Abstract
Although rare within the context of 30 000 species of extant fishes, scale-feeding as an ecological strategy has evolved repeatedly across the teleost tree of life. Scale-feeding (lepidophagous) fishes are diverse in terms of their ecology, behaviour, and specialized morphologies for grazing on scales and mucus of sympatric species. Despite this diversity, the underlying ontogenetic changes in functional and biomechanical properties of associated feeding morphologies in lepidophagous fishes are less understood. We examined the ontogeny of feeding mechanics in two evolutionary lineages of scale-feeding fishes: Roeboides, a characin, and Catoprion, a piranha. We compare these two scale-feeding taxa with their nearest, non-lepidophagous taxa to identify traits held in common among scale-feeding fishes. We use a combination of micro-computed tomography scanning and iodine staining to measure biomechanical predictors of feeding behaviour such as tooth shape, jaw lever mechanics and jaw musculature. We recover a stark contrast between the feeding morphology of scale-feeding and non-scale-feeding taxa, with lepidophagous fishes displaying some paedomorphic characters through to adulthood. Few traits are shared between lepidophagous characins and piranhas, except for their highly-modified, stout dentition. Given such variability in development, morphology and behaviour, ecological diversity within lepidophagous fishes has been underestimated.
Keywords: lepidophagy, Characiformes, grazing, paedomorphosis, mucophagy, pterygophagy
1. Introduction
Better minds than ours have pointed out the link between feeding morphology and diet, and many of the archetypes in evolutionary study are examples of the powerful links between anatomy, performance and ecology. There are some cases that imply that the greater the specialization the more finely-tuned the morphology. There are cichlids in African rift lakes that allegedly specialize in eating the eyes of other cichlids [1,2]. These fishes and sympatric scale-eating cichlids maintain a handedness polymorphism that allows half the population to deliver nasty surprises from the right side of prey while the other half operates on the left side [3]. Ecomorphological studies rely on this link, though when linking shape to diet across an ecosystem there seems to be considerable noise in the system [4,5]. Measuring morphology may be fraught with certain errors, but it may be that the very concept of a narrow dietary niche is quite often to blame. For example, molluscivory represents a near monophagous dietary specialization associated with a narrow suite of morphological characters: large jaw muscles, and robust teeth and jaws [6,7]. But diet and morphology suffer a serious mismatch when the snail-slurping snake Sibon is grouped ecologically with mollusc-mauling myliobatine stingrays [8,9]; the former ratchet snails from their shells with elongate, gracile lower jaws, while the latter crush prey outright with stout jaws fused at the symphysis and pavement-like dentition.
Ecological and morphological specialization are intimately tied in parasitic organisms. Parasites and parasitoids specialize on particular prey species, and their morphologies and life histories are intimately tied to the life cycle of their hosts. Vertebrate parasites are rare relative to those in other animal lineages, but a diversity of fishes specialize in feeding on the mucus and scales of other fishes. Lepidophagous fishes are represented by at least 37 genera of fishes, with this strategy evolving multiple times independently [10,11]. The success of this feeding strategy may lie in it being the carnivorous equivalent of grazing: a fish can be parasitized many times and simply grow the scales and mucus back. Scale-feeders are common in tropical marine and freshwater systems where prey species density and richness are high [3,12–15]. These vertebrate parasites have been associated with some of the fastest rates of morphological evolution recorded in vertebrates, presumably given radically specialized behaviours and morphologies [11].
As with other parasites, scale-feeders show changes in autecology with different stages in their life history and the density of their prey. The degree of lepidophagy within and between species ranges from facultative to obligatory, as well as varying with ontogeny and seasonality, in relation to prey density [16–18]. For example, crescent grunters (Terapon jarbua) feed increasingly more on scales in addition to whole fishes, crustaceans and ectoparasites, throughout their ontogeny [19]. Other lepidophagous fishes consume only scales, and do so throughout their entire ontogeny, such as Bahamian pupfishes (Cyprinodon desquamator) [11] and the bucktooth tetra (Exodon paradoxus) [20]. The phylogenetic diversity of these scale-feeding fishes raises the question: are all lineages converging on similar feeding morphologies and behaviours for scale-feeding? Are scale-feeders overtly similar as a guild and is lepidophagy an ecologically-singular construct?
Using both sister-species and ontogenetic comparisons, we assess developmental and functional themes in lepidophagous characiform fishes (figure 1). We used micro-computed tomography (μCT) scanning, coupled with iodine-enhanced contrast staining (diceCT) [22] to examine two independent lineages of scale feeding characiform fishes from the Neotropics. We compared the scale-eating characin (Roeboides affinis) and the closely related (and similarly sized) ixha (Charax cf. pauciradiatus). The ixha is a dietary generalist its whole life while the aptly named scale-eating characin does just that from transformation to adulthood [23–25]. We also compared the obligate, lifelong scale eater Catoprion mento (wimple piranha) and the lobe-toothed piranha (Pygopristis denticulata), which eats scales when young, but transitions to more complete consumption of other fishes and plants as adults [26–28] (figure 2). This system gives us two species that only eat scales and one species that only eats scales as a juvenile to compare with a non-scale-feeding species and a non-scale-feeding adult. Our objectives were: (i) to contrast the gross morphology of scale-feeding (Catoprion and Roeboides) with non-scale-feeding relatives (Pygopristis and Charax, respectively); (ii) to determine whether some aspects of feeding morphology augment feeding performance in lepidophagous taxa; (iii) to assess morphological convergence in the lepidophagous fishes; and (iv) to assess whether the lepidophagous ‘niche’ in these two lineages is a useful construct.
Figure 1.
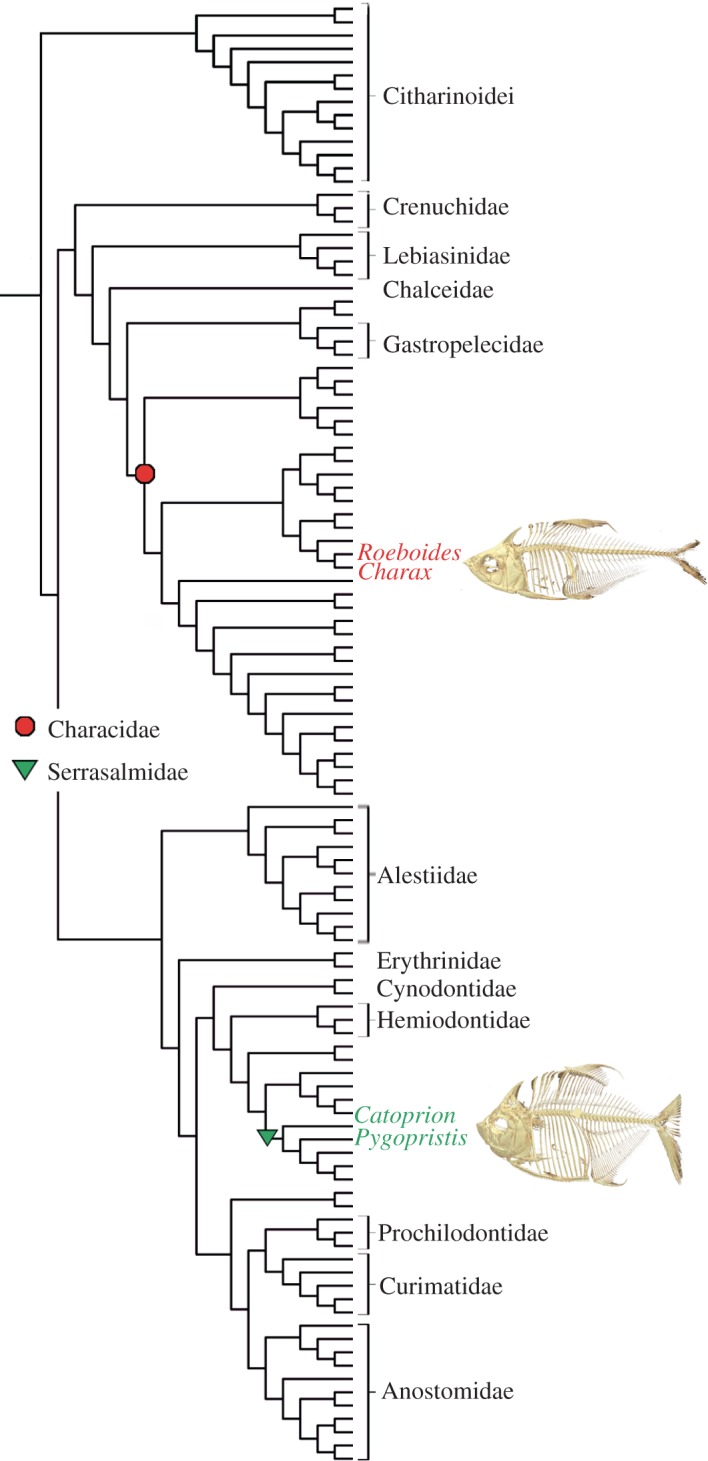
Phylogeny of characiform fishes, modified from Arcila et al. [21] to show the evolutionary separation of serrasalmid scale-feeders from characin scale-feeders.
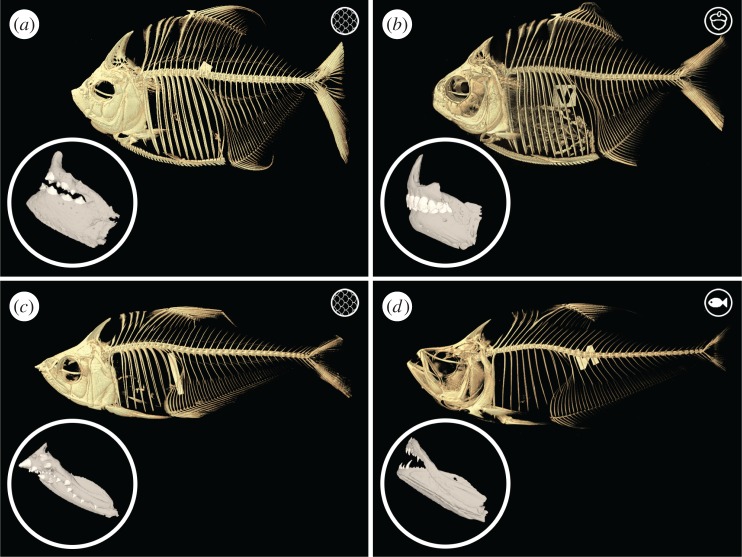
Figure 2.
Lateral view of the reconstructed μCT scans for the four characiform species used in this study. In the lower left corner of each panel is the isolated jaw of each species. (a) Catoprion mento, (b) Pygopristis denticulata, (c) Roeboides affinis, (d) Charax cf. pauciradiatus.
2. Methods
2.1. Specimen acquisition and micro-CT scanning
We used μCT scanning to visualize and measure the cranial morphology of ontogenetic series of four species. Catoprion mento (n = 11) specimens were loaned from the Royal Ontario Museum (Toronto, CA) and Roeboides affinis (n = 8), Pygopristis denticulata (n = 8), and Charax cf. pauciradiatus (n = 8) specimens were obtained from the Auburn University Museum (Auburn, USA). For iodine-contrast staining of each ontogenetic series, we used a subset (n = 6–8) of the previously scanned specimens. Prior to scanning, specimens were tagged with a radiopaque label and then fishes of similar sizes were wrapped in 70% ethanol-soaked cheesecloths and packed tightly into a PLA (polylactic acid)-plastic cylinder.
Specimens were scanned using the Bruker Skyscan 1173 at the Karel F. Liem Bio-Imaging Center at Friday Harbor Laboratories at 65 kV and 123 µA with a voxel size ranging from 17.1 to 33.5 µm. The nature of CT scanning only allows X-ray imaging to capture dense material such as bone, dentine and enamel; however, soft tissues such as muscle and tendon can be visualized through contrast-staining with a chemical agent such as iodine. We modified the iodine contrast-staining method of Gignac & Kley [22], to visualize muscle tissue. All specimens were soaked in an aqueous solution of 3% Lugol's iodine (i.e. 0.75% I2 and 1.5% of KI) for 12 h or until specimens were completely stained. Specimens were then patted dry and prepared for CT scanning as described above.
2.2. Functional morphology of the feeding apparatus
Reconstructed scans were converted to .dcm format and exported into the CT segmentation program, Horos (The Horos Project, 2015 http://www.horosproject.org/). We used the 3D-MPR mode in Horos to measure functional aspects of the cranial morphology of these fishes, including: (i) tooth aspect-ratio for comparing tooth shape and robustness; (ii) occlusional offset, an indicator of slicing or crushing jaw action; (iii) anterior (AMA) and posterior jaw mechanical advantage (PMA), measurements of jaw leverage or force transmittance to prey (electronic supplementary material, figure S1); (iv) jaw adductor muscle cross-sectional area (CSA), a proxy for muscle force generation; and (v) second moment of area of the jaws, a proxy for jaw stiffness in either vertical or lateral bending.
Tooth aspect ratio was calculated as the maximum tooth height divided by its perpendicular maximum tooth width. We measured three undamaged, mature teeth that were representative of overall tooth shape. To measure occlusional offset, we first drew a line from the tip of the anterior most tooth to the posterior most tooth in the lower jaw [29]. Then we measured the orthogonal distance from that axis to the jaw joint. We measured mechanical advantage (a ratio which evaluates trade-offs between jaw leverage and jaw-closing speed), in piranhas from the jaw joint to the insertion point of the adductor mandibulae tendon [30]. However, for the characids we approximated the centre of the concave area in the mandible and used that as the insertion point as muscle-scarring was not immediately obvious. Anterior and posterior out-lever were measured from the jaw joint to the middle of the anterior and posterior tooth, respectively. After iodine-staining, we could clearly observe and segment the primary jaw adductor muscles in Horos. We looked at the primary jaw closing muscle, the adductor mandibulae (only the alpha division), and measured the cross-sectional area of those muscles [31]. For measurements of muscle CSA, we first determined myofibre directionality, made a digital slice through the muscle perpendicular to fibre direction at the estimated centre of muscle mass, and finally measured this area with the polygon tool in Horos. Jaw height and width were measured at two different regions (0%, just adjacent to symphysis, and 90%, at jaw joint) along the central axis of the lower jaw. For calculations of second moment of area, jaw height was considered the major axis while jaw width was considered the minor axis. These values were used in the equation:
where a is jaw height (major axis length) and b is jaw width (minor axis length). We used size-corrected jaw height as a proxy for jaw stiffness in the PCA.
2.3. Statistical analysis
We used analysis of variance (ANOVA) to contrast gross trends in skull skeletal architecture and muscle CSA among lepidophagous and non-lepidophagous taxa over their ontogeny. We size-corrected these data by regressing each morphological trait against each fish's standard length (SL) for ANOVA, calculated the residuals, and used these values as our size-corrected morphometric measures. Since ratios and angular measurements are proportions, and therefore naturally size-corrected, we did not transform mechanical advantage or aspect ratios [32]. We also examined the scaling relationships of how the above, measured morphometric traits changed over ontogeny using reduced major axis regression (RMA) [33] using the lmodel2 package [34]. We used the smatr 3 package [35] to confirm significant differences between these ontogenetic slopes. Scaling data were log-transformed prior to scaling analyses, excluding ratios as above [32]. We also visualized the functional feeding morphospace for all species using a principal components analysis. All statistical tests were analysed using R (www.r-project.org)
3. Results
3.1. Differences in feeding morphology between sister taxa
The two piranhas differed significantly for most traits, including tooth aspect ratio, anterior mechanical advantage, posterior mechanical advantage, jaw length, occlusional offset and adductor muscle CSA. ANOVAs of tooth aspect ratio showed that P. denticulata had longer and narrower teeth than Catoprion, which had stouter more spatulate teeth (0.96 ± 0.04 s.e. versus 0.85 ± 0.02 s.e. respectively, p = 0.013), at all sizes. Catoprion deviated from more scissor-like jaw action, exhibiting greater occlusional offset (3.72 mm ± 0.47 s.e.) relative to Pygopristis (2.59 mm ± 0.46 s.e., p = 0.004). Catoprion (10.0 mm ± 1.27 s.e.) have longer jaws than Pygopristis (6.04 mm ± 0.89 s.e., p < 0.001), which is reflected in Catoprion having lower jaw leverage at the anterior-most tooth compared to Pygopristis. The average AMA of Pygopristis (0.62 ± 0.01 s.e.) was similar to that of Catoprion (0.60 ± 0.01 s.e., 0.03, p = 0.035); however, in Pygopristis jaw leverage at the posterior of the jaws (PMA) (1.19 ± 0.02 s.e.) was less than that of Catoprion (1.45 ± 0.02 s.e., p < 0.001). Second moment of area for the jaws at the symphysis did not differ (1.04 ± 0.03 s.e. versus 0.29 ± 0.02 s.e., p = 0.43), but differed at the jaw joint (0.06 ± 0.03 s.e., versus 0.17 ± 0.09 s.e., p = 0.001) in Catoprion and Pygopristis. Finally, Pygopristis had significantly larger jaw adducting muscles than Catoprion (12.6 mm2 ± 3.2 s.e., 6.2 mm2 ± 1.9 s.e. respectively, p = 0.001).
Compared to the stark morphological contrast between Catoprion and Pygopristis, Roeboides and Charax did not display overt distinctions in feeding morphology. Only tooth aspect ratio, PMA and jaw length differed between these sister characid species. Charax generally had narrower, pointed teeth (2.22 ± 0.11 s.e.) than Roeboides (0.868 ± 0.01 s.e., p < 0.0001), which had broader, more robust teeth. Jaw length was greater in Charax over Roeboides (Charax: 10.32 ± 1.16 s.e., Roeboides: 8.64 mm ± 0.91 s.e., p < 0.001), evident in the noticeable overbite in the latter characid species. Occlusional offset (0.09) and anterior mechanical advantage (p = 0.089) were statistically indistinguishable between Roeboides and Charax. However, Charax had greater posterior mechanical advantage (8.66 ± 0.4 s.e.) relative to Roeboides (0.683 ± 0.02 s.e., p = 0.003). Second moment of area for the jaws differed at both the symphysis (0.03 ± 0.01 s.e. versus 0.01 ± 0.01 s.e., p = 0.023) and the jaw joint (0.06 ± 0.03 s.e., versus 0.18 ± 0.06 s.e., p < 0.001) in Roeboides and Charax. Charax also had significantly larger adductor muscles than Roeboides (2.29 mm2 ± 0.52 s.e., 3.07 ± 0.84 s.e., respectively, p = 0.006).
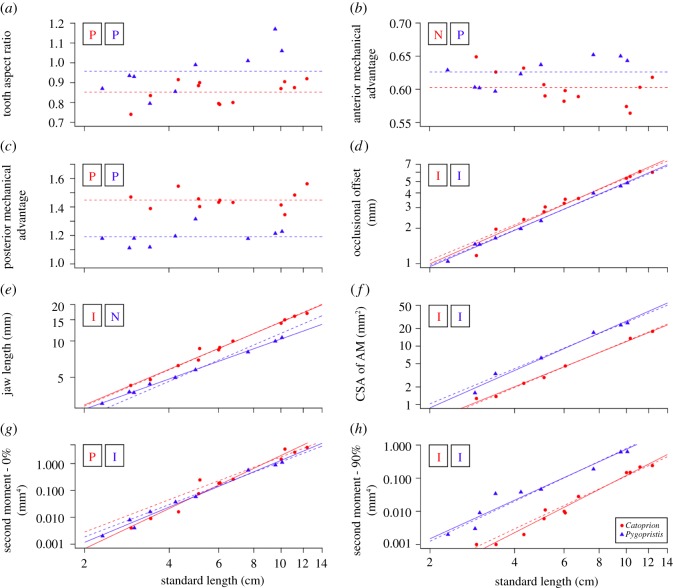
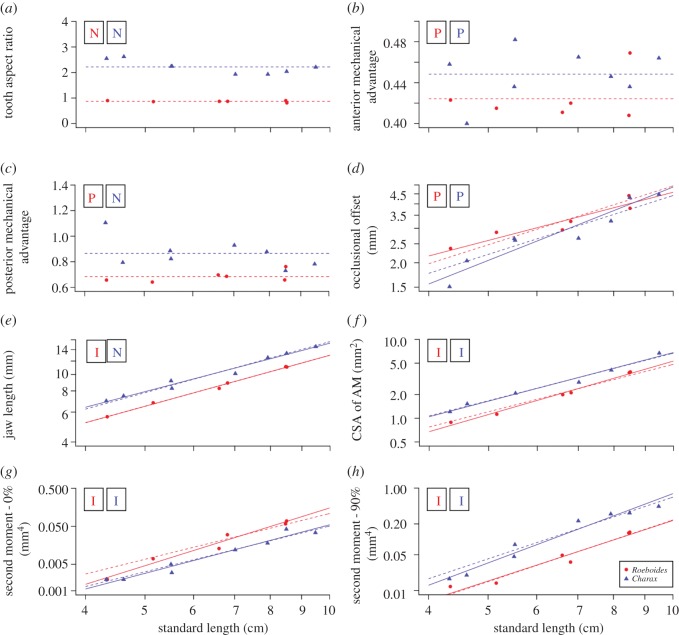
3.2. Intraspecific ontogenetic change in feeding morphology
Tooth aspect ratio scaled with positive allometry in both piranha species over ontogeny (Catoprion: slope = 0.15, Pygopristis: slope = 0.21; table 1, figure 3). However, the manner in which the teeth occlude did not deviate across ontogeny; occlusional offset showed isometric growth for both piranha species (Catoprion: slope = 1.05, Pygopristis: slope = 1.02; table 1, figure 3). In Catoprion, jaw length scaled isometrically, while jaw length in Pygopristis scaled with negative allometry, shortening relative to body length over ontogeny (Catoprion: slope = 0.98, Pygopristis: slope = 0.83; table 1, figure 3). While anterior mechanical advantage scaled with negative allometry in C. mento, in P. denticulata jaw leverage scaled positively (Catoprion: slope = −0.08, Pygopristis: slope = 0.063; table 1, figure 3). However, the slopes of these anterior mechanical advantage lines were not significantly different (p = 0.79; table 1). Conversely, mechanical advantage at the rear of the jaws scaled with positive allometry in both C. mento and P. denticulata (Catoprion: slope = 0.09, Pygopristis: slope = 0.09, figure 3). Scaling of second moment of area at the symphysis differed between piranhas, scaling with positive allometry in Catoprion but not in Pygopristis (Catoprion: slope = 4.96, Pygopristis: slope = 4.36, figure 3), but did not differ at the jaw joint (isometry; Catoprion: slope = 4.33, Pygopristis: slope = 3.86). Finally, the cross-sectional area of the jaw muscles grew isometrically in both C. mento and P. denticulata (Catoprion: slope = 1.95, Pygopristis: slope = 2.13; table 1, figure 3).
Table 1.
Scaling of jaw morphology over ontogeny in Catoprion mento and Pygopristis denticulata. For scaling scenarios, I = isometric growth, P = positive allometry and N = negative allometry.
| independent variables | r2 | isometric slope | intercept | slope | confidence intervals | p | scaling scenario | Δ slope | elevation? (p =) | shift? |
|---|---|---|---|---|---|---|---|---|---|---|
| Tooth Aspect RatioC | 0.243 | 0 | 0.622 | 0.125 | 0.070–0.224 | 5.16 × 10−2 | P | 0.16 | <0.001 | 0.5 |
| Tooth Aspect RatioP | 0.654 | 0 | 0.632 | 0.211 | 0.127–0.349 | 4.17 × 10−3 | P | |||
| Ant. MAC | 0.386 | 0 | 0.700 | −0.053 | (−0.090)–(−0.031) | 1.54 × 10−2 | N | 0.79 | 0.007 | 0.5 |
| Ant. MAP | 0.612 | 0 | 0.566 | 0.039 | 0.023–0.067 | 6.37 × 10−3 | P | |||
| Post. MAC | 0.004 | 0 | 1.206 | 0.132 | 0.068–0.254 | 4.20 × 10−1 | P | 0.72 | <0.001 | <0.001 |
| Post. MAP | 0.206 | 0 | 1.020 | 0.111 | 0.054–0.231 | 1.10 × 10−1 | P | |||
| Occlusional OffsetC | 0.958 | 1 | −0.737 | 1.057 | 0.916–1.220 | 1.60 × 10−8 | I | 0.68 | 0.01 | 0.15 |
| Occlusional OffsetP | 0.995 | 1 | −0.770 | 1.026 | 0.961–1.095 | 1.66 × 10−9 | I | |||
| Jaw LengthC | 0.986 | 1 | 0.397 | 0.983 | 0.905–1.067 | 5.94 × 10−11 | I | 0.001 | <0.001 | 0.06 |
| Jaw LengthP | 0.998 | 1 | 0.419 | 0.834 | 0.799–0.870 | 7.65 × 10−11 | N | |||
| Muscle CSAC | 0.993 | 2 | −1.998 | 1.946 | 1.770–2.139 | 6.50 × 10−7 | I | 0.28 | <0.001 | 0.53 |
| Muscle CSAP | 0.983 | 2 | −1.608 | 2.127 | 1.781–2.541 | 5.16 × 10−5 | I | |||
| Second Moment-0C | 0.960 | 4 | −10.735 | 4.964 | 4.311–5.751 | 1.33 × 10−8 | P | 0.13 | 0.65 | 0.20 |
| Second Moment-0P | 0.984 | 4 | −9.779 | 4.365 | 3.901–4.885 | 7.44 × 10−8 | I | |||
| Second Moment-90C | 0.974 | 4 | −12.090 | 4.331 | 3.868–4.849 | 1.44 × 10−9 | I | 0.30 | <0.001 | 0.84 |
| Second Moment-90P | 0.942 | 4 | −9.186 | 3.861 | 3.121–4.776 | 6.79 × 10−6 | I |
Figure 3.
Reduced-major axis regressions of feeding morphology traits and standard length in Catoprion mento, and Pygopristis denticulata over ontogeny. (a) Tooth aspect ratio, (b) anterior mechanical advantage, (c) posterior mechanical advantage, (d) occlusional offset, (e) jaw length, (f) jaw adductor cross-sectional area, (g) second moment of area at the jaw joint (0%), (h) second moment of area at the jaw symphysis (90%). The dotted lines indicate the predicted isometric curve. Boxes represent the scaling pattern displayed by each species: N = negative allometry, P = positive allometry, I = isometric growth. Scale-feeders are outlined in red, their non-lepidophagous relatives outlined in blue.
For Roeboides and Charax, tooth aspect ratio scaled with negative allometry in both species (Roeboides: slope = −0.14, Charax: slope = −0.39; table 2, figure 4). Posterior mechanical advantage scaled with positive allometry in Roeboides and with negative allometry in Charax (Roeboides: slope = 0.23, Charax: slope = −0.43; table 2, figure 4), but the difference between these slopes was not found to be significantly different (p = 0.79). Jaw length grew isometrically in both species of characid fishes (Roeboides: slope = 0.99, Charax: slope = 0.94, figure 4). Scaling of jaw second moment of area scaled isometrically and did not differ at either the symphysis (Roeboides: slope = 5.05, Charax: slope = 4.24; table 2, figure 4) or the jaw joint (Roeboides: slope = 3.92, Charax: slope = 4.49). The adductor muscles of Roeboides and Charax also grew isometrically (Roeboides: slope = 2.25, Charax: slope = 2.04; table 2, figure 4).
Table 2.
Scaling of jaw morphology over ontogeny in Roeboides affinis and Charax cf. pauciradiatus. For scaling scenarios, I = isometric growth, P = positive allometry and N = negative allometry.
| independent variables | r2 | isometric slope | intercept | slope | confidence intervals | p | scaling scenario | Δ slope | elevation? (p =) | shift? |
|---|---|---|---|---|---|---|---|---|---|---|
| Tooth Aspect RatioR | 0.164 | 0 | 1.098 | −0.123 | (−0.355)–(−0.043) | 2.13 × 10−1 | N | 0.002 | <0.001 | <0.001 |
| Tooth Aspect RatioC | 0.601 | 0 | 3.870 | −0.891 | (−1.615)–(−0.491) | 1.19 × 10−2 | N | |||
| Ant. MAR | 0.108 | 0 | 0.268 | 0.084 | 0.028–0.248 | 2.62 × 10−1 | P | 0.98 | 0.13 | 0.31 |
| Ant. MAC | 0.061 | 0 | 0.289 | 0.086 | 0.036–0.203 | 2.77 × 10−1 | P | |||
| Post. MAR | 0.361 | 0 | 0.381 | 0.162 | 0.062–0.422 | 1.04 × 10−1 | P | 0.12 | 0.003 | 0.008 |
| Post. MAC | 0.303 | 0 | 1.604 | −0.399 | (−0.851)–(−0.187) | 7.87 × 10−2 | N | |||
| Occlusional OffsetR | 0.837 | 1 | −1.786 | 2.712 | 1.589–4.629 | 5.28 × 10−3 | P | 0.09 | 0.06 | 0.64 |
| Occlusional OffsetC | 0.889 | 1 | −3.625 | 3.546 | 2.556–4.919 | 2.25 × 10−4 | P | |||
| Jaw LengthR | 0.995 | 1 | 0.263 | 0.999 | 0.909–1.098 | 4.08 × 10−6 | I | 0.49 | <0.001 | 0.62 |
| Jaw LengthC | 0.977 | 1 | 0.543 | 0.949 | 0.815–1.105 | 2.04 × 10−6 | N | |||
| Muscle CSAR | 0.989 | 2 | −3.513 | 2.251 | 1.942–2.608 | 2.43 × 10−5 | I | 0.31 | <0.001 | 0.82 |
| Muscle CSAC | 0.982 | 2 | −2.791 | 2.045 | 1.697–2.466 | 6.35 × 10−5 | I | |||
| Second Moment-0R | 0.964 | 4 | −13.512 | 5.051 | 3.896–6.548 | 2.43 × 10−4 | I | 0.22 | <0.001 | 0.61 |
| Second Moment-0C | 0.955 | 4 | −12.679 | 4.245 | 3.437–5.244 | 1.48 × 10−5 | I | |||
| Second Moment-90R | 0.948 | 4 | −10.489 | 3.923 | 2.869–5.364 | 5.25 × 10−4 | I | 0.38 | <0.001 | 0.55 |
| Second Moment-90C | 0.960 | 4 | −10.592 | 4.492 | 3.683–5.478 | 1.02 × 10−5 | I |
Figure 4.
Reduced-major axis regressions of feeding morphology traits and standard length in Roeboides affinis, and Charax cf. pauciradiatus over ontogeny. (a) Tooth aspect ratio, (b) anterior mechanical advantage, (c) posterior mechanical advantage, (d) occlusional offset, (e) jaw length, (f) jaw adductor cross-sectional area, (g) second moment of area at the jaw joint (0%), (h) second moment of area at the jaw symphysis (90%). The dotted lines indicate theoretical isometric growth. Boxes represent the scaling pattern displayed by each species: N = negative allometry, P = positive allometry, I = isometric growth. Scale-feeders are outlined in red, their non-lepidophagous relatives outlined in blue.
3.3. Similarities between lepidophagous taxa
Of the ontogenetic jaw mechanics traits shared between species pairs, both serrasalmids and characids, only isometric growth of the lower jaw and CSA of the adductor muscle were similar between scale-feeding fishes. Between C. mento and R. affinis, a similar trait found in common was tooth aspect ratio, which had values that were similar to each other (0.85 ± 0.02 s.e. and 0.86 ± 0.01 s.e., respectively), as well as these teeth being significantly stouter than their non-scale-eating counterparts. Additionally, the CSAs of the scale-feeding fishes were significantly smaller than their respective sister taxa.
Characid and serrasalmid species pairs showed elevational changes between their respective regression lines, i.e. where the ontogenetic trajectory of one species was higher than its sister taxon. Pygopristis and Charax had significantly more cuspidate teeth throughout their ontogeny relative to Catoprion and Roeboides (respectively), although the slopes of these relationships were indistinguishable between sister taxa (tables 1 and 2). A similar pattern was evident for muscle CSA, where non-lepidophagous taxa had conspicuously larger muscle masses consistently over ontogeny than their scale-feeding counterparts (tables 1 and 2). No other morphological similarities were apparent among the two lepidophagous taxa.
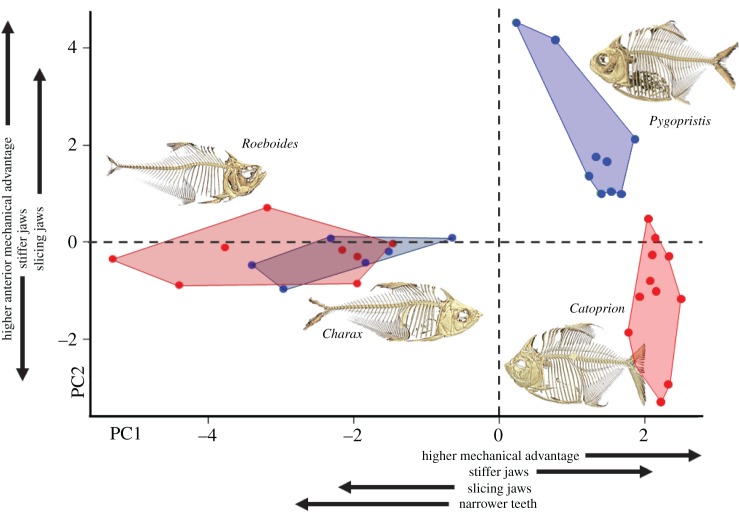
Scale-feeding taxa functionally resemble their sister taxon more closely than other scale-feeders. Roeboides and Charax largely overlap in their trait values, having similar mechanical advantages and jaw morphologies. The results of the PCA (electronic supplementary material, table S1) show that while individuals of Catoprion and Pygopristis generally do not overlap functionally, Roeboides and Charax have largely similar mechanical configurations (figure 5). The PCA loadings showed trends corroborated by ANOVA and regression results, e.g. Charax generally had narrower, more pointed teeth than Roeboides, which had broader, more robust teeth. Both characins had narrower teeth relative to the serrasalmid taxa. The average AMA of Pygopristis was greater than that of Catoprion; however, both serrasalmids had greater mechanical advantage than either characin taxa. Pygopristis had jaw occlusion suitable for slicing action, while Catoprion had jaws built for gripping or crushing. Both scale-feeders however had more robust jaws (more material distributed around a neutral axis in the Y-plane) than their non-lepidophage cousins.
Figure 5.
A principal component analysis of functional feeding traits from all characiform species in this study. Convex hulls are drawn around each species, points are individual specimens. Scale-feeder hulls are outlined in red, their non-lepidophagous relatives outlined in blue.
4. Discussion
4.1. Is scale-feeding an ecomorphological monolith?
Mechanically and functionally, the lepidophagous fishes we examined have little in common. There is no archetypal morphology for scale-feeding as seen for some other dietary strategists like hard prey crushers (robust teeth and jaws, large jaw muscles), piscivores (large epaxial muscles, protrusible or tube-like mouths) and herbivores (multicuspid teeth, grinding dentition, long gut tract). Instead we find three different morphologies, one specialized for bodily ramming into prey to dislodge small scales (Roeboides), another which feed orally on small scales as a juvenile (Pygopristis) but move on to other prey as an adult, and a third which appears to feed on large scales consistently throughout its ontogeny (Catoprion) [23,36]. Roeboides' morphospace largely overlaps with its sister taxon Charax, with only a opisthognathous jaw covered by stout teeth distinguishing the two. This shared morphospace is not the same as that of the piranhas, which have completely non-overlapping morphospaces (figure 5). The many-to-one mapping scheme can be used to explain morphological diversity in ecological guilds [5], resulting from functional equivalency in phylogenetically-conserved systems, yet here we find different functional outcomes mismatched to an overly-broad ecological category.
We believe these examples of lepidophagy are in fact very different niches from a functional and dietary point of view. The guts of Catoprion and Roeboides are packed with scales, but scales of very different size and number (figure 6). Catoprion eat large scales throughout their ontogeny [28,36]; in one specimen with 19 scales in its stomach, the average scale size was 80% the estimated maximum gape (assuming a gape angle of 120° [36]), and 14% larger than the length of the lower jaw. Larger scales are often found on larger fishes, which may be attractive to scale-feeders which retain a lepidophagous ecology as large adults [19]. Given the sort of niche partitioning diversity evident in ‘narrow-niche’ fishes like wood-eating catfishes [37], why should we not expect similar patterns to be replicated in the numerous scale-feeding fishes inhabiting similar habitats? Scale-feeding fishes typically inhabit an ecological niche-continuum spanning mucophages, and presumably pterygophages (perhaps even ectoparasite feeders [19,38]), facilitating specialization on any of these nuances of vertebrate ectoparasitism.
Figure 6.
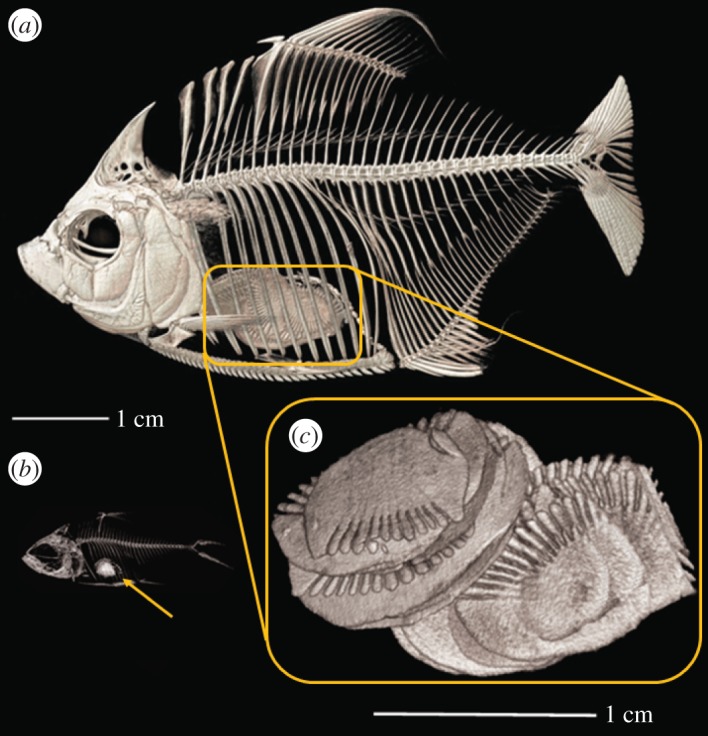
Scales in the gut of Catoprion mento. Adult (a) and juvenile (b) fishes have large scales (c) in their stomachs.
Catoprion is one of the few fish we know of that specialize in scales that are very large relative to their gape throughout ontogeny (figure 6, see Terapon jarbua [19]). As juveniles, wimple piranhas are one of many neotropical lepidophagous freshwater fishes, but as adults they alone among scale-feeders exploit the scales of comparably large prey [19,36,38]. This stands in contrast to lepidophagous characins, which consume small scales at small sizes and even when these characins reach larger sizes, continue to eat greater quantities of small scales rather than larger ones [19,39]. Adult Catoprion are also distinguished from most other scale-feeders (and other piranhas) in being a solitary predator, while even large Roeboides and Terapon are found interspersed among their prey or in shoals with conspecifics [38,40]. Juvenile lepidophagous characins occasionally mimic their prey, and are almost always found in schools. In contrast, adult Catoprion control specific territories [27,41], reflecting the need for access to large, mobile prey fishes that pass through these territories. The very traits that make piranhas excellent predators on fishes and fruits [42,43], wide gape, fast jaw closure, strong jaws, are exapted in Catoprion to lever scales from large fishes.
4.2. Development of scale-feeding morphologies
Ontogenetic slopes between lepidophage and non-lepidophage relatives show distinct differences in slope elevation while exhibiting few differences in actual slope. This pattern is consistent with the hypothesis that static allometries between species are difficult to evolve while allometric slope elevations are more readily evolvable [44–46]. In theory, natural selection can act more readily on slope elevations in static allometries because differences in elevation reflect differences in relative trait sizes within population means. Roeboides and Charax exhibit indistinguishable allometric slopes for tooth aspect ratio; however, the allometric elevation (intercept) shows lepidophagous Roeboides have markedly stouter teeth than piscivorous Charax. A similar pattern exists between Catoprion and Pygopristis with regards to tooth shape and posterior jaw leverage (mechanical advantage); Catoprion have markedly greater allometric elevation. These differences likely reflect the need of Catoprion for stout, robust teeth to remove scales and a greater posterior jaw leverage to close the jaw at extreme gape angles [36].
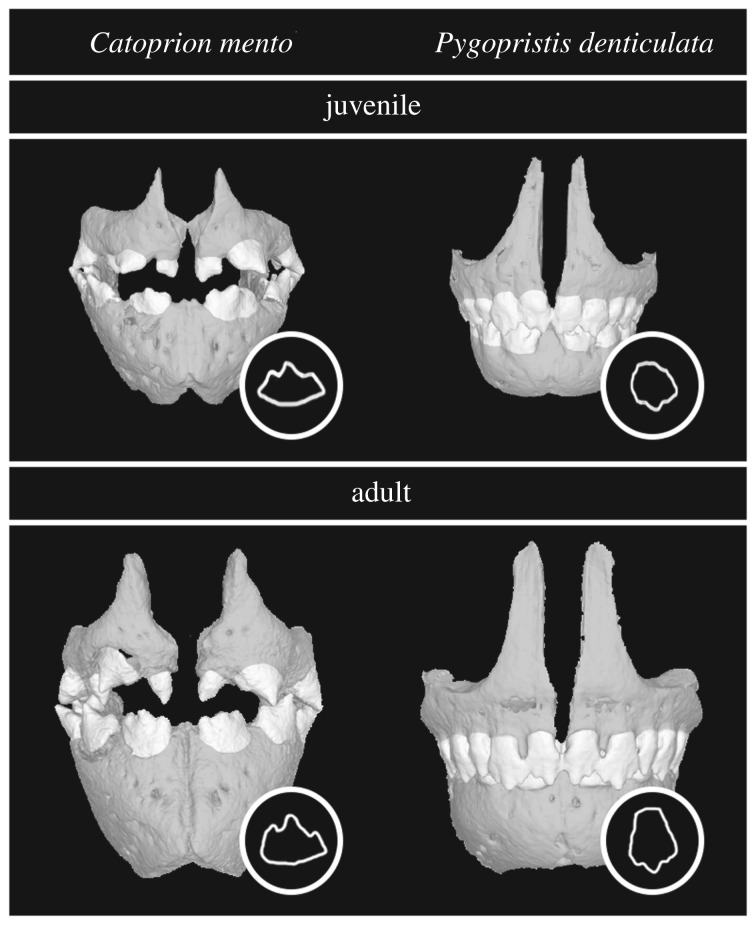
We find evidence for paedomorphosis (retention of juvenilized morphology) (sensu [47,48]) in the evolution and development of several associated feeding morphologies in Catoprion compared with its close generalist relative, Pygopristis. At juvenile stages both species exhibit similar tooth shapes. However, later in development Pygopristis grow longer teeth while Catoprion exhibit less pronounced dental growth (figure 7). The retention of juvenile tooth morphology in Catoprion is consistent with paedomorphosis. These juvenilized teeth may aid in lepidophagous habits as they exhibit a smaller aspect ratio, suggesting that the teeth are more resistant to breakage [43].
Figure 7.
Comparison of tooth shape in Catoprion mento and Pygopristis denticulata as juveniles and as adults. Tooth outlines in lower right corner of each species' panel.
We might expect that these observed patterns of mosaic heterochrony allow scale-feeding fishes with diverse ancestral bauplans to converge on similar end phenotypes (i.e. odontocetes and crocodylians [49]). This is not the case, for across diverse lineages with diverse ancestral bauplans, lepidophagous fishes and other dietary specialists (e.g. compare piscivores [50,51]), fail to converge on similar functional, behavioural, or even ecological outcomes. Rather than invoke many-to-one mapping as a panacea for when morphology cannot predict ecology, we maintain that this mismatch lies instead with broad-brush attempts to reduce the functional diversity in natural history to ecological placeholders like guilds and trophic levels. Given that scale-feeding fishes exhibit variability in both the extent (ecology) and duration (ontogeny) of lepidophagy, these fishes offer a potent system for examining morphological and ecological specialization, and what relationships exist (or don't) between these paradigms.
4.3. A behavioural hypothesis for tooth form and function in lepidophagous fishes
Our data agree with prior studies, all demonstrating that shared morphological adaptations for scale-feeding (at least among Characiformes) involve specialized dentition [41,52]. Catoprion and Roeboides both attack their prey head on at roughly 90° angles, typically using ram attacks to dislodge scales [17,36]. African scale-feeding cichlids, Perissodus straeleni and Perissodus microlepis, have recurved laminar teeth that they use to laterally pry scales from prey [20,52]. These species maintain mouth-to-prey contact as they rotate along the long axis of their body to remove scales. Similarly, juvenile Oligoplites use hook-shaped teeth to attack prey from the rear, scraping scales parallel to the long axis of their prey's body [13,41]. The major difference between these two types of scale-feeding behaviours, perpendicular ram-feeding and orthogonal scraping, are distinguished by the tooth shape they require. Ram feeding requires stout teeth that withstand the impact, while scraping is facilitated by recurved teeth which pry scales from prey.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We dedicate this manuscript to D. Taphorn, a mentor and friend, as well as a prodigious source of information on neotropical fishes. We especially thank D. Stacey, E. Holm and H. Lopez-Fernandez from the Royal Ontario Museum, D. Werneke and J. Armbruster from the Auburn University Museum, and R. Robins at the Florida Museum of Natural History for providing specimens and advice. We also thank A. von Hagel, S. Farina, C. Wells and A. Cairns for technical support and advice. We thank two anonymous reviewers for their comments, which made this manuscript much stronger. We also thank Friday Harbor Laboratories for a stimulating learning environment and the camaraderie of the FHL Zoobot Quarter Program for Spring 2017.
Ethics
No special collecting permit or ‘Animal Care Protocol’ was required; specimens used in this study have been deposited in museums, and have been deceased for decades. No fieldwork was required for this study.
Data accessibility
Our code and data are deposited at Dryad (http://dx.doi.org/10.5061/dryad.q4157) [53]. CT scan data are available using the following MorphoSource identifiers: Catoprion mento (S6825, S8029, M16103, M16136, M17551), Pygopristis denticulata (M16105–16113), Roeboides affinis as R. thurni (M17594–17599, S9555) and Charax sp. as C. pauciradiatus (M20028).
Authors' contributions
M.A.K. and K.E. conceived the study; J.M.H. carried out morphological measurements and data collection, participated in data analysis, created figures and tables, and drafted the first version of manuscript; M.A.K. and A.P.S. coordinated the study; M.A.K. led computed tomography scanning and reconstruction, assisted with data collection and led data analysis, created figures, and drafted the final version of manuscript with A.P.S., J.M.H., and K.E. All authors gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
M.A.K. was supported by a Friday Harbor Laboratories Postdoctoral Fellowship and a NSF PRFB, DBI for Expanding Museum collections. J.M.H. was supported by a Friday Harbor Laboratories Mary Gates Scholarship. K.E. came to the labs on a Wainwright Fellowship. A.P.S. is supported by NSF DEB-1701665 and IOS-1256602. All scanning was done using the Bruker Skyscan 1173, which is supported by an endowment for the Karel Liem Memorial Bio-Imaging facility.
References
- 1.Liem KF, Osse JWM. 1975. Biological versatility, evolution, and food resource exploitation in African cichlid fishes. Am. Zool. 15, 427–454. (doi:10.1093/icb/15.2.427) [Google Scholar]
- 2.Wickler W. 1966. Ein Augen fressender Buntbarsch. Naturmuseum am Frankfurt. 96, 311–315. [Google Scholar]
- 3.Lee HJ, Kusche H, Meyer A. 2012. Handed foraging behavior in scale-eating cichlid fish: its potential role in shaping morphological asymmetry. PLoS ONE 7, e44670 (doi:10.1371/journal.pone.0044670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wainwright PC, Alfaro ME, Bolnick DI, Hulsey CD. 2005. Many-to-one mapping of form to function: a general principle in organismal design? Int. Comp. Biol. 45, 256–262. (doi:10.1093/icb/45.2.256) [DOI] [PubMed] [Google Scholar]
- 5.Young RL, Haselkorn TS, Badyaev AV. 2007. Functional equivalence of morphologies enables morphological and ecological diversity. Evolution 61, 2480–2492. (doi:10.1111/j.1558-5646.2007.00210.x) [DOI] [PubMed] [Google Scholar]
- 6.Grubich J. 2003. Morphological convergence of pharyngeal jaw structure in durophagous perciform fish. Biol. J. Linn. Soc. 80, 147–165. (doi:10.1046/j.1095-8312.2003.00231.x) [Google Scholar]
- 7.Schaerlaeken V, Holanova V, Boistel R, Aerts P, Velensky P, Rehak I, Andrade DV, Herrel A. 2012. Built to bite: feeding kinematics, bite forces, and head shape of a specialized durophagous lizard, Dracaena guianensis (Teiidae). J. Exp. Zool. Part A 317, 371–381. (doi:10.1002/jez.1730) [DOI] [PubMed] [Google Scholar]
- 8.Gans C. 1983. Snake feeding strategies and adaptations: conclusion and prognosis. Am. Zool. 23, 455–460. (doi:10.1093/icb/23.2.455) [Google Scholar]
- 9.Summers AP. 2000. Stiffening the stingray skeleton-an investigation of durophagy in myliobatid stingrays (Chondrichthyes, Batoidea, Myliobatidae). J. Morphol. 243, 113–126. (doi:10.1002/(SICI)1097-4687(200002)243:2<113::AID-JMOR1>3.0.CO;2-A) [DOI] [PubMed] [Google Scholar]
- 10.Martin CH, Wainwright PC. 2011. Trophic novelty is linked to exceptional rates of morphological diversification in two adaptive radiations of Cyprinodon pupfish. Evolution. 65, 2197–2212. (doi:10.1111/j.1558-5646.2011.01294.x) [DOI] [PubMed] [Google Scholar]
- 11.Martin CH, Wainwright PC. 2013. On the measurement of ecological novelty: scale-eating pupfish are separated by 168 my from other scale-eating fishes. PloS ONE 8, e71164 (doi:10.1371/journal.pone.0071164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lima MRL, Bessa E, Krinski D, Carvalho LN. 2012. Mutilating predation in the Cheirodontinae Odontostilbe pequira (Characiformes: Characidae). Neotrop. Ichthyol. 10, 361–368. (doi:10.1590/S1679-62252012000200011) [Google Scholar]
- 13.Major PF. 1973. Scale feeding behavior of the leatherjacket, Scomberoides lysan and two species of the genus Oligoplites (Pisces: Carangidae). Copeia 1, 151–154. (doi:10.2307/1442377) [Google Scholar]
- 14.Mok H-K. 1978. Scale-feeding in Tydemania navigatoris (Pices: Triacanthodidae). Copeia 78, 338 (https://doi.org/10.2307/144357) [Google Scholar]
- 15.Nakae M, Sasaki K. 2002. A scale-eating triacanthodid, Macrorhamphosodes uradoi: prey fishes and mouth ‘handedness’ (Tetraodontiformes, Triacanthoidei). Ichthyol. Res. 49, 2–5. (doi:10.1007/s102280200001) [Google Scholar]
- 16.Peterson CC, Winemiller KO. 1997. Ontogenetic diet shifts and scale-eating in Roeboides dayi, a Neotropical characid. Environ. Biol. Fish 49, 111–118. (doi:10.1023/A:1007353425275) [Google Scholar]
- 17.Peterson CC, McIntyre P. 1998. Ontogenetic diet shifts in Roeboides affinis with morphological comparisons. Environ. Biol. Fish 53, 105–110. (doi:10.1023/A:1007487326990) [Google Scholar]
- 18.Evans KM, Crampton WGR, Albert JS. 2017. Taxonomic revision of the deep channel electric fish genus Sternarchella (Teleostei: Gymnotiformes: Apteronotidae), with descriptions of two new species. Neotrop. Ichthyol. 15, 296 (doi:10.1590/1982-0224-20160168) [Google Scholar]
- 19.Whitfield AK, Blaber SJM. 1978. Scale-eating habits of the marine teleost Terapon jarbua. J. Fish Biol. 12, 61–70. (doi:10.1111/j.1095-8649.1978.tb04151.x) [Google Scholar]
- 20.Hata H, Yasugi M, Hori M. 2011. Jaw laterality and related handedness in the hunting behavior of a scale-eating characin, Exodon paradoxus. PLoS ONE 6, e29349 (doi:10.1371/journal.pone.0029349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arcila D, et al. 2017. Genome-wide interrogation advances resolution of recalcitrant groups in the tree of life. Nat. Ecol. Evol. 1, 0020 (doi:10.1038/s41559-016-0020) [DOI] [PubMed] [Google Scholar]
- 22.Gignac PM, Kley NJ. 2014. Iodine-enhanced micro-CT imaging: methodological refinements for the study of the soft-tissue anatomy of post-embryonic vertebrates. J. Exp. Zool. Part B 322, 166–176. (doi:10.1002/jez.b.22561) [DOI] [PubMed] [Google Scholar]
- 23.Hahn NS, Pavanelli CS, Okada EK. 2000. Dental development and ontogenetic diet shifts of Roeboides paranensis Pignalberi (Osteichthyes, Characinae) in pools of the upper Rio Paraná floodplain (state of Paraná, Brazil). Rev. Bras. Biol. 60, 93–99. (doi:10.1590/S0034-71082000000100012) [DOI] [PubMed] [Google Scholar]
- 24.Novakowski GC, Fugi R, Hahn NS. 2004. Diet and dental development of three species of Roeboides (Characiformes: Characidae). Neotrop. Ichthyol. 2, 157–162. (doi:10.1590/S1679-62252004000300008) [Google Scholar]
- 25.Albrecht MP, Reis VCS, Caramaschi ÉP. 2013. Resource use by the facultative lepidophage Roeboides affinis (Günther, 1868): a comparison of size classes, seasons and environment types related to impoundment. Neotrop. Ichthyol. 11, 387–394. (doi:10.1590/S1679-62252013005000007) [Google Scholar]
- 26.Vieira I, Gery J. 1979. Crescimento diferencial e nutrição em Catoprion mento (Characoidei). Peixe lepidófago da Amazônia. Acta Amazonica 9, 143–146. (doi:10.1590/1809-43921979091143) [Google Scholar]
- 27.Sazima I. 1988. Territorial behaviour in a scale-eating and a herbivorous Neotropical characiform fish. Rev. Bras. Biol. 48, 189–194. [Google Scholar]
- 28.Nico L. G. 1991. Trophic ecology of piranhas (Characidae: Serrasalminae) from savanna and forest regions in the Orinoco River basin of Venezuela. Unpublished Ph.D. dissertation, University of Florida, Gainesville. [Google Scholar]
- 29.Anderson PS. 2009. Biomechanics, functional patterns, and disparity in Late Devonian arthrodires. Paleobiology 35, 321–342. (doi:10.1666/0094-8373-35.3.321) [Google Scholar]
- 30.Datovo A, Castro RM. 2012. Anatomy and evolution of the mandibular, hyopalatine, and opercular muscles in characiform fishes (Teleostei: Ostariophysi). Zoology 115, 84–116. (doi:10.1016/j.zool.2011.09.008) [DOI] [PubMed] [Google Scholar]
- 31.Arbour JH, López-Fernández H. 2013. Ecological variation in South American geophagine cichlids arose during an early burst of adaptive morphological and functional evolution. Proc. R. Soc. B 280, 20130849 (doi:10.1098/rspb.2013.0849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfaller JB, Gignac PM, Erickson GM. 2011. Ontogenetic changes in jaw-muscle architecture facilitate durophagy in the turtle Sternotherus minor. J. Exp. Biol. 214, 1655–1667. (doi:10.1242/jeb.048090) [DOI] [PubMed] [Google Scholar]
- 33.Kolmann MA, Huber DR, Motta PJ, Grubbs RD. 2015. Feeding biomechanics of the cownose ray, Rhinoptera bonasus, over ontogeny. J. Anat. 227, 341–351. (doi:10.1111/joa.12342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legendre P. 2014. lmodel2: Model II Regression. R package version 1.7-2.
- 35.Warton DI, Duursma RA, Falster DS, Taskinen S. 2012. smatr 3–an R package for estimation and inference about allometric lines. Methods Ecol. Evol. 3, 257–259. (doi:10.1111/j.2041-210X.2011.00153.x) [Google Scholar]
- 36.Janovetz J. 2005. Functional morphology of feeding in the scale-eating specialist Catoprion mento. J. Exp. Biol. 208, 4757–4768. (doi:10.1242/jeb.01938) [DOI] [PubMed] [Google Scholar]
- 37.Lujan NK, German DP, Winemiller KO. 2011. Do wood-grazing fishes partition their niche?: morphological and isotopic evidence for trophic segregation in Neotropical Loricariidae. Funct. Ecol. 25, 1327–1338. (doi:10.1111/j.1365-2435.2011.01883.x) [Google Scholar]
- 38.Sazima I, Machado FA. 1990. Underwater observations of piranhas in western Brazil. Environ. Biol. Fish 28, 17–31. (doi:10.1007/BF00751026) [Google Scholar]
- 39.Roberts TR. 1970. Scale-eating American characoid fishes with special reference to Probolodus heterostomus. Proc. Calif. Acad. Sci. 38, 383–390. [Google Scholar]
- 40.Sazima I. 2002. Juvenile snooks (Centropomidae) as mimics of mojarras (Gerreidae), with a review of aggressive mimicry in fishes. Environ. Biol. Fish 65, 37–45. (doi:10.1023/A:1019654721236) [Google Scholar]
- 41.Sazima I. 1983. Scale-eating in characoids and other fishes. Environ. Biol. Fish 9, 87–101. (doi:10.1007/BF00690855) [Google Scholar]
- 42.Correa SB, Winemiller KO, López-Fernández H, Galetti M. 2007. Evolutionary perspectives on seed consumption and dispersal by fishes. AIBS Bull. 57, 748–756. (doi:10.1641/B57090) [Google Scholar]
- 43.Grubich JR, Huskey S, Crofts S, Orti G, Porto J. 2012. Mega-Bites: extreme jaw forces of living and extinct piranhas (Serrasalmidae). Sci. Rep. 2, 1009 (doi:10.1038/srep01009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huxley JS. 1924. Constant differential growth-ratios and their significance. Nature 114, 895–896. (doi:10.1038/114895a0) [Google Scholar]
- 45.Smith JM, Burian R, Kauffman S, Alberch P, Campbell J, Goodwin B, Lande R, Raup D, Wolpert L. 1985. Developmental constraints and evolution: a perspective from the Mountain Lake conference on development and evolution. Q. Rev. Biol. 60, 265–287. (doi:10.1086/414425) [Google Scholar]
- 46.Egset CK, Hansen TF, Le Rouzic A, Bolstad GH, Rosenqvist G, Pélabon C. 2012. Artificial selection on allometry: change in elevation but not slope. J. Evol. Biol. 25, 938–948. (doi:10.1111/j.1420-9101.2012.02487.x) [DOI] [PubMed] [Google Scholar]
- 47.Alberch P, Gould SJ, Oster GF, Wake DB. 1979. Size and shape in ontogeny and phylogeny. Paleobiology 5, 296–317. (doi:10.1017/S0094837300006588) [Google Scholar]
- 48.Evans KM, Waltz BT, Tagliacollo VT, Chakrabarty P, Albert JS. 2017. Why the short face? Developmental disintegration of the neurocranium drives convergent evolution in Neotropical electric fishes. Ecol. Evol. 7, 1783–1801. (doi:10.1002/ece3.2704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCurry MR, Evans AR, Fitzgerald EMG, Adams JW, Clausen PD, McHenry CR. 2017. The remarkable convergence of skull shape in crocodilians and toothed whales. Proc. R. Soc. B 284, 20162348 (doi:10.1098/rspb.2016.2348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collar DC, O'Meara BC, Wainwright PC, Near TJ. 2009. Piscivory limits diversification of feeding morphology in centrarchid fishes. Evolution 63, 1557–1573. (doi:10.1111/j.1558-5646.2009.00626.x) [DOI] [PubMed] [Google Scholar]
- 51.Habegger ML, Motta PJ, Huber DR, Deban SM. 2011. Feeding biomechanics in the Great Barracuda during ontogeny. J. Zool. 283, 63–72. (doi:10.1111/j.1469-7998.2010.00745.x) [Google Scholar]
- 52.Takahashi R, Moriwaki T, Hori M. 2007. Foraging behaviour and functional morphology of two scale-eating cichlids from Lake Tanganyika. J. Fish Biol. 70, 1458–1469. (doi:10.1111/j.1095-8649.2007.01423.x) [Google Scholar]
- 53.Kolmann MA, Huie JM, Evans K, Summers AP. 2018. Data from: Specialized specialists and the narrow niche fallacy: a tale of scale-feeding fishes. Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.q4157) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Kolmann MA, Huie JM, Evans K, Summers AP. 2018. Data from: Specialized specialists and the narrow niche fallacy: a tale of scale-feeding fishes. Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.q4157) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Our code and data are deposited at Dryad (http://dx.doi.org/10.5061/dryad.q4157) [53]. CT scan data are available using the following MorphoSource identifiers: Catoprion mento (S6825, S8029, M16103, M16136, M17551), Pygopristis denticulata (M16105–16113), Roeboides affinis as R. thurni (M17594–17599, S9555) and Charax sp. as C. pauciradiatus (M20028).