Abstract
The United States and numerous other countries worldwide are currently experiencing a public health crisis due to the abuse of illicitly manufactured fentanyl (IMF) and its analogues. This manuscript describes the development of a liquid chromatography-tandem mass spectrometry-based method for the multiplex detection of N = 24 IMF analogues and metabolites in whole blood at concentrations as low as 0.1–0.5 ng mL–1. These available IMFs were fentanyl, norfentanyl, furanyl norfentanyl, remifentanil acid, butyryl norfentanyl, remifentanil, acetyl fentanyl, alfentanil, AH-7921, U-47700, acetyl fentanyl 4-methylphenethyl, acrylfentanyl, para-methoxyfentanyl, despropionyl fentanyl (4-ANPP), furanyl fentanyl, despropionyl para-fluorofentanyl, carfentanil, (±)-cis-3-methyl fentanyl, butyryl fentanyl, isobutyryl fentanyl, sufentanil, valeryl fentanyl, para-fluorobutyryl fentanyl, and para-fluoroisobutyryl fentanyl. Most IMF analogues (N = 22) could be easily distinguished from one another; the isomeric forms butyryl/isobutyryl fentanyl and para-fluorobutyryl/para-fluoroisobutyryl fentanyl could not be differentiated. N = 13 of these IMF analogues were quantified for illustrative purposes, and their forensic quality control standards were also validated for limit of detection (0.017–0.056 ng mL–1), limit of quantitation (0.100–0.500 ng mL–1), selectivity/sensitivity, ionization suppression/enhancement (87–118%), process efficiency (60–95%), recovery (64–97%), bias (<20%), and precision (>80%). This flexible, time- and cost-efficient method was successfully implemented at the Montgomery County Coroner’s Office/Miami Valley Regional Crime Laboratory in Dayton, Ohio, where it aided in the analysis of N = 725 postmortem blood samples collected from February 2015 to November 2016.
Introduction
Fentanyl is a synthetic opioid that was developed for pharmaceutical use in 1960 by Paul Janssen in Belgium.1−4 Since its introduction into the United States in 1970, fentanyl has rapidly become a leading analgesic and anesthetic agent due to its 50–100 times higher potency than that of morphine, shorter onset, and quicker absorption by the human body.3,4 Fentanyl causes depression of the respiratory and central nervous system in a dose-dependent manner. Over the past few years, increased availability and abuse of illicitly manufactured fentanyl (IMF) and its analogues emerged as a significant threat to public health in the United States and other countries.5−10 Ohio is one of several U.S. states that was gravely impacted by the opioid epidemic; the number of IMF-related overdose deaths increased by 526% between 2013 and 2015.11 Even more alarming is the fact that new IMF analogues are being synthesized in Asian countries and marketed on a regular basis across United States and Europe in an attempt to stay ahead of regulations.12−14 Many of these analogues have increased potency compared with IMF. For example, carfentanil or the so called “elephant tranquilizer” entered the U.S. market in July 2016 and is known to be 100 times more potent than fentanyl.15,16 From July to November 2016, over 80% of all carfentanil positive cases in the United States (i.e., N = 451 cases) were reported in Ohio.17
The U.S. Drug Enforcement Agency (DEA) has responded to this epidemic by declaring IMF a public health safety factor on March 18th, 2015.8 Unfortunately, IMF and its analogues are not always part of routine toxicology testing in the United States. Thus, there is an urgent need for developing sensitive, multiplex detection methods that could be easily modified to include newly emerging IMF analogues. A successful method was reported in 2017 by the Miami-Dade County Medical Examiner Department, where an ultrahigh performance liquid chromatography ion trap mass spectrometry system with MSn capabilities (UHPLC-Ion-Trap-MSn) was employed for the qualitative identification of N = 13 IMF analogues (i.e., acetyl fentanyl, alfentanil, β-hydroxythiofentanyl, butyryl fentanyl, carfentanil, despropionyl fentanyl, fentanyl, furanyl fentanyl, norfentanyl, 4-fluoroisobutyryl fentanyl, 4-fluorobutyryl fentanyl, sufentanil, and U-47700) in postmortem samples.18
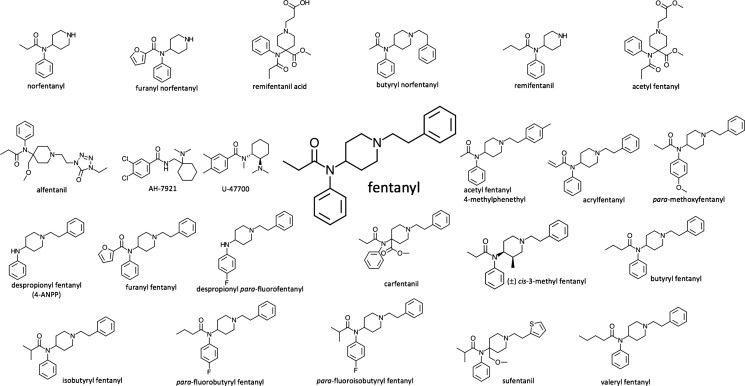
The key aim of this study is to describe the development and validation of a new liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based method for the multiplex detection of N = 24 IMF analogues, metabolites, and synthetic opioids. The IMF analogues were selected on the basis of previous forensic reports and their presence on the Dark Web:19 (1) norfentanyl, (2) furanyl norfentanyl, (3) remifentanil acid, (4) butyryl norfentanyl, (5) remifentanil, (6) acetyl fentanyl, (7) alfentanil, (8) AH-7921, (9) U-47700, (10) acetyl fentanyl 4-methylphenethyl, (11) acrylfentanyl, (12) fentanyl, (13) para-methoxyfentanyl, (14) despropionyl fentanyl (4-ANPP), (15) furanyl fentanyl, (16) despropionyl para-fluorofentanyl, (17) carfentanil, (18) (±)-cis-3-methyl fentanyl, (19) butyryl fentanyl, (20) isobutyryl fentanyl, (21) para-fluorobutyryl fentanyl, and (22) para-fluoroisobutyryl fentanyl, (23) sufentanil, and (24) valeryl fentanyl (Figure 1). U-47700 is not an analogue of fentanyl and is not approved as a pharmaceutical agent, but it is typically included in fentanyl studies because of its similar, potent analgesic activity and combination with IMF in cases of overdose deaths.20 AH-7921 is also a synthetic opioid analgesic that was placed into schedule I of the U.S. Controlled Substances Act in 2016.21 It is usually incorporated in fentanyl-related studies due to its structure being similar to that of IMF and potency comparable to that of morphine.22
Figure 1.
Molecular structure of N = 24 IMF analogues, metabolites, and synthetic opioids used for the development of the LC-MS/MS-based method.
In this study, LC-MS/MS is the analytical method of choice because of its common use in numerous forensic and toxicology laboratories across the nation.23 LC has become the leading separation technique in chromatography due to its flexibility, accuracy, and efficiency. Although LC achieves the physical separation of multiple components in a mixture, MS offers information about their structural identity. The addition of tandem MS technology further improves the specificity and accuracy of the detection method. The triple-quadrupole mass spectrometry (QQQ) capability of the selected system facilitates the simultaneous identification and quantification of fentanyl analogues. QQQ performs a true multiple-reaction monitoring (MRM) mode scan because both mass analyzers can simultaneously monitor quantitative and qualitative transition ions. Running dynamic MRM24,25 is desired for rapid and simple quantifications due to its dynamic/noble range and sensitivity.26 Pairing LC-MS/MS with solid phase extraction (SPE)27 allows for the identification and quantification of IMF analogues from postmortem blood.
Experimental Section
Materials
High-performance liquid chromatography (HPLC) grade water and acetonitrile (ACN) were purchased from Honeywell (Morris Plains, NJ). Formic acid (88%), methanol, ammonium formate, potassium phosphate monobasic-sodium hydroxide buffer solution (phosphate-buffered saline (PBS), pH 6.0), glacial acetic acid, ammonium hydroxide, isopropanol, and methylene chloride were obtained from Fisher Scientific (Pittsburgh, PA). Certified reference standards of acetyl fentanyl, acetyl norfentanyl, alfentanil, sufentanil, fentanyl, and norfentanyl were acquired from both Cerilliant (Round Rock, TX) and Lipomed (Cambridge, MA). Butyryl fentanyl and (±)-cis-3-methyl fentanyl were procured from both Lipomed and Cayman Chemical. Butyryl norfentanyl, para-fluorofentanyl, para-fluorobutyryl fentanyl, furanyl fentanyl, furanyl norfentanyl, valeryl fentanyl, acrylfentanyl, isobutyryl fentanyl, despropionyl para-fluorofentanyl, 4-ANPP, U-47700, 4-fluoroisobutyryl fentanyl, para-methoxyfentanyl, acetyl fentanyl 4-methylphenethyl analogue, and AH-7921 were purchased from Cayman Chemical (Ann Arbor, MI). Remifentanil and remifentanil metabolite were obtained from Cerilliant. Internal standards were acetyl fentanyl-13C6, fentanyl-d5, and norfentanyl-d5 from Cerilliant. Carfentanil was donated by DEA. Clean screen drugs of abuse (DAU) SPE columns were acquired from United Chemical Technologies Worldwide Monitoring (Bristol, PA).
Instrumentation
Two different LC-MS/MS systems (Agilent Technologies, Santa Clara, CA) were employed for validation purposes: (1) a 1200 series LC system (Binary HPLC Pump, high-performance autosampler, and vacuum degasser) equipped with a 6410 triple quadrupole, and (2) an HPLC 1260 Infinity system (binary pumps, a six-port valve, and high-performance autosampler) coupled to a 6420 triple quadrupole HPLC-MS/MS system. The analytical column on both instruments was a Raptor biphenyl LC column (150.0 mm x 3.0 mm, 2.7 μm) that was purchased from Restek (Bellefonte, PA). SPE was done on a UCT Positive Pressure Manifold.
Preparation of Calibration and Quality Control Solutions
Stock standards and stock controls of 1 and 100 ng mL–1 were prepared for all IMF analogues by dilution of the purchased certified reference material in methanol and were stored at −4 °C for up to 3 months. All standards (0.1–50.0 ng mL–1) and quality controls (0.35, 2.5, and 25.0 ng mL–1) were made by serial dilution from stocks directly into treated blank whole blood (see Biological Matrices). The quality control concentrations were selected to fit the low (quality control low concentration (QCLO)), medium (medium concentration (QCMED)), and high (high concentration (QCHI)) ends of the calibration range. Additional controls included blank water and blank whole blood. All standards and quality controls excluding blank water were spiked with three internal standards to a final concentration of 10.0 ng mL–1. The norfentanyl-d5 (stock of 100.0 μg mL–1), fentanyl-d5 (100.0 μg mL–1), and acetyl fentanyl-13C6 (50.0 μg mL–1) working internal standards were prepared by 5–10-fold volumetric dilution of stock internal standard to 100.0 ng mL–1 in methanol. 2H1 and 13C6 internal standards were selected for use due to their structural similarity and physicochemical properties compared to those of the IMFs. Fentanyl-d5 was used as the internal standard for all IMF analogues without a stable, labeled internal standard on the market due to the limited availability of most analogues and the structural similarities to fentanyl. Controls (triplicate) and calibration standards were extracted daily. Post extraction controls (spiked after separation) and neat controls (directly evaporated and not extracted) were also made for method validation purposes.
Biological Matrices
Whole blood free of pathogens was obtained from the Community Blood Center, Dayton, OH. Blank whole blood was preserved with sodium fluoride (1%) and was refrigerated (∼4 °C) or frozen (−10 to −20°C). Before use, the acquired blood was analyzed for over 70 potential contaminants and drugs of abuse (Table S1) by running a blank sample through multiple extractions and quantifications. Verified whole blood was diluted with water at a 1:1 ratio. Because of limited blood supply, the product was diluted to extend the amount of blood needed for each analysis; however, proficiency blind tests were carried out to demonstrate accurate analyte quantitation for accreditation purposes.
Sample Preparation and Solid Phase Extraction
Calibrants, controls, and samples were treated the same throughout all experiments for method validation. Briefly, 1.0 mL of whole blood was added to 4.0 mL of PBS and 2.0 mL of water in a 16 x 125 Pyrex Screw Cap Tube. Each sample was then spiked with 100.0 μL of internal standard. Calibrators and controls were administered to additional stock solutions, resulting in seven calibration concentrations (0.1, 0.25, 0.5, 1.0, 5.0, 10.0, and 50.0 ng mL–1) and three quality controls (0.35, 2.5, and 25.0 ng mL–1). Afterward, calibrants, controls, and samples were vortexed and centrifuged at 3000 rpm (1811g) for 10 min to remove particulate matter prior to SPE.
The isolation of IMFs was selectively achieved using CLEAN SCREEN DAU columns (United Chemical Technologies Bristol, PA). Desired drugs were selectively eluted by maintaining the pH of reagents and column close to 6.0 through the addition of PBS buffer. Briefly, SPE columns were preconditioned and activated with 3.0 mL of methanol, washed with 3.0 mL of water, and conditioned to pH 6.0 with PBS. Slight positive pressure (∼10 psi) was employed for each wash using a UCT Positive Pressure Manifold.
Calibrants, controls, and samples were loaded into the SPE columns, which were then washed with 3.0 mL of water, 1.0 mL of 1.0 M of acetic acid, and 3.0 mL of methanol to remove potential interferences. The cationic IMFs were eluted with 3.0 mL of a v/v/v methylene chloride/isopropanol/ammonium hydroxide mixture (78:20:2). The eluate was collected and evaporated at 40 °C under a stream of air. Analytes were then reconstituted with 100.0 μL of methanol and injected into LC-MS/MS.
Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS)
Separation of fentanyl analytes was achieved with a Raptor biphenyl analytical column heated to 40 °C. Mobile phase A (MPA) consisted of 10.0 mM ammonium formate and 0.1% formic acid in water. Mobile phase B (MPB) was made of 0.1% formic acid in ACN. MPA and MPB were held for 2 min at 90/10%. MPA was gradually ramped down from 90 to 10% over 6 min, then held for 0.5 min at 10/90%, and finally returned to 90/10% in 0.1 min, and was held for the remainder of the time. A total run time of 13.5 min ensured the elution of analytes and the equilibration of the column.
Electrospray ionization in a positive ion scan mode was selected for MS measurements. Source parameters were maintained for nitrogen gas temperature (350 °C), gas flow (12.0 L min–1), and capillary voltage (4000 V). Detection was accomplished by using a dynamic MRM scan function. Precursor and product ions were identified using the Optimizer software (Agilent) and manual determination (Table 1).
Table 1. Precursor Ions along with Their Qualitative and Quantitative Transitions for All IMF Analogues (N = 24) and Internal Standardsa.
| peak | analyte | quant transition (m/z) | qualifier transitions(m/z) | fragmentor (V) | collision energy (V) |
|---|---|---|---|---|---|
| 1 | norfentanyl-d5 | 238.4–84.1 | 238.4–55.2 | 106 | 16, 44 |
| 2 | norfentanyl | 233.4–84.1 | 233.4–94.0, 233.4–55.2 | 106 | 16, 36, 44 |
| 3 | furanyl norfentanyl | 271.4–84.1 | 271.4–95.0 | 106 | 16, 44 |
| 4 | remifentanil acid | 363.4–53.2 | 363.4–81.1 | 111 | 72, 44 |
| 5 | butyryl norfentanyl | 247.3–84.1 | 247.3–94.0, 247.3–55.2 | 106 | 16, 32, 44 |
| 6 | remifentanil | 377.5–317.0 | 377.5–345.0 | 25 | 15 |
| 7 | acetyl fentanyl | 323.0–105.0 | 323.0–188.0 | 141 | 20, 40 |
| 8 | acetyl fentanyl13C6 | 329.4–105.0 | 329.4–77.1 | 136 | 44, 96 |
| 9 | alfentanil | 417.5–165.0 | 417.5–99.0, 417.5–77.1 | 131 | 36, 40, 100 |
| 14 | AH-7921 | 329.0–95.1 | 329.0–284.0 | 111 | 20, 36 |
| 10 | U-47700 | 329.0–81.0 | 329.0–204.0 | 120 | 36, 25 |
| 11 | acetyl fentanyl 4-methylphenethyl | 337.5–119.0 | 337.5–91.1 | 136 | 36, 72 |
| 12 | acrylfentanyl | 335.5–105.0 | 335.5–77.1, 335.5–51.2 | 141 | 44, 92, 140 |
| 15 | fentanyl-d5 | 342.5–105.0 | 342.5–77.1 | 141 | 44, 100 |
| 13 | fentanyl | 337.5–188.0 | 337.5–105.0 | 131 | 20, 44 |
| 17 | para-methoxyfentanyl | 367.6–105.0 | 367.6–77.1, 367.6–51.2 | 136 | 44, 108, 160 |
| 16 | 4-ANPP | 281.4–105.1 | 284.4–77.2, 281.4–51.3 | 116 | 36, 76, 124 |
| 18 | furanyl fentanyl | 375.1–105.0 | 375.1–188.2 | 125 | 40, 25 |
| 19 | despropionyl para-fluorofentanyl | 299.4–105.0 | 299.4–77.1, 299.4–51.2 | 111 | 36, 88, 88 |
| 22 | carfentanil | 395.2–113.0 | 395.2–105.0, 395.2–77.1 | 131 | 36, 56, 112 |
| 20 | (±)-cis-3-methyl fentanyl | 351.5–202.1 | 351.5–105.0 | 150 | 20, 48 |
| 21 | butyryl/isobutyryl fentanyl | 351.2–188.1 | 351.2–105.1 | 146 | 24, 48 |
| 23 | para-fluorobutyryl/para-fluoroisobutyryl fentanyl | 369.2–105.1 | 369.2–188.1, 369.2–77.1 | 141 | 44, 24, 108 |
| 24 | sufentanil | 387.6–111.0 | 387.3–238.2, 387.6–132.0 | 121 | 44, 36, 36 |
| 25 | valeryl fentanyl | 365.5–105.0 | 365.5–77.1, 365.5–51.2 | 136 | 44, 112, 164 |
N = 3, in bold.
LC-MS/MS Assay Validation
Validation followed method development and occurred daily over 5 days. It included a batch of seven calibrators, controls in triplicate, a negative blood blank, and a water blank. The limit of detection (LOD), limit of quantitation (LOQ), bias, precision (coefficient of variation, % CV), linearity, matrix effects, recovery, carryover, and any potential interferences were determined within the validation period.
Data Analysis
The software used for data analysis was MassHunter Qualitative and Quantitative analysis. Data was plotted in Excel 2016 and Origin 8 software.
The development of the LC-MS/MS method required the adaptation of the following: (1) SPE extractions for separation of the analytes of interest from interferences inherent in biological matrices, (2) LC for further improvement in sensitivity and specificity, and (3) MS/MS for MRM transitions specific to each IMF analogue and analogue quantification at sub ng mL–1 concentrations.
SPE Extractions were performed according to the United Chemical Technologies extraction method (10.5)26 for N = 9 IMF analogues in urine (fentanyl, alfentanil, carfentanil, sufentanil, 3-methyl fentanyl, para-fluorofentanyl, α-methyl fentanyl, thianfentanil, and lofentanil). This gas chromatography–mass spectrometry (GC–MS) method was successfully adapted for the extraction of all N = 24 IMF analogues in whole blood specimens.
LC Optimization
First, two columns were tested: C-18 and biphenyl. The C-18 column proved inefficient for the separation of all IMF analogues, whereas the biphenyl column was found to generate an improved signal-to-noise ratio and separation. Thus, the biphenyl column was selected for method validation. Three mobile phase mixtures (methanol and formic acid, ammonium formate and formic acid in water (MPA), and formic acid in ACN (MPB)) were explored to provide the best separation of IMF analogues in the shortest amount of time. Exploratory work deemed methanol and formic acid as unsuitable because separation of IMF analogues could not be achieved within acceptable time frames (<20 min) and corresponding chromatography exhibited poor signal-to-noise ratios under the studied conditions. Following this, MPA and MPB mixtures were deemed acceptable for a gradient method by achieving time efficient separation (13.5 min). A flow rate of 0.400 mL min–1 was selected to accommodate the maximum column pressure on both LC-MS/MS systems. The gradient change of mobile phases was then optimized from 90% MPA/10% MPB to 10% MPA/90% MPB to achieve a total run time of 13.5 min per sample and to avoid overloading the column (Figure S1).
MS/MS Optimization
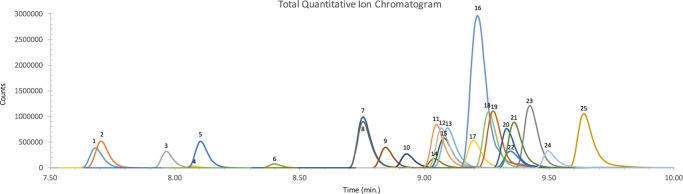
Electrospray ionization mode paired with tandem quadrupole mass spectrometry was employed for MRM transitions, which were optimized for high sensitivity of each IMF analogue. Briefly, precursor-ion and product-ion transitions for each IMF analogue and internal standards (Table 1) were mostly determined using the Agilent Optimizer software. Manual adjustment of the fragmentor and collision energy voltage was done when the software adjustment led to low sensitivity for the qualifier transitions ((±)-cis-3-methyl fentanyl, U-47700, and remifentanil). MRM transitions were identified by the highest sensitivity and specific discrimination between coeluting analogues (e.g., AH-7921 and U-47700 in Table 1). Separation and identification between butyryl fentanyl and isobutyryl fentanyl and para-fluorobutyryl fentanyl and 4-fluoroisobutyryl fentanyl could not be achieved under current conditions. Thus, they were classified as butyryl/isobutyryl and para-fluoroisobutyryl fentanyl/para-fluorobutyryl fentanyl. Using this method, the isomeric IMF analogues can be detected but not distinguished from each other. Thus, Figure 2 shows only the quantitative transitions of N = 22 fentanyl analogues and N = 3 internal standards. The acquisition method report is provided in the Supporting Information.
Figure 2.
LC-MS/MS ion chromatogram of a high calibrator. Each peak represents the quantitative transition ion (qualitative transition ion not shown). Fentanyl analogue and internal standard peak identities: (1) norfentanyl-d5, (2) norfentanyl, (3) furanyl norfentanyl, (4) remifentanil acid, (5) butyryl norfentanyl, (6) remifentanil, (7) acetyl fentanyl, (8) acetyl fentanyl-13C6, (9) alfentanil, (10) U-47700, (11) acetyl fentanyl 4-methylphenethyl, (12) acrylfentanyl, (13) fentanyl, (14) AH-7921, (15) fentanyl-d5, (16) 4-ANPP, (17) para-methoxyfentanyl, (18) furanyl fentanyl, (19) despropionyl para-fluorofentanyl, (20) (±)-cis-3-methyl fentanyl, (21) butyryl/isobutyryl fentanyl, (22) carfentanil, (23) para-fluorobutyryl/para-fluoroisobutyryl fentanyl, (24) sufentanil, and (25) valeryl fentanyl. Separation between butyryl/isobutyryl and para-fluoroisobutyryl fentanyl/para-fluorobutyryl fentanyl was not achieved due to isomerism.
Method Validation
The directed assay was validated by determining the limit of detection (LOD), limit of quantitation (LOQ), selectivity and specificity, recovery, ion suppression/enhancement, process efficiency, bias, and precision. All analyses were performed after a 5 day validation period. LOD, lower limit of quantitation (LLOQ), upper limit of quantitation, bias, and precision were calculated over five replicates from five consecutive days.
Limit of Detection (LOD)
LODs for IMF analogues are listed in Table 2. Evaluation of LOD for most IMF analogues (excluding fentanyl) was carried out by using a linear calibration curve model. LOD was estimated using eq 1
| 1 |
where sy is the standard deviation of the y-intercept and Avgm is the average of the calibration slopes.
Table 2. Retention Times, Limit of Detection (LOD), Lower Limit of Quantitation (LLOQ), and Linear Ranges for IMF Analogues (N = 22) along with Corresponding Internal Standardsa.
| analyte | internal standard | retention time (min) | LOD (ng mL–1) | LLOQ (ng mL–1) | linear range (ng mL–1) |
|---|---|---|---|---|---|
| norfentanyl | norfentanyl-d5 | 7.62 | 0.038 | 0.100 | 0.100–50.0 |
| furanyl norfentanyl | norfentanyl-d5 | 7.90 | 0.058 | 0.250 | 0.250–10.0 |
| remifentanil acid | norfentanyl-d5 | 7.99 | 0.100 | 0.500 | 0.500–10.0 |
| butyryl norfentanyl | norfentanyl-d5 | 8.04 | 0.044 | 0.100 | 0.100–10.0 |
| remifentanil | fentanyl-d5 | 8.33 | 0.053 | 0.100 | 0.100–10.0 |
| acetyl fentanyl | acetyl fentanyl-13C6 | 8.68 | 0.017 | 0.100 | 0.100–10.0 |
| alfentanil | fentanyl-d5 | 8.77 | 0.048 | 0.100 | 0.100–10.0 |
| AH-7921 | fentanyl-d5 | 8.96 | 0.042 | 0.100 | 0.100–10.0 |
| U-47700 | fentanyl-d5 | 8.85 | 0.019 | 0.100 | 0.100–10.0 |
| acetyl fentanyl 4-methylphenethyl | fentanyl-d5 | 8.97 | 0.037 | 0.100 | 0.100–10.0 |
| acrylfentanyl | fentanyl-d5 | 8.99 | 0.034 | 0.100 | 0.100–10.0 |
| fentanyl | fentanyl-d5 | 9.01 | 0.050 | 0.100 | 0.100–50.0 |
| para-methoxyfentanyl | fentanyl-d5 | 9.11 | 0.056 | 0.100 | 0.100–10.0 |
| 4-ANPP | fentanyl-d5 | 9.13 | 0.025 | 0.100 | 0.100–10.0 |
| furanyl fentanyl | fentanyl-d5 | 9.17 | 0.029 | 0.100 | 0.100–10.0 |
| despropionyl para-fluorofentanyl | fentanyl-d5 | 9.19 | 0.016 | 0.100 | 0.100–10.0 |
| carfentanil | fentanyl-d5 | 9.26 | 0.050 | 0.100 | 0.100–10.0 |
| (±)-cis-3-methyl fentanyl | fentanyl-d5 | 9.24 | 0.048 | 0.250 | 0.250–10.0 |
| butyryl/isobutyryl fentanyl | fentanyl-d5 | 9.27 | 0.026 | 0.100 | 0.100–10.0 |
| para-fluorobutyryl/para-fluoroisobutyryl fentanyl | fentanyl-d5 | 9.33 | 0.042 | 0.100 | 0.100–10.0 |
| sufentanil | fentanyl-d5 | 9.42 | 0.100 | 0.250 | 0.250–10.0 |
| valeryl fentanyl | fentanyl-d5 | 9.54 | 0.047 | 0.100 | 0.100–10.0 |
N = 3.
LOD of fentanyl (quadratic fit, 1/x) was determined by evaluating the calibration standards 1/2, 1/5, and 1/10 of the lowest calibrator (i.e., 0.05, 0.025, and 0.01 ng mL–1) for the lowest fentanyl concentration with an acceptable signal-to-noise ratio. The standard that exhibited a signal five times greater than the background noise was then selected as the LOD for fentanyl.
Limit of Quantitation (LOQ)
LODs represent the lowest quantity that can be distinguished from a blank, whereas LOQs define our range of quantitation for the assay. The lowest limit of quantitation (LLOQ) was chosen to be the lowest nonzero calibrator that demonstrated acceptable bias and precision (<20, >80%), along with reproducible chromatography. The upper limit of quantitation (ULOQ) was selected as the highest calibrator within the calibration range (i.e., 10.0 ng mL–1 for all IMF analogues except for fentanyl and norfentanyl at 50.0 ng mL–1).
Selectivity/Specificity
All IMF analogues were evaluated for endogenous and exogenous interferences. Endogenous interferences were evaluated (N = 5) daily with whole blood previously screening negative for targeted IMF analogues. Negative blanks were extracted daily to assess false positive results due to potential matrix interferences. All negative blanks revealed no interferences were present that could result in a false positive identification.
Exogenous interferences were measured with solutions containing N = 70 commonly detected analytes in toxicology laboratories (Table S1). Verification of selectivity included extracting each commonly detected analyte at the concentration level specified in Table S1 and spiking with 0.35 ng mL–1 targeted IMF analytes in whole blood. Specificity was addressed by analyzing all N = 70 nontargeted analytes in whole blood without the addition of IMF analogues. False positives were not detected with nontargeted analytes, but large concentrations of benzodiazepine (2500 ng mL–1) were found to interfere with AH-7921 and U-47700. However, these concentrations are much larger than usually seen in normal assays.
Recovery
SPE extraction recoveries were determined by analyzing post extraction spikes against regular extractions. Recoveries were determined using LOCTRL, MEDCTRL, and HICTRL (N = 3). The average recoveries for LOCTRL, MEDCTRL, and HICTRL were 84 ± 19, 78 ± 12, and 94 ± 4.1%, respectively, for all N = 21 nonisomeric IMF analogues (excluding butyryl/isobutyryl fentanyl and para-fluorobutyryl/para-fluoroisobutyryl fentanyl). Recovery ranges for LOCTRL, MEDCTRL, and HICTRL were 38–140, 33–96, and 91–97%, respectively. All recovery values can be found in Table S2.
Ionization Suppression/Enhancement (ISE)
Ion suppression and enhancement (ISE) was evaluated using post extraction additions that were compared with neat standards. The signal response exhibited minor changes in most IMF analogues. Remifentanil acid exhibited the lowest ISE (<45%). The detection of each analyte was not affected by ISE. All ISE values can be found in Table S3.
Process Efficiency
The total process efficiency was determined for each IMF analogue by comparison of neat standards against regular extractions. The process efficiency for LOCTRL, MEDCTRL, and HICTRL were 80 ± 13, 76 ± 12, and 89 ± 1.3%, respectively, for all IMF analogues. Process efficiency ranges for LOCTRL, MEDCTRL, and HICTRL were 45–104, 41–91, and 88–90%, respectively. All process efficiency values can be found in Table S4.
Statistical quantitation of each IMF analogue followed immediately after qualitative evaluation. Structural isomers that coeluted with each other were only qualitatively determined. Quantitative determination of drugs is normally important for toxicological analyses; however, taking into account the paucity of data available on IMFs, the qualitative identification of an IMF is more important than its quantity. All other IMF analogues were evaluated for bias and precision to meet acceptable criteria.28
Bias and Precision
Intra- and interday bias and precision were assessed with the help of quality control samples containing all IMF analogues (0.35, 2.5, and 25.0 ng mL–1 of IMF analogues). Intraday bias and precision were expressed as the largest calculated bias and precision for each of the 5 days of the validation period. All other bias and precision values fell below the maximum intraday value (Table 3). Any IMF analogue not meeting acceptable criteria (bias < 20% and precision > 80%) was defined as qualitative only.
Table 3. Intra- and Interday Bias and Precision for All IMF Analogues Excluding the Isomeric IMFsa.
| bias
(%) |
precision (% CV) |
|||||
|---|---|---|---|---|---|---|
| analyte | expected concentration (ng mL–1) | mean (ng mL–1) | intraday (n = 3) | interday(n = 15) | intraday (n = 3) | interday (n = 15) |
| norfentanyl | 0.350 | 0.3652 ± 0.011 | 8.6 | 4.3 | 97.7 | 97.0 |
| 2.5 | 2.722 ± 0.17 | 18.0 | 8.9 | 94.3 | 93.5 | |
| 25 | 27.13 ± 0.67 | 10.0 | 8.5 | 98.3 | 97.4 | |
| furanyl norfentanyl | 0.350 | 0.3179 ± 0.023 | 17.0 | 9.2 | 86.0 | 92.5 |
| 2.5 | 2.351 ± 0.21 | 9.3 | 6.0 | 78.0 | 91.0 | |
| remifentanil acid | 0.350 | 0.3211 ± 0.052 | 14.0 | 8.3 | 72.0 | 83.0 |
| 2.5 | 2.327 ± 0.32 | 17.0 | 6.9 | 83.0 | 86.0 | |
| butyryl norfentanyl | 0.350 | 0.3630 ± 0.027 | 17.0 | 3.7 | 94.3 | 92.4 |
| 2.5 | 2.602 ± 0.20 | 18.0 | 4.1 | 94.1 | 92.0 | |
| remifentanil | 0.350 | 0.3353 ± 0.017 | 8.7 | 4.2 | 94.1 | 94.7 |
| 2.5 | 2.339 ± 0.17 | 12.0 | 6.4 | 94.0 | 92.6 | |
| acetyl fentanyl | 0.350 | 0.3478 ± 0.015 | 6.4 | 0.63 | 97.9 | 95.7 |
| 2.5 | 2.579 ± 0.21 | 17.0 | 3.2 | 93.7 | 91.6 | |
| alfentanil | 0.350 | 0.3210 ± 0.019 | 12.0 | 8.3 | 90.9 | 93.8 |
| 2.5 | 2.373 ± 0.15 | 9.4 | 5.1 | 92.9 | 93.6 | |
| AH-7921 | 0.350 | 0.2722 ± 0.11 | 65.0 | 22.0 | –32.0 | 59.0 |
| 2.5 | 1.761 ± 0.65 | 55.0 | 30.0 | 4.0 | 62.0 | |
| U-47700 | 0.350 | 0.3466 ± 0.038 | 13.0 | 0.97 | 87.0 | 89.0 |
| 2.5 | 2.292 ± 0.15 | 14.0 | 8.3 | 92.0 | 93.3 | |
| acetyl fentanyl4-methylphenethyl | 0.350 | 0.3628 ± 0.015 | 8.8 | 3.7 | 94.7 | 95.8 |
| 2.5 | 2.556 ± 0.15 | 9.0 | 2.2 | 92.7 | 93.9 | |
| acrylfentanyl | 0.350 | 0.3507 ± 0.014 | 5.0 | 0.20 | 94.9 | 96.0 |
| 2.5 | 2.502 ± 0.095 | 4.4 | 0.096 | 96.1 | 96.1 | |
| fentanyl | 0.350 | 0.3326 ± 0.015 | 13.0 | 5.0 | 98.2 | 95.3 |
| 2.5 | 2.458 ± 0.15 | 8.0 | 1.7 | 95.1 | 93.5 | |
| 25 | 24.77 ± 1.5 | 6.2 | 0.92 | 88.0 | 93.6 | |
| para-methoxyfentanyl | 0.350 | 0.3598 ± 0.018 | 9.1 | 2.8 | 92.5 | 94.7 |
| 2.5 | 2.533 ± 0.12 | 7.5 | 1.3 | 93.8 | 95.2 | |
| 4-ANPP | 0.350 | 0.3306 ± 0.043 | 20.0 | 5.5 | 76.0 | 87.0 |
| 2.5 | 2.373 ± 0.32 | 20.0 | 5.1 | 84.0 | 86.0 | |
| furanyl fentanyl | 0.350 | 0.3426 ± 0.015 | 4.4 | 2.1 | 94.6 | 95.5 |
| 2.5 | 2.487 ± 0.12 | 5.4 | 0.53 | 95.3 | 95.0 | |
| despropionyl para-fluorofentanyl | 0.350 | 0.2819 ± 0.044 | 23.0 | 19.0 | 71.0 | 84.0 |
| 2.5 | 2.006 ± 0.33 | 37.0 | 20.0 | 81.0 | 83.0 | |
| carfentanil | 0.350 | 0.3281 ± 0.011 | 9.7 | 6.3 | 96.6 | 96.5 |
| 2.5 | 2.366 ± 0.11 | 9.9 | 5.3 | 91.9 | 95.1 | |
| (±)-cis-3-methyl fentanyl | 0.350 | 0.3419 ± 0.030 | 18.0 | 2.3 | 94.1 | 91.0 |
| 2.5 | 2.418 ± 0.24 | 20.0 | 3.3 | 94.0 | 90.0 | |
| butyryl/isobutyryl fentanyl | 0.350 | 0.4488 ± 0.20 | 86.0 | 28.0 | 26.0 | 54.0 |
| 2.5 | 3.348 ± 1.4 | 95.0 | 34.0 | 31.0 | 55.0 | |
| para-fluorobutyryl/para-fluoroisobutyryl fentanyl | 0.350 | 0.3665 ± 0.042 | 19.0 | 4.7 | 92.1 | 88.0 |
| 2.5 | 2.709 ± 0.26 | 19.0 | 8.4 | 96.3 | 90.1 | |
| sufentanil | 0.350 | 0.2903 ± 0.035 | 30.0 | 17.0 | 87.0 | 88.0 |
| 2.5 | 2.074 ± 0.16 | 26.0 | 17.0 | 93.3 | 91.9 | |
| valeryl fentanyl | 0.350 | 0.3492 ± 0.015 | 6.0 | 0.23 | 95.3 | 95.7 |
| 2.5 | 2.480 ± 0.11 | 5.1 | 0.80 | 95.9 | 95.3 | |
Isomeric IMFs include butyryl, isobutyryl fentanyl, para-fluorobutyryl fentanyl, and FIBF. Underlined IMF analogues refer to successful quantitation that met acceptable criteria.
Results and Discussion
The LC-MS/MS method developed in this study allows for the multiplex detection of N = 24 IMF analytes with good sensitivity and a short sample run time (13.5 min). Quantitated IMF analogues (N = 13) passed all evaluations. These were norfentanyl, butyryl norfentanyl, remifentanil, acetyl fentanyl, alfentanil, U-47700, acetyl fentanyl 4-methylphenethyl, acrylfentanyl, fentanyl, para-methoxyfentanyl, furanyl fentanyl, carfentanil, and valeryl fentanyl. All analytes had an LOD ≤ 0.100 ng mL–1 and a maximum LLOQ of 0.500 ng mL–1 (the lowest LLOQ value being 0.100 ng mL–1).
Casework
Since its development and validation in January 2017, the LC-MS/MS method was successfully utilized in the IMF analysis of N = 725 blood samples at the Montgomery County Coroner’s Office (MCCO) in Dayton, Ohio. The postmortem samples were collected from accidental drug overdose death cases that occurred between February 2015 and November 2016. The MCCO laboratory provides postmortem forensic toxicology services to approximately 30 of Ohio’s 88 counties. The following N = 10 IMF analogues were found to be present in the analyzed samples: (±)-cis-3-methyl fentanyl, 4-ANPP, acetyl fentanyl, carfentanil, despropionyl para-fluorofentanyl, fentanyl, furanyl fentanyl, furanyl norfentanyl, norfentanyl, and U-47700. Table 4 summarizes the total number of times each IMF analogue was detected across the N = 725 whole blood samples. Fentanyl (N = 662, 91%) and its metabolite, norfentanyl (N = 582, 80%), were the most commonly encountered in the examined cases. Furthermore, N = 82 cases (11%) tested positive for 4-ANPP, which is an impurity related to the synthesis of fentanyl and also a metabolite of fentanyl. There were also 40 acetyl fentanyl (6%), 39 furanyl fentanyl (5%), and 22 carfentanil (3%) positive cases.
Table 4. Total Number of Times each IMF Analogue was Detected in the N = 725 Cases of Unintentional Drug Overdose Death.
| IMF analogue | number of times detected |
|---|---|
| (±)-cis-3-methyl fentanyl | 1 |
| 4-ANPP | 82 |
| acetyl fentanyl | 40 |
| carfentanil | 22 |
| despropionyl fluorofentanyl | 1 |
| fentanyl | 662 |
| furanyl fentanyl | 39 |
| furanyl norfentanyl | 2 |
| norfentanyl | 582 |
| U-47700 | 3 |
The analysis of more recent accidental overdose cases at MCCO laboratory that occurred in between January and February 2017, identified N = 13 IMF analogues (fentanyl, acrylfentanyl, furanyl fentanyl, carfentanil, norfentanyl, despropionyl fentanyl (4-ANPP), despropionyl para-fluorofentanyl, furanyl norfentanyl, acetyl fentanyl, butyryl/isobutyryl fentanyl, butyryl norfentanyl, fluorobutyryl/fluoroisobutyryl fentanyl, and U-47700).29
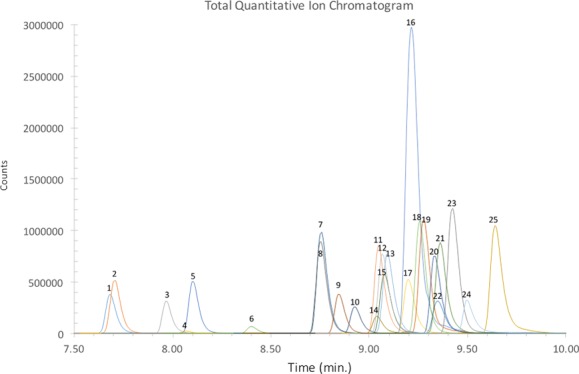
Figure 3 illustrates the recent, multiplex detection of N = 8 IMF analogues from a single whole blood sample at MCCO along with their corresponding concentrations: norfentanyl (0.47 ng mL–1), U-47700 (0.50 ng mL–1), fentanyl (1.2 ng mL–1), and furanyl fentanyl (1.5 ng mL–1). Because of cases like this, it is critical to incorporate such flexible methods into the routine toxicological analysis at forensic laboratories worldwide. A second manuscript is in production, in which N = 725 cases will be discussed in detail.
Figure 3.
Quantitative ion chromatogram of an accidental overdose case from late 2016. Abbreviations are as follows: (1) norfentanyl-d5, (2) norfentanyl, (8) acetyl fentanyl 13C6, (10) U-47700, (13) fentanyl, (15) fentanyl-d5, (16) 4-ANPP, (18) furanyl fentanyl, (19) despropionyl para-fluorofentanyl, (21) butyryl/isobutyryl fentanyl, and (23) para-fluorobutyryl/para-fluoroisobutyryl fentanyl. Inset shows the low-response count region of IMF analogues.
Several previous studies8−10,30−35 have already demonstrated the capabilities of the LC-MS/MS-based analytical method in detecting IMF analogues, in the 0.050–0.500 ng mL–1 concentration range.10 However, the LOQ was determined to be the lowest calibrator at 0.100 ng mL–1, which is equivalent to the LOQ value of this study. To the best of our knowledge, these LC-MS/MS studies on human blood detected at most N = 17 IMF analogues and homologues with a 35 min scan time but without quantitation.30 Furthermore, those LC-MS/MS methods that offered quantitation did not tackle more than N = 9 IMF analogues.35 As the frequency of opioid abuse cases is drastically increasing both in the forensic and clinical world, both the qualitative identification and quantitation of such analytes is becoming equally important.36 The LC-MS/MS method of this study will address this deficit by facilitating both the identification (N = 22) and quantification (N = 13 for illustrative purposes) of IMF analogues and metabolites (total of N = 24 of the most commonly encountered IMFs in human blood) down to 0.100 ng mL–1, i.e., the lowest LOQ reported to date according to our knowledge. Additionally, this LC-MS/MS method can be easily adapted to accommodate newly emerging IMFs in various drug analysis settings and with the shortest screening time (13.5 min) under the studied conditions.
Other analytical methods, such as gas chromatography–mass spectrometry (GC–MS)37 and thermal desorption direct analysis in real time mass spectrometry,38 have also been explored. Although successful in the qualitative detection of N = 17 and quantitation of N = 4 IMF analogues and metabolites, these methods had greater LODs (0.08–0.351 ng mL–1) and LLOQs (0.500 ng mL–1) than the ones described in this LC-MS/MS method, namely, 0.017–0.050 and 0.100 ng mL–1, respectively, for all N = 13 quantitated IMF analogues.
Conclusions
An LC-MS/MS-based method was developed for the multiplex detection of N = 24 IMF analogues and metabolites in postmortem blood at sub ng mL–1 concentrations. It was successfully implemented at the Montgomery County Coroner’s Office/Miami Valley Regional Crime Laboratory in Dayton, Ohio, where it aided in the analysis of N = 725 postmortem blood samples collected from accidental drug overdose death cases. This forensic work demonstrated the cost- and time-efficiency of the newly developed IMF detection method. In addition to employing commercially available, inexpensive supplies and common forensic instrumentation, the method requires 13.5 min scan time for a single sample and 5–10 min for quantitative and qualitative analysis. The LC-MS/MS-based protocol can be easily adapted by forensic laboratories worldwide; it is currently undergoing modifications to incorporate the addition of four new IMF analogues (β-hydroxythiofentanyl, para-fluorofentanyl, tetrahydrofuran fentanyl, and cyclopropyl fentanyl) at the Montgomery County Coroner’s Office.
Acknowledgments
This work was supported by the National Institute on Drug Abuse (NIH/NIDA #R21DA042757), the Montgomery County Coroner’s Office/Miami Valley Regional Crime Laboratory, and the Department of Chemistry at WSU. The authors would like to thank Robert Carlson, Ph.D., for the useful discussions and Dr. Kent Harshbarger and the Montgomery County Coroner’s Office staff for technical support with the method development and implementation.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01536.
Mobile phase gradient versus time change plot (Figure S1), the drugs commonly encountered in forensic toxicology work (Table S1), the IMF recovery percentages (Table S2), the ionization suppression and enhancement percentages (Table S3), the process efficiency percentages (Table S4), and the LC-MS/MS acquisition method report (PDF)
Author Contributions
All authors contributed to the writing of this manuscript and the design of the studies. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Rosenblum A.; Marsch L. A.; Joseph H.; Portenoy R. K. Opioids and the Treatment of Chronic Pain: Controversies, Current Status, and Future Directions. Exp. Clin. Psychopharmacol. 2008, 16, 405–416. 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather L. E. Clinical Pharmacokinetics of Fentanyl and its Newer Derivatives. Clin. Pharmacokinet. 1983, 8, 422–446. 10.2165/00003088-198308050-00004. [DOI] [PubMed] [Google Scholar]
- Suzuki J.; El-Haddad S. A review: Fentanyl and non-pharmaceutical fentanyls. Drug Alcohol Depend. 2017, 171, 107–116. 10.1016/j.drugalcdep.2016.11.033. [DOI] [PubMed] [Google Scholar]
- Stanley T. H. The history and development of the fentanyl series. J. Pain Symptom Manage. 1992, 7, S3–S7. 10.1016/0885-3924(92)90047-L. [DOI] [PubMed] [Google Scholar]
- Stanley T. H. The Fentanyl Story. J. Pain 2014, 15, 1215–1226. 10.1016/j.jpain.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Henderson G. L. Fentanyl-related deaths: demographics, circumstances, and toxicology of 112 cases. J. Forensic Sci. 1991, 36, 422–433. 10.1520/JFS13045J. [DOI] [PubMed] [Google Scholar]
- Kronstrand R.; Druid H.; Holmgren P.; Rajs J. A cluster of fentanyl-related deaths among drug addicts in Sweden. Forensic Sci. Int. 1997, 88, 185–195. 10.1016/S0379-0738(97)00068-6. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Agency DEA Issues Nation Wide Alert on Fentanyl as Threat to Health and Public Safety. https://www.dea.gov/divisions/hq/2015/hq031815.shtml (accessed March 19, 2017).
- Seither J.; Reidy L. Confirmation of Carfentanil, U-47700 and Other Synthetic Opioids in a Human Performance Case by LC-MS-MS. J. Anal. Toxicol. 2017, 41, 493–497. 10.1093/jat/bkx049. [DOI] [PubMed] [Google Scholar]
- Sofalvi S.; Schueler H. E.; Lavins E. S.; Kaspar C. K.; Brooker I. T.; Mazzola C. D.; Dolinak D.; Gilson T. P.; Perch S. An LC-MS-MS Method for the Analysis of Carfentanil, 3-Methylfentanyl, 2-Furanyl Fentanyl, Acetyl Fentanyl, Fentanyl and Norfentanyl in Postmortem and Impaired-Driving Cases. J. Anal. Toxicol. 2017, 41, 473–483. 10.1093/jat/bkx052. [DOI] [PubMed] [Google Scholar]
- Peterson A. B.; et al. Increases in Fentanyl-Related Overdose Deaths - Florida and Ohio, 2013-2015. Morb. Mortal. Wkly. Rep. 2016, 65, 844–849. 10.15585/mmwr.mm6533a3. [DOI] [PubMed] [Google Scholar]
- Helander A.; Backberg M.; Beck O. Intoxications involving the fentanyl analogues acetylfentanyl, 4-methoxybutyrfentanyl and furanylfentanyl: results from the Swedish STRIDA project. Clin. Toxicol. 2016, 54, 324–32. 10.3109/15563650.2016.1139715. [DOI] [PubMed] [Google Scholar]
- Poklis J.; Poklis A.; Wolf C.; Mainland M.; Hair L.; Devers K.; Chrostowski L.; Arbefeville E.; Merves M.; Pearson J. Postmortem tissue distribution of acetyl fentanyl, fentanyl and their respective nor-metabolites analyzed by ultrahigh performance liquid chromatography with tandem mass spectrometry. Forensic Sci. Int. 2015, 257, 435–441. 10.1016/j.forsciint.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr A. L. A.; Friscia M.; Papsun D.; Kacinko S. L.; Buzby D.; Logan B. K. Analysis of Novel Synthetic Opioids U-47700, U-50488 and Furanyl Fentanyl by LC-MS/MS in Postmortem Casework. J. Anal. Toxicol. 2016, 40, 709–717. 10.1093/jat/bkw086. [DOI] [PubMed] [Google Scholar]
- Sherba T.Fentanyl, and the Deadlier Carfentanil, Now Outpacing Heroin Sales in Many Areas. http://mha.ohio.gov/Portals/0/assets/Research/OSAM-TRI/Fentanyl-Carfentanil-OSAM-O-Gram_March2017.pdf (accessed March 22, 2017).
- Drug Enforcement Agency Carfentanil: A Dangerous New Factor in the U.S. Opioid Crisis. https://www.dea.gov/divisions/hq/2016/hq092216_attach.pdf (accessed March 22, 2017).
- Head J.Synthetic Drug Threats in the United States: 2017 Update. https://ndews.umd.edu/sites/ndews.umd.edu/files/ndews-webinar-feb-15-2017-synthetic-drug-threats-jill-m-head-dea.pdf (accessed March 22, 2017).
- Shoff E. N.; Zaney M. E.; Kahl J. H.; Hime G. W.; Boland D. M. Qualitative Identification of Fentanyl Analogues and Other Opioids in Postmortem Cases by UHPLC-Ion Trap-MSn. J. Anal. Toxicol. 2017, 41, 484–492. 10.1093/jat/bkx041. [DOI] [PubMed] [Google Scholar]
- Popper N.Opioid Dealers Embrace the Dark Web to Send Deadly Drugs by Mail. https://www.nytimes.com/2017/06/10/business/dealbook/opioid-dark-web-drug-overdose.html (accessed Aug 10, 2017).
- Anderson L. A.U-47700 (Pink) Drug: Effects, Hazards & Warnings. https://www.drugs.com/illicit/u-47700.html (accessed Aug 10, 2017).
- Schedules of Controlled Substances: Placement of AH-7921 Into Schedule I. https://www.deadiversion.usdoj.gov/fed_regs/rules/2016/fr0414_4.htm (accessed April 14, 2017).
- Katselou M.; Papoutsis I.; Nikolaou P.; Spiliopoulou C.; Athanaselis S. AH-7921: the list of new psychoactive opioids is expanded. Forensic Toxicol. 2016, 34, 199. 10.1007/s11419-015-0301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. L.; Vorce S. P.; Holler J. M.; Shimomura E.; Magluilo J.; Jacobs A. J.; Huestis M. A. Modern instrumental methods in forensic toxicology. J. Anal. Toxicol. 2007, 31, 237–253. 10.1093/jat/31.5.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson E.; Andersson M.; Stephanson N.; Beck O. Validation of direct injection electrospray LC-MS/MS for confirmation of opiates in urine drug testing. J. Mass Spectrom. 2007, 42, 881–889. 10.1002/jms.1219. [DOI] [PubMed] [Google Scholar]
- Edinboro L. E.; Backer R.; Poklis A. Direct analysis of opiates in urine by liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2005, 29, 704–710. 10.1093/jat/29.7.704. [DOI] [PubMed] [Google Scholar]
- Anderson L.; Hunter C. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol. Cell. Proteomics 2006, 5, 573–588. 10.1074/mcp.M500331-MCP200. [DOI] [PubMed] [Google Scholar]
- Telepchak M. J.; August T. F.; Chaney G.. Forensic and Clinical Applications of Solid Phase Extraction; Humana Press: Totowa, NJ 07512, 2004; Vol. 1, pp 370. [Google Scholar]
- Scientific Working Group for Forensic Toxicology (SWGTOX) Standard Practices for Method Validation in Forensic Toxicology. J. Anal. Toxicol. 2013, 37, 452–474.. 10.1093/jat/bkt054. [DOI] [PubMed] [Google Scholar]
- Daniulaityte R.; Juhascik M. P.; Strayer K. E.; Sizemore I. E.; Harshbarger K. E.; Antonides H. M.; Carlson R. R. Overdose Deaths Related to Fentanyl and Its analogues - Ohio, January-February 2017. Morb. Mortal. Wkly. Rep. 2017, 66, 904–908. 10.15585/mmwr.mm6634a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie I. S.; Iio R. Use of multiple-reaction monitoring ratios for identifying incompletely resolved fentanyl homologs and analogues via ultra-high-pressure liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 1515–1519. 10.1016/j.chroma.2008.12.097. [DOI] [PubMed] [Google Scholar]
- Verplaetse R.; Tytgat J. Development and validation of a sensitive ultra performance liquid chromatography tandem mass spectrometry method for the analysis of fentanyl and its major metabolite norfentanyl in urine and whole blood in forensic context. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2010, 878, 1987–1996. 10.1016/j.jchromb.2010.05.033. [DOI] [PubMed] [Google Scholar]
- Shaner R. L.; Kaplan P.; Hamelin E. I.; Bragg W. A.; Johnson R. C. Comparison of two automated solid phase extractions for the detection of ten fentanyl analogues and metabolites in human urine using liquid chromatography tandem mass spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2014, 962, 52–58. 10.1016/j.jchromb.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner R. L.; Schulze N. D.; Seymour C.; Hamelin E. I.; Thomasa J. D.; Johnsona R. C. Quantitation of fentanyl analogues in dried blood spots by flow-through desorption coupled to online solid phase extraction tandem mass spectrometry. Anal. Methods 2017, 9, 3876–3883. 10.1039/C7AY00532F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumba V. A.; Di Rago M.; Peka M.; Drummer O. H.; Gerostamoulos D. The analysis of 132 novel psychoactive substances in human hair using a single step extraction by tandem LC/MS. Forensic Sci. Int. 2017, 279, 192–202. 10.1016/j.forsciint.2017.08.031. [DOI] [PubMed] [Google Scholar]
- Gergov M.; Nokua P.; Vuori E.; Qjanpera I. Simultaneous screening and quantification of 25 opioid drugs in post-mortem blood and urine by liquid chromatography-tandem mass spectrometry. Forensic Sci. Int. 2009, 186, 36–43. 10.1016/j.forsciint.2009.01.013. [DOI] [PubMed] [Google Scholar]
- America’s Opioid Epidemic and Its Effect On The Nation’s Commericially-Insured Population. https://www.bcbs.com/the-health-of-america/reports/americas-opioid-epidemic-and-its-effect-on-the-nations-commercially-insured (accessed Nov 25, 2017).
- Strano-Rossi S.; Alvarez I.; Tabernero M. J.; Cabarcos P.; Fernandez P.; Bermejo A. M. Determination of fentanyl, metabolite and analogues in urine by GC/MS. J. Appl. Toxicol. 2011, 31, 649–654. 10.1002/jat.1613. [DOI] [PubMed] [Google Scholar]
- Sisco E.; Verkouteren J.; Staymates J.; Lawrence J. Rapid detection of fentanyl, fentanyl analogues, and opioids for on-site or laboratory based drug seizure screening using thermal desorption DART-MS and ion mobility spectrometry. Forensic Chem. 2017, 4, 108–115. 10.1016/j.forc.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.