Abstract
Introduction
In preparation for future clinical trials, we determined the reliability, relationship to measures of disease severity, and consistency across sites of the 6 minute walk test (6MWT) in patients with facioscapulohumeral muscular dystrophy (FSHD).
Methods
Genetically defined and clinically affected FSHD participants at 2 sites performed the 6MWT, the Timed Up and Go, and the 30 foot Go/Timed 10 meter test as measures of mobility using standard procedures.
Results
Eight-six participants representing the full range of severity performed the 6MWT. The mean 6MWT distance was 404.3 meters (SD 123.9), with no difference between sites. The 6MWT was reliable (n=25, intraclass correlation coefficient= 0.99) and demonstrated moderate to strong correlations with lower extremity strength, functional outcomes, and FSHD Clinical Score.
Discussion
The 6MWT is reliable and is associated with other measures of FSHD disease severity. Future directions include assessing its sensitivity to disease progression.
Search Terms: Neuromuscular disease, Facioscapulohumeral muscular dystrophy, clinical trials, outcome measures, 6 Minute Walk Test
Introduction
Facioscapulohumeral muscular dystrophy (FSHD) is one of the most common forms of adult muscular dystrophy.1 It is an autosomal dominant condition with incomplete penetrance where affected individuals experience progressive weakness that affects the facial, scapular, trunk, and limb muscles.2 Over time, weakness results in impaired functional abilities; however, most individuals remain ambulatory until later in the disease course, and approximately 20% of individuals require the use of a wheelchair for mobility by their sixth decade.1 Advances in our understanding of the molecular pathophysiology underlying the disease process have identified potential targets for therapy.3 As the FSHD research community focuses on symptomatic and disease-directed therapies, there is a pressing need to develop reliable, sensitive, and clinically meaningful functional outcome measures for clinical trials. This need has been highlighted in 2 international clinical trial preparedness FSHD workshops.4, 5
The 6 minute walk test (6MWT) is a measure of functional exercise capacity that originated in studies of patients with cardiac and respiratory conditions.6 Using a standard protocol, individuals are asked to walk as far possible in 6 minutes. The 6MWT has been widely used as an outcome measure in neuromuscular conditions including spinal muscular atrophy,7 Duchenne muscular dystrophy (DMD),8 inclusion body myositis,9 myotonic dystrophy,10 and Pompe disease,11 and it was a primary outcome for a study gaining regulatory approval of enzyme replacement therapy for adult-onset Pompe disease.12
The objective of this study was to determine 6MWT distance and it’s variability in a symptomatically diverse group of genetically defined FSHD participants while assessing the consistency of results across 2 sites using a similar protocol. Through this research we also assessed the test-retest reliability and relationship of the 6MWT to other measures of disease severity and mobility.
Methods
Prospective cross-sectional studies were performed using separate protocols at the University of Rochester Medical Center (URMC) and the Kennedy Krieger Institute (KKI) between 2012 and 2015. Data from the 2 sites were combined for analysis. These studies were approved by Institutional Review Boards at both sites, and all participants gave informed consent.
Participants
Participants were recruited from both sites using existing neuromuscular databases, or directly from clinic. Participants were > 18 years of age, had genetic confirmation of a 4q deletion diagnostic of FSHD1,13 were independently ambulatory, and were clinically affected based on examination by a neuromuscular physician. Participants who were not able to perform the functional testing or were unable to give informed consent were excluded.
Participants came into the clinic or Clinical Research Center for a single day visit and performed the 6MWT, the Timed Up and Go (TUG), the 30 foot Go (performed at URMC) or the 10 meter/ walk run (performed at KKI), and manual muscle testing (performed at URMC) using standard procedures. Participants at URMC returned within 3 weeks for interval reliability testing.
Measures
Functional Assessments
The 6MWT was performed using a standardized protocol that followed the American Thoracic Society guidelines.14 Both sites used a straight course, but the course length varied between the 2 sites; a 40 meter course was used at URMC, and a 50 meter course was used at KKI. A prior study showed equivalency of different straight course lengths between 15–50 meters in calculating 6MWT distances.14,15 Additionally, the distance walked during the first and last 2 minutes of the 6MWT was recorded (URMC only).
The TUG is a measure of functional mobility and balance where the individual stands from a standard height chair, walks 3 meters, turns around, and returns to the seated position. The TUG is a reliable measure of mobility that has also been correlated with a patient’s fall risk.16,17
The 30 foot Go is a test of maximal performance. Individuals stand at the start line and are shown the finish line. They are asked to traverse 30 feet as fast as they are able to do so safely. The time from the word “go” to both feet crossing the finish line is recorded. The 30 foot Go has shown to be a reliable test in individuals with FSHD.18 The timed 10 meter walk/run is performed in a similar fashion, however over a slightly longer distance of 10 meters.19
Clinical Severity
Disease severity was documented using the FSHD Clinical Score. The FSHD Clinical Score is a 15 point ordinal scale made up of subscales for the face, shoulders, arms, abdomen, and distal and proximal lower extremities; a score of 0 indicates no muscle weakness, and 15 indicates severe muscle weakness.20
Strength Assessment
Manual muscle testing was performed using standard muscle testing procedures on 16 bilateral muscle groups, neck flexion, and neck extension.18,21 The muscle groups tested were shoulder abductors and external rotators, elbow flexors and extensors, wrist flexors and extensors, common finger extension, thumb flexors, hip flexors and extensors, hip abductors and adductors, knee flexors and extensors, ankle dorsiflexors and plantar flexors, and neck flexors and extensors. Strength assessment was performed by a trained physical therapist. Muscles were graded 0–5 using a modified Medical Research Council scale.18
Statistical Analyses
Descriptive statistics [mean, standard deviation (SD), frequencies] were used to summarize the data. Differences between sites were tested using 2 sample t-tests for continuous variables or Fisher exact tests for frequencies. Interclass Correlation Coefficients (ICC) and the lower 95% confidence limits were calculated to determine test-retest reliability. Standard Pearson or Spearman (for non-normal distributions) correlation coefficients were used to determine the relationship between the 6MWT and clinical severity, lower extremity strength, and other measures of mobility. The minimal detectible change 95% (MDC95) was defined as .22,23 As there were differences in age between sites, an adjusted analysis of the mean 6MWT between sites was obtained using a generalized linear model with 6MWT as the dependent variable, and site, gender, age, severity, and mutation as independent variables. Lastly, the degree to which lower extremity strength contributed to 6MWT distance was determined using regression analysis. All statistical tests were 2-sided, and P<0.05 were used for significance. Statistics were performed using the IBM SPSS statistical package (version 23).
Results
A total of 86 participants (42% woman, mean age 49.1 years) representing a broad range of clinical severity participated in the study (FSHD Clinical Score range 1–12, Table 1). The sites were comparable with regard to gender, clinical severity, and D4Z4 residual fragment size. Participants at KKI were 6.7 years younger (P=0.04).
Table 1.
Participant demographics
| Demographic | URMC N=35 |
KKI N=51 |
Combined N=86 |
P-value |
|---|---|---|---|---|
| Mean Age Years (range; SD) | 53.1 (22–68; 11.7) | 46.4 (18–84; 16.7) | 49.1 (18–84; 15.2) | 0.04 |
| Gender Number Women(%) | 12 (34) | 24 (47) | 36 (42) | 0.27 |
| Mean D4Z4 residual fragment kb (range; SD) | 25.1 (13–41; 6.3) | 22.9 (14–36; 5.7) | 23.7 (13–41; 6.0) | 0.11 |
| Mean FSHD Clinical Score (range; SD) | 7.0 (2–12; 2.9) | 5.9 (1–12; 3.1) | 6.4 (1–12; 3.0) | 0.11 |
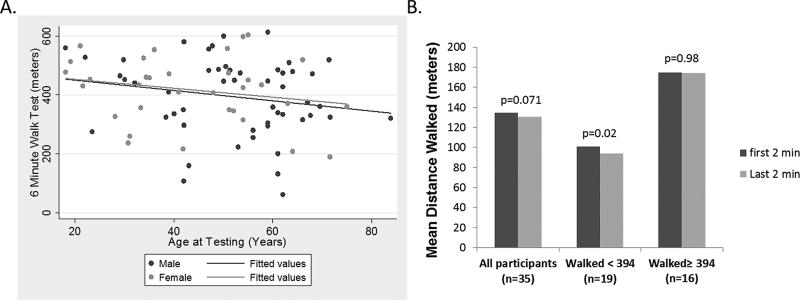
The combined mean distance walked during the 6MWT was 404.3 meters, SD 123.9; URMC 394.4 meters, SD 140.4; KKI 4411.1 meters, SD 112.4, P=0.54. There were no differences in 6MWT distances related to age, or between men and women (Figure 1A). An adjusted analysis for potential differences in age, gender, severity, and mutation also did not reveal a difference in 6MWT distances between sites (URMC 412.5 meters, SD 105.5; KKI 406.9 meters, SD 103.5, P=0.82). To further investigate the utility of the 6MWT for use as a potential outcome measure in FSHD, the minimal detectable change (MDC95) was calculated. The MDC95 was 34.3 meters, which represents the magnitude of change at which one could be 95% certain the change exceeds measurement error.
Figure 1.
A. There was no relationship between 6MWT distance and age for adult patients with FSHD (r=0.20 P=0.07). There was no difference in performance based on gender. B. Mean difference in the distance walked during the first and last 2 minutes of the 6MWT. Participants who performed below the mean 6MWT distance showed fatigue when comparing the first 2 minutes to the last 2 minutes. P-values are taken from single sample paired t-test.
The change in the distance walked between the beginning of the test versus the end has been indicative of fatigue in some neuromuscular conditions.7 We found no difference between the distance walked during the first 2 minutes of the 6MWT and the last 2 minutes. However participants who performed less than the mean 6MWT distance showed a drop of 6.8 meters between the first and last 2 minutes of the test (n=19, P=0.02, Figure 1B).
The test-retest reliability (baseline versus < 3 weeks) of the 6MWT was excellent, with an ICC of 0.99 (lower confidence limit 0.98, n=25).
The 6MWT had moderate to strong relationships with other measures of FSHD disease severity (Table 2). The 6MWT was strongly correlated with a combined lower extremity MMT score (ρ= 0.79; P <0.0001), and lower extremity strength accounted for approximately 47% of the variability seen in the 6MWT distances in a generalized linear regression (R2=0.47, P<0.0001). In addition, the 6MWT was associated with other tests of mobility. It was inversely correlated with timed functional tasks, including the TUG (ρ= −0.81; P<0.0001), the 30 foot Go (ρ= −0.88; P<0.0001), and the timed 10 meter walk/run (ρ=−0.78; P<0.0001).
Table 2.
Relationship of 6MWT to disease severity and other measures of mobility
| 6MWT | |||
|---|---|---|---|
| Measure | UR (ρ; P value) |
KKI (ρ; P value) |
Combined Sites (ρ; P value) |
| FSHD Clinical Score | −0.74; <0.0001 | −0.42; 0.009* | −0.57; <0.0001 |
| TUG | −0.89; <0.0001 | −0.73; <0.0001ǂ | −0.81; <0.0001 |
| 30 foot GO/10MT | −0.88; <0.0001 | −0.78; <0.0001† | −0.83; <0.0001 |
FSHD Clinical Score available for 38 of 51 subjects
10 MT available for 50 of 51 subjects
TUG available for 48 of 51 subjects
Discussion
We found the 6MWT to be reliable and its performance consistent when compared across 2 sites using similar protocols. The 6MWT had moderate to strong correlations with other measures of FSHD disease severity, including measures of strength and function. Combined lower extremity strength appeared to account for approximately half of the variability seen in distance. While fatigue during the test was not a major factor for the whole population (as based on a comparison between the distance walked in the first vs. last 2 minutes), it may be a factor in those participants who walk < 400 meters. A below 400 meter 6-minute walk distance may be a necessary inclusion criterion for any future study looking to use the 6MWT to study fatigue and impaired endurance in FSHD.
Although debate exists regarding what a small change in a measure like the 6MWT might mean to the patient, it has become established as a common outcome for neuromuscular clinical trials. The amount of change considered to be meaningful to patients is usually defined statistically by determining the minimally clinically important difference (MCID). The most common approach for determining the MCID uses an anchoring technique to determine the smallest change in an outcome that corresponds to patients stating they are improved. The MCID for the 6MWT has been defined for many diseases, and the scale of this change has been relatively consistent, ranging from 35–55 meters in the cardiopulmonary literature.27, 28–30 In Duchenne muscular dystrophy the MCID was estimated to be approximately 30 meters.8 While the MCID cannot be determined from a cross-sectional study, statistical measures can be used to determine the MDC95, which was 34.3 meters. This is a value that is similar to prior estimates of the MCID in other diseases.
One possible detriment to using the 6MWT has been the perception that the test potentially detects changes smaller than what is clinically meaningful. In the key registration trial of enzyme-replacement therapy for adult-onset Pompe disease, the size of the effect on the 6MWT was only 28 meters; some questioned whether this was enough to be considered a meaningful improvement despite simultaneous improvement in respiratory function. An outcome measure like the 6MWT may be important for FSHD, especially if changes predict future improvements or are associated with improvements across multiple other clinically relevant outcomes. Indeed, for drugs designed to alter the disease course, a small change in functional performance in a task inherently important, like walking, may be important when deciding to continue long-term therapy.
More specifically for FSHD a functional task with a broader dynamic range that was sensitive to small changes in function is important for early efficacy studies. To date standard timed functional measures, like the 30 foot Go, time to stand from seated position, and time to climb 4 stairs were not sensitive to disease progression in FSHD, for periods of time as long as 3 years.31 The 6MWT has many appealing qualities for a disease like FSHD, which is mainly a disease of purely skeletal muscle dysfunction. The longer length of the test allows for measurement of both of strength and muscle fatigue. Lower extremity motor strength accounts for about half of the variability in the 6MWT in FSHD, and while there was not marked fatigue across the whole group, a subgroup walked less than the mean and experienced fatigue over the course of the test. The 6MWT distance correlates with FSHD disease severity, so the participants who walked less than the mean are typically more severely affected. This suggests that there may be a cut-off in the 6MWT which corresponds to underlying severity where fatigue becomes a significant component of performance on the 6MWT. This may help inform inclusion criteria or stratification strategies for future clinical trials using the 6MWT as a primary outcome.
In DMD a major challenge to using a functional measure like the 6MWT has been the complex relationship between increasing motor function which occurs as young boys develop and decreasing motor function associated with disease. In particular, for DMD, it was the lack of understanding of the natural history of the 6MWT in DMD which arguably has muddied the interpretation of the effectiveness of potential new therapies. This was perhaps most evident in the Ataluren randomized controlled study, which was negative for its primary outcome, but which suffered from a higher than anticipated loss of ambulation in boys over the course of the study, in both the treated and placebo groups.32 In this study a sub-analysis of boys who were > 7 years of age, and who walked < 350 meters were identified as a responder group. These same observations have been confirmed in subsequent natural history studies which identified a ‘sweet spot’ for more consistent rates of decline in the 6MWT for boys > 7 and walking 200–400 meters, which have become standard early entry criteria for newer exon-skipping trials in DMD.25,27 FSHD, unlike DMD, is predominately a late-adolescent or adult onset muscular dystrophy, so developmental change is not a major issue for clinical trial planning. Nevertheless, similar to DMD, understanding the natural history of 6MWT in FSHD is important. This study is a first step in that process. Here, we show that the 6MWT in FSHD is reliable, abnormal, and relates to other important measures of disease progression.
Limitations to this study include the small sample size and evaluation of the test at only 2 study sites. Ultimately understanding the relationship between gender, age, baseline functional status, mutation, and change over time will be necessary for efficient clinical trial planning. The 6MWT is a test of both strength and endurance, and endurance is tied to cardiopulmonary status. Unlike Pompe disease or DMD, where respiratory involvement can be early, in FSHD respiratory involvement is typically late and follows weakness.34 However, this may be both a limitation but also an advantage for FSHD, as changes in the 6MWT are more likely to directly reflect changes in skeletal muscle function than cardiopulmonary status.
In conclusion, we have documented excellent test-retest reliability of the 6MWT in our sample of individuals with FSHD. The relationship between the 6MWT and measures of disease severity and other measures of mobility were moderate to strong, supporting concurrent validity of this outcome measure. Lastly, the 6MWT was administered and performed consistently across 2 sites. Further, exploration of the sensitivity to change over time and establishing the degree of change that is clinically meaningful to individuals with FSHD will be critical to determining the utility of the 6MWT as an outcome measure in FSHD.
Acknowledgments
Study Funding: The project described in this publication was supported by the following: the FSH Society (FSHS-82012), NIAMS #U01AR065119, and NICHD #U54HD060848. Additional support provided by the University of Rochester CTSA award number UL1 RR024160 from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Statland’s work on this project was supported by a NCATS grant awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research # KL2TR000119.
We would like to thank all the patients and their family members who were the impetus for this study.
Dr. Chad Heatwole receives grant funding from the National Institutes of Health (NIAMS) and is the PI of U01AR065119. He also receives funding through the FDA and the Cure SMA foundation. He is the founder and CEO of the Neuromuscular Quality of Life Institute. He receives royalties for the Myotonic Dystrophy Health Index. He has provided consultation to Biogen, aTyr, and Acceleron Pharma.
Dr. Jeffrey Statland’s work on this project was supported by an NCATS grant awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research # KL2TR000119. Dr. Statland is a consultant for Cydan, Third Rock Ventures, and Acceleron, and served on the advisory board for Bristol Myer Squibb and Sarepta.
Dr. Tawil is a consultant for Acceleron, aTyr and Novartis.
Abbreviations
- FSHD
Facioscapulohumeral dystrophy
- DMD
Duchenne Muscular Dystrophy
- ICC
Intraclass Correlation Coefficient
- KKI
Kennedy Krieger Institute
- MCID
Minimal Clinically Important Difference
- MDC
Minimally Detectable Change
- URMC
University of Rochester Medical Center
- SD
Standard Deviation
- TUG
Timed Up and Go
- 6MWT
6 Minute Walk Test
Footnotes
Disclosures:
Katy Eichinger is a consultant for Ionis Pharmaceuticals.
References
- 1.Padberg GW. (Thesis) Leiden. The Netherlands: Leiden University; 1982. Facioscapulohumeral Disease. [Google Scholar]
- 2.Tawil R, van der Maarel S. Facioscapulohumeral muscular dystrophy. Muscle Nerve. 2006;34:1–15. doi: 10.1002/mus.20522. [DOI] [PubMed] [Google Scholar]
- 3.van der Maarel SM, Tawil R, Tapscott SJ. Facioscapulohumeral muscular dystrophy and DUX4: Breaking the silence. Trends Mol Med. 2011;17(5):252–258. doi: 10.1016/j.molmed.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tawil R, Shaw DW, van der Maarel SM, Tapscott SJ. Clinical trial preparedness in facioscapulohumeral dystrophy: Outcome measures and patient access: 8–9 April 2013, Leiden, the Netherlands. Neuromuscul Disord. 2014;24(1):79–85. doi: 10.1016/j.nmd.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Tawil R, Padberg GW, Shaw DW, van der Maarel SM, Tapscott SJ FSHD Workshop Participants. Clinical trial preparedness in facioscapulohumeral muscular dystrophy: Clinical, tissue, and imaging outcome measures: 29–30 May 2015, Rochester, New York. Neuromuscul Disord. 2016;26:181–186. doi: 10.1016/j.nmd.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119(1):256–270. doi: 10.1378/chest.119.1.256. [DOI] [PubMed] [Google Scholar]
- 7.Montes J, McDermott MP, Martens WB, Dunaway S, Glanzman AM, Riley S, et al. Six-minute walk test demonstrates motor fatigue in spinal muscular atrophy. Neurology. 2010;74(10):833–838. doi: 10.1212/WNL.0b013e3181d3e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henricson E, Abresch R, Han JJ, Nicorici A, Goude Keller E, de Bie E, et al. The 6-minute walk test and person-reported outcomes in boys with duchenne muscular dystrophy and typically developing controls: Longitudinal comparisons and clinically-meaningful changes over one year. PLoS Curr. 2013;5 doi: 10.1371/currents.md.9e17658b007eb79fcd6f723089f79e06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowes LP, Alfano L, Viollet L, Rosales XQ, Sahenk Z, Kaspar BK, et al. Knee extensor strength exhibits potential to predict function in sporadic inclusion-body myositis. Muscle Nerve. 2012;45(2):163–168. doi: 10.1002/mus.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kierkegaard M, Tollback A. Reliability and feasibility of the six minute walk test in subjects with myotonic dystrophy. Neuromuscular Disorders. 2007;17(11–12):943–949. doi: 10.1016/j.nmd.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Lachmann R, Schoser B. The clinical relevance of outcomes used in late-onset pompe disease: Can we do better? Orphanet J Rare Dis. 2013;8 doi: 10.1186/1750-1172-8-160. 160-1172-8-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Ploeg AT, Clemens PR, Corzo D, Escolar DM, Florence J, Herson S, et al. A randomized study of alglucosidase alfa in late-onset pompe's disease. N Engl J Med. 2010;362(15):1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 13.Tawil R, van der Maarel S, Padberg GW, van Engelen BG. 171st ENMC international workshop: Standards of care and management of facioscapulohumeral muscular dystrophy. Neuromuscul Disord. 2010 Jul;20(7):471–5. doi: 10.1016/j.nmd.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 14.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 15.Sciurba F, Criner GJ, Lee SM, Mohsenifar Z, Shade D, Slivka W, et al. Six-minute walk distance in chronic obstructive pulmonary disease: Reproducibility and effect of walking course layout and length. Am J Respir Crit Care Med. 2003;167(11):1522–1527. doi: 10.1164/rccm.200203-166OC. [DOI] [PubMed] [Google Scholar]
- 16.Podsiadlo D, Richardson S. The timed "up & go": A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 17.Shumway-Cook A, Brauer S, Woollcott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80:896–903. [PubMed] [Google Scholar]
- 18.Personius K, Pandya S, King W, Tawil R, McDermott MP The FSH DY Group. Facioscapulohumeral dystrophy natural history study: Standardization of testing procedures and reliability of measurements. Phys Ther. 1994;74:253–263. doi: 10.1093/ptj/74.3.253. [DOI] [PubMed] [Google Scholar]
- 19.Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: Reference values and determinants. Age Ageing. 1997;26(1):15–19. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- 20.Lamperti C, Fabbri G, Vercelli L, D'Amico R, Frusciante R, Bonifazi E, et al. A standardized clinical evaluation of patients affected by facioscapulohumeral muscular dystrophy: The FSHD clinical score. Muscle Nerve. 2010;42(2):213–217. doi: 10.1002/mus.21671. [DOI] [PubMed] [Google Scholar]
- 21.Kendall FP, McCreary EK, Provance PG. Muscles, testing and function : With posture and pain. Baltimore, Md: Williams & Wilkins; 1993. [Google Scholar]
- 22.Beckerman H, Roebroeck ME, Lankhorst GJ, Becher JG, Bezemer PD, Verbeek AL. Smallest real difference, a link between reproducibility and responsiveness. Qual Life Res. 2001;10(7):571–578. doi: 10.1023/a:1013138911638. [DOI] [PubMed] [Google Scholar]
- 23.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. 2005;19(1):231–240. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- 24.Amato AA, Sivakumar K, Goyal N, David WS, Salajegheh M, Praestgaard J, et al. Treatment of sporadic inclusion body myositis with bimagrumab. Neurology. 2014;83(24):2239–2246. doi: 10.1212/WNL.0000000000001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendell JR, Rodino-Klapac LR, Sahenk Z, Roush K, Bird L, Lowes LP, et al. Eteplirsen for the treatment of duchenne muscular dystrophy. Ann Neurol. 2013;74(5):637–647. doi: 10.1002/ana.23982. [DOI] [PubMed] [Google Scholar]
- 26.Voit T, Topaloglu H, Straub V, et al. Safety and efficacy of drisapersen for the treatment of Duchenne muscular dystrophy (DEMAND II): An exploratory, randomised, placebo-controlled phase 2 study. Lancet Neurol. 2014;13(10):987–996. doi: 10.1016/S1474-4422(14)70195-4. [DOI] [PubMed] [Google Scholar]
- 27.McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Atkinson L, et al. The 6-minute walk test in Duchenne/Becker muscular dystrophy: Longitudinal observations. Muscle Nerve. 2010;42(6):966–974. doi: 10.1002/mus.21808. [DOI] [PubMed] [Google Scholar]
- 28.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 29.Rasekaba T, Lee AL, Naughton MT, Williams TJ, Holland AE. The six-minute walk test: A useful metric for the cardiopulmonary patient. Intern Med J. 2009;39(8):495–501. doi: 10.1111/j.1445-5994.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 30.Tang A, Eng JJ, Rand D. Relationship between perceived and measured changes in walking after stroke. J Neurol Phys Ther. 2012;36(3):115–121. doi: 10.1097/NPT.0b013e318262dbd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.A prospective, quantitative study of the natural history of facioscapulohumeral muscular dystrophy (FSHD): Implications for therapeutic trials. the FSH-DY group. Neurology. 1997;48(1):38–46. doi: 10.1212/wnl.48.1.38. [DOI] [PubMed] [Google Scholar]
- 32.Bushby K, Finkel R, Wong B, et al. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve. 2014;50(4):477–487. doi: 10.1002/mus.24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Statland JM, Tawil R. Risk of functional impairment in facioscapulohumeral muscular dystrophy. Muscle Nerve. 2014;49(4):520–527. doi: 10.1002/mus.23949. [DOI] [PubMed] [Google Scholar]
- 34.Scully MA, Eichinger KJ, Donlin-Smith CM, Tawil R, Statland JM. Restrictive lung involvement in facioscapulohumeral muscular dystrophy. Muscle Nerve. 2014;50(5):739–743. doi: 10.1002/mus.24218. [DOI] [PMC free article] [PubMed] [Google Scholar]