Abstract
Background and Aims:
A risk of tracheal mucosa injury induced by subglottic suctioning has been raised. Therefore, this prospective randomized study aims to compare the effect of continuous suctioning of subglottic secretions versus intermittent suctioning of subglottic secretions (CSSS vs. ISSS) secretions on tracheal mucosa in front of the suctioning port of the endotracheal tube.
Patients and Methods:
Patients requiring intubation or reintubation in Intensive Care Unit with an expected ventilation duration > 24 h were eligible. Participants received CSSS at −20 mmHg or ISSS at −100 mmHg during 15 s and no suction during 8 s. The effect on tracheal mucosa in front of the suction port was assessed after intubation (T0) and before extubation (T1) using bronchoscopy. Tracheal mucosa damages were graded into five categories (no injury, erythema, edema, ulceration, or necrosis). The occurrence (no injury observed at T0 but present at T1) or the worsening (injury observed at T0 exacerbating at T1) was studied.
Results:
Seventy-three patients were included and 53 patients (CSSS, n = 26 and ISSS, n = 27) were evaluable on the primary endpoint. The occurrence or worsening of tracheal mucosal damages did not differ between the two groups (CSSS, n = 7 [27%] vs. ISSS, n = 5 [17%], P = 0.465). Daily average volume of suctioned secretion was higher with ISSS (74 ± 100 ml vs. 20 ± 25 ml, P < 0.001). Impossibility to aspirate was higher with CSSS (0.14 ± 0.16 per day vs. 0.03 ± 0.07 per day, P < 0.001).
Conclusions:
Our results suggest that tracheal mucosal damages did not differ between CSSS and ISSS. The aspirated volume was higher and impossibility to aspirate was lower with ISSS.
Clinical Trial Registration:
ClinicalTrials.gov Identifier: NCT01555229.
Keywords: Intensive care, intubation, mechanical ventilation, subglottic secretion drainage, tracheal injury
INTRODUCTION
The subglottic secretion drainage above the cuff of an endotracheal tube is recognized as an effective method to prevent ventilator-associated pneumonia (VAP) in critically ill patients.[1,2,3] Nevertheless, some concerns have been raised on the risk of tracheal mucosa damage induced by suctioning both in animal and in critically ill patients.[4,5,6,7,8] The aim of this study was to compare the incidence of endotracheal lesion induced by suctioning with the latest generation of endotracheal tube and to compare the effect of continuous suctioning of subglottic secretions (CSSS) or intermittent suctioning of subglottic secretions (ISSS) on tracheal mucosa damage in intensive care-ventilated patients.
PATIENTS AND METHODS
This was a single-center, randomized study, performed in a Surgical Intensive Care Unit (ICU) at a university hospital in France. Patients requiring intubation or re-intubation and mechanical ventilation in the ICU with an expected ventilation duration of more than 24 h and nonpregnant patients were eligible. Exclusion criterion was patients who had a previous tracheal injury. Eligible patients were orally intubated with an endotracheal tube allowing suction above the cuff (internal diameter = 7.5 Mallinckrodt™ TaperGuard Evac oral tracheal tube, Covidien, MA, USA). The main objective was to compare the effect of CSSS to ISSS on tracheal mucosa damage by fiber-optic bronchoscopy. This study did not evaluate potential lesions secondary to the cuff of the endotracheal tube on tracheal mucosa. Accordingly, only the tracheal zone in front of the subglottic suctioning port was explored. Secondary end points included the mean daily volume of suctioned secretions, the mean daily number of difficulties (defined as a failure to aspirate resolved with rinse of the suction lumen of the endotracheal tube) or impossibility to aspirate (impossibility to aspirate not resolved despite rinsing), the incidence of VAP, and the occurrence of postextubation laryngeal edema.
Approval of the Committee for Protection of People in Biomedical Research of Rennes (date of acceptance: October 5, 2010) and written informed consent from each participant or next of kin were obtained. The patients were randomized to receive CSSS at −20 mmHg or ISSS (suction at −100 mmHg during 15 ± 3 s and no suction during 8 ± 3 s) with an automatic suctioning unit (Intermittent suction unit, Ohio Medical, IL, USA). Randomization was computer generated with a 1:1 ratio.
Evaluation of tracheal injury and management of the endotracheal tube
The degree of tracheal mucosa damage was determined using fiber-optic bronchoscopy after intubation (T0) and before extubating (T1). The occurrence (no injury observed at T0 but present at T1) or the worsening (injury observed at T0 exacerbating at T1) was studied. Bronchoscopy was performed through the endotracheal tube and allowed a visualization of the tracheal mucosa in front of the suctioning port as previously described.[6] The severity was graded into five categories (no injury, erythema, edema, ulceration, or necrosis). Fiber-optic bronchoscope was performed by three trained pulmonologists blinded of the study arm, with suctioning being stopped at the moment of the bronchoscopy. The exact position of the endotracheal tube and the pressure of the cuff were checked daily and maintained between 20 and 30 cmH2O to avoid inadvertent mobilization and under- or overinflation cuff, respectively. If a tracheostomy was required, the bronchoscopy was performed before its realization.
Data collection
The following parameters were collected: age, sex, severity assessed according to the Simplified Acute Physiologic Score II and the Sequential Organ Failure Assessment score during the 24 h following intubation, reasons for ICU hospitalization, and intubation. Moreover, the number of intubations before randomization during the current hospitalization and the duration of intubation between T0 and T1 were recorded.
Sample size and statistical analysis
Based on published data reporting suction dysfunction during subglottic suctioning, we hypothesized an expected rate of 40% of participants with mucosal damage using CSSS.[6,9] We calculated that a sample size of 126 patients (63 per group) was required for the study to have 80% power to show a 50% relative reduction of mucosal damage with ISSS at a one-sided alpha level of 0.05. Statistical analysis was performed using SAS statistical software version 9.4 (SAS Institute, Cary, NC). Qualitative variables were compared between the two groups with the Chi-square of the Fisher's exact test when needed, and continuous variables were compared using the Student's t-test or the Wilcoxon rank-sum test as appropriate. All P values were one-sided, and P < 0.05 was considered statistically significant.
RESULTS
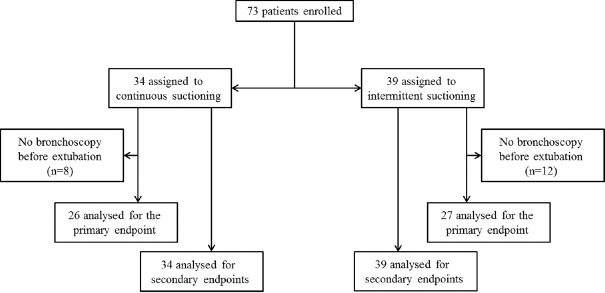
From August 2011 to January 2015, a total of 80 participants were randomized, 7 of them withdrew informed consent and 73 were included (CSSS, n = 34 and ISSS, n = 39). Recruitment was prematurely stopped by the study sponsor due to study duration, and low recruitment due to three periods (total 6 months) of out of stock of endotracheal tubes and two periods during which pulmonologists were unavailable (total 3 months). Baseline characteristics are provided in Table. Of these, twenty participants had no fiber-optic bronchoscopy at T1, before extubating (8 and 12 in the CSSS and ISSS groups, respectively), unavailability of the pneumologist (n = 13), self-extubating (n = 3), or other causes (n = 4). Consequently, 53 participants were analyzed on the primary end point (CSSS, n = 26 and ISSS, n = 27) [Figure 1]. The occurrence or worsening of mucosal damage was observed in 12 (23%) participants and did not differ between the two groups (CSSS, n = 7 [27%] vs. ISSS, n = 5 [17%], P = 0.465). Individual data are presented in Figure 2. Two participants developed local tracheal mucosa ulcerations in the CSSS group.
Table 1.
Baseline characteristics
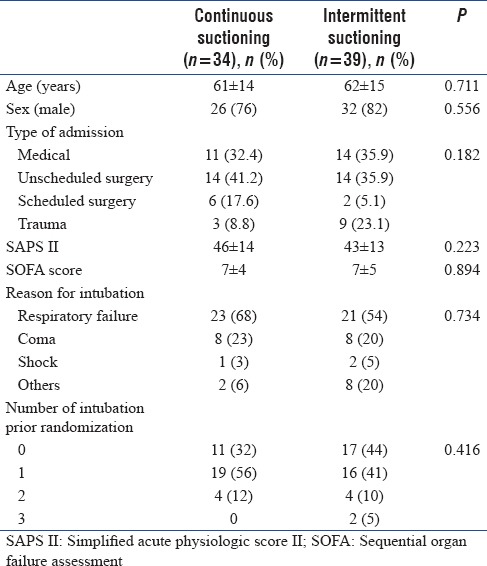
Figure 1.
Patients analyzed for the primary end point (tracheal mucosal damage) and for secondary end points
Figure 2.
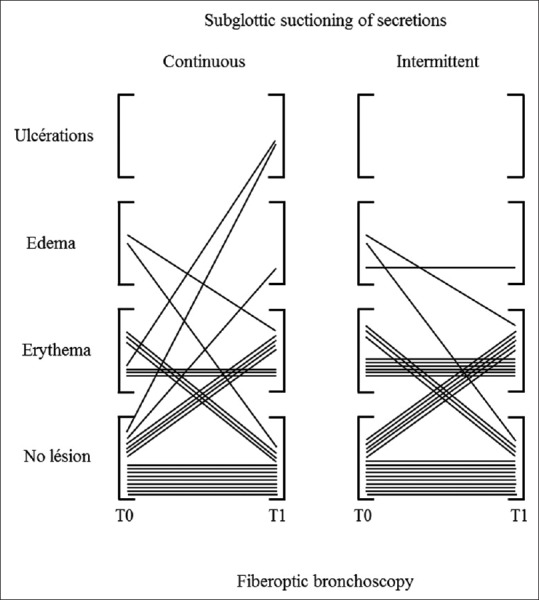
Individuals data of tracheal mucosa damage in front of the suctioning port after intubation (T0) and prior extubation (T1) in continuous and intermittent subglottic suctioning groups
The mean ± standard deviation daily volume of suctioned secretion was significantly higher with ISSS as compared to CSSS (74 ± 100 [interquartile range: 20–75] ml vs. 20 ± 25 [interquartile range: 6–30] ml, respectively, P < 0.001). Mean daily difficulties to aspirate through the suction lumen of the endotracheal tube did not differ between the two groups (CSSS group, 0.40 ± 0.54 per day and ISSS group, 0.16 ± 0.26 per day, P = 0.102), whereas mean daily impossibility to aspirate was significantly higher with CSSS as compared with ISSS (0.14 ± 0.16 per day vs. 0.03 ± 0.07 per day, respectively, P < 0.001). The duration of intubation between T0 and T1 and the incidence of VAP did not differ between the CSSS and ISSS groups (13 ± 8 vs. 11 ± 6 days, P = 0.386, and 4/34 [12%] vs. 2/39 [5%], P = 0.407, respectively). Postextubation laryngeal edema was not observed in the two groups.
DISCUSSION
In our study, the global incidence of mucosa tracheal damage when subglottic secretion drainage was applied with the TaperGuard Evac oral tracheal tube was 23%, but the modality of suction (continuous vs. intermittent) did not influence the occurrence of tracheal mucosa injuries. To the best of our knowledge, this is the first study which has evaluated continuous versus intermittent subglottic suctioning in human. Continuous suctioning was associated with more frequent impossibility to aspirate and lower volume of suctioned secretions. Postextubation laryngeal edema was not observed. This result has been largely confirmed in larger studies, but the absence of postextubation laryngeal edema does not signify that tracheal injuries induced by suctioning are not present.[1,3]
In a prospective randomized study realized in sheep, Berra et al. showed that CSSS at ≤20 mmHg with a 8 mm Hi-Lo Evac Mallinckrodt endotracheal tube-induced macroscopic tracheal injuries in all animals, notably necrosis and/or hemorrhage in the area of the subglottic port of the endotracheal tube.[4] Nevertheless, despite efforts to mimic human's tracheal orientation, it cannot be excluded that the tracheal anatomy of sheep favored a tight contact between the subglottic port and tracheal mucosa, and our results do not support this observation in humans.
In critically ill patients, it has been reported an incidence of 40% of postextubation laryngeal edema with continuous suctioning but representing only two of the five patients who were extubated.[5] Since this publication, postextubation edema associated with continuous subglottic suctioning has been reported ranging from 2.9% to 10.1% in larger populations but did not differ from control groups without suctioning.[1,3] With regard to the high frequency of suction dysfunction (19/40 ICU patients), fiber-optic bronchoscope examination revealed that, in 85% of cases, dysfunction was attributed to an obstruction of the subglottic port related to suctioned mucosa raising concerns about the safety of the method.[6] Two observations of tracheoesophageal fistula in severe trauma patients intubated with a Mallinckrodt Hi-Lo Evac and suspected to be in relation to the site of subglottic suctioning have been reported.[7] In the same way, a recent report performed in six patients intubated with a 8 mm Mallinckrodt Hi-Lo Evac endotracheal tube in which an intermittent suctioning at −125 mmHg during 15 s was applied, showed on computed tomography-scan an entrapment of the tracheal mucosa into the subglottic port of the tube.[8] In our study, intermittent suctioning provided a significant 3-fold increase in volume of suctioned secretions, and this observation may be related to a lower rate obstruction of the subglottic port. Although this point cannot be proved because not specifically explored, this hypothesis is likely and reinforced by the higher rate of impossibility to aspirate in the continuous suctioning group. This finding may be interesting in clinical practice because the more secretions are removed; the less will pass the endotracheal cuff.
The incidence of tracheal mucosa damage we observed was lower than expected. This can be explained by the utilization of the latest generation of TaperGuard endotracheal tube used in our study in comparison to older studies. Indeed, in the latest generation, the subglottic port was closer to the cuff and the suction lumen larger to limit tracheal mucosa injury and obstruction, respectively. As the number of patients included was also lower than calculated, a lack of statistical power cannot be ruled out to explain our negative result on mucosal damage. In fact, with an incidence of tracheal of 23%, to test the hypothesis that the absolute difference between groups observed for the primary endpoint was real, a total of 212 participants would be needed to show a statistical difference with an 80% power. Finally, the -100 mmHg applied in the intermittent group may appear too high, but it was the level of aspiration recommended by the manufacturer, and at least two studies have used such aspiration level.[10,11]
CONCLUSIONS
Our results suggest that intermittent suctioning did not reduce mucosal damage as compared with continuous suctioning. Nevertheless, the aspirated volume was higher and impossibility to aspirate was lower with intermittent suctioning.
Financial support and sponsorship
The study was supported by a grant from Rennes University hospital (2009 “Comité de la Recherche Clinique”).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Damas P, Frippiat F, Ancion A, Canivet JL, Lambermont B, Layios N, et al. Prevention of ventilator-associated pneumonia and ventilator-associated conditions: A randomized controlled trial with subglottic secretion suctioning. Crit Care Med. 2015;43:22–30. doi: 10.1097/CCM.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 2.Muscedere J, Rewa O, McKechnie K, Jiang X, Laporta D, Heyland DK, et al. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: A systematic review and meta-analysis. Crit Care Med. 2011;39:1985–91. doi: 10.1097/CCM.0b013e318218a4d9. [DOI] [PubMed] [Google Scholar]
- 3.Lacherade JC, De Jonghe B, Guezennec P, Debbat K, Hayon J, Monsel A, et al. Intermittent subglottic secretion drainage and ventilator-associated pneumonia: A multicenter trial. Am J Respir Crit Care Med. 2010;182:910–7. doi: 10.1164/rccm.200906-0838OC. [DOI] [PubMed] [Google Scholar]
- 4.Berra L, De Marchi L, Panigada M, Yu ZX, Baccarelli A, Kolobow T, et al. Evaluation of continuous aspiration of subglottic secretion in an in vivo study. Crit Care Med. 2004;32:2071–8. doi: 10.1097/01.ccm.0000142575.86468.9b. [DOI] [PubMed] [Google Scholar]
- 5.Girou E, Buu-Hoi A, Stephan F, Novara A, Gutmann L, Safar M, et al. Airway colonisation in long-term mechanically ventilated patients. Effect of semi-recumbent position and continuous subglottic suctioning. Intensive Care Med. 2004;30:225–33. doi: 10.1007/s00134-003-2077-4. [DOI] [PubMed] [Google Scholar]
- 6.Dragoumanis CK, Vretzakis GI, Papaioannou VE, Didilis VN, Vogiatzaki TD, Pneumatikos IA, et al. Investigating the failure to aspirate subglottic secretions with the Evac endotracheal tube. Anesth Analg. 2007;105:1083–5. doi: 10.1213/01.ane.0000278155.19911.67. [DOI] [PubMed] [Google Scholar]
- 7.Harvey RC, Miller P, Lee JA, Bowton DL, MacGregor DA. Potential mucosal injury related to continuous aspiration of subglottic secretion device. Anesthesiology. 2007;107:666–9. doi: 10.1097/01.anes.0000282083.83319.5f. [DOI] [PubMed] [Google Scholar]
- 8.Spapen H, Suys E, Nieboer K, Stiers W, De Regt J. Automated intermittent aspiration of subglottic secretions and tracheal mucosa damage. Minerva Anestesiol. 2013;79:316–7. [PubMed] [Google Scholar]
- 9.Rello J, Soñora R, Jubert P, Artigas A, Rué M, Vallés J, et al. Pneumonia in intubated patients: Role of respiratory airway care. Am J Respir Crit Care Med. 1996;154:111–5. doi: 10.1164/ajrccm.154.1.8680665. [DOI] [PubMed] [Google Scholar]
- 10.Bouza E, Pérez MJ, Muñoz P, Rincón C, Barrio JM, Hortal J, et al. Continuous aspiration of subglottic secretions in the prevention of ventilator-associated pneumonia in the postoperative period of major heart surgery. Chest. 2008;134:938–46. doi: 10.1378/chest.08-0103. [DOI] [PubMed] [Google Scholar]
- 11.Smulders K, van der Hoeven H, Weers-Pothoff I, Vandenbroucke-Grauls C. A randomized clinical trial of intermittent subglottic secretion drainage in patients receiving mechanical ventilation. Chest. 2002;121:858–62. doi: 10.1378/chest.121.3.858. [DOI] [PubMed] [Google Scholar]