Abstract
Most pathogenic Clostridium difficile produce two major exotoxins TcdA and TcdB, in the absence of which the bacterium is non-pathogenic. While it is important to investigate the role of each toxin in the pathogenesis of C. difficile infection (CDI) using isogenic strains, it is impossible to precisely control the expression levels of individual toxins and exclude bacterial factors that may contribute to the toxins' effects during infection. In this study, we utilized an acute intestinal disease model by injecting purified toxins directly into mouse cecum after a midline laparotomy. We evaluated the physical condition of mice by clinical score and survival, and the intestinal tissue damage and inflammation by histology. Depending on the dose of the toxins, mice developed mild to severe colitis, experienced diarrhea or rapidly died. We found that both purified TcdA and TcdB were able to induce clinical disease, intestinal inflammation, and tissue damage that resembled CDI. TcdA was significantly faster in inducing intestinal inflammation and tissue damage, and was approximately five times more potent than TcdB in terms of inducing severe gut disease and death outcomes in mice. Moreover, we found that the two toxins had significant synergistic effects on disease induction. Comparison of the in vivo toxicity of TcdB from clinical strains revealed that TcdB from an epidemic RT 027 strain was more toxic than the others. Our study thus demonstrates that both TcdA and TcdB, independent of other factors from C. difficile bacterium, are able to cause disease that resembles CDI and highlights the importance of targeting both toxins for vaccines and therapeutics against the disease.
Keywords: Clostridium difficile, Toxin A, Toxin B, Pathogenesis, Cecum
1. Introduction
Clostridium difficile infection (CDI) is an antibiotic-associated disease characterized by life-threatening diarrhea and colitis, reports of which have increased globally in the past several decades. In United States, the incidence of CDI in hospitalized patients or that leads to hospitalization has risen dramatically with more than 250, 000 cases per year [1]. Since the emergence of hypervirulent strains, the mortality of CDI has increased 400% between 2000 and 2007 and the infection has led to 14,000 annual deaths in US [1]. Two exotoxins, toxin A (TcdA) and toxin B (TcdB), secreted by the bacteria are the primary causes of CDI. Both toxins are multidomain proteins and share a similar molecular mode of action [2,3]. The toxicity of either toxin is mainly mediated via their glucosyltransferases that glucosylate Rho GTPase family members of target cells and lead to pathogenesis in hosts [4–7].
The relative role of TcdA and TcdB in CDI pathogenesis has been a focus of investigation. Studies using the intestinal loop model in animals and human intestinal organoids demonstrated that TcdA, but not TcdB, induced intestinal tissue damage and inflammation [8–13] whereas a study using human colonic explants showed that TcdB was more potent than TcdA in inducing human epithelial barrier damage [14]. These arguments led to a hypothesis that the differential effects of TcdB in animal and human intestines may be due to the expression of toxin receptors [15]. More recently, the relative roles of TcdA and TcdB in pathogenesis were examined in hamster [16,17] or mouse [18] infection models using isogenic strains that express one of either toxin; these studies found that the genetically engineered strains expressing TcdB alone caused more severe disease. Besides the two major toxins TcdA and TcdB, C. difficile may produce other virulence factors that have been proposed to play roles in pathogenesis, such as binary toxin (CDT) [19–21] or potentially other unknown toxin co-factors [22].
In this study, we evaluated the relative roles of TcdA and TcdB in disease pathogenesis by injecting a defined amount of highly purified toxins directly into the mouse cecum, monitoring mouse clinical symptoms and survival, and assessing tissue damage and inflammation. Our results indicated that individually, both toxins are pathogenic and capable of inducing disease that mimics clinical symptoms of CDI in animals and humans. We also compared the in vivo toxicity of TcdB variants including TcdB from VPI 10463, M68 and clinic isolated RT 027 strains. Our data further demonstrated that TcdA and TcdB have a significant synergistic effect on inducing tissue damage and inflammation when present together in the mouse intestine.
2. Materials and methods
2.1. Mice
Six to eight week-old CD1 mice were purchased from Envigo (Frederick, Maryland). All mice were housed in dedicated pathogen-free facilities in groups of 5 mice per cage under the same conditions. Food, water, bedding, and cages were autoclaved. 10 mice were used for each experiment group and the experiment was repeated once. For each group, 5 mice were used for survival studies, and the other 5 mice were used for histopathological studies. All procedures involving mice were conducted under protocols approved by the Institutional Animal Care and Use Committee.
2.2. Toxins
Full-length wild-type TcdA and TcdB from VPI 10463 strain (RT 087, toxinotype 0 [23]), and TcdB from strain M68 (TcdBM68, RT 017, toxinotype VIII [23]) were recombinant toxins expressed in Bacillus megaterium and purified from total crude extracts using the same method as described previously [24]. TcdB from a clinical isolate [25] (TcdB027, RT 027, toxinotype III [23]) was a gift from Dr. Xinhua Chen at Harvard University.
2.3. Cecum toxin injection surgery
Mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) injected intramuscularly. A midline laparotomy was performed and the cecum, ileum and colon were exposed. An insulin syringe (29G) was inserted into the ileocecal junction and 100 µl of PBS or toxin was injected into the cecum. The gut was then returned to the abdomen, and the incision was closed with silk sutures.
2.4. Clinical scoring
The clinical scores of the mice were evaluated by 2 experimenters independently 24 h post-surgery. The clinical score system was modified from a previous study [26], and included five criteria (activity level, posture, appearance of the coat, appearance of eyes and nose, and diarrhea). Each parameter was graded on a scale from 0 up to 4 and added together to generate a score with a maximum value of 15 (Table 2). A normal mouse would score 0 and a mouse found dead would be scored as 15. Mice with a score equal to or higher than 11 were considered moribund and were euthanized. Weight loss was not included in our scale since even the PBS treated mice lost weight when measured 24 h post-surgery, which was most likely the result of less food consumption after the surgery; however, these control mice recovered after 16–24 h and resumed their activities including eating, climbing and grooming.
Table 2.
Criteria for monitor physical conditions of micea.
| Category | Scores | ||||
|---|---|---|---|---|---|
|
|
|||||
| 0 | 1 | 2 | 3 | 4 | |
| Activity | Normal | Alert/slow moving | Lethargic/Shaky | Inactive unless Prodded | Not moving |
| Posture | Normal | Back slanted | Hunched | Hunched/nose down | |
| Coat | Normal | Piloerection | Rough skin | Very ruffled/puff/ungroomed | |
| Diarrhea | Normal | Hard to produce stool and looked Normal | Wet stained tail/red rectal/liquid stool | ||
| Eye/Nose | Normal | Squinted ½ closed | Squinted/discharge | Closed/discharge | |
Total possible score = 15; Normal = 0; Found dead = 15.
Mice with score equal or higher than 11 were euthanized.
Clinical score = sum of all parameter scores.
2.5. Intestinal inflammation and histopathology
Four to five mice from each treatment group were sacrificed 24 h post-surgery. Cecum tissues were collected from each mouse for histopathological analysis. One portion was fast frozen for myeloperoxidase assay, the rest portion was flushed with PBS and fixed with 4% Phosphate Buffered Formalin. The fixed tissues were sectioned and stained with hematoxylin and eosin by the EM/Histology Lab, Department of Pathology, University of Maryland Baltimore. Overall damage was analyzed by two pathologists who were blinded to the identity of each sample. Damage scores were based on five criteria each graded on a scale from 0 to 3 (normal, mild, moderate to severe) and added together to generate a score with a maximum value of 15. The criteria included were epithelial cell and architectural disruption, hemorrhagic congestion, mucosal edema, mucosal depletion, and inflammatory cell infiltration and inflammation.
2.6. Myeloperoxidase (MPO) assay
To measure neutrophil myeloperoxidase (MPO) activity, a portion of the resected cecum was freeze-dried and homogenized in 1 mL of 50 mM pH 6.0 potassium phosphate buffer containing 0.5% hexadecyl trimethyl ammonium bromide. The tissues were disrupted with both sonication and freeze-thaw cycles, and then centrifuged. The supernatant was aliquoted and its protein concentration was measured (Nanodrop 2000C, ThermoScientific). MPO activity in the supernatants was determined using TMB peroxidase substrate (KPL) followed by measurement of the absorbance of the samples at 450 nm using a plate reader. MPO activity (in units per milligram of total protein) was calculated according to the standard curve generated using purified MPO from human leukocytes (Sigma).
2.7. Cell rounding assay
The toxicity of sera and pleural fluids were assessed by cell rounding assays. Vero cells (ATCC) seeded in 96-well plates were treated with either serum or pleural fluid samples. Cell rounding was visualized by phase-contrast microscopy. Each sample was tested in triplicate for overall cell rounding.
2.8. Statistical analysis
Clinical score, histology score, and MPO were analyzed using Two-tailed Welch's correction unpaired t-test to determine statistical differences. 72 h post-surgery survival curves for mice in different treatment groups were analyzed to compare the sensitivity to different doses of TcdA and/or TcdB. In this analysis, mice with scores 11 or greater according to the criteria set forth under “Clinical scoring” were considered moribund and euthanized. The log-rank Mantel-Cox test was used to determine if the survival curves of the various groups were statistically different.
3. Results
3.1. Cecum injection of TcdA induced dose-dependent disease
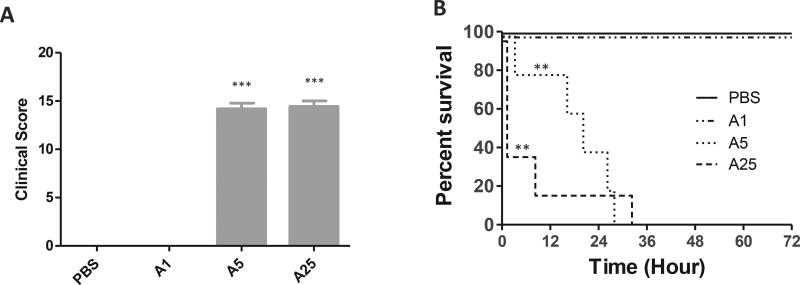
Following the injection of TcdA, disease progression was monitored by clinical scores. A dose-response was clearly observed between groups. Mice treated with 1 µg of TcdA appeared to be normal when examined 24 h post-surgery (Fig. 1A). Their coats and eyes were similar to PBS treated mice, and their posture and activity level were comparable with the PBS group as well. When the dose of TcdA was increased to 5 µg, most mice died within 24 h and none of them survived after 36 h post-injection (Fig. 1B). Death occurred even faster in the 25 µg TcdA treated group, with 60% of mice dying within 2 h post-injection (Fig. 1B). Interestingly, diarrhea was only observed in 25 µg-TcdA treated mice. Those who quickly developed watery diarrhea survived longer. Since mice that died within 24 h of toxin injection were assigned the maximum score, the clinical scores from the two groups that received higher doses of TcdA reached near maximum, significantly higher than the PBS group (Fig. 1 A, B).
Fig. 1. Physical condition and survival after various single doses of TcdA.
A) Clinical scores of mice treated with various doses of TcdA for 24 h. The scores were assessed according to the criteria in Table 2; B) Survival curve. Mice were monitored for survival for 72 h post toxin injection. Mice with a score equal to or higher than 11 were considered moribund and were euthanized. (n = 10 per group) A1: TcdA 1 µg; A5: TcdA 5 µg; A25: TcdA 25 µg *: p < 0.05, vs PBS; **: p < 0.01, vs PBS; ***: p < 0.001, vs PBS.
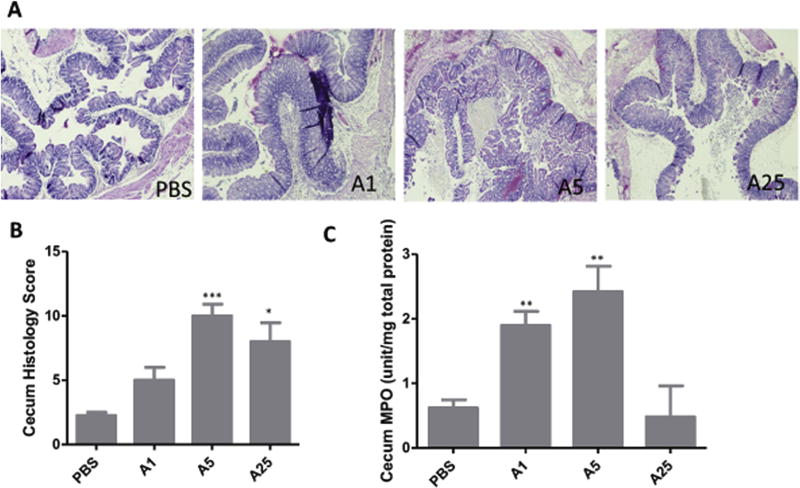
Mice treated with different doses of TcdA also showed different levels of intestinal epithelial tissue damage. Although the 1 µg TcdA group appeared normal clinically, gut tissues collected 24 h post injection showed some histopathological damage: mild to moderate hemorrhagic congestion and inflammatory cell infiltration, with mild to severe mucosal edema (Fig. 2A). However, the average histological score of this group was not significantly different from the PBS treated group (Fig. 2B). Tissues from the 5 µg TcdA group showed severe hemorrhagic congestion, inflammatory cell infiltration and mucosal edema (Fig. 2A). Compared to the 5 µg TcdA group, 25 µg TcdA caused milder damage based on these three histological criteria. That the histological damage was not fully developed may be due to the quick death of the mice (Figs. 2B and 1A). MPO score showed a similar pattern, as the activity of the 25-µg group is less than the 5 µg TcdA group (Fig. 2C). Since most of the mice in the 25 µg TcdA group died within 2 h of toxin injection, the timespan may not permit a significant influx of neutrophil.
Fig. 2. Tissue damage by TcdA.
A) H&E stained ceca. Tissues were collected from mice exposed to TcdA after 24 h and fixed in 4% formalin for H&E staining. B) Average histopathological scores of 4–5 mice with each TcdA treatment; C) MPO activity assay. A small portion of cecum from each mouse was collected and lysed for MPO assay. A1: TcdA 1 µg; A5: TcdA 5 µg; A25: TcdA 25 µg *: p < 0.05, vs PBS; **: p < 0.01, vs PBS; ***: p < 0.001, vs PBS.
3.2. Cecum injection of TcdB induced a dose-dependent disease
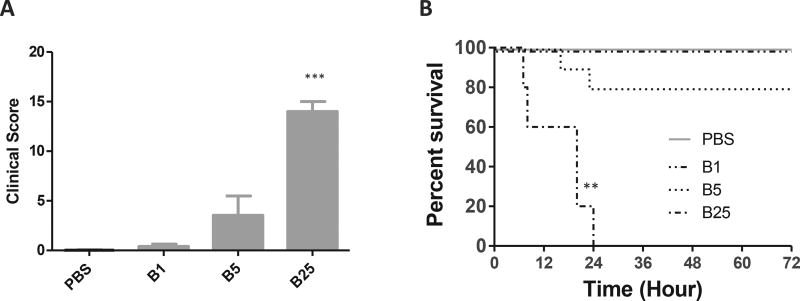
Mice from the 1 µg of TcdB group all appeared normal when examined 24 h post surgery (Fig. 3A). Two mice died within 24 h of toxin injection while all the other mice appeared to be normal in the group of mice injected with 5 µg TcdB (Fig. 3A); hence there were no statistical differences in the clinical score and survival curve between mice in the 5 µg TcdB group and the PBS group (Fig. 3A and B). All mice injected with 25 µg TcdB died within 24 h of injection (Fig. 3B).
Fig. 3. Physical condition and survival after various single doses of TcdB.
A) Clinical scores of mice treated with various doses of TcdB for 24 h. The scores were assessed according to the criteria in Table 2; B) Survival curve. Mice were monitored for survival for 72 h. Mice with a score equal to or higher than 11 were considered moribund and were euthanized (n = 10 per group) B1: TcdB 1 µg; B5: TcdB 5 µg; B25: TcdB 25 µg *: p < 0.05, vs PBS; **: p < 0.01, vs PBS; ***: p < 0.001, vs PBS.
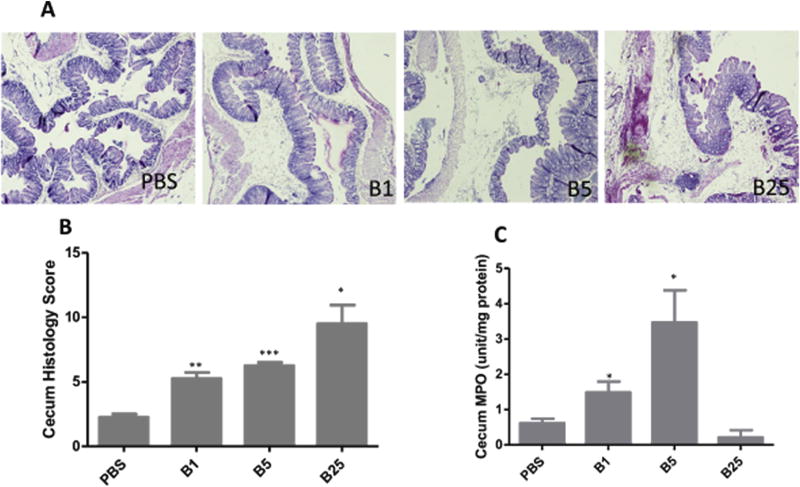
Similar to the TcdA injected group, although most mice looked and behaved normal following 1 µg and 5 µg TcdB injection, some histological damage can be observed. Mild hemorrhagic congestion, mild to moderate inflammatory cell infiltration as well as severe mucosal edema were observed in the tissues from both 1 µg and 5 µg TcdB groups, with more severe histopathological changes in the latter group (Fig. 4A and B). In the 25 µg group, these histological parameters reached severe levels and about half of the epithelial tissues showed damage including epithelial depletion and extensive muscular hemorrhage. The cecum MPO scores after TcdB treatment showed a toxin dose-dependent response pattern among lower dose groups whereas the 25-µg group showed a lesser degree of MPO activity (Fig. 4C).
Fig. 4. Tissue damage by TcdB.
A) H&E stained ceca. Tissues were collected from mice exposed to TcdB after 24 h and fixed in 4% formalin for H&E staining. B) Average histopathological scores of 4–5 mice with each TcdB treatment; C) MPO activity assay. A small portion of cecum from each mouse was collected and lysed for MPO assay. B1: TcdB 1 µg; B5: TcdB 5 µg; B25: TcdB 25 µg *: p < 0.05, vs PBS; **: p < 0.01, vs PBS; ***: p < 0.001, vs PBS.
3.3. TcdA and TcdB showed a synergetic effect on mouse intestines
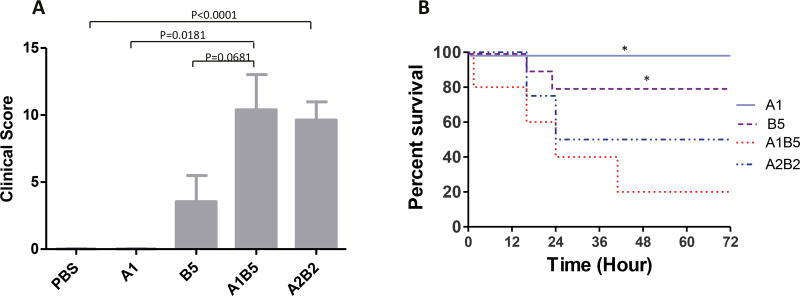
In single toxin treatment experiments, neither 1 µg TcdA nor 5 µg TcdB caused discernible clinical symptoms in surviving mice although their tissues exhibited histopathologic changes. In order to investigate whether TcdA and TcdB have synergistic effects on disease pathogenesis, groups of mice were injected with mixtures of both toxins. Synergistic effects of the two toxins were observed including more severe clinical symptoms, higher mortality and more extensive tissue damage (Figs. 5 and 6). 80% of mice treated with 1 µg TcdA plus 5 µg TcdB (A1B5) died within 40 h, which is significantly higher than the mortality rates of mice treated with either 1 µg TcdA (0%) or 5 µg TcdB (20%) (Fig. 5B). Moreover, mice injected with both TcdA and TcdB rapidly developed clinical symptoms while groups of mice injected with individual toxins appeared to be either normal or have significantly less severe disease (Fig. 5A). Histopathologically, ceca from mice injected with both toxins also exhibited significantly higher tissue damage, inflammation, and histology scores than ceca from mice injected with individual toxins (Fig. 6A and B). In addition to edema and inflammatory cell infiltration, significant hemorrhage occurred in the epithelia, which was not seen in single low-dose toxin groups (Figs. 2A and 4A).
Fig. 5. Synergistic effects of TcdA and TcdB.
Mice were injected with individual toxins (TcdA or TcdB) or their mixtures. The clinical scores at 24 h (A) and survival curves for 72 h (B) are exhibited. Mice with a score (Table 2) equal to or higher than 11 were considered moribund and were euthanized (n = 10 per group) A1: TcdA 1 µg; B5: TcdB 5 µg; A1B5: 1 µg TcdA + 5 µg TcdB; A2B2: 2 µg TcdA + 2 µg TcdB. *: p < 0.05, vs A1B5.
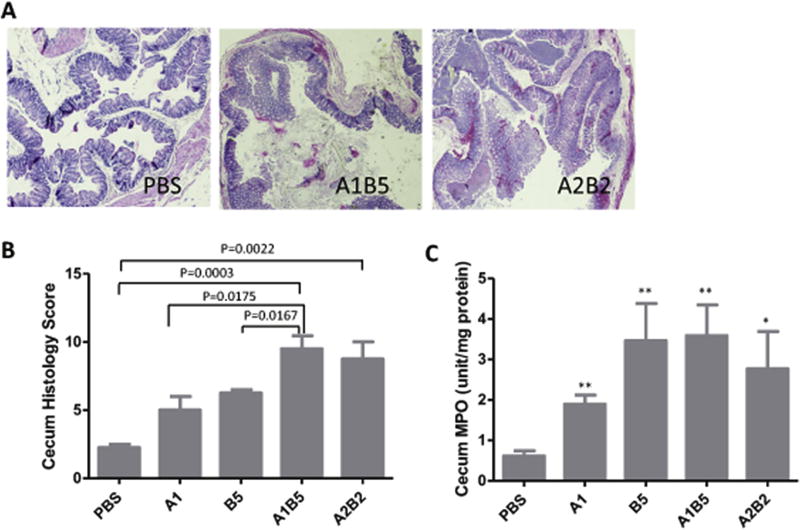
Fig. 6. Synergistic effects of TcdA and TcdB.
A) H&E stained ceca. Tissues were collected from mice exposed to mixtures of TcdA and TcdB after 24 h and fixed in 4% formalin for H&E staining. B) Average histopathological scores of 4–5 mice from each treatment. C) MPO activity assay. A small portion of cecum from each mouse was collected and lysed for MPO assay. A1: TcdA 1 µg; B5: TcdB 5 µg; A1B5: 1 µg TcdA + 5 µg TcdB; A2B2: 2 µg TcdA + 2 µg TcdB. *: p < 0.05, vs PBS; **: p < 0.01, vs PBS.
Next, we investigated the effects of an equal ratio of toxins (2 µg TcdA plus 2 µg TcdB (A2B2)). Similar effects were seen in A2B2 treated mice as in A1B5 treated mice (Figs. 5 and 6). Surprisingly, hemorrhage was more extensive from mucosal to muscle layer in A2B2 treated cecum, which may be due to increased TcdA (Fig. 6A). The MPO activity assay showed that a significant neutrophil influx was induced by both combinations of TcdA and TcdB. Compared with single toxin treated groups, the presence of both toxins resulted in more severe systemic pleural accumulation in the chest (Table 1). The results here demonstrated that C. difficile toxins worked synergistically to induce a more severe disease.
Table 1.
The presence of toxins in systemic effusions after cecum injectiona.
| TcdA positive serum/P.E. |
TcdB positive serum/P.E. |
|
|---|---|---|
| TcdA 1 µg | 1/1 | – |
| TcdA 5 µg | 3/4 | – |
| TcdA 25 µg | 2/2 | – |
| TcdB 1 µg | – | – |
| TcdB 5 µg | – | 2/2 |
| TcdB 25 µg | – | 3/4 |
| TcdA 2µg/TcdB 2 µg | 7/7 | 4/7 |
| TcdA 1µg/TcdB 5 µg | 4/4 | 3/4 |
7 mice were used for each toxin treatment. Total number of mice with toxin in serum or pleural effusion (P. E.) among 7 mice were shown. The presence of toxin was detected by a cell rounding assay.
3.4. Comparison of the in vivo toxicity of TcdB variants
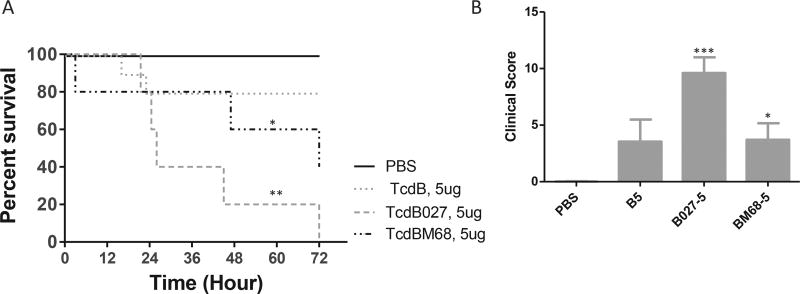
Since TcdB variants were frequently identified in clinical strains, we examined the in vivo toxicity of selected variants. 5 µg of TcdB (RT 087, toxinotype 0), TcdB027 (RT 027, toxinotype III) or TcdBM68 (RT 017, toxinotype VIII) were injected into cecum. All the mice succumbed to TcdB027 within 72 h while 40% of the TcdBM68 treated mice survived (Fig. 7A). 5 µg of TcdB only induced mild disease that 80% of the mice survived (Fig. 7A). The disease induced by TcdB027 was rapidly developed since the average clinical score at 24 h was about 9 compared to 3 in TcdB or TcdBM68 groups (Fig. 7B). The data suggested that hypervirulent TcdB027 more potently initiated disease than the other TcdB variants.
Fig. 7. Comparison of the in vivo toxicity of TcdB variants.
A) Clinical scores of mice treated with TcdB variants for 24 h. The scores were assessed according to the criteria in Table 2; B) Survival curve. Mice were monitored for survival for 72 h. Mice with a score equal to or higher than 11 were considered moribund and were euthanized (n = 10 per group) B5: TcdB 5 µg; B027-5: TcdB027 5 µg; BM68-5: TcdBM68 5 µg *: p < 0.05, vs PBS; **: p < 0.01, vs PBS; ***: p < 0.001, vs PBS.
4. Discussion
The large clostridial toxins TcdA and TcdB are the major virulence factors of C. difficile. Most pathogenic C. difficile strains produce both TcdA and TcdB during the infection, but their relative roles in disease pathogenesis are not well known [8,14,16,17]. No conclusive studies have been reported to clarify the role of individual toxins in CDI manifestation. In terms of determining the pathogenic role of TcdA and TcdB, the infection model is not able to exclude other factors from C. difficile that may affect the progression of disease. The complexity of infection, which includes the level of bacteria colonization, possible unidentified cofactors, and the amount of either toxin produced in the gastrointestinal (GI) tract, hinders the clarification of the roles of the individual toxins in disease pathogenesis. The amount of each toxin produced in the GI tract after bacterial colonization may vary greatly; however, the increased virulence of an epidemic NAP1/BI/027 strain was attributed to its increased secretion of both toxins in vitro [27,28]. In humans, C. difficile colonizes the large intestine and in the mouse infection model, disease mainly occurs in the cecum and colon [29–31]. To avoid the limitations described above, we directly injected defined amounts of purified toxins into mouse ceca to induce disease. Utilizing this cecum injection model, we describe here an overall profile of virulent potency of TcdA and TcdB in the GI tract. In this study, both toxins were capable of inducing disease independently, although earlier studies showed that TcdB might not be the primary virulence factor in animal intestines [8]. The pathogenic responses of the hosts including inflammation, tissue damage and clinical symptoms caused by either toxin were similar to responses observed from C. difficile infected mice [29–31]. Since either toxin alone or their variants were demonstrated to trigger disease, it is evident that both are virulence factors responsible for C. difficile infection. Therefore, treatments against both toxins were necessary for therapeutic interventions.
In this study, we correlated the severity of disease to the amount of toxin present in the GI tract. Apparently, the presence of 5 µg of either toxin was required to develop clinical symptoms in the mouse model. However, TcdA more potently induced disease in this particular model. 5 µg of TcdA was lethal while 5 µg of TcdB only caused 20% death. Even though TcdB027 was most virulent toxin B in this study, it took a longer time to reach the endpoint of death at 5 µg when compared with TcdA. In vitro studies revealed that TcdB is a potent cytotoxin that causes cytopathic and cytotoxic effects in cultured cells within hours, even at a picogram concentrations [32,33]. However, TcdA is approximately 1000 times less cytotoxic to most cultured cells than TcdB in vitro [34], but previous in vivo studies showed that only TcdA was an enterotoxin [8,35–37], suggesting that toxins' behaviors in vivo and in vitro may vary. Human α-defensin was demonstrated to protect host from TcdB induced disease [38]. Therefore, the defenses against toxins, including antibody response from the host immune system, may affect the potency of the toxins and play a certain role in disease progression [39–42]. TcdA seemed to induce more acute responses since high doses of TcdA killed most of the animals within 12 h, while the deaths caused by TcdB at the same dose were delayed for several hours (Figs. 1 and 3). This further supports that TcdA was more potent than TcdB in this model, although the host transcriptional responses to TcdA and TcdB are reportedly similar [43]. But the rapid death caused by TcdA may also be due to shock; however, a few of the mice treated with the highest dose of TcdA quickly produced watery diarrhea which may have flushed out some of the excess toxin and allowed them to survive for a few extra hours, but they still succumbed to death in less than 48 h like those receiving a fifth of the dose. Therefore, the different potency of toxins in vivo and in vitro emphasizes the importance of an in vivo study to understand the virulence of TcdA and TcdB.
In an earlier study, TcdA and TcdB were hypothesized to have synergistic effects based on that TcdB would not damage intestine unless TcdA first disrupted the intestinal epithelium [8]. In the cecum injection model, although either toxin was capable of inducing disease, we did observe the synergistic effects in terms of disease severity. Two different ratios of TcdA and TcdB were tested in this study since the relative amount of secretion of the two toxins during C. difficile infection was unclear. Although both combinations of toxins had synergistic effects, the increased amount of TcdB appeared to aggravate the disease. It is possible that the presence of small amount of TcdA accelerated the effects of TcdB although a higher dose TcdB alone was pathogenic.
To summarize this study, utilizing a cecum injection model, we excluded the impact from C. difficile bacteria and its products other than TcdA and TcdB and demonstrated that both TcdA and TcdB in the GI tract were pathogenic. TcdA more potently evoked CDI-like diseases in cecum injection mouse model and the synergistic effects of the toxins were significant.
Acknowledgments
This work was supported by National Institutes of Health [R01AI088748, R01DK084509, R56AI99458, R43AI129044, R01AI132207 and U19 AI109776]. We thank Ashley Saint Fleur for writing assistance.
References
- 1.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jank T, Aktories K. Structure and mode of action of clostridial glucosylating toxins: the ABCD model. Trends Microbiol. 2008;16:222–229. doi: 10.1016/j.tim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Pruitt RN, Chambers MG, Ng KK, Ohi MD, Lacy DB. Structural organization of the functional domains of Clostridium difficile toxins A and B. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13467–13472. doi: 10.1073/pnas.1002199107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Just I, Wilm M, Selzer J, Rex G, von Eichel-Streiber C, Mann M, et al. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J. Biol. Chem. 1995;270:13932–13936. doi: 10.1074/jbc.270.23.13932. [DOI] [PubMed] [Google Scholar]
- 5.Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Shi L, Yang Z, Zhang Y, Perez-Cordon G, Huang T, et al. Critical roles of Clostridium difficile toxin B enzymatic activities in pathogenesis. Infect. Immun. 2015;83:502–513. doi: 10.1128/IAI.02316-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Z, Zhang Y, Huang T, Feng H. Glucosyltransferase activity of Clostridium difficile Toxin B is essential for disease pathogenesis. Gut microbes. 2015;6:221–224. doi: 10.1080/19490976.2015.1062965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyerly DM, Saum KE, MacDonald DK, Wilkins TD. Effects of Clostridium difficile toxins given intragastrically to animals. Infect. Immun. 1985;47:349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lima AA, Lyerly DM, Wilkins TD, Innes DJ, Guerrant RL. Effects of Clostridium difficile toxins A and B in rabbit small and large intestine in vivo and on cultured cells in vitro. Infect. Immun. 1988;56:582–588. doi: 10.1128/iai.56.3.582-588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell TJ, Ketley JM, Haslam SC, Stephen J, Burdon DW, Candy DC, et al. Effect of toxin A and B of Clostridium difficile on rabbit ileum and colon. Gut. 1986;27:78–85. doi: 10.1136/gut.27.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Triadafilopoulos G, Pothoulakis C, O'Brien MJ, LaMont JT. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology. 1987;93:273–279. doi: 10.1016/0016-5085(87)91014-6. [DOI] [PubMed] [Google Scholar]
- 12.Lonnroth I, Lange S. Toxin A of Clostridium difficile: production, purification and effect in mouse intestine. Acta pathologica, microbiologica, et immunologica Scandinavica Section B. Microbiology. 1983;91:395–400. doi: 10.1111/j.1699-0463.1983.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 13.Leslie JL, Huang S, Opp JS, Nagy MS, Kobayashi M, Young VB, et al. Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect. Immun. 2015;83:138–145. doi: 10.1128/IAI.02561-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savidge TC, Pan WH, Newman P, O'Brien M, Anton PM, Pothoulakis C. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology. 2003;125:413–420. doi: 10.1016/s0016-5085(03)00902-8. [DOI] [PubMed] [Google Scholar]
- 15.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 16.Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–1181. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711–U97. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 18.Carter GP, Chakravorty A, Pham Nguyen TA, Mileto S, Schreiber F, Li L, et al. Defining the roles of TcdA and TcdB in localized gastrointestinal disease, systemic organ damage, and the host response during Clostridium difficile infections. mBio. 2015;6:e00551. doi: 10.1128/mBio.00551-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckert C, Emirian A, Le Monnier A, Cathala L, De Montclos H, Goret J, et al. Prevalence and pathogenicity of binary toxin-positive Clostridium difficile strains that do not produce toxins A and B. New microbes new Infect. 2015;3:12–17. doi: 10.1016/j.nmni.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen A. Clostridium difficile toxins: mediators of inflammation. J. innate Immun. 2012;4:149–158. doi: 10.1159/000332946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerding DN, Johnson S, Rupnik M, Aktories K. Clostridium difficile binary toxin CDT: mechanism, epidemiology, and potential clinical importance. Gut microbes. 2014;5:15–27. doi: 10.4161/gmic.26854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter GP, Rood JI, Lyras D. The role of toxin A and toxin B in the virulence of Clostridium difficile. Trends Microbiol. 2012;20:21–29. doi: 10.1016/j.tim.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Rupnik M, Janezic S. An update on Clostridium difficile toxinotyping. J. Clin. Microbiol. 2016;54:13–18. doi: 10.1128/JCM.02083-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang G, Zhou B, Wang J, He X, Sun X, Nie W, et al. Expression of recombinant Clostridium difficile toxin A and B in Bacillus megaterium. BMC Microbiol. 2008;8:192. doi: 10.1186/1471-2180-8-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cloud J, Noddin L, Pressman A, Hu M, Kelly C. Clostridium difficile strain NAP-1 is not associated with severe disease in a nonepidemic setting. Clin. gastroenterology hepatology official Clin. Pract. J. Am. Gastroenterological Assoc. 2009;7:868–873 e2. doi: 10.1016/j.cgh.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Warren CA, van Opstal EJ, Riggins MS, Li Y, Moore JH, Kolling GL, et al. Vancomycin treatment's association with delayed intestinal tissue injury, clostridial overgrowth, and recurrence of Clostridium difficile infection in mice. Antimicrob. agents Chemother. 2013;57:689–696. doi: 10.1128/AAC.00877-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 28.Akerlund T, Persson I, Unemo M, Noren T, Svenungsson B, Wullt M, et al. Increased sporulation rate of epidemic Clostridium difficile Type 027/NAP1. J. Clin. Microbiol. 2008;46:1530–1533. doi: 10.1128/JCM.01964-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, et al. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135:1984–1992. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Pawlowski SW, Calabrese G, Kolling GL, Platts-Mills J, Freire R, AlcantaraWarren C, et al. Murine model of Clostridium difficile infection with aged gnotobiotic C57BL/6 mice and a BI/NAP1 strain. J. Infect. Dis. 2010;202:1708–1712. doi: 10.1086/657086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun X, Wang H, Zhang Y, Chen K, Davis B, Feng H. Mouse relapse model of Clostridium difficile infection. Infect. Immun. 2011;79:2856–2864. doi: 10.1128/IAI.01336-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S, Shi L, Yang Z, Feng H. Cytotoxicity of Clostridium difficile toxin B does not require cysteine protease-mediated autocleavage and release of the glucosyltransferase domain into the host cell cytosol. Pathogens Dis. 2013;67:11–18. doi: 10.1111/2049-632X.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Shi L, Li S, Yang Z, Standley C, Yang Z, et al. A segment of 97 amino acids within the translocation domain of Clostridium difficile toxin B is essential for toxicity. PloS one. 2013;8:e58634. doi: 10.1371/journal.pone.0058634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaves-Olarte E, Weidmann M, Eichel-Streiber C, Thelestam M. Toxins A and B from Clostridium difficile differ with respect to enzymatic potencies, cellular substrate specificities, and surface binding to cultured cells. J. Clin. investigation. 1997;100:1734–1741. doi: 10.1172/JCI119698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun X, Savidge T, Feng H. The enterotoxicity of Clostridium difficile toxins. Toxins. 2010;2:1848–1880. doi: 10.3390/toxins2071848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly CP, Becker S, Linevsky JK, Joshi MA, O'Keane JC, Dickey BF, et al. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J. Clin. investigation. 1994;93:1257–1265. doi: 10.1172/JCI117080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyerly DM, Lockwood DE, Richardson SH, Wilkins TD. Biological activities of toxins A and B of Clostridium difficile. Infect. Immun. 1982;35:1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giesemann T, Guttenberg G, Aktories K. Human alpha-defensins inhibit Clostridium difficile toxin B. Gastroenterology. 2008;134:2049–2058. doi: 10.1053/j.gastro.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Olson A, Diebel LN, Liberati DM. Effect of host defenses on Clostridium difficile toxin-induced intestinal barrier injury. J. trauma acute care Surg. 2013;74:983–989. doi: 10.1097/TA.0b013e3182858477. discussion 9–90. [DOI] [PubMed] [Google Scholar]
- 40.Bauer MP, Nibbering PH, Poxton IR, Kuijper EJ, van Dissel JT. Humoral immune response as predictor of recurrence in Clostridium difficile infection. Clin. Microbiol. Infect. official Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014;20:1323–1328. doi: 10.1111/1469-0691.12769. [DOI] [PubMed] [Google Scholar]
- 41.Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–193. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 42.Yu H, Chen K, Wu J, Yang Z, Shi L, Barlow LL, et al. Identification of toxemia in patients with Clostridium difficile infection. PloS one. 2015;10:e0124235. doi: 10.1371/journal.pone.0124235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Auria KM, Kolling GL, Donato GM, Warren CA, Gray MC, Hewlett EL, et al. In vivo physiological and transcriptional profiling reveals host responses to Clostridium difficile toxin A and toxin B. Infect. Immun. 2013;81:3814–3824. doi: 10.1128/IAI.00869-13. [DOI] [PMC free article] [PubMed] [Google Scholar]