Abstract
Background
Scientific guidelines recommend the National Institutes of Health Stroke Scale for ischemic stroke assessment. However, many nurses find “slim” National Institutes of Health Stroke Scale versions or the Glasgow Coma Scale easier to use.
Objective
To compare 3 “slim” versions of the National Institutes of Health Stroke Scale and the Glasgow Coma Scale with the full National Institutes of Health Stroke Scale.
Methods
Components of the full National Institutes of Health Stroke Scale and Glasgow Coma Scale were abstracted from records of consecutive stroke patients. Items were subtracted from the full National Institutes of Health Stroke Scale, with items contained in “slim” versions retained. False-negative rates for neurological disability were calculated for the “slim” versions and the Glasgow Coma Scale.
Results
Data were collected from 172 acute stroke patients (median [interquartile range] 6 [3–12] for National Institutes of Health Stroke Scale, 15 [12–15] for Glasgow Coma Scale): 143 (83%) were ischemic stroke patients (27% posterior circulation strokes) and 29 (17%) were intracerebral hemorrhage patients. The value of “slim” scales and the Glasgow Coma Scale declined in a stepwise manner as the full National Institutes of Health Stroke Scale decreased because of false-negative results despite the presence of a measurable disabling deficit. False-negative rates were 5% to 19% on “slim” versions and 56% with the Glasgow Coma Scale.
Conclusions
Use of “slim” scales, and in particular the Glasgow Coma Scale, substantially decreases the value of a structured neurological assessment, particularly in patients with low National Institutes of Health Stroke Scale scores.
The National Institutes of Health Stroke Scale (NIHSS) was developed as a method to quantify neurological disability in patients with acute ischemic stroke who were enrolled in clinical trials of reperfusion therapies.1 Use of the scale provides a reliable measure of neurological deficits, whether the scores are obtained directly through in-person examination, gathered indirectly via telemedicine, or calculated from documented clinical findings in medical records.2–22 Since 1996, use of the NIHSS has become the standard of care for ongoing neurological assessment of ischemic stroke in US stroke centers, and its use is supported by the Brain Attack Coalition guidelines,23 the American Stroke Association (ASA) guidelines24 for care of patients with acute ischemic stroke, and the ASA nursing scientific statement25 for care of patients with acute stroke.
Despite these recommendations, concerns commonly voiced by nursing staff about use of the full NIHSS include the time required to complete the neurological examination and the difficulty in mastering performance and interpretation of some components of the examination, which are more complex than components of other neurological scales such as the Glasgow Coma Scale (GCS). Although some researchers26–29 have systematically studied methods to shorten the NIHSS yet retain detection of important neurological disabilities and an ability to predict outcome, many nursing units have independently developed unstudied “slim” versions of the NIHSS to provide ease of use and reduced workload. The most common of the slim versions used by nurses is a GCS-like version that retains level of consciousness and motor function measures, but adds language testing. Gochan and Fisher30 have advocated use of this limited measure set solely when repetitive assessments are ordered more frequently than every 8 hours for patients in stable condition. Additionally, some institutions continue using the GCS as a substitute nursing examination for patients with ischemic stroke, although no data exist documenting the validity of the GCS in detecting neurological deficit in stroke patients. We sought to understand the relationship and agreement between the full NIHSS and 3 slim versions cited in the literature and the GCS in patients with ischemic stroke or intracerebral hemorrhagic to determine the value of the slim nursing assessments in detecting neurological disability.
Methods
Permission was obtained from the Committee for the Protection of Human Subjects at the Comprehensive Stroke Center, University of Alabama at Birmingham, Birmingham, Alabama, to perform a retrospective analysis of NIHSS and GCS scores on a convenience sample of stroke patients admitted to the stroke service during a 4-month period. Inclusion criteria for the study included age greater than 19 years, documentation in the medical record of both an NIHSS and a GCS score, and either a primary admitting diagnosis of acute ischemic stroke verified by diffusion-weighted imaging findings on magnetic resonance imaging or intracerebral hemorrhage as verified by noncontrast computed tomography.
Slim versions of the full NIHSS selected for the study (see Table) were derived from the literature as follows: version 1 (subtraction of items 1B, 1C, 5A, 5B, 7, 8, 11)26; version 2 (subtraction of items 1A 1B, 1C, 4, 5A, 5B, 7, 8, 10, 11)26; and version 3 (subtraction of items 1B, 1C, 2, 3, 4, 7, 8, 10, 11).30 Elements of the full NIHSS not included in each of the 3 slim versions were subtracted from the true total NIHSS score to determine scores for slim versions 1, 2, and 3. Total possible scores were zero to 23 for slim version 1, zero to 16 for slim version 2, zero to 21 for slim version 3, and zero to 42 for the full NIHSS.
The individual component scores for the full NIHSS and GCS were abstracted from medical records and were verified for accuracy against both the documented corresponding clinical examination and the stroke center data repository system. Data were collected and verified by 4 NIHSS-certified practitioners: 1 nurse who abstracted medical record data; 1 nurse and 1 physician who verified the agreement and accuracy of the NIHSS with the documented clinical examination and stroke registry findings; and the senior investigator who, along with biostatisticians, verified overall accuracy of data entry and slim scale calculations. Discrepancies in scores were vetted by the physician director of the stroke center and the senior investigator to ensure accuracy of all assessment scores.
Three “slim” versions, the Glasgow Coma Scale, and the National Institutes of Health Stroke Scale were compared.
Statistical analyses were performed by using SPSS, version 15.0. software (SPSS Inc). Continuous data are presented as means and standard deviations or as medians and interquartile ranges (IQRs). Rates of false-negative scores, whereby a slim version total score was zero and the full NIHSS indicated the presence of a neurological disability, were calculated.
Results
A total of 239 medical records were screened for inclusion in the study; of these, 172 met the criteria for inclusion. The mean age of the patients was 62 years (SD, 15), and 51% were women. Of the 172 patients included in the study, 143 (83%) had ischemic stroke, and 29 (17%) had intracerebral hemorrhagic stroke. Among the 143 with ischemic stroke, 39 (27%) had posterior circulation stroke.
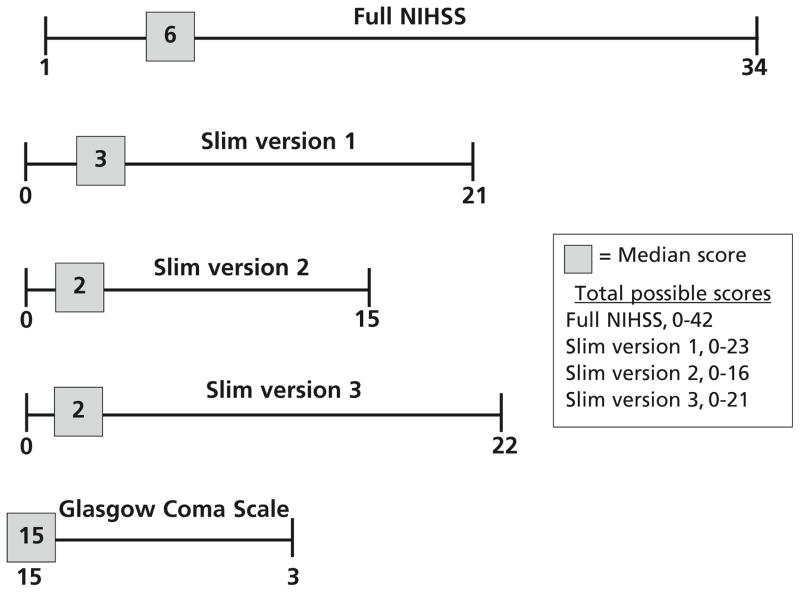
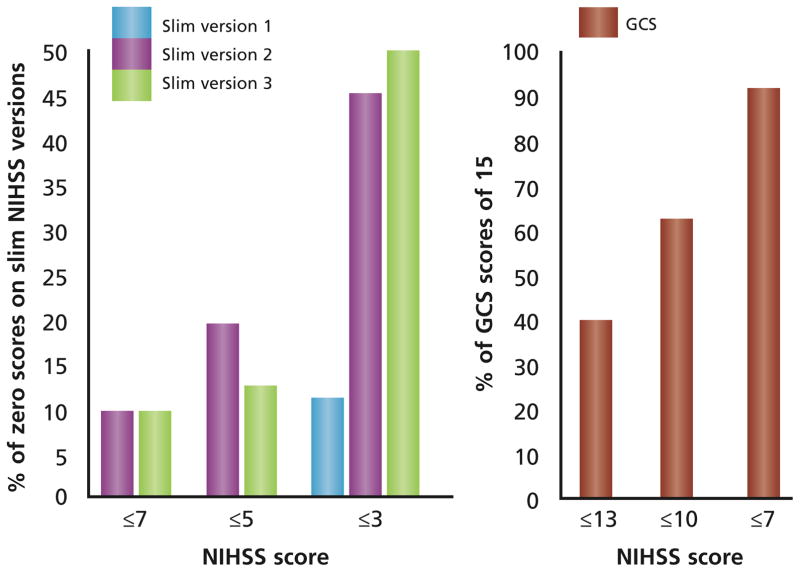
The median full NIHSS score was 6 (IQR, 3–12), and the median GCS score was 15 (IQR, 12–15). Figure 1 shows the ranges and median scores for each slim version in relation to the range and median score of the full NIHSS and the GCS. The value of using the slim-NIHSS versions and the GCS declined in a stepwise manner as the full NIHSS score decreased because of false-negative (ie, “Slim-NIHSS” = 0; GCS = 15) results despite the presence of a measurable disabling deficit on the full NIHSS (Figure 2). Slim version 1 incorrectly categorized 8 of 172 patients (5%) as having no neurological deficit when the full NIHSS measured actual neurological deficits, whereas slim versions 2 and 3 both incorrectly categorized 33 of 172 patients (19%) as having no neurological deficit when the full NIHSS showed neurological deficits. The GCS incorrectly categorized 97 of 172 patients (56%) as having no neurological deficit, when neurological disability was detectable on the full NIHSS. With a full NIHSS score of less than 7, slim versions 2 and 3 had 10% false-negative results, whereas the GCS had 90% false-negative results. Slim version 1 demonstrated an ability to detect some aspects of neurological disability until the full NIHSS score totaled 3 or less.
Figure 1.
Ranges and medians of each slim version and the Glas-gow Coma Scalea compared with the full National Institutes of Health Stroke Scale (NIHSS).
aPresented inverse to the NIHSS and slim version ranges because a score of 15 is considered normal.
Figure 2.
Poor agreement of slim versions and the Glasgow Coma Scale (GCS) with the full National Institutes of Health Stroke Scale (NIHSS). As the full NIHSS score decreases, the rate of false-negative scores on the 3 slim versions and the Glasgow Coma Scale increases.
Discussion
Our findings underscore the value of using the full NIHSS to detect neurological disability at key intervals in the treatment and hospitalization of patients with acute stroke, particularly patients with low NIHSS scores for whom the substitution of a slim version may result in false-negative results. Although scores on the NIHSS may not indicate findings obtained with a classical neurological examination, such as hand clumsiness and gait disturbances, the scores do provide standardization of examination across disciplines and allow quantification of neurological disability. Additionally, in acute disability-threatening circumstances, completion of a thorough classical examination is impractical; for example, gait testing is not feasible in most patients with stroke during the hyperacute phase of care.
A common complaint about use of the NIHSS among nursing staff is the length of time required to complete the examination. However, Shafqat et al8 found that completion of the NIHSS takes 9.7 minutes via telemedicine compared with 6.5 minutes with direct in-person completion. For nurses for whom the NIHSS is new, the examination may take slightly longer, but speed and accuracy in performance are well supported by training and practice,31–35 making arguments related to the time required to complete the neurological examination inappropriate. Once a score on the full NIHSS has been obtained, use of a customized version that focuses solely on the abnormal elements detected with the full NIHSS, or use of the 8-item slim version 1,26 which provided the best results in our study, might be suitable alternatives in time-sensitive circumstances if a shortened examination time is required.
Another common complaint of nurses is that mastery of the complex neurological tests for extraocular movement (item 2), visual fields (item 3), ataxia (item 7), and extinction (item 11) in the full NIHSS is difficult. Although version 1 was the best of the slim versions we tested, nurses who wish to eliminate difficult test elements would find little advantage in using this version because it retains many of the more complex assessment components commonly found objectionable. In other words, mastery of the full NIHSS supports an ability to appropriately perform the slim version 1. Therefore, we support the ASA nursing scientific statement,25 which recommends that registered nurses who provide care to patients with acute stroke should know how to use the full NIHSS.
Use of the full NIHSS for both the initial assessment and assessments repeated at key intervals also supports consistency of interdisciplinary communication among nurses and physicians in their written descriptions and verbal discussions of stroke disabilities. Accuracy in communication is essential to patients’ safety because it ensures interdisciplinary awareness of key findings and the potential need for emergency intervention, further indicating the importance of having nurses adhere to use of a valid and reliable standardized method for assessment of patients with acute stroke. Because of the low rates in the United States of treatment with intravenous tissue plasminogen activator in patients with acute ischemic stroke, nursing units that choose to use slim versions of the NIHSS must clearly communicate this choice to physicians. Otherwise, physicians may mistake a truly disabled patient as deficit-free or as having a nondisabling stroke, particularly because mild or resolving deficits are often used as reasons not to treat patients with intravenous tissue plasminogen activator. Additionally, low NIHSS scores that may be missed when slim versions are used should not be assumed to reflect minimal neurological deficit that does not meet criteria for treatment with tissue plasminogen activator. Scores of 3 or less may include neurological findings such as visual field cuts and cortical blindness, hemineglect, or pure expressive aphasias that are highly disabling and should be recognized as warranting treatment with intravenous tissue plasminogen activator.
Whether or not a patient is a candidate for reperfusion therapy, all patients with ischemic stroke should have the full NIHSS when they are admitted to obtain a baseline measure, throughout their hospital stay to document improvement or deterioration in their neurological status, and at discharge to document hospital outcome. However, use of the full NIHSS for neurological assessments conducted every 15 to 30 minutes to meet standard-of-care requirements in patients undergoing intravenous or intra-arterial reper-fusion treatments may not be realistic. Although current guidelines do not specify what components of the neurological assessment should be used during these frequent repeat assessments, we propose that the following assessment practice be adopted:
Use the full NIHSS to obtain a baseline score before reperfusion therapy is started.
Obtain full NIHSS scores a minimum of every 2 hours for the first 24 hours during reperfusion; after that obtain full NIHSS scores at least every 4 hours, increasing the time between assessments as dictated by changes in a patient’s status.
During neurological assessments done at 15- to 30-minute intervals, use a customized NIHSS that focuses on the patient’s disabilities. For example, if the patient has weakness of the right upper extremity, the face, and the lower extremity along with expressive aphasia, the examination should focus on these elements. Alternatively, the slim version 1 might be considered, although depending on the neurological territory involved, this slim version may or may not contain elements specific to the patient’s signs and symptoms.
At any time, if a patient’s neurological status improves or deteriorates, a full NIHSS score should be obtained and documented, and the frequency of neurological assessments should be reconsidered.
We also found that use of the GCS as an assessment measure in patients with acute stroke provides little information about neurological disability and important changes in the clinical examination that warrant notification of the stroke team and the potential need for intervention. This finding was clearly evident in our study from both the median GCS score of 15 and the high degree of discordance between full NIHSS scores as high as 13, of which 40% were in patients with normal GCS scores. We conclude that institutions that use the GCS as their primary assessment measure for patients with ischemic stroke or diagnoses associated with a high risk for stroke (eg, transient ischemic attack, carotid revascularization), should reevaluate their practice and adopt use of the full NIHSS and the strategies for frequent repeated assessments that we propose.
We did not address the validity of the full NIHSS in patients with strokes due to intracerebral hemorrhage. However, our findings indicate that use of the GCS alone may not reflect the severity of neurological deficits regardless of stroke subtype. Future research on whether the NIHSS may be a valid standardized neurological assessment in patients with intracerebral hemorrhage is clearly warranted. Because the intracerebral hemorrhage score focuses on prediction of mortality and should be obtained near the time of hemorrhage, concurrent NIHSS assessments may provide a complimentary picture of evolving neurological disability in patients with intracerebral hemorrhage.
In conclusion, our findings support the Brain Attack Coalition guidelines23 and ASA scientific statements24,25 for use of the full NIHSS to quantify neurological deficit in patients with acute stroke. Nurses’ use of the full NIHSS provides important information on the severity of the stroke, promotes comparisons with NIHSS examinations performed by other interdisciplinary members of the stroke team, and allows detection and prompt communication of deficits that might otherwise not be evident when a slim version of the NIHSS is the sole assessment used. Although guidelines currently do not specify what scale should be used for high-frequency assessments after reperfusion therapy, we propose that use of customized versions of the NIHSS would provide the best alternative for detecting neurological change in a time-sensitive manner.
Table.
Examination components of the 3 “slim” versions of the National Institutes of Health Stroke Scale selected for study
| Items retained or removed from the full scale | Slim version | ||
|---|---|---|---|
| 1a | 2a | 3b | |
| Retained | Level of consciousness item 1A (wakefulness) Best gaze item 2 Visual fields item 3 Facial weakness item 4 Motor legs items 6A and 6B Language item 9 Dysarthria item 10 |
Best gaze item 2 Visual fields item 3 Motor legs items 6A and 6B Language item 9 |
Level of consciousness item 1A (wakefulness) Motor arms items 5A and 5B Motor legs items 6A and 6B Language item 9 |
| Removed | Level of consciousness items 1B (questions) and 1C (commands) Motor arms items 5A and 5B Limb ataxia item 7 Sensory item 8 Extinction item 11 |
Level of consciousness items 1A (wakefulness), 1B (questions), and 1C (commands) Facial weakness item 4 Motor arms items 5A and 5B Limb ataxia item 7 Sensory item 8 Dysarthria item 10 Extinction item 11 |
Level of consciousness items 1B (questions) and 1C (commands) Best gaze item 2 Visual fields item 3 Facial weakness item 4 Limb ataxia item 7 Sensory item 8 Dysarthria item 10 Extinction item 11 |
Footnotes
To purchase electronic or print reprints, contact The InnoVision Group, 101 Columbia, Aliso Viejo, CA 92656. Phone, (800) 899-1712 or (949) 362-2050 (ext 532); fax, (949) 362-2049; reprints@aacn.org
FINANCIAL DISCLOSURES
None reported.
eLetters
Now that you’ve read the article, create or contribute to an online discussion on this topic. Visit www.ajcconline.org and click “Submit a response” in either the full-text or PDF view of the article.
Contributor Information
Brandon R. Nye, BSN Honors students at University of Alabama at Birmingham (UAB) School of Nursing when this study was conducted.
Christina E. Hyde, BSN Honors students at University of Alabama at Birmingham (UAB) School of Nursing when this study was conducted.
Georgios Tsivgoulis, Assistant professor at the UAB Comprehensive Stroke Center and in the Department of Neurology, Democritus University of Thrace School of Medicine, Alexandroupolis, Greece.
Karen C. Albright, Assistant professor at the UAB Comprehensive Stroke Center and a doctoral student in the UAB School of Public Health.
Andrei V. Alexandrov, Professor and director of the UAB Comprehensive Stroke Center.
Anne W. Alexandrov, Assistant dean and professor, Doctor of Nursing Practice program coordinator, and NET SMART program director at UAB School of Nursing and UAB Comprehensive Stroke Center.
References
- 1.Brott T, Adams H, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol. 1989;46(6):660–662. doi: 10.1001/archneur.1989.00520420080026. [DOI] [PubMed] [Google Scholar]
- 3.D’Olhaberriague L, Litvan I, Mitsias P, Mansbach HH. A reappraisal of reliability and validity studies in stroke. Stroke. 1996;27:2331–2336. doi: 10.1161/01.str.27.12.2331. [DOI] [PubMed] [Google Scholar]
- 4.Tilley BC, Marler J, Geller NL, et al. Use of a global test for multiple outcomes in stroke trials with application to the National Institute of Neurological Disorders and Stroke t-PA stroke trial. Stroke. 1996;27:2136–2142. doi: 10.1161/01.str.27.11.2136. [DOI] [PubMed] [Google Scholar]
- 5.Woo D, Broderick JP, Kothari RU, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA Stroke Study Group. Stroke. 1999;30(11):2355–2359. doi: 10.1161/01.str.30.11.2355. [DOI] [PubMed] [Google Scholar]
- 6.Lyden P, Lu M, Jackson C, et al. Underlying structure of the National Institutes of Health Stroke Scale: results of a factor analysis. NINDS tPA Stroke Trial Investigators. Stroke. 1999;30(11):2347–2354. doi: 10.1161/01.str.30.11.2347. [DOI] [PubMed] [Google Scholar]
- 7.Craig JJ, McConville JP, Patterson VH, Wootton R. Neurological examination is possible using telemedicine. J Telemed Telecare. 1999;5:177–181. doi: 10.1258/1357633991933594. [DOI] [PubMed] [Google Scholar]
- 8.Shafqat S, Kvedar JC, Guanci MM, Chang Y, Schwamm LH. Role for telemedicine in acute stroke: feasibility and reliability of remote administration of the NIH stroke scale. Stroke. 1999;30:2141–2145. doi: 10.1161/01.str.30.10.2141. [DOI] [PubMed] [Google Scholar]
- 9.Meyer BC, Lyden PD, Al-Khoury L, et al. Prospective reliability of the STRokE DOC wireless/site independent telemedicine system. Neurology. 2005;64(6):1058–1060. doi: 10.1212/01.WNL.0000154601.26653.E7. [DOI] [PubMed] [Google Scholar]
- 10.LaMonte MP, Bahouth MN, Hu P, et al. Telemedicine for acute stroke: triumphs and pitfalls. Stroke. 2003;34:725–728. doi: 10.1161/01.STR.0000056945.36583.37. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Lee SB, Pardue C, et al. Remote evaluation of acute ischemic stroke: reliability of National Institutes of Health Stroke Scale via telestroke. Stroke. 2003;34:e188–e191. doi: 10.1161/01.STR.0000091847.82140.9D. [DOI] [PubMed] [Google Scholar]
- 12.Handschu R, Littmann R, Reulbach U, et al. Telemedicine in emergency evaluation of acute stroke: interrater agreement in remote video examination with a novel multimedia system. Stroke. 2003;34:2842–2846. doi: 10.1161/01.STR.0000102043.70312.E9. [DOI] [PubMed] [Google Scholar]
- 13.Hess DC, Wang S, Hamilton W, et al. REACH: clinical feasibility of a rural telestroke network. Stroke. 2005;36(9):2018–2020. doi: 10.1161/01.STR.0000177534.02969.e4. [DOI] [PubMed] [Google Scholar]
- 14.Schwamm LH, Rosenthal ES, Hirshberg A, et al. Virtual TeleStroke support for the emergency department evaluation of acute stroke. Acad Emerg Med. 2004;11(11):1193–1197. doi: 10.1197/j.aem.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Schwamm LH, Holloway RG, Amarenco P, et al. A review of the evidence for use of telemedicine within stroke systems of care. Stroke. 2009;40:1–19. doi: 10.1161/STROKEAHA.109.192360. [DOI] [PubMed] [Google Scholar]
- 16.Audebert HJ, Wimmer ML, Hahn R, et al. TEMPIS Group. Can telemedicine contribute to fulfill WHO Helsingborg Declaration of specialized stroke care? Cerebrovasc Dis. 2005;20(5):362–369. doi: 10.1159/000088064. [DOI] [PubMed] [Google Scholar]
- 17.Audebert HJ, Kukla C, Vatankhah B, et al. Comparison of tissue plasminogen activator administration management between Telestroke Network hospitals and academic stroke centers: the Telemedical Pilot Project for Integrative Stroke Care in Bavaria/Germany. Stroke. 2006;37:1822–1827. doi: 10.1161/01.STR.0000226741.20629.b2. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Gross H, Lee SB, et al. Remote evaluation of acute ischemic stroke in rural community hospitals in Georgia. Stroke. 2004;35:1763–1768. doi: 10.1161/01.STR.0000131858.63829.6e. [DOI] [PubMed] [Google Scholar]
- 19.Meyer BC, Raman R, Rao R, et al. The STRokE DOC trial technique: “video clip, drip and/or ship”. Int J Stroke. 2007;2:281–287. doi: 10.1111/j.1747-4949.2007.00153.x. [DOI] [PubMed] [Google Scholar]
- 20.Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7:787–795. doi: 10.1016/S1474-4422(08)70171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasner SE, Chalela JA, Luciano JM, et al. Reliability and validity of estimating the NIH Stroke Scale score from medical records. Stroke. 1999;30:1534–1537. doi: 10.1161/01.str.30.8.1534. [DOI] [PubMed] [Google Scholar]
- 22.Lindsell CJ, Alwell K, Moomaw CJ, et al. Validity of a retrospective National Institutes of Health Stroke Scale scoring methodology in patients with severe stroke. J Stroke Cerebrovasc Dis. 2005;14:281–283. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Alberts MA, Hademenos G, Latchaw RE, et al. Brain Attack Coalition. Recommendations for the establishment of primary stroke centers. JAMA. 2000;283:3102–3109. doi: 10.1001/jama.283.23.3102. [DOI] [PubMed] [Google Scholar]
- 24.Adams HP, Jr, Del Zoppo G, Alberts MJ, et al. American Heart Association; American Stroke Association Stroke Council; Clinical Cardiology Council; Cardiovascular Radiology and Intervention Council; Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 25.Summers D, Leonard A, Wentworth D, et al. Comprehensive overview of nursing and interdisciplinary care of the acute ischemic stroke patient. Stroke. 2009;40:2911–2944. doi: 10.1161/STROKEAHA.109.192362. [DOI] [PubMed] [Google Scholar]
- 26.Tirschwell D, Longstreth WT, Becker KJ, et al. Shortening the NIH Stroke Scale for use in the prehospital setting. Stroke. 2002;33(12):2801–2806. doi: 10.1161/01.str.0000044166.28481.bc. [DOI] [PubMed] [Google Scholar]
- 27.Lyden PD, Lu M, Levine SR, Brott TG, Broderick J. A modified National Institutes of Health Stroke Scale for use in stroke clinical trials: preliminary reliability and validity. Stroke. 2001;32:1310–1317. doi: 10.1161/01.str.32.6.1310. [DOI] [PubMed] [Google Scholar]
- 28.Meyer BC, Hemmen TM, Jackson CM, Lyden PD. Modified National Institutes of Health Stroke Scale for use in stroke clinical trials: prospective reliability and validity. Stroke. 2002;33:1261–1266. doi: 10.1161/01.str.0000015625.87603.a7. [DOI] [PubMed] [Google Scholar]
- 29.Kothari R, Hall K, Brott T, Broderick J. Early stroke recognition: developing an out-of-hospital NIH stroke scale. Acad Emerg Med. 1997;4:986–990. doi: 10.1111/j.1553-2712.1997.tb03665.x. [DOI] [PubMed] [Google Scholar]
- 30.Gochan S, Fisher A. Neurologic assessment by nurses using the National Institutes of Health Stroke Scale: implementation of best practice guidelines. Can J Neurosci Nurs. 2008;30(3):31–42. [PubMed] [Google Scholar]
- 31.Goldstein LB, Samsa GP. Reliability of the National Institutes of Health Stroke Scale: extension to non-neurologists in the context of a clinical trial. Stroke. 1997;28(2):307–310. doi: 10.1161/01.str.28.2.307. [DOI] [PubMed] [Google Scholar]
- 32.Dewey HM, Donnan GA, Freeman EJ, et al. Interrater reliability of the National Institutes of Health Stroke Scale: rating by neurologists and nurses in a community-based stroke incidence study. Cerebrovasc Dis. 1999;9:323–327. doi: 10.1159/000016006. [DOI] [PubMed] [Google Scholar]
- 33.Spilker J, Kongable G, Barch C, et al. Using the NIH Stroke Scale to assess stroke patients: the NINDS rt-PA Stroke Study Group. J Neurosci Nurs. 1997;29:384–392. doi: 10.1097/01376517-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Schmulling S, Grond M, Rudolf J, Kiencke P. Training as a prerequisite for reliable use of NIH Stroke Scale. Stroke. 1998;29(6):1258–1259. doi: 10.1161/01.str.29.6.1258. [DOI] [PubMed] [Google Scholar]
- 35.Lyden P, Brott T, Tilley B, et al. Improved reliability of the NIH Stroke Scale using video training: NINDS TPA Stroke Study Group. Stroke. 1994;25(11):2220–2226. doi: 10.1161/01.str.25.11.2220. [DOI] [PubMed] [Google Scholar]