Introduction
The human insulin receptor is encoded by a single gene with 22 exons, and it is a membrane protein composed of two α and two β subunits (1). Binding of insulin to the extracellular α subunit causes a conformational change, leading to the activation of tyrosine kinase at the intracellular β subunit as well as the autophosphorylation of the receptor, followed by the activation of signal transduction cascades. Mutations in the gene encoding the insulin receptor (INSR) result in the insulin-resistant syndromes such as Leprechaunism, also known as the Donohue syndrome, as well as the Rabson-Mendenhall syndrome and type A insulin resistance (IR) (2). The former two syndromes are autosomal recessive disorders and characterized by severe phenotypes including intrauterine and postnatal growth retardation, dysmorphic features, altered glucose homeostasis, and early mortality. On the other hand, type A IR is an autosomal dominant disorder and is characterized by IR, acanthosis nigricans (AN), and hyperandrogenism such as polycystic ovarian syndrome (PCOS). To date, more than 100 disease-causing mutations have already been reported (2). Among them, approximately half of the mutations were identified in type A IR. Type B IR is an autoimmune disorder characterized by the presence of the insulin receptor antibody. Here, we described a clinical course from childhood to puberty in a Japanese girl with type A IR due to a novel nonsense mutation in INSR.
Case Report
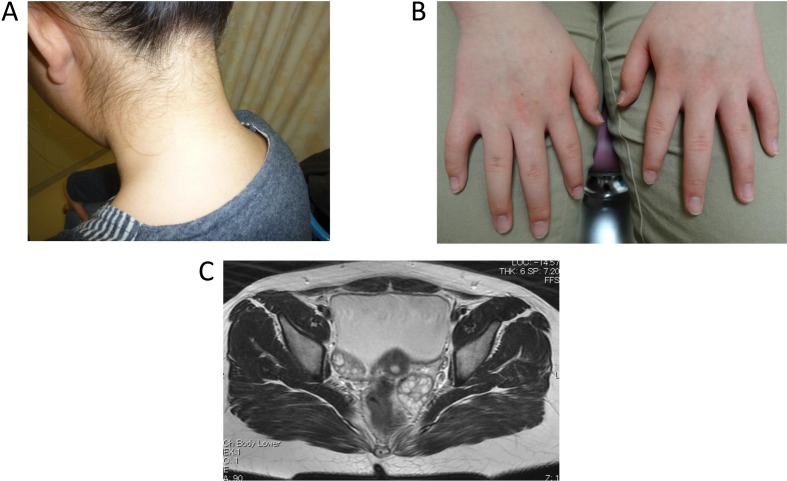
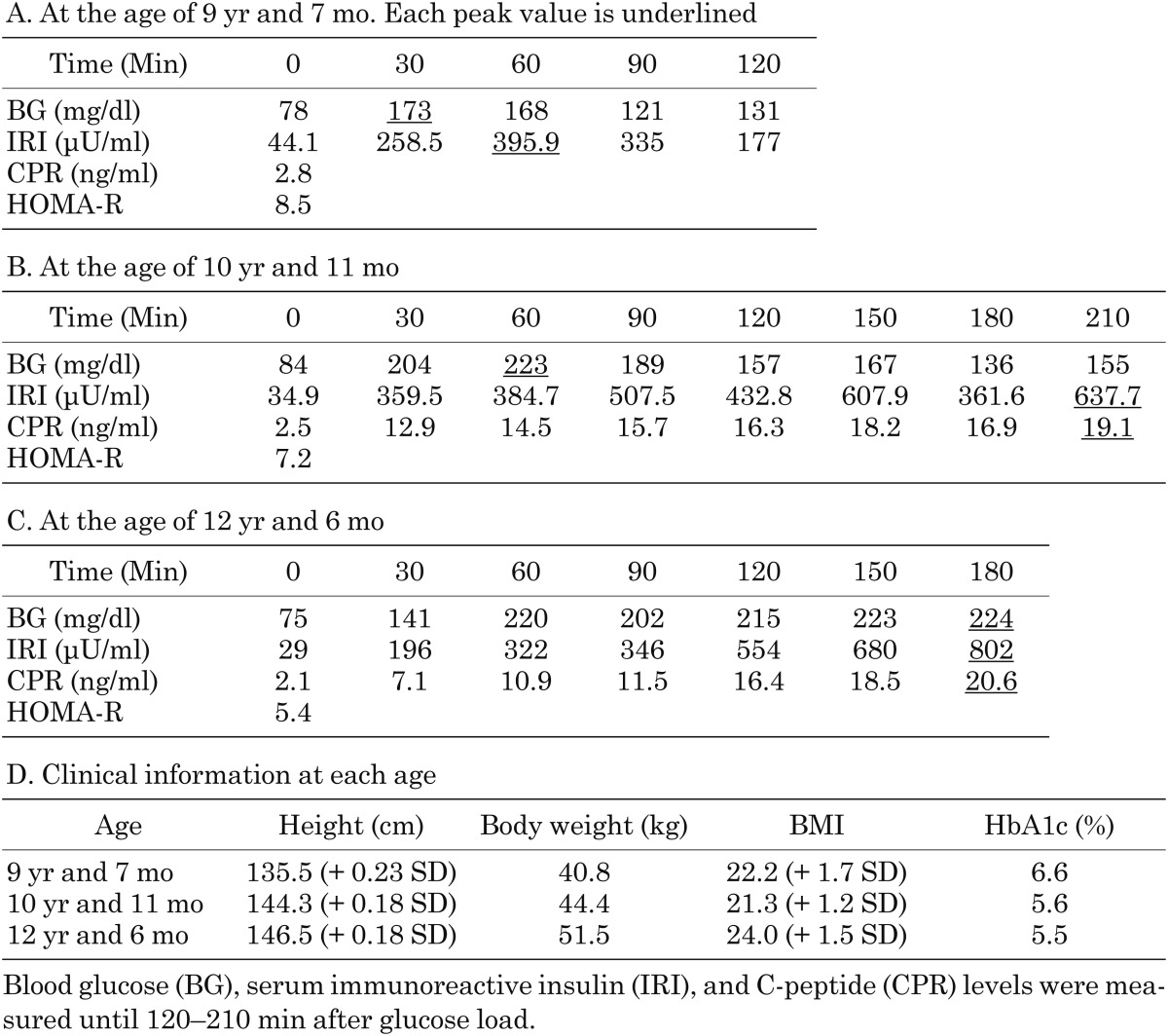
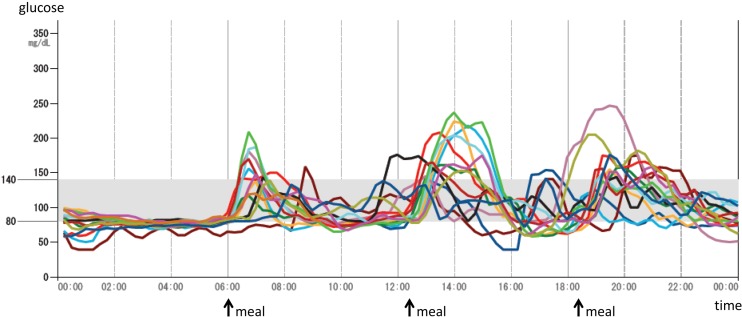
The patient is a 12-yr-old Japanese girl who was the only child of unrelated Japanese parents. Her mother is healthy, but her father was diagnosed with adult onset impaired glucose tolerance and is now taking an oral hypoglycemic agent. After the pregnancy was established by in vitro fertilization, the patient was born at 36 wk of gestation by emergency cesarean section due to fetal distress. She was small for her gestational age (SGA), with a birth weight and height of 1894 g (−2.8 SD) and 42.5 cm (−2.8 SD), respectively. After birth, hypoglycemia of unknown origin was prolonged for a few weeks. At the age of 8 yr, she had an operation for leg length discrepancy with genu valgum. At the age of 9 yr, hyperglycemia (220 mg/dl) was noticed at the preoperative examination of the second operation for plate removal, and she was referred to our hospital for the assessment of hyperglycemia. Her weight and height were 40.8 kg (BMI 22.2, + 1.7 SD) and 135.5 cm (+ 0.23 SD), respectively. Her physical findings were not remarkable except for moderate obesity with striae distensae and absence of one permanent tooth. She was not mentally retarded, and a dysmorphic face, acanthosis nigricans (AN) (Fig. 1A, B), hirsutism, or clitoromegaly was not recognized. She was at a pubertal stage of B-1 and PH-1. An oral glucose tolerance test (OGTT) revealed prolonged high insulin secretion although blood glucose (BG) levels were normal at 120 min (131 mg/dl) (Table 1A, D). HbA1c levels were elevated (6.6%, NGSP; National Glycohemoglobin Standardization Program), and the homeostasis model assessment of IR (HOMA-R), an index of IR, was high at 8.5. Based on these findings, she was diagnosed with type 2 diabetes mellitus with moderate obesity. Exercise therapy, diet therapy, and medical therapy with metformin (250 mg per d) were initiated. However, severe IR remained (insulin levels after meal were between 309.3 and 921.7 μU/ml), even though obesity and the HbA1c levels (5.6%, NGSP) were improved. At the age of 10 and 12 yr, we repeatedly performed the OGTT extended until 210 min after glucose load (Table 1B, C, D). Both insulin levels and blood glucose levels became more prolonged and higher with age. The insulin loading test showed a drop in BG levels from 82 mg/dl (basal) to 47 mg/dl (at 30 min). Both anti-insulin and anti-insulin receptor antibodies were negative, and hyperproinsulinemia was not recognized either. From these data, insulin antibody syndrome, type B IR, or hyperproinsulinemia was excluded. She is now 12 yr old and still has hyperinsulinemia (casual insulin levels were between 24.8 and 428.6 μU/ml), although the HbA1c levels have been maintained between 5.1–5.5% (NGSP). Hypoglycemia was not observed. Continuous glucose monitoring (CGM) by FreeStyle Libre Pro system (Abbott Japan, Kyoto) showed elevations in postprandial glucose levels (Fig. 2). She continued her medical therapy with metformin (250 mg per day). She had menarche at the age of 10 yr shortly after breast development at the same age, and her pubertal stage is now at B-4 and PH-4. However, she recently had oligomenorrhea. Hormonal data showed elevations in basal LH levels, the LH to FSH ratio (LH 13.28 mIU/ml, FSH 4.18 mIU/ml, estradiol 33 pg/ml), testosterone levels (73 ng/dl), and dehydroepiandrosterone sulfate (DHEAs) levels (295 μg/dl). Pelvic MRI showed polycystic ovaries (Fig. 1C). She was then diagnosed with PCOS. Virilism, hirsutism, or AN was not recognized.
Fig. 1.
A and B. Photos of the neck and fingers. Acanthosis nigricans was not recognized in the neck (A), the knuckles (B), or other areas such as the axilla. C. Pelvic magnetic resonance imaging (MRI) findings. Pelvic MRI showed bilateral swollen polycystic ovaries.
Table 1. Oral glucose tolerance test.
Fig. 2.
Continuous glucose monitoring (CGM) at each day for 14 d. CGM by FreeStyle Libre Pro system (Abbott Japan, Kyoto) showed an elevation in postprandial glucose levels.
Mutational Analysis
Genomic DNA was extracted from the peripheral blood leukocytes of the patient. First, we analyzed the gene encoding insulin (INS) by Sanger sequencing, but no mutations were found in INS. Next, whole-exome sequencing was conducted. Targeted enrichment was performed using an Ion AmpliSeq Exome RDY Kit. Exon-enriched DNA libraries were sequenced on the Ion Proton. After screening causative genes for IR or hyperinsulinemia (INSR, LMNA, TBC1D4, PPARG+PP1R3A, AGPAT2, BSCL2, CAV1, PTRF, LMNA, PPARG, AKT2, PLIN1, PSMB8, ZMPSTE24, DSMB8, ABCC8, KCNJ11, GLUD1, GCK, HADH1, UCP1, MCT1, HNF4A, HNF1A, HK1, and PGM1), a novel heterozygous nonsense mutation in exon 2 of INSR (RefSeq NM_000208.3, c.610C>T [p.(Gln205Ter)]) was identified, which causes a premature termination at residue 205 in the α subunit of INSR. This mutation was not found in any databases, including the Exome Aggregation Consortium (ExAC) database version 0.3, dbSNP137, and the Human Genetic Variation Database (HGVD) (a reference database of genetic variations in the Japanese population; URL: http://www.genome.med.kyoto-u. ac.jp/SnpDB). This study was approved by the Ethics Committee of Tohoku University School of Medicine and performed after obtaining written informed consent from the parents of the patient. We could not obtain samples from parents. Therefore, we could not clarify whether this mutation was paternal, maternal, or of de novo origin.
Discussion
Various mutations in INSR have been reported (2), and AN is commonly observed in most patients with type A IR. However, in our case, AN was not recognized despite the presence of severe hyperinsulinemia and PCOS. AN is characterized by hyperpigmented, velvety, irregular plaques typically in intertriginous areas. The direct and indirect activations of the IGF-1 receptors by hyperinsulinemia trigger the proliferation of fibroblasts and keratinocytes, leading to the development of skin lesions (3). It is reported that the extent and severity of AN could parallel the degree of IR (4). In the present case, the reason for the lack of AN is unknown, but we speculate that IGF-1 resistance in skin cells at a receptor or post-receptor level or an inhibitory action of the mutant insulin receptor on IGF-1 receptor signaling in skin cells could contribute to this condition. Analysis of more cases without AN is needed to elucidate this phenomenon.
There has been no report on precocious puberty in type A IR. However, in our case, menarche occurred at the age of 10 yr shortly after breast development, showing rapid progression of puberty. In children with SGA, insulin resistance and hyperinsulinemia followed by postnatal catch-up growth is thought to be linked to the progression of puberty (5). It has also been reported that insulin sensitizer therapy (metformin) was associated with slower pubertal development in SGA children (5). Therefore, we presume that being born SGA, rapid weight gain from early childhood, and exacerbation of IR itself might advance the timing of menarche in our case.
We repeatedly performed the OGTT during puberty. Both stimulated insulin and blood glucose levels were high and prolonged with age, although fasting blood glucose and HbA1c levels remained within a normal range. CGM also showed an elevation of postprandial blood glucose levels. In addition, the patient already presented with PCOS at the age of 12 yr, reflecting severe IR. Puberty itself might be a cause of deterioration in IR as well as IR itself might advance puberty. Huang et al. (2014) also reported increments of IR with the progression of puberty in siblings with type A IR (6). Therefore, appropriate management by diet, exercise, and medicine might be more important, especially during puberty.
In conclusion, we reported an adolescent Japanese girl with type A IR. We believe that type A IR should be reclassified as a differential diagnosis for severe IR, even though AN is not recognized. Furthermore, the follow-up with periodic OGTT is important as an indicator for glycemic control, especially during puberty.
References
- 1.Ebina Y, Ellis L, Jarnagin K, Edery M, Graf L, Clauser E, et al. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell 1985;40: 747–58. doi: 10.1016/0092-8674(85)90334-4 [DOI] [PubMed] [Google Scholar]
- 2.Ardon O, Procter M, Tvrdik T, Longo N, Mao R. Sequencing analysis of insulin receptor defects and detection of two novel mutations in INSR gene. Mol Genet Metab Rep 2014;1: 71–84. doi: 10.1016/j.ymgmr.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phiske MM. An approach to acanthosis nigricans. Indian Dermatol Online J 2014;5: 239–49. doi: 10.4103/2229-5178.137765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng HY. Acanthosis nigricans in obese adolescents: prevalence, impact, and management challenges. Adolesc Health Med Ther 2016;8: 1–10. doi: 10.2147/AHMT.S103396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verkauskiene R, Petraitiene I, Albertsson Wikland K. Puberty in children born small for gestational age. Horm Res Paediatr 2013;80: 69–77. doi: 10.1159/000353759 [DOI] [PubMed] [Google Scholar]
- 6.Huang Z, Liu J, Ma L, Wan X, He X, Fang D, et al. Glucose metabolism, insulin sensitivity and β-cell function in type A insulin resistance syndrome around puberty: a 9-year follow-up. Horm Metab Res 2014;46: 65–72. [DOI] [PubMed] [Google Scholar]