Abstract
We present here a new CryoEM grid boxes storage system designed to simplify sample labeling, tracking and retrieval. The system is based on the crystal pucks widely used by the x-ray crystallographic community for storage and shipping of crystals. This system is suitable for any cryoEM laboratory, but especially for large facilities that will need accurate tracking of large numbers of samples coming from different sources
Keywords: CryoTEM, automation, grid storage, pucks
Introduction
Over the past several decades (1), and more dramatically over the past 5 years (2), single particle cryo electron microscopy (cryoEM) has established itself as an essential tool for three dimensional structure determination of molecules of biological interest. Continuous advances in hardware, software and sample preparation and handling have enabled high-resolution analysis of samples ranging in size from megadaltons to less than 70K (3) (4) (5) (6). Some structures can now be determined to sub-3Å resolutions (7) (8) (9) (5) (10), and molecular complexity and flexibility is no longer seen as a major obstacle to structure determination, but rather as an asset that can be exploited to fully understand the behavior of proteins under near-native conditions (11).
Statistics from the EM map deposition database1 clearly show that in the past 8 years there has been a dramatic increase in the number of maps released and their associated publications2; structures released in 2014–2016 account for more than half of the total structures present in the database. As the full potential of the technique is increasingly understood, access to cryoEM microscopes is becoming a more and more sought after asset for academic and industrial laboratories worldwide. While larger institutions can afford to establish their own EM laboratories, smaller institutions will probably rely on more central user facilities, following the established trends with synchrotrons for x-ray data collection.
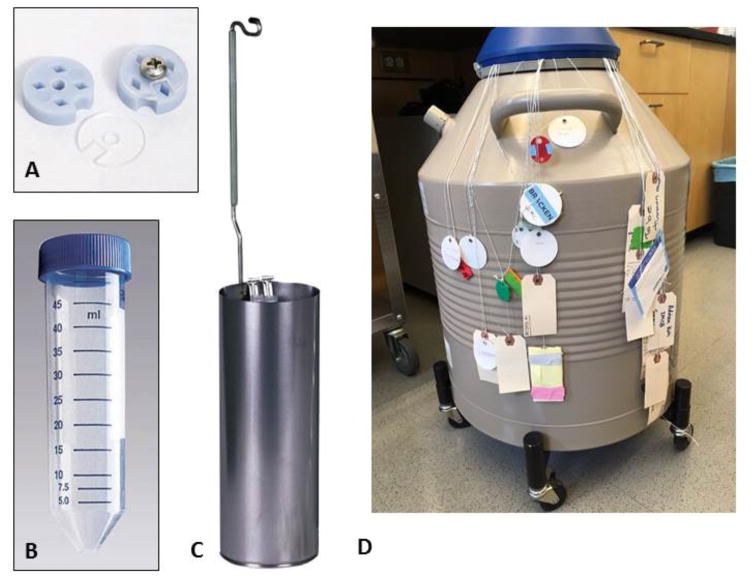
One issue that both individual labs and, to a greater extent, larger facilities will need to address is the inevitable increase in the number of projects and samples that will need to be handled at any given time. A robust and routine method for sample labeling, tracking, storage and retrieval will be critically important for accurately associating individual samples with the correct experimental data. The current method used to store cryoEM grids in most practicing labs and facilities is to store a number of individual grid boxes, each typically holding 4 individual sample grids (see figure 1a) inside 50ml conical falcon tubes (figure 1b) which are placed into canisters (figure 1c) and lowered into long-term liquid nitrogen storage dewars (figure 1d). Each falcon tube is inserted and retrieved from the canister inside the dewar by means of a string threaded and knotted through the lid of the tube which is typically identified by some kind of label attached by a variety of means to the end of the string that hangs out of the dewar (figure 1d). As multiple grid boxes can be stored in a single tube and multiple tubes are stored in the same dewar this can create a practical and logistical nightmare (figure 1) for the organization, retrieval and management of cryoEM samples. The strings often become tangled, loose or may snap, leading to the loss of the conical tube inside the dewar; the labels can wear off or become detached. Furthermore, identification of the correct grid box among several identical boxes within the same tube can be difficult or confusing and this can also lead to loss of samples.
Figure 1.
a) Typical cryo-grid box; each box has four positions. b) 50ml conical falcon tube. c) Sample canister for storage of the falcon tubes. d) Long term liquid nitrogen storage dewars; each tube in the dewar is attached to a string, and identified by labels attached to the same string
For facilities that will be handling proprietary data from a variety of different clients (for example industrial support facilities) as well as larger facilities with many external users, the current method of grid storage will not only be a major inconvenience but may become a legal liability, since it may lead to sample loss and also does not ensure an adequate separation between samples coming from different sources.
Storage pucks for cryoEM
For many years now, crystallography labs and large x-ray facilities have been using a puck system to store and track crystals frozen for data collection, for example, the Rigaku ACTOR magazine system3, or the Unipuck system4. Although both were designed to facilitate automated sample handling both within individual laboratory and at synchrotron facilities5, they proved to be equally effective for simple crystal storage and shipping. Another type of storage system used throughout Europe is the SPINE system6.
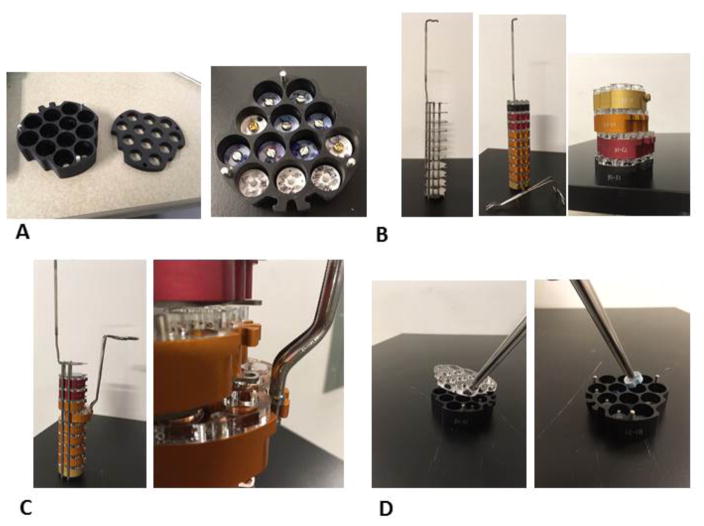
Drawing from the experience with these crystallography solutions, we have designed a modified magazine system (figure 2a), based on the Rigaku magazine system, suitable for storage of cryoEM grid boxes in individual compartments within a larger metal rack (figure 2b) that is then inserted into a standard wide neck LN2 dewar. The pucks are constructed of anodized aluminum, to ensure that they are durable and remain frost free. They hold their temperature (~−170°C) allowing sufficient transfer time from the storage dewar to any other container that may be used to handle the grid boxes. Pucks can be manipulated with specialized tongs (figure 2c), characterized by a hemostat-style closure and tips that fit into the puck slots to ensure secure handling. Pucks can be color-coded, labeled or barcoded for easy classification and accurate record keeping and tracking. Removal of the grid boxes from the pucks can be done using standard tweezers (figure 2d).
Figure 2.
a) View of the modified magazine system described in the text; 12 cryoEM grid boxes can be stored in individual compartments. b) The metal rack used to store the pucks. Each puck is color-coded, and engraved with a unique code for easy classification and tracking. c) Pucks can be manipulated with specialized tongs with a hemostat-style closure and tips that fit into the puck slots to ensure secure handling. d) Removal of the puck cover and the grid boxes from the pucks can be done using standard tweezers.
Up to 10 pucks can be stored in an individual rack (figure 2b), which can then be stored under liquid nitrogen in a standard dewar (for example, Taylor-Wharton HC20, HC34, HC35, and VHC35 storage dewars). The bottom of each puck is magnetic, to prevent it from slipping out of the storage rack. X-ray crystals are usually stored in loops with a magnetic base, and are thus held in place while in the puck. As grid boxes are not magnetic, and may potentially float away while in the LN2, a simple cover has been designed to fit on top of the pucks (Figure 2e). The cover is held in place by three small pins and it is easily removable. Holes in the cover allow the puck to fill with LN2. The design of the rack is also compatible with standard CX100 (or equivalent) shipping dewars, thus facilitating transportation of samples between facilities.
This novel puck storage system has been tested at The National Resource for Automated Molecular Microscopy and the Simons Electron Microscopy Center at the New York Structural Biology Center. No increase of grid contamination was observed for samples stored in the new puck system as compared to those stored in the traditional Falcon tubes. Each of the pucks is engraved with a unique code (currently the batch code followed by a number, see figure 2b). As a result, labeling, storage and retrieval of the boxes is much easier, faster and much less prone to error or loss of sample.
Since these dewars can hold 6 racks, up to 720 individual cryoEM grids can be stored in a single dewar. In practice we often do not use the top two positions of the rack, closest to the top of the storage dewar, since that would require that the LN2 level be topped off almost daily to avoid frost formation. This approach still leaves 8 pucks, 96 positions, 576 cryoEM samples, available for storage.
Use of the pucks is currently tracked in a centralized manner using an Excel spread-sheet, and it is assumed that each user keeps track of his/her puck content. It will be very straightforward to adapt this to a database driven position tracking system (dewar/rack/puck/position/grid box slot) well suited to a large lab or central user facility. For example, at Merck crystals are tracked using an internal database that allows the user to associate each sample (crystal) to a specific position of a specific puck in a specific cane in the user’s storage dewar. Although at the moment this is done manually by the user, it would be probably possible to associated barcodes to each of the components, and use a scanner to accomplish the tracking.
The puck design was reverse engineered from the existing Rigaku pucks. The top plate and hook for the storage rack were purchased from Rigaku. All other parts were commercially available or fabricated by Rotech Tool & Mold (824 Fairfield Ave, Kenilworth, NJ 07033). Designs can be made available upon request. We encourage others to use these as a starting point to develop similar or more sophisticated system for cryoEM grid storage.
Acknowledgments
The authors would like to thanks Z. Zhang, V. Dandey (National Resource for Automated Molecular Microscopy (NRAMM) - NYSBC), and K. Jordan (Simons Electron Microscopy Center (SEMC) - NYSBC) for testing the pucks and providing valuable feedbacks; B. Carragher and C. S. Potter (NRAMM & SEMC – NYSBC) for continuous support. NRAMM is supported by a grant from the National Institute of General Medical Sciences (9 P41 GM103310) from the National Institutes of Health; SEMC is supported by the Simons Foundation Grant 349247.
Footnotes
References
- 1.The development of cryo-EM into a mainstream structural biology technique. Nogales E. Nat Meth. 2016;13:24–27. doi: 10.1038/nmeth.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The resolution revolution. Kühlbrandt W. Science. 2014;343:1443–1444. doi: 10.1126/science.1251652. 6178. [DOI] [PubMed] [Google Scholar]
- 3.Cryo-electron microscopy and the amazing race to atomic resolution. Binshtein E, Ohi MD. Biochemistry. 2015 May 26;54:3133–3141. doi: 10.1021/acs.biochem.5b00114. 20. [DOI] [PubMed] [Google Scholar]
- 4.Overview and future of single particle electron cryomicroscopy. Henderson R. Arch Biochem Biophys. 2015 Sep 1;581:19–24. doi: 10.1016/j.abb.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 5.Resolution advances in cryo-EM enable application to drug discovery. Subramaniam S, et al. Curr Opin Struct Bio. 2016 Dec;41:194–202. doi: 10.1016/j.sbi.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cryo electron microscopy to determine the structure of macromolecular complexes. Carroni M, Saibil HR. Methods. 2016 Feb 15;95:78–85. doi: 10.1016/j.ymeth.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antibody-Based Affinity Cryoelectron Microscopy at 2.6-angstrom Resolution. Yu G, et al. Structure. 2016;24:1984–1990. doi: 10.1016/j.str.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Structure and assembly model for the Trypanosoma cruzi 60S ribosomal subunit. Liu Z, et al. Proc Natl Acad Sci USA. 2016;113:12174–12179. doi: 10.1073/pnas.1614594113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.2.3 A resolution cryo-EM structure of human p97 and mechanism of allosteric inhibition. Banerjee S, et al. Science. 2016;351:871–875. doi: 10.1126/science.aad7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Single-Particle CryoEM Analysis at Near-Atomic Resolution from Several Thousand Asymmetric Subunits. Passos DO, Lyumkis D. J Struct Biol. 2015;192:235–244. doi: 10.1016/j.jsb.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Conformational changes studied by cryo-electron microscopy. Saibil HR. Nat Struct Biol. 2000;7:711–714. doi: 10.1038/78923. [DOI] [PubMed] [Google Scholar]