Abstract.
The principal treatment for children and adolescents with type 2 diabetes is dietary and exercise management. However, the blood glucose levels of some patients receiving this treatment fail to improve; thus, pharmacological treatment is eventually required. The pathophysiology of type 2 diabetes in pediatric patients appears to be similar to that in adults; thus, the range of antidiabetic drugs used in adults is likely to be effective in pediatric patients as well. However, in the majority of countries, including Japan, only metformin, glimepiride, and insulin have been approved for use in pediatric patients. Indeed, the evidence for the usefulness of antidiabetic drugs other than metformin and insulin in children and adolescents is limited at this time. Therefore, the efficacy and safety of various antidiabetic drugs, including DPP-4 inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors, which are used in adult patients, should be evaluated in the pediatric population in a large number of centers worldwide. In addition, it is critical that researchers and clinicians establish treatment guidelines for children and adolescents with type 2 diabetes in all racial groups worldwide.
Keywords: pediatric patients, type 2 diabetes, glycemic control, pharmacological treatment
Introduction
In recent years, concerns regarding type 2 diabetes in the pediatric population have risen because more children and adolescents have been diagnosed with type 2 diabetes worldwide. Furthermore, type 2 diabetes has a greater incidence among children and adolescents in certain racial minority groups, particularly Asians and Native Pacific Islanders. For example, in Hong Kong, 90% of youth-onset diabetes is type 2 diabetes; in Taiwan the equivalent figure is 50%, while it is nearly 60% in Japan (1). In this regard, several Japanese studies have reported the incidence of type 2 diabetes among schoolchildren (7–15 yr of age) in major cities in Japan, relying on data from a urine glucose screening program carried out in schools (2, 3). These reports have estimated that the overall annual incidence of type 2 diabetes is 2.5–3.5 cases per 100,000 schoolchildren who participate. Moreover, a Tokyo Study showed that the incidence in junior high school children (13–15 yr of age) was about six times higher than that in primary school children (7–12 yr of age; incidence: 0.9 vs. 6.5 per 100,000 schoolchildren per year, P < 0.0001) (2, 3). Accordingly, the incidence of type 2 diabetes in Japan is thought to be substantially higher than that in the United States and Europe. However, the most recent SEARCH for Diabetes in Youth (SEARCH) Study (4) reported a much higher rate among pediatric individuals residing in the United States, indicating that the incidence of type 2 diabetes significantly increased between 2002 and 2012. Specifically, the incidences per 100,000 in the United States in 2012 were 3.9 in non-Hispanic Whites, 32.6 in non-Hispanic Blacks, 18.2 in Hispanics, 12.2 in Asians or Pacific Islanders, and 46.5 in Native Americans. For all racial groups, these rates were considerably higher than those reported in Japanese schoolchildren (2, 3). Therefore, it is critical that researchers and clinicians establish guidelines for the treatment of type 2 diabetes in the pediatric population of all racial groups worldwide (1).
Therapeutic Approaches in Children and Adolescents with Type 2 Diabetes
The principal treatment in children and adolescents with type 2 diabetes is dietary and exercise management. Lifestyle change and education on diet and physical activity are essential for all pediatric patients with type 2 diabetes (1). Treatment is considered successful when excessive body weight gain ceases, normal linear growth occurs, emotional conditions are controlled, and hyperglycemia improves. Lifestyle modification that aims to reduce body weight, such as the initiation of a well-balanced diet and adequate physical activity, is usually recommended as an initial therapeutic approach.
In dietary management, a relatively modest diet regimen is recommended. That is, caloric restriction of 5–10% of the energy requirement of age-matched, healthy children, with adequate composition of energy source. Stricter restriction of food intake impairs childhood physical development and is likely to lead to patient withdrawal from the regimen over time. Patients’ families are encouraged to change their dietary habits in accordance with the same healthy dietary recommendations: reduced carbohydrates, sugar, and total and saturated fat intake, as well as increased fiber intake and physical activity (5). Sugar-containing soft drinks and juices should be eliminated from the patients’ diets. Fast food intake should be limited. With regards to exercise management, regular exercise improves glycemic control, reduces cardiovascular events, and reduces body weight (6). Reduction of sedentary time is essential, so television, video games, and computer-related activities should be restricted (7). Physical activity, such as walking to school and to the shops, using the stairs instead of an elevator, and helping with the housework, should be promoted as an everyday affair. Admittedly, it is difficult to maintain diet and exercise regimens consistently. Therefore, family support is essential, as is ongoing education on behavioral changes conducted by a medical team that includes a dietician and a psychologist. All family members, not just patients, should be involved in this education (1).
Pharmacological Treatment of Pediatric Patients with Type 2 Diabetes
Some patients fail to improve their blood glucose levels through dietary and exercise management alone, and eventually require pharmacological treatment. In patients who are unable to change their lifestyle, or in those who make lifestyle changes but continue to have suboptimal glycemic control, a variety of antidiabetic drugs are now available. Because the pathophysiology of type 2 diabetes in pediatric patients appears to be similar to that in adults, it is likely that such drugs are effective in pediatric patients as well. However, only metformin, glimepiride (a sulfonylurea), and insulin have been approved for use in pediatric patients in the majority of countries, including Japan (1). The evidence for the usefulness of antidiabetic drugs other than metformin and insulin is limited at this time. Nonetheless, several clinical trials of newer antidiabetic drugs are underway in pediatric patients with type 2 diabetes.
Initial Pharmacological Treatment of Children and Adolescents with Type 2 Diabetes
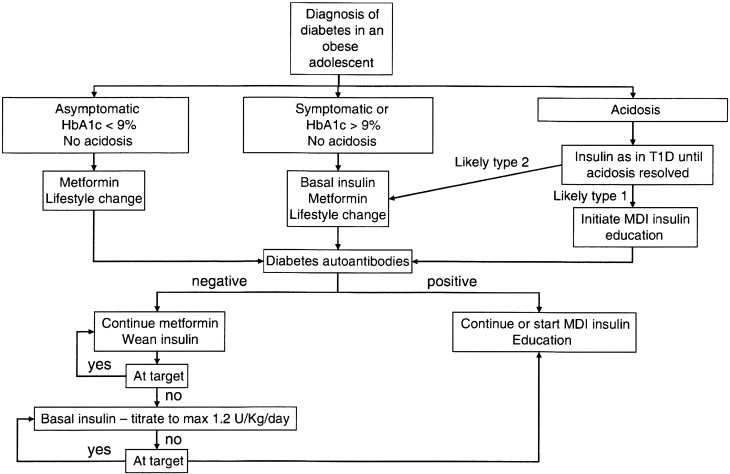
The International Society for Pediatric and Adolescent Diabetes (ISPAD) has recommended metformin as a first-choice antidiabetic drug in children and adolescents with type 2 diabetes (1). Metformin is useful in the majority of children and adolescents with type 2 diabetes, because over 80% of these patients are obese and have insulin resistance (1,2,3). If patients fail to sustain optimal glycemic levels, addition of basal insulin to the treatment regimen should be strongly considered (1). Figure 1 indicates the approach to initial and subsequent treatment of pediatric patients with type 2 diabetes, as proposed by ISPAD (1). The initial treatment strategy depends on the clinical symptoms, severity of hyperglycemia, and presence of ketosis/ketoacidosis. If the patient shows an HbA1c < 9.0% and no symptoms, metformin monotherapy is the treatment of choice. The initial dose should be 500–1,000 mg b.i.d., and this should be titrated to 1,000–2,000 mg b.i.d. If the patient is metabolically unstable, insulin should be administered, at least initially. Basal insulin (0.25–0.50 units/kg) is the starting dose, and this often achieves metabolic control. Generally, metformin can be initiated at the same time as insulin, unless acidosis exists. Transition from insulin to metformin monotherapy can usually be achieved over 2–6 wk by decreasing the basal insulin dose. Thereafter, most patients’ glucose levels can be controlled by metformin monotherapy during the early stages of the disease (1).
Fig. 1.
Approaches to initial and subsequent treatment of pediatric patients with type 2 diabetes. Reproduced with permission from John Wiley & Sons, Inc. from Zeitler P, et al. Type 2 diabetes in the child and adolescent. ISPAD Clinical Practice Consensus Guidelines 2014 Compendium. Pediatr Diabetes 2014; 15 (Suppl. 20): 26-46 (Ref 1).
The goal of this initial treatment should an HbA1c level of < 6.5% (1). If the patient fails to reach this target after 3–4 wk of metformin monotherapy, basal insulin (up to 1.2 units/kg) should be added to the treatment regimen. If the target is still not attained using metformin plus basal insulin, prandial bolus insulin should be used and titrated until the target HbA1c level of < 6.5% is reached (Figure 1) (1).
Use of Various Antidiabetic Drugs
Various antidiabetic drugs are used in addition to or instead of metformin and/or insulin. Such drugs might be beneficial for glycemic control in pediatric patients with type 2 diabetes. However, few studies have addressed the use of these drugs in such patients, and they are generally not approved for use in pediatric population (1).
Metformin
Metformin, a biguanide, reduces hepatic glucose output by decreasing gluconeogenesis. It also increases insulin-stimulated glucose uptake by muscle and fat. Metformin does not promote insulin secretion; thus, it carries little or no risk of hypoglycemia when used as a monotherapy. In addition, body weight either decreases or remains stable during metformin monotherapy, and plasma lipid profiles improve (8, 9).
Metformin has been approved for pediatric use worldwide, and it is recognized as a first-line antidiabetic drug (1). The “Treatment Options for Type 2 Diabetes in Adolescents and Youth” (TODAY) study demonstrated that most pediatric patients with recent onset type 2 diabetes can attain their target HbA1c level of < 8.0%. In one study, 90.9% of these patients had an HbA1c level of < 8.0%, 77.9% had a level of < 7.0%, and 46.4% had a level of < 6.0%, with a short median run-in time of 71 d of metformin monotherapy and standard diabetic education (10). However, the TODAY study later reported that participants often required additional antidiabetic therapy, such as rosiglitazone (a thiazolidinedione) to maintain optimal glycemic control for a longer period (11, 12).
Intestinal symptoms are the most common side effect of metformin. These reduce over time and with an appropriate treatment schedule. In addition, vitamin B12 deficiency may occur in patients with anemia and peripheral neuropathy (13). Lactic acidosis is extremely rare and is usually restricted to patients with renal or hepatic impairment, or cardiac insufficiency (1).
α-glucosidase inhibitors (not approved for use in patients < 18 yr of age)
α-glucosidase inhibitors work by inhibiting the absorption of carbohydrates in the small intestine. They also mitigate the postprandial rise of plasma glucose and improve glycemic control, particularly in patients at an early stage of diabetes (1). The most common side effect is flatulence, which makes the treatment unacceptable to most adolescents.
Sulfonylureas (glimepiride is approved for use in pediatric patients)
Sulfonylureas promote endogenous insulin secretion by binding to receptors on the K+/ATP channel complex, causing K+ channels to close. Obesity is likely to be aggravated when this drug is used inappropriately in patients under insufficient dietary management. Moreover, sulfonylureas may accelerate the loss of β-cell function and eventually of glycemic control when used in monotherapy.
One limited clinical trial of a sulfonylurea, glimepiride, has been carried out in pediatric patients. This drug has a lower binding affinity to sulfonylurea receptors (K+/ATP channels), but it does exert extrapancreatic effects, such as decreased glucose output from the liver and enhanced sensitivity of peripheral tissues to insulin (14). One study reported that glimepiride was similar to metformin in terms of improving glycemic control when used as a monotherapy in pediatric patients with type 2 diabetes (15).
Thiazolidinediones (not approved for use in patients < 18 yr of age)
Thiazolidinediones increase insulin sensitivity in muscle, adipose tissue, and liver, and they have a greater effect on muscle glucose uptake than metformin. They bind to nuclear protein, activating γ-peroxisome proliferator activator receptors. Rosiglitazone, a thiazolidinedione, is not used in Japan, but pioglitazone is widely used. The side effects of thiazolidinediones include weight gain, anemia, and fluid retention (congestive heart failure). Severe liver toxicity has not been reported with newer thiazolidinediones (1).
In the TODAY study, addition of rosiglitazone to a metformin treatment regimen decreased the risk of developing an insulin requirement by 23% (12). On the other hand, pioglitazone was reported to lower LDL levels more than rosiglitazone.
DPP-4 inhibitors and GLP-1 receptor agonists (not approved for use in patients < 18 yr of age)
Glucagon-like peptide-1 (GLP-1), a gut-deprived hormone secreted from L-cells in the small intestine, enhances insulin secretion in proportion with plasma glucose levels. It also lowers raised plasma glucose by suppressing glucagon secretion, prolonging gastric emptying, and promoting satiety (16). It is rapidly degraded by dipeptidyl peptidase-4 (DPP-4).
GLP-1 associated drugs, such as GLP-1 enhancers (also known as DPP-4 inhibitors: sitagliptin, vildagliptin, alogliptin, linagliptin, teneligliptin, anagliptin, and saxagliptin in Japan) and GLP-1 mimetics (GLP-1 receptor agonists: liraglutide, exenatide, lixisenatide, dulaglutide), have recently been introduced. Side effects include intestinal symptoms such as diarrhea, nausea, and vomiting, as well as infrequent headache and dyspepsia. Although the efficacy and safety of these new drugs are well documented and they are used widely in adults, few studies have been carried out in the pediatric population. Nonetheless, several trials are currently underway (17).
SGLT2 inhibitors (not approved for use in patients < 18 yr of age)
Sodium-glucose co-transporter-2 (SGLT2) inhibitors (ipragliflozin, dapagliflozin, luseogliflozin, tofogliflozin, canagliflozin, and empagliflozin) inhibit proximal renal tubular reabsorption of glucose, leading to increased urinary glucose output, reduction in plasma glucose, and body weight loss. As a result, β-cell function and peripheral insulin action improve, and glucose toxicity is reduced. Furthermore, energy metabolism adapts to the relative glucose deficiency, leading to increased lipolysis in fat cells (18). Side effects include increased prevalence of genital mycotic and urinary tract infections, and a risk of dehydration (18). These new antidiabetic drugs are now widely used in adults, but no studies have addressed the use of SGLT2 inhibitors in pediatric patients.
Insulin
Insulin is the oldest hypoglycemic agent used in the treatment of diabetes. In the 1980s, oral hypoglycemic drugs were almost completely prohibited for use in pediatric patients, and insulin was the only hypoglycemic agent approved for pediatric use. Nowadays, less than a third of pediatric patients receive insulin therapy. Indeed, the ISPAD guidelines recommend once-daily injection of a long-acting insulin analogue, such as insulin levemir, insulin glargine, or insulin degludec, at bedtime or before breakfast. This approach supports basal insulin secretion. Prandial insulins, such as insulin aspart, insulin lispro, or insulin glulisine, act more rapidly. After treatment initiation with such insulins, they should be titrated to attain optimal glycemic control. They may be used once daily —before the largest meal— or at each meal (1). Patients who have completely lost endogenous insulin secretary capacities eventually progress to intensive insulin regimens; i.e., multiple daily injections or pump therapy, such as is used in the treatment of type 1 diabetes. It has been reported that non-obese children and adolescents with type 2 diabetes tend to require more insulin treatment over time to achieve optimal glycemic control, as their β-cell function gradually declines (19).
Expanding Treatment Options for Children and Adolescents with Type 2 Diabetes: Current Problems and Proposed Solutions
In its position statement, the American Diabetes Association (ADA) proposed a therapeutic approach to treating adult patients with type 2 diabetes (20). Specifically, the association recommended metformin monotherapy as a first-line antidiabetic drug, except when there are contraindications, such as renal insufficiency and problematic side effects. In patients with metformin contraindications or intolerance, various antidiabetic drugs, such as DPP-4 inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors, have been proposed in dual or triple therapies to more expeditiously attain the target HbA1c level. Furthermore, combination therapy using injectable drugs is more effective when hyperglycemia is severe, particularly if catabolic features are present. In one meta-analysis, glycemic control or risk of cardiovascular or all-cause mortality did not differ among the available antidiabetic drugs (21). However, only a few studies have used antidiabetic drugs in the pediatric population, and, other than metformin, glimepiride, and insulin, such drugs are generally not approved for use in the pediatric population (1).
The NICHD Diabetes Working Group proposed trials of early combination therapy in adolescents with type 2 diabetes (17). In these trials, subjects receiving metformin monotherapy and who have well-controlled blood glucose levels would be randomized to receive combination therapy with either metformin plus a placebo or metformin plus an experimental agent. Indeed, the TODAY studies did prefer to use early combination therapy in pediatric patients with type 2 diabetes, rather than wait for the failure of metformin monotherapy (10,11,12).
In our own clinic, we have used various antidiabetic drugs in pediatric patients with obese and non-obese type 2 diabetes (22). In obese patients, metformin monotherapy or combination therapy with additional medications is frequently used. Some patients more than 10 yr of age receive new antidiabetic drugs, including DPP-4 inhibitors and GLP-1 receptor agonists, which yield safe and effective glycemic control. In other patients, blood glucose levels can be controlled using (1) twice daily injections of premixture insulin, or (2) basal insulin with oral antidiabetic drugs, mainly metformin. SGLT2 inhibitors have recently been used in patients more than 10 yr of age. These drugs are thought to improve blood glucose levels and weight loss without any problematic side effects. Conversely, non-obese patients are frequently treated using (1) insulin alone or (2) insulin in combination with additional antidiabetic drugs, followed by sulfonylureas. In contrast, obese patients tend to require insulin treatment during the early stage of the disease (within 5 yr of diagnosis); this requirement develops alongside decreases in endogenous insulin secretion. GLP-1-associated drugs seem to positively affect blood glucose levels without leading to hypoglycemia recurrence in cases more than 10 yr of age. In fact, liraglutide, a GLP-1 receptor agonist, can be used at a daily dose of 0.3–0.6 mg in pediatric patients, which is lower than the recommended maintenance daily dose of 0.9 mg in adult patients (data not in published). Possible antidiabetic drugs for use in obese and non-obese pediatric patients with type 2 diabetes are shown in Table 1.
Table 1. Possible antidiabetic drugs for obese and non-obese pediatric patients with type.
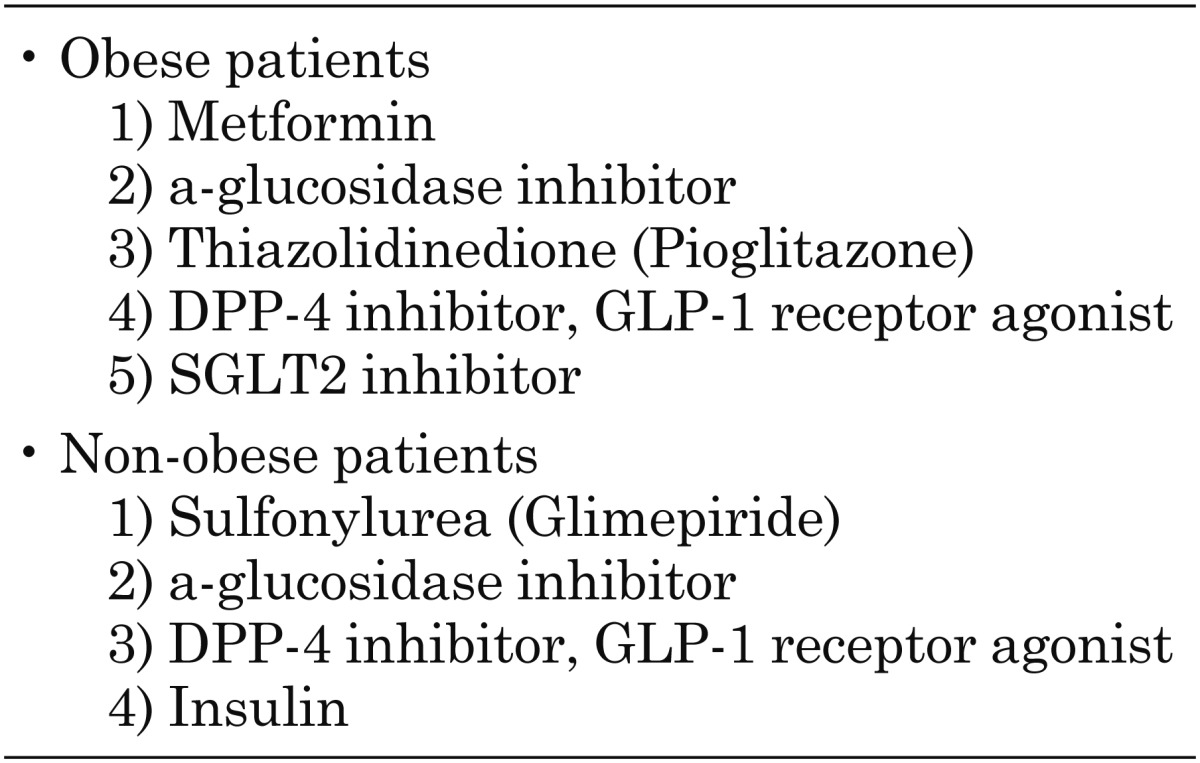
In conclusion, all antidiabetic drugs used regularly in adult patients, including DPP-4 inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors, would contribute to glycemic control without any problematic side effects in pediatric patients of more than 10 yr of age. The indications for these drugs may differ somewhat between obese and non-obese patients with type 2 diabetes. In future, the efficacy and safety of these agents in the pediatric population should be evaluated in a large number of centers worldwide.
Conflict of interest: The author declares no conflicts of interests to this work.
References
- 1.Zeitler P, Fu J, Tandon N, Nadeau K, Urakami T, Barrett T, et al. International Society for Pediatric and Adolescent Diabetes. ISPAD Clinical Practice Consensus Guidelines 2014. Type 2 diabetes in the child and adolescent. Pediatr Diabetes 2014;15(Suppl 20): 26–46. doi: 10.1111/pedi.12179 [DOI] [PubMed] [Google Scholar]
- 2.Urakami T, Kubota S, Nitadori Y, Harada K, Owada M, Kitagawa T. Annual incidence and clinical characteristics of type 2 diabetes in children as detected by urine glucose screening in the Tokyo metropolitan area. Diabetes Care 2005;28: 1876–81. doi: 10.2337/diacare.28.8.1876 [DOI] [PubMed] [Google Scholar]
- 3.Urakami T, Morimoto S, Nitadori Y, Harada K, Owada M, Kitagawa T. Urine glucose screening program at schools in Japan to detect children with diabetes and its outcome-incidence and clinical characteristics of childhood type 2 diabetes in Japan. Pediatr Res 2007;61: 141–5. doi: 10.1203/pdr.0b013e31802d8a69 [DOI] [PubMed] [Google Scholar]
- 4.Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. SEARCH for Diabetes in Youth Study. Incidence trends of type 1 and type 2 diabetes among youths, 20022012. N Engl J Med 2017;376: 1419–29. doi: 10.1056/NEJMoa1610187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Kim Y. Effects of exercise alone on insulin sensitivity and glucose tolerance in obese youth. Diabetes Metab J 2013;37: 225–32. doi: 10.4093/dmj.2013.37.4.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhuper S, Buddhe S, Patel S. Managing cardiovascular risk in overweight children and adolescents. Paediatr Drugs 2013;15: 181–90. doi: 10.1007/s40272-013-0011-y [DOI] [PubMed] [Google Scholar]
- 7.Chahal H, Fung C, Kuhle S, Veugelers PJ. Availability and night-time use of electronic entertainment and communication devices are associated with short sleep duration and obesity among Canadian children. Pediatr Obes 2013;8: 42–51. doi: 10.1111/j.2047-6310.2012.00085.x [DOI] [PubMed] [Google Scholar]
- 8.Cuse K, DeFronzo RA. Metformin: a review of its metabolic effects. Diabetes Res 1998;6: 89–131. [Google Scholar]
- 9.Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 2002;25: 89–94. doi: 10.2337/diacare.25.1.89 [DOI] [PubMed] [Google Scholar]
- 10.Laffel L, Chang N, Grey M, Hale D, Higgins L, Hirst K, et al. TODAY Study Group. Metformin monotherapy in youth with recent onset type 2 diabetes: experience from the prerandomization run-in phase of the TODAY study. Pediatr Diabetes 2012;13: 369–75. doi: 10.1111/j.1399-5448.2011.00846.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arslanian S. TODAY study. Treatment effects on insulin sensitivity and b-cell function in TODAY. Pediatr Diabetes 2012;13(Suppl 17): 1–14. [Google Scholar]
- 12.Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, et al. TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366: 2247–56. doi: 10.1056/NEJMoa1109333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration Metformin-containing Drugs: Drugs Safety Communication-Revised Warnings for Certain Patients With Reduced Kidney Function (Internet). Available from http://fda.gov/Safety/MedWatch/Safetyinformation/SafetyAlertsforHumanMedicalProducts/ucm494829.htm?sorce=govdelivery&utm_medium=email&utm_source=govdelivery.
- 14.Campbell RK. Glimepiride: role of a new sulfonylurea in the treatment of type 2 diabetes mellitus. Ann Pharmacother 1998;32: 1044–52. doi: 10.1345/aph.17360 [DOI] [PubMed] [Google Scholar]
- 15.Gottschalk M, Danne T, Vlajnic A, Cara JF. Glimepiride versus metformin as monotherapy in pediatric patients with type 2 diabetes: a randomized, single-blind comparative study. Diabetes Care 2007;30: 790–4. doi: 10.2337/dc06-1554 [DOI] [PubMed] [Google Scholar]
- 16.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368: 1696–705. doi: 10.1016/S0140-6736(06)69705-5 [DOI] [PubMed] [Google Scholar]
- 17.Tamborlane WV, Haymond MW, Dunger D, Shankar R, Gubitosi-Klug R, Bethin K, et al. NICHD Diabetes Working Group. Expanding treatment options for youth with type 2 diabetes: Current problems and proposed solutions: A white paper from the NICHD Diabetes Working Group. Diabetes Care 2016;39: 323–9. doi: 10.2337/dc15-1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashiwagi A, Maegawa H. Metabolic and hemodynamic effects of sodium-dependent glucose cotransporter 2 inhibitors on cardio-renal protection in the treatment of patients with type 2 diabetes mellitus. J Diabetes Investig 2017;8: 416–27. doi: 10.1111/jdi.12644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urakami T, Kuwabara R, Habu M, Okuno M, Suzuki J, Takahashi S, et al. Clinical characteristics of non-obese children with type 2 diabetes mellitus without involvement of β-cell autoimmunity. Diabetes Res Clin Pract 2013;99: 105–11. doi: 10.1016/j.diabres.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association. 8. Pharmacologic Approaches to Glycemic Treatment. Diabetes Care 2017;40(Suppl 1): S64–74. [DOI] [PubMed] [Google Scholar]
- 21.Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: A meta-analysis. JAMA 2016;316: 313–24. doi: 10.1001/jama.2016.9400 [DOI] [PubMed] [Google Scholar]
- 22.Urakami T, Kuwabara R, Habu M, Yoshida A, Okuno M, Suzuki J, et al. Pharmacologic treatment strategies in children with type 2 diabetes mellitus. Clin Pediatr Endocrinol 2013;22: 1–8. doi: 10.1297/cpe.22.1 [DOI] [PMC free article] [PubMed] [Google Scholar]