Abstract
Background:
There is limited data regarding the microbiology of Intensive Care Unit (ICU)-acquired infections, such as ventilator-associated pneumonia (VAP), catheter-associated urinary tract infections (CAUTI), and catheter-related bloodstream infections (CRBSI) from India.
Objectives:
To explore the microbiology and resistance patterns of ICU-acquired infections and evaluate their outcomes.
Materials and Methods:
This was a multicenter observational study, conducted by Indian Society of Critical Care Medicine (MOSER study) between August 2011 and October 2012. Patients in the ICU ≥48 h with any ICU-acquired infection within 14 days of index ICU stay were included. Patient demographics, relevant clinical, and microbiological details were collected. Follow-up until hospital discharge or death was done, and 6-month survival data were collected.
Results:
Of the 381 patients included in the study, 346 patients had 1 ICU infection and 35 had more than one ICU infection. Among patients with single infections, 223 had VAP with Acinetobacter being the most common isolate. CAUTI was seen in 42 patients with Klebsiella as the most common organism. CRBSI was seen in 81 patients and Klebsiella was the most common causative organism. Multidrug resistance was noted in 87.5% of Acinetobacter, 75.5% of Klebsiella, 61.9% of Escherichia coli, and 58.9% of Pseudomonas isolates, respectively. Staphylococcus constituted only 2.4% of isolates. Mortality rates were 26%, 11.9%, and 34.6% in VAP, CAUTI, and CRBSI, respectively.
Conclusion:
VAP is the most common infection followed by CRBSI and CAUTI. Multidrug-resistant Gram-negative bacteria are the most common organisms. Staphylococcus aureus is uncommon in the Indian setting.
Keywords: Catheter-associated urinary tract infections, catheter-related bloodstream infections, Gram-negative bacteria, Intensive Care Unit-acquired infections, multidrug resistance, ventilator-associated pneumonia
INTRODUCTION
Incidence of nosocomial infections in the Intensive Care Unit (ICU) has been reported to be about 2–5 times higher than in the general inpatient hospital population.[1] These infections add substantial costs, morbidity, and mortality to the patients. The epidemiology, microbiology, and impact of ICU-acquired ICU infections such as ventilator-associated pneumonia (VAP), catheter-associated urinary tract infections (CAUTI), and catheter-related bloodstream infections (CRBSI) have been extensively studied and reported in the western literature.[2] There is increasing evidence to suggest that rates of ICU-acquired infections may even be higher in developing countries.[1] In the INDICAPS study, 12.2% patients developed an infection in the ICU, with an overall mortality of 28.4%.[3]
There is limited literature regarding common ICU-acquired infections in the Indian setting, and hence, western guidelines are being extrapolated for the management of these infections. The regional variations in the incidence, microbiology, and resistance patterns within India are not well understood yet. Local societal guidelines to prevent and treat these infections cannot be made without proper evaluation of region-specific epidemiology. Hence, we sought to perform a multicenter, epidemiological prospective data collection evaluating the microbiology, resistance patterns, and outcomes of ICU infections.
Objectives
To explore the microbiology and resistance patterns of ICU-related infections (VAP, CAUTI, and CRBSI) in India. The study had a secondary objective to evaluate the outcomes associated with these common ICU-acquired infections.
MATERIALS AND METHODS
Study design
This study was a prospective, multicenter, observational study conducted in medical/surgical ICUs across India (MOSER study) conducted between August 2011 and October 2012.
Study population
The study included only patients with ICU stay ≥48 h and any one of the infections – VAP, CAUTI, or CRBSI. Only index ICU admission for that hospitalization was included. Patients <18 years or >70 years, with index ICU stay <48 h, re-admissions to the ICU within the same hospitalization, HIV serology positivity, burns and solid organ or bone-marrow transplant patients were excluded.
As this is an observational study exploring the epidemiology of bacterial infections in the ICU, this study did not require any power analysis or a definitive sample size. However, we aimed to recruit a total of 400 individuals during the study period.
Study procedure
Indian Society of Critical Care Medicine (ISCCM) formed a core research panel of intensivists who coordinated the conduct of this study. Twenty ICUs across India were selected and invited for participation in this study as per the discretion of the research committee of ISCCM, out of which fifteen ICUs finally participated.
Ethics committee approval was obtained from all study centers. Due to the observational nature of this study, waiver for informed consent was requested and obtained at some study centers, while other centers obtained informed consent from the patients before enrolling them into the study.
Definition of Intensive Care Unit infections
The ICU infections were defined according to the standard definition of the United States centers for disease control and prevention (CDC) as follows:[4,5]
Ventilator-associated pneumonia
Clinical suspicion of pneumonia in a patient mechanically ventilated for >48 h and new or changing chest X-ray infiltrate with 2 out of the following 3 clinical findings – new onset fever, leukocytosis, and purulent tracheal secretions with or without microbiological diagnosis (endotracheal culture, bronchoscopic, or nonbronchoscopic BAL culture).
Catheter-associated urinary tract infection
Urinary tract infection (≥105 colony forming units in urine culture) in a catheterized patient with the presence of systemic signs and symptoms (fever, chills, and hypotension) and the absence of an alternative focus of infection.
Blood stream infections
Growth of pathogenic bacteria or fungi (that are not related to another site of infection) from one or more blood cultures. When the blood culture yields a potential skin contaminant (coagulase-negative Staphylococcus, diphtheroids, or Bacillus spp.), more stringent criteria were used to diagnose a BSI. In such cases, the presence of more than one positive culture and the presence of systemic signs and symptoms (fever, chills, and hypotension) and the absence of an alternative focus of infection were considered essential for diagnosis.
Catheter-related blood stream infections
CRBSI was defined as any BSI in a patient with a central venous catheter where no other source was identifiable or any BSI where blood and central venous catheter tip cultures showed the same organism.
Data collected
Clinical data
Data collection was done by allotted study personnel at all sites. Demographic data, diagnosis on admission, APACHE II, and type of admission (medical vs. surgical) were collected. Risk factor profile including history of diabetes mellitus, steroid use, and preadmission systemic antibiotic use were recorded. For each infection, the specific clinical presentation details (i.e., presence of fever, leukocytosis, and hypotension) were collected.
Laboratory data
Microbiological data including site of culture, organism with species, and sensitivity pattern with specific focus on incidence of extended-spectrum beta lactamases (ESBL), multidrug-resistant Acinetobacter and Pseudomonas, methicillin-resistant Staphylococcus aureus (MRSA), carbapenem-resistant Enterobacteriaceae, and vancomycin-resistant enterococci were collected. For fungal infections, speciation and sensitivities when available were obtained. First sample (tracheal aspirate, urine and blood) of patients tested within 48 h of ICU admission was omitted in order to exclude community-acquired infections at the time of ICU admission.
Laboratory evidence such as total counts, culture reports, and other investigations such as X-ray findings were correlated with the clinical findings such as temperature, pulse rate, blood pressure, and any other specific symptoms to differentiate infection or colonization. Site investigator was the final deciding authority who differentiated colonization from a true infection.
Date of insertion and removal of all relevant invasive tubes and lines were recorded. To ensure diagnosis as per guidelines, data were collected on clinical, radiographic, and microbiological findings.
Outcome data
All patients were followed till hospital discharge or death. Outcome measures such as duration of mechanical ventilation, ICU mortality, hospital mortality, ICU length of stay (LOS), and hospital LOS (hospital LOS) were obtained. Six-month survival of patients discharged alive were collected by telephonic contact of patient or family when feasible.
Data entry and auditing
All data were collected from the patients’ medical records using study-specific data collection form. A website with the data collection instrument was created for the purpose of this study. Access to the website was individualized to each center with a site-specific username and password. Each site was allowed to enter, access and edit only their data. All data were entered by the site investigators into this online database. Data sharing between centers was prohibited. Data from one site were blinded to investigators from other sites to maintain confidentiality and avoid any reporting bias. Only the overall study investigator and study coordinator were granted access to the entire database. Website hosting, monitoring, and maintenance were done by an independent professional agency. Random audit of at least 10% of charts was done at each site for ensuring quality/accuracy of the data collected and entered. A second round of quality check/audit was done by the primary investigator. Any discrepancies/queries were raised with the respective site investigator and clarified. Data were summarized by routine descriptive statistics to describe the patterns, sensitivity, and resistance of the organisms.
RESULTS
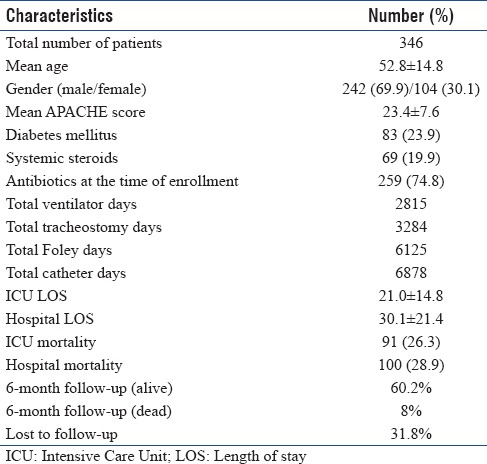
A total of 381 patients were included in the study. When there was more than one organism (polymicrobial) in the same episode of infection, the patients were categorized as having single infection for the purpose of analysis. Patients with more than one infection such as VAP and/or CAUTI and/or CRBSI were categorized as multiple infections and were not included in the current analysis. Based on this categorization, out of the total 381 patients, 346 patients had a single ICU-acquired infection during the study period (males 242 [69.9%] and females 104 [30.1%]). The demographic details of the patients are presented in Table 1.
Table 1.
Patient demographics
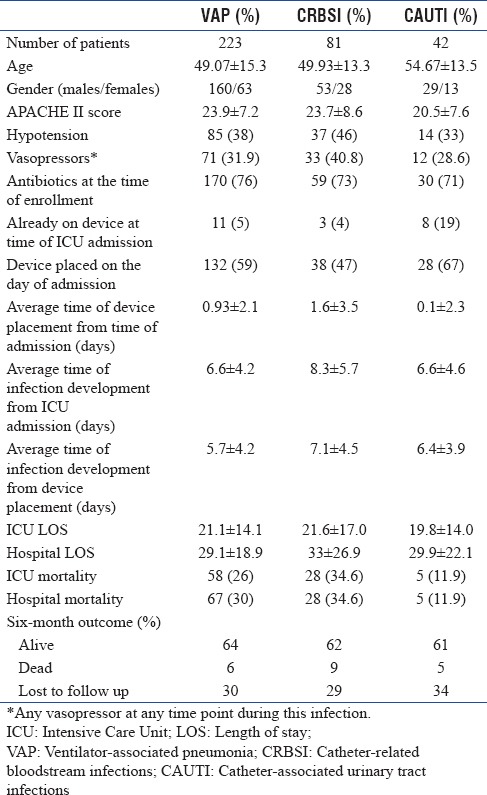
The most frequently diagnosed infection was VAP, followed by CRBSI and CAUTI. Details of each infection are presented in Table 2.
Table 2.
Patient characteristics with Intensive Care Unit-acquired infections
Ventilator-associated pneumonia
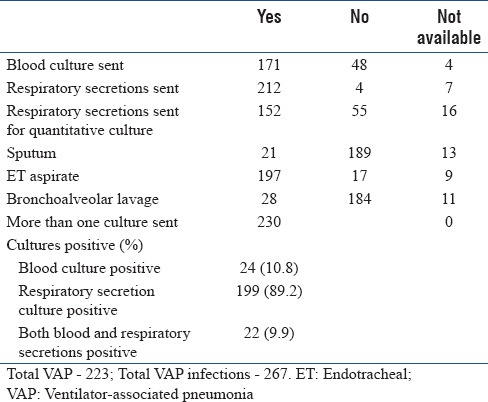
A total of 223 patients had VAP, and the total number of VAP infections was 267, including those that were polymicrobial. Relevant details of VAP are summarized in Table 2. Blood culture was sent for 171 patients and respiratory culture was sent for 212 patients [Table 3a].
Table 3a.
Ventilator-associated pneumonia – culture details
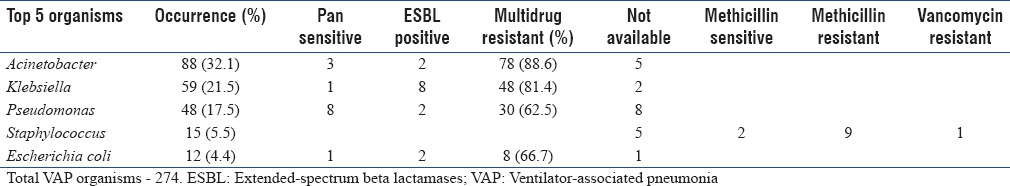
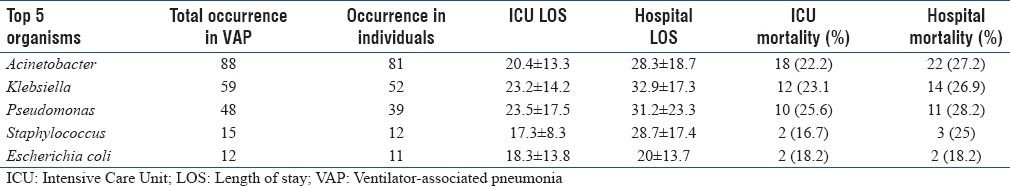
A total of 274 organisms were isolated, including polymicrobial infections. The most common five organisms isolated from VAP patients are presented in Table 3b along with their resistant patterns. Multidrug resistance was very prevalent – 88.6% of Acinetobacter and 81.4% of Pseudomonas species were multidrug resistant. These strains were sensitive only to colistin.
Table 3b.
Ventilator-associated pneumonia organisms – resistance pattern
Overall ICU and hospital mortality in patients with VAP was 26% and 30%, respectively. Organism-specific outcomes have been summarized in Table 3c.
Table 3c.
Ventilator-associated pneumonia organisms and mortality
Catheter-related blood stream infections
A total of 81 patients had CRBSI and the total number of infections was 86, including polymicrobial infections. Details related to CRBSI are summarized in Table 2.
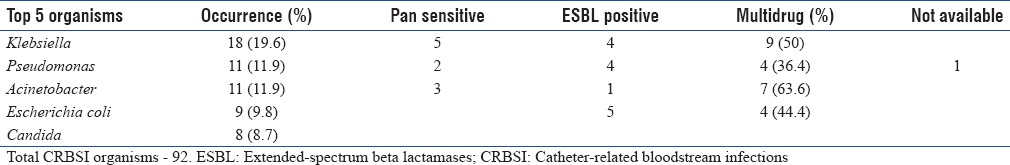
Blood culture was sent for 77 patients and catheter tip culture was sent for 39 patients [Table 4a]. A total of 92 pathogens were isolated from blood in 81 patients including those who had polymicrobial CRBSI. The top 5 organisms isolated from patients who had CRBSI are presented in Table 4b along with their resistant patterns. Multidrug resistance was seen in 50% of Klebsiella, 36.4% of Pseudomonas and 63.6% of Acinetobacter species, and were sensitive only to colistin.
Table 4a.
Catheter-related bloodstream infections – culture details
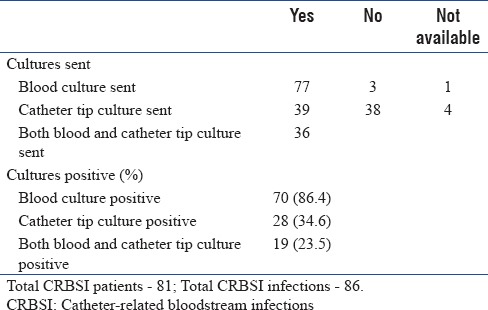
Table 4b.
Catheter-related bloodstream infections organisms – resistance pattern
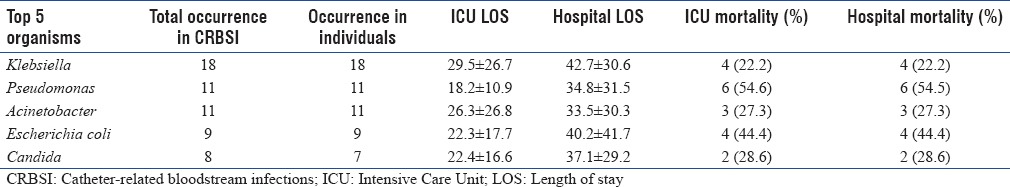
Overall ICU and hospital mortality in CRBSI patients was 34.6%, highest of all the 3 infections. Organism-specific outcome is summarized in Table 4c.
Table 4c.
Catheter-related bloodstream infections organisms and mortality
Catheter-associated urinary tract infection
There were 42 patients with CAUTI with 43 CAUTI infections, including a polymicrobial infection. Table 2 summarizes the clinical details of CAUTI.
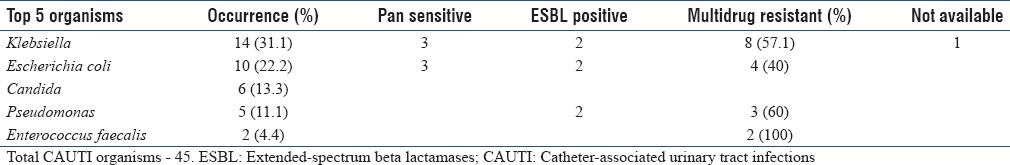
Blood culture was sent for 32 patients and urine culture was sent for 40 patients [Table 5a]. Most common organisms isolated from CAUTI patients are presented in Table 5b along with their resistant patterns.
Table 5a.
Catheter-associated urinary tract infections – culture details
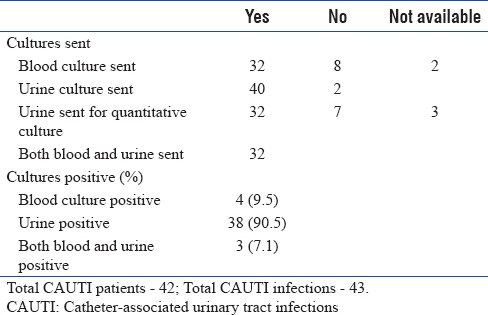
Table 5b.
Catheter-associated urinary tract infections organisms – resistance pattern
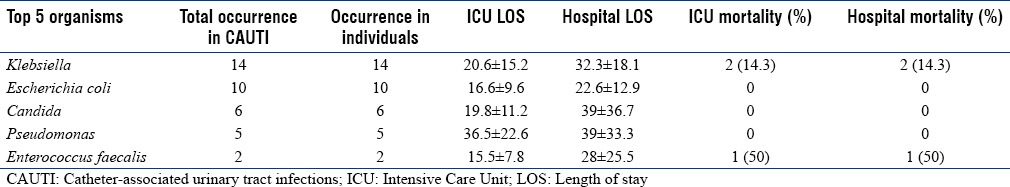
Overall ICU and hospital mortality in CAUTI patients was 11.9%. Mortality rate in Klebsiella was 14.3%. Organism-specific outcomes are summarized in Table 5c.
Table 5c.
Catheter-associated urinary tract infections organisms and mortality
Multidrug resistance pattern
Gram-negative bacilli contributing to the ICU infections were 91.7% and Gram-positive cocci were 8.2%. Multidrug resistance was very prevalent among Gram-negative isolates. While isolates in VAP were highly multidrug resistant, comparatively, they were less multidrug resistant in CAUTI and CRBSI. Of the Gram-negative isolates, 34% were ESBL producers. The major ESBL producer was Klebsiella (41.1%), followed by Escherichia coli (26.4%) and Pseudomonas (23.5%). Overall, mortality was high in CRBSI, compared to the other two infections.
Outcome
The overall mean ICU LOS was 21.0 ± 14.8 and mean hospital LOS was 30.1 ± 21.4 days. The mean ICU LOS for VAP, CRBSI, and CAUTI were 21.1 ± 14.1, 21.6 ± 17.0, and 19.8 ± 14.0, respectively. The overall ICU mortality was 26.3% and hospital mortality was 28.9%.
In the 6-month follow up, 60.2% were alive, 8% were dead, and 31.8% were lost to follow-up [Table 1]. Six-month outcome details of each infection are presented in Table 2.
DISCUSSION
Infection surveillance is an essential prerequisite for quality care and prevention and proper management of ICU-acquired infections. Incidence of ICU-acquired infections may vary from setting to setting, type of ICU, patient population, and the definitions used to identify these infections.[6] Knowing the incidence and the resistance patterns in our native environment will help develop specific guidelines for prevention and management of ICU-acquired infections.
In India, the rate of ICU-acquired infections shows variations and has great treatment implications. Our study results are concordant to other studies. Rosental et al. in their multicenter study on device-associated nosocomial infections in 55 ICUs of 8 developing countries, including India, reported that ventilator-associated pneumonia posed the greatest risk (41% of all device-associated infections) followed by bloodstream infections (BSIs) (30%) and catheter-associated urinary tract infections (29%).[1] The same author in another study of ICUs as part of the international infection control consortium from seven Indian cities reported that the overall infection rates were 10.46/1000 ventilator days for VAP, 7.92/1000 catheter days for CLABSI, and 1.41/1000 catheter days for CAUTI.[7]
Habibi et al., in their paper on epidemiology of nosocomial infections in their hospital in northern India, reported higher rates of infection with pneumonia in 77% patients, urinary tract infection in 24%, and BSI in 24%.[8] This study was done in a medical ICU and hence may have found a higher rate of VAP.
Although these results are comparable to the results of our study, several other studies in India have reported different rates of infections. Data from the United States National Nosocomial Infections Surveillance System showed that nosocomial pneumonia constituted 31%, urinary tract infections (UTIs) 23%, and primary BSIs 14%.[9]
Kamat et al. in their study on nosocomial isolates in their teaching hospital in Goa have shown that urinary tract infection was the most common nosocomial infection in their setting (26.6%), followed by surgical site infection (23.7%), wound infection (23%), and nosocomial pneumonia (18.3%).[10] This study was however done in patients in medical and surgical wards hence not reporting VAP rates. In another single-center study[11] that looked at the epidemiology and risk factors of healthcare-associated infections in ICUs in North India, CRBSI (13.5%) was the most common healthcare-associated infection followed by UTI (10.8%) and VAP (6.2%). Similarly, one study in North India by Agarwal et al. reported different rates of infection with pneumonia in 23%, bacteremia in 7.5%, urinary tract infections in 1.5%, and catheter-related BSIs in 1%.[12]
In concordance with our results, several studies have reported that more than half of the nosocomial infections occurring in the Indian ICU are due to Gram-negative bacteria.[9,10,13] The detection of Candida species in 13% of the isolates in our study is also consistent with other studies.[14] Some studies have reported relatively smaller percentages; in one study, ninety-seven percent of the isolates were bacterial, while the others were fungal.[10] INDICAPS, a study that was conducted to explore the organizational aspects, case mix, and practices in Indian ICUs, reported 68.9% Gram-negative organisms, 15.9% Gram-positive organisms, 7.5% fungi, 2.4% mycobacteria, 1.7% viruses, and 1.1% malarial parasites.[3]
Several studies have shown that isolates of Acinetobacter, Pseudomonas and Klebsiella and E. coli were the dominant pathogens in ICU-acquired infections.[8,10,12] The study of Agarwal et al. reported majority of infections with Gram-negative bacilli in respiratory ICU. They found Acinetobacter species followed by Pseudomonas aeruginosa to be the most common organisms causing pneumonia.[12] However, in our study, Acinetobacter was followed by Klebsiella as the common organisms causing VAP.
Although an Indian study on CRBSI in the ICU patients reported Staphylococcus to be the most common pathogen (isolated in 25.9%), followed by Gram-negatives,[15] in our study, Gram-positive bacteria comprised only of a very small proportion of all organisms.
All isolates of Acinetobacter, Pseudomonas, Klebsiella, and E. coli were resistant to the third-generation cephalosporins.[8] Almost 70% of the isolates were resistant to all the antibiotics for which susceptibility was tested except polymyxin. This could be attributed to the fact that over 70% of patients in our study already received some empiric antibiotics at the time of enrollment making them a higher risk for developing multidrug resistance.
With regard to Gram-positive infections, although S. aureus was very uncommon, proportion of MRSA in our study was 60% as against 71.4% and 84% reported in other studies.[1,10]
The differences in the findings of various studies may be attributed to factors such as difference in the criteria for patient selection, the case mix, ICU type, rate of device utilization, patient:nurse ratio, infection control practices and compliance and infection detection criteria. Furthermore, results from a single-center study may differ from that of a multicenter study as the patients from a single institution can present with different risk of infection in the context of differing case mix, severity of illness, and utilization rates of invasive devices.[9]
Various study authors have cited LOS as being an important reason for the development of infection.[8,12] The longer the patients stay in ICU, longer will be the time period of insertion of devices and more are the chances of getting colonized with multidrug-resistant bacteria. This could not be assessed in our study as we did not collect information on LOS of patients without infections. However, there were no significant difference among the LOS of VAP, CAUTI, and CRBSI.
Mortality rate for patients with device-associated infections ranged from 35.2% for BSI to 44.9% for ventilator-associated pneumonia[1] very comparable to that of our study.
One of the main strengths of our study is that ours is the first multicenter study to look into the epidemiology of ICU-acquired infections in India. Our study included a wide spectrum of institutions encompassing government and private sectors, teaching and non-teaching facilities and had varied organizational characteristics such as nurse:patient ratio. Hence, our study results likely represent the epidemiology of these infections in our setting. Only institutions with a standard microbiology laboratory were included and hence our data on resistance patterns are likely to be more robust. The limitations of our study are that details of patients without ICU infections were not collected, and hence, rates of VAP, CRBSI, and CAUTI could not be calculated. Second, the site investigators were left to decide colonization versus Infection, and hence, our study could have potentially overdiagnosed or underdiagnosed infections.
CONCLUSION
VAP is the most common infection followed by CRBSI and CAUTI. The most common organisms are multidrug-resistant Gram-negative bacteria, namely Acinetobacter, Klebsiella, and Pseudomonas. Gram-positive infections and MRSA form a small minority of infections. National and regional guidelines based on local microbiological data and resistant patterns need to be developed, as Western guidelines may not be applicable to treat ICU infections at tertiary care hospitals in India.
Financial support and sponsorship
Research Grant funded by MSD Pharmaceuticals India Private Limited, Mumbai, to Indian Society of Critical Care Medicine (ISCCM).
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors acknowledge the contribution of Dr. Lakshmi Ranganathan (PhD), Apollo Hospitals, Chennai (Overall Study Coordinator) and the participating sites: Dr. Sushma Gurav, Ruby Hall Clinic, Dr. Prakash Sasthri, Sir Gangaram hospital, Dr. Yatin Mehta, Medanta (Medicity), Dr. Atul Kulkarni, Tata Memorial Hospital, Dr. Narendar Rungta, Rungta Hospitals, Dr. Mrinal Sircar, Fortis Hospital, Noida, Dr. YP Singh, Max Superspeciality Hospitals (Patparganj), Dr. Subhash Todi, AMRI Hospitals, Dr. Simran Singh, PD Hinduja Hospital, Dr. Deven Janeja, Max Super Speciality Hospital (Saket).
REFERENCES
- 1.Rosenthal VD, Maki DG, Salomao R, Moreno CA, Mehta Y, Higuera F, et al. Device-associated nosocomial infections in 55 Intensive Care Units of 8 developing countries. Ann Intern Med. 2006;145:582–91. doi: 10.7326/0003-4819-145-8-200610170-00007. [DOI] [PubMed] [Google Scholar]
- 2.Fridkin SK, Welbel SF, Weinstein RA. Magnitude and prevention of nosocomial infections in the Intensive Care Unit. Infect Dis Clin North Am. 1997;11:479–96. doi: 10.1016/s0891-5520(05)70366-4. [DOI] [PubMed] [Google Scholar]
- 3.Divatia JV, Amin PR, Ramakrishnan N, Kapadia FN, Todi S, Sahu S, et al. Intensive care in India: The indian intensive care case mix and practice patterns study. Indian J Crit Care Med. 2016;20:216–25. doi: 10.4103/0972-5229.180042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Types of Healthcare-associated Infections HAI CDC. [Last accessed on 2017 Sep 25]. Available from: https://www.cdc.gov/hai/infectiontypes.html .
- 5.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Gastmeier P, Sohr D, Just HM, Nassauer A, Daschner F, Rüden H, et al. How to survey nosocomial infections. Infect Control Hosp Epidemiol. 2000;21:366–70. doi: 10.1086/501774. [DOI] [PubMed] [Google Scholar]
- 7.Mehta A, Rosenthal VD, Mehta Y, Chakravarthy M, Todi SK, Sen N, et al. Device-associated nosocomial infection rates in Intensive Care Units of seven Indian cities. Findings of the International Nosocomial Infection Control Consortium (INICC) J Hosp Infect. 2007;67:168–74. doi: 10.1016/j.jhin.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Habibi S, Wig N, Agarwal S, Sharma SK, Lodha R, Pandey RM, et al. Epidemiology of nosocomial infections in medicine intensive care unit at a tertiary care hospital in Northern India. Trop Doct. 2008;38:233–5. doi: 10.1258/td.2008.070395. [DOI] [PubMed] [Google Scholar]
- 9.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical Intensive Care Units in the United States. Infect Control Hosp Epidemiol. 2000;21:510–5. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 10.Kamat U, Ferreira A, Savio R, Motghare D. Antimicrobial resistance among nosocomial isolates in a teaching hospital in Goa. Indian J Community Med. 2008;33:89–92. doi: 10.4103/0970-0218.40875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta P, Rani H, Chauhan R, Gombar S, Chander J. Health-care-associated infections: Risk factors and epidemiology from an Intensive Care Unit in Northern India. Indian J Anaesth. 2014;58:30–5. doi: 10.4103/0019-5049.126785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal R, Gupta D, Ray P, Aggarwal AN, Jindal SK. Epidemiology, risk factors and outcome of nosocomial infections in a Respiratory Intensive Care Unit in North India. J Infect. 2006;53:98–105. doi: 10.1016/j.jinf.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in Intensive Care Units. JAMA. 2009;302:2323–9. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 14.Pittet D, Wenzel RP. Nosocomial bloodstream infections. Secular trends in rates, mortality, and contribution to total hospital deaths. Arch Intern Med. 1995;155:1177–84. doi: 10.1001/archinte.155.11.1177. [DOI] [PubMed] [Google Scholar]
- 15.Kaur M, Gupta V, Gombar S, Chander J, Sahoo T. Incidence, risk factors, microbiology of venous catheter associated bloodstream infections – A prospective study from a tertiary care hospital. Indian J Med Microbiol. 2015;33:248–54. doi: 10.4103/0255-0857.153572. [DOI] [PubMed] [Google Scholar]


