–429. doi: 10.1016/j.tem.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma ZG, Yuan YP, Xu SC, Wei WY, Xu CR, Zhang X, Wu QQ, Liao HH, Ni J, Tang QZ. CTRP3 attenuates cardiac dysfunction, inflammation, oxidative stress and cell death in diabetic cardiomyopathy in rats. Diabetologia. 2017;60:1126–1137. doi: 10.1007/s00125-017-4232-4. [DOI] [PubMed] [Google Scholar]
- 38.Tsao SM, Yin MC. Antioxidative and antiinflammatory activities of asiatic acid, glycyrrhizic acid and oleanolic acid in human bronchial epithelial cells. J Agric Food Chem. 2015;63:3196–3204. doi: 10.1021/acs.jafc.5b00102. [DOI] [PubMed] [Google Scholar]