Abstract
BACKGROUND
Human papillomavirus (HPV) vaccines can potentially prevent greater than 90% of cervical and anal cancers as well as a substantial proportion of vulvar, vaginal, penile, and oropharyngeal cancers caused by certain HPV types. Because more than 38,000 HPV-associated cancers are diagnosed annually in the United States, current studies are needed to understand how relative survival varies for each of these cancers by certain demographic characteristics, such as race and age.
METHODS
The authors examined high-quality data from 27 population-based cancer registries covering approximately 59% of the US population. The analyses were limited to invasive cancers that were diagnosed during 2001 through 2011 and followed through 2011 and met specified histologic criteria for HPV-associated cancers. Five-year relative survival was calculated from diagnosis until death for these cancers by age, race, and sex.
RESULTS
The 5-year age-standardized relative survival rate was 64.2% for cervical carcinomas, 52.8% for vaginal squamous cell carcinomas (SCCs), 66% for vulvar SCCs, 47.4% for penile SCCs, 65.9% for anal SCCs, 56.2% for rectal SCCs, and 51.2% for oropharyngeal SCCs. Five-year relative survival was consistently higher among white patients compared with black patients for all HPV-associated cancers across all age groups; the greatest differences by race were observed for oropharyngeal SCCs among those aged <60 years and for penile SCCs among those ages 40 to 49 years compared with other age groups.
CONCLUSIONS
There are large disparities in relative survival among patients with HPV-associated cancers by sex, race, and age. HPV vaccination and improved access to screening (of cancers for which screening tests are available) and treatment, especially among groups that experience higher incidence and lower survival, may reduce disparities in survival from HPV-associated cancers.
Keywords: human papillomavirus (HPV) cancer, HPV-associated cancer, HPV-associated cancer, relative survival
INTRODUCTION
Human papillomavirus (HPV) infection is the most common sexually transmitted infection among men and women in the United States, with approximately 14 million new infections occurring each year.1 Persistent infections with high-risk HPV (including HPV types 16, 18, 31, 33, 45, 52, and 58) cause nearly all cervical cancers and a substantial proportion of other cancers.2,3 HPV-associated cancers can be defined as invasive cancers at anatomic sites (cervix, vulva, vagina, penis, oropharynx, anus, and rectum) with cell types in which HPV DNA is frequently identified—all carcinomas of the cervix, including adenocarcinomas and squamous cell carcinomas (SCCs), and SCCs only for the other anatomic sites. A recent genotyping study estimated that HPV DNA was present in 91% of cervical cancers, 75% of vaginal cancers, 69% of vulvar cancers, 91% of anal cancers, 63% of penile cancers, and 71% oropharyngeal cancers.4 However, cancer registries do not routinely collect information on the presence of HPV DNA in cancer tissues. Therefore, to estimate the number of cancers attributed to HPV, the Centers for Disease Control and Prevention (CDC) multiplied the number of HPV-associated cancers by the percentage of each cancer type attributable to HPV based on the genotyping study. On the basis of these attributable fractions, 79% of the 38,793 “HPV-associated cancers” diagnosed annually in the United States during 2008 through 2012 could be attributed to HPV.5
Many studies to date have focused on cervical cancers, because almost all of these cancers are associated with HPV infection. Furthermore, cervical and oropharyngeal cancers account for greater than one-half of all HPV-associated cancers.5 However, in recent years, considerable attention has been given to oropharyngeal cancers and the role of HPV infection in these cancers. Although many studies have focused on the incidence of HPV-associated cancers, very few have examined survival, and most of these have focused on cervical and oropharyngeal cancers.3,5–8 Survival reports on less common HPV-associated cancers, such as vaginal, vulvar, penile, anal, and rectal malignancies, are lacking. Thus, to better understand survival in HPV-associated cancers in the United States along with any disparities by sex, race, or age that may exist, the objective of this study was to examine survival among patients diagnosed with HPV-associated cancers using data from the CDC’s National Program of Cancer Registries (NPCR) from 2001 through 2011.
MATERIALS AND METHODS
The NPCR data set includes cancer incidence data from central cancer registries funded by the NPCR in 45 states, the District of Columbia, and Puerto Rico.9 Data on all new diagnoses of cancer from patient records at such medical facilities as hospitals, physicians’ offices, therapeutic radiation facilities, freestanding surgical centers, and pathology laboratories are reported to central cancer registries, which collate these data and use state vital records to collect information about any cancer deaths that were not reported as cases. The central cancer registries use uniform data items and codes as documented by the North American Association of Central Cancer Registries. These data are submitted annually in November to the CDC and are combined into 1 data set. Cancer registries demonstrate that data are of high quality by meeting US Cancer Statistics publication criteria. Information on primary site and histology is coded according to the International Classification of Diseases for Oncology, Third Edition and categorized according to the revised Surveillance, Epidemiology, and End Results (SEER) recodes dated January 27, 2003, which define standard groupings of primary cancer sites (available at; https://seer.cancer.gov/siterecode accessed December, 2016). The data include information about diagnostic confirmation of each case, including whether histologic type was determined by microscopic examination of tumor tissue.
For the current analysis we used survival data for invasive cancer cases diagnosed during 2000 through 2011 using the NPCR November 2014 data submission.10 We limited our analysis to registries that met the NPCR data-quality standards for inclusion in US Cancer Statistics publications11 and also performed active patient follow-up or linkage with the National Death Index. These 27 registries represent 59% of the US population.
We limited our analysis to invasive cancers that were microscopically confirmed and met specified histologic criteria for HPV-associated cancers using the same framework as in prior studies.7,12 Cancers were classified by anatomic site using International Classification of Diseases for Oncology, Third Edition codes.13 All epithelial carcinomas (histology codes 8010-8671 and 8940-8941) were included for cervical cancers (site codes C53.0-C53.9).14 For other sites, we included SCCs (histology codes 8050-8084 and 8120-8131) for sites in the vulva (site codes C51.0-C51.9), vagina (site code C52.9), penis (site codes C60.0-60.9), anus and rectum (site codes C21.0-C21.9 and C20.9), and certain subsites of the oropharynx (site codes C01.9, C02.4, C02.8, C05.1, C05.2, C09.0, C09.1, C09.8, C09.9, C10.0, C10.1, C10.2, C10.3, C10.4, C10.8, C10.9, C14.0, C14.2, and C14.8).5 Oropharyngeal subsites (including the base of tongue; pharyngeal tonsils, anterior and posterior tonsillar pillars, and glossotonsillar sulci; anterior surface of soft palate and uvula; and lateral and posterior pharyngeal walls) were based on sites in which HPV DNA is frequently identified.4,5,7,12,14–17 Although rectal SCC was not included in the genotyping study,4 we included it in this analysis, because recent studies indicate that rectal SCCs, although rare, are similar to anal SCCs and probably misclassified anal SCCs, and are likely to be HPV-associated.3,18
By using sex-specific and state-specific life tables for all races and black and white race from the National Center for Health Statistics, we calculated 5-year relative survival percentages for patients diagnosed during 2001 through 2011 on all those who had follow-up through 2011. Relative survival was defined as the ratio of the observed all-cause survival in a group of individuals with cancer to the expected all-cause survival of the general population. For this study, 5-year relative survival was calculated using the Ederer II relative survival method.19 Relative survival was calculated for each of the HPV-associated cancers by age at diagnosis, race, sex, and SEER Summary Stage 2000 (local, regional, distant). For patients who had multiple HPV-associated cancers diagnosed during the study period, all cancers were included in the analysis. All analyses were age-standardized using the International Cancer Survival Standard.20 Survival was calculated only for white and black racial groups, because expected life tables were not available for other racial/ethnic groups. SEER*Stat software version 8.3.2 was used for analysis.21
Survival estimates were calculated as a ratio and estimates with counts <25 suppressed. Formal statistical testing for pairwise comparisons was not performed to avoid multiple comparison problems. Ninety-five percent confidence intervals (95% CIs) are provided to allow for informal comparisons of survival estimates. Although examining overlap between CIs to determine differences in estimates is conservative, the sample sizes from more than 10 years of national data are generally large for most groups.
RESULTS
In total, 220,211 HPV-associated cancers were diagnosed during 2001 through 2011, and the majority consisted of oropharyngeal SCCs (OPSCCs) and cervical carcinomas (36.4% and 36.1%, respectively) followed by anal SCCs (11.8%) (Table 1). When comparing survival by cancer site, 5-year age-standardized relative survival was highest for vulvar and anal SCCs (66% and 65.9%, respectively) and lowest for penile and oropharyngeal SCCs (47.4% and 51.2%, respectively) compared with other sites (Table 2).
TABLE 1.
Population Characteristics for Patients Diagnosed With Human Papillomavirus-Associated Cancer: National Program of Cancer Registries, 2001–2011a
| HPV-Associated Cancers | Total No. | Total No. (%) | % White | % Women | % Localized Stage at Diagnosisb |
|---|---|---|---|---|---|
| Cervical carcinoma | 79,425 | 36.1 | 77.2 | 100 | 47.1 |
| Vaginal SCC | 4871 | 2.2 | 80.9 | 100 | 37.5 |
| Vulvar SCC | 19,345 | 8.8 | 88.7 | 100 | 57.5 |
| Penile SCC | 6248 | 2.8 | 85.4 | — | 35.0 |
| Anal SCC | 26,026 | 11.8 | 86.3 | 63.6 | 50.8 |
| Rectal SCC | 4145 | 1.9 | 85.5 | 68.2 | 43.3 |
| Oropharyngeal SCC | 80,151 | 36.4 | 86.7 | 20.8 | 15.9 |
| Total | 220,211 | 100.0 | 83.0 | 32.7c | 36.4 |
Abbreviations: HPV, human papillomavirus; SCC, squamous cell carcinoma.
Data were compiled from 27 population-based cancer registries that participate in the National Program of Cancer Registries, meet the data-quality standards for inclusion in US Cancer Statistics, and meet the criteria for inclusion in the survival data set, which covers approximately 59% of the US population.
Localized cancers are the those identified only in the part of the body where they started.
The percentages of cancers that occurred among men and women are indicated
TABLE 2.
Age-Standardized 5-Year Relative Survival for Human Papillomavirus-associated cancers by Stage of Cancer at Diagnosis, Age, Race, and Sex: National Program of Cancer Registries, 2001–2011a
| HPV-Associated Cancers | Total | Localizedb | Regionalc | Distantd | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| No. | % Survival | 95% CI | No. | % Survival | 95% CI | No. | % Survival | 95% CI | No. | % Survival | 95% CI | |
| Cervical carcinomae | 79,425 | 64.2 | 63.8–64.7 | 37,449 | 86.0 | 85.3–86.7 | 28,409 | 55.3 | 54.5–56.1 | 9049 | NA | NA |
| Age at diagnosis, yf | ||||||||||||
| <40 | 21,212 | 82.3 | 81.7–82.9 | 13,493 | 94.0 | 93.5–94.4 | 5460 | 64.3 | 62.9–65.7 | 1120 | 23.7 | 20.9–26.6 |
| 40–49 | 21,159 | 74.2 | 73.5–74.9 | 11,136 | 91.9 | 91.3–92.5 | 7108 | 61.6 | 60.2–62.8 | 2015 | 20.9 | 18.9–23.0 |
| 50–59 | 16,009 | 64.5 | 63.6–65.4 | 6132 | 87.9 | 86.8–88.9 | 6640 | 58.8 | 57.3–60.2 | 2426 | 19.2 | 17.4–21.1 |
| ≥60 | 21,075 | 52.4 | 51.5–53.3 | 6696 | 80.3 | 78.8–81.7 | 9207 | 48.1 | 46.7–49.4 | 3489 | 13.2 | 11.8–14.7 |
| Race | ||||||||||||
| White | 61,061 | 64.8 | 64.2–65.3 | 29,689 | 86.5 | 85.7–87.3 | 21,348 | 55.6 | 54.7–56.6 | 6784 | NA | NA |
| Black | 12,819 | 57.0 | 55.8–58.1 | 5112 | 80.1 | 78.2–81.8 | 5131 | 50.0 | 48.1–51.9 | 1748 | NA | NA |
| Vaginal SCCg | 4871 | 52.8 | 50.9–54.6 | 1829 | 65.7 | 62.7–68.5 | 1786 | 53.7 | 50.6–56.7 | 693 | 20.7 | 16.9–24.7 |
| Age at diagnosis, yf | ||||||||||||
| <40 | 165 | 62.7 | 54.1–70.2 | 76 | 83.3 | 71.9–90.3 | 58 | 48.4 | 33.2–62.0 | NR | NR | NR |
| 40–49 | 506 | 66.6 | 61.7–71.1 | 210 | 78.5 | 71.2–84.1 | 185 | 67.0 | 58.4–74.1 | 65 | 22.9 | 12.0–35.9 |
| 50–59 | 909 | 62.5 | 58.6–66.1 | 340 | 76.3 | 70.1–81.3 | 337 | 60.9 | 54.3–66.8 | 121 | 32.0 | 22.2–42.3 |
| ≥60 | 3298 | 46.4 | 44.0–48.7 | 1204 | 59.3 | 55.2–63.1 | 1206 | 48.7 | 44.8–52.5 | 490 | 16.3 | 12.2–21.1 |
| Race | ||||||||||||
| White | 3932 | 52.6 | 50.6–54.6 | 1498 | 65.1 | 61.7–68.2 | 1427 | 54.1 | 50.6–57.4 | 559 | 19.9 | 15.8–24.4 |
| Black | 745 | 50.8 | 46.1–55.3 | 256 | 67.2 | 58.2–74.7 | 297 | 51.1 | 43.3–58.4 | 109 | NA | NA |
| Vulvar SCCg | 19,345 | 66.0 | 65.1–67.0 | 11,125 | 79.0 | 77.7–80.3 | 6561 | 52.5 | 50.9–54.1 | 799 | 16.5 | 13.2–20.2 |
| Age at diagnosis, yf | ||||||||||||
| <40 | 1035 | 84.5 | 81.8–86.9 | 692 | 91.9 | 89.1–94.0 | 253 | 71.1 | 64.4–76.8 | 35 | 26.3 | 11.3–44.1 |
| 40–49 | 2770 | 85.9 | 84.3–87.4 | 1822 | 92.2 | 90.5–93.7 | 755 | 75.9 | 72.0–79.3 | 82 | 33.0 | 20.7–45.8 |
| 50–59 | 3468 | 79.8 | 78.1–81.4 | 2103 | 88.7 | 86.8–90.4 | 1103 | 70.4 | 67.0–73.5 | 134 | 26.4 | 17.1–36.6 |
| ≥60 | 12,117 | 57.5 | 56.2–58.8 | 6532 | 73.6 | 71.7–75.4 | 4451 | 41.8 | 39.7–43.8 | 548 | 10.9 | 7.8–14.8 |
| Race | ||||||||||||
| White | 17,141 | 66.3 | 65.2–67.3 | 9888 | 79.4 | 78.0–80.7 | 5830 | 52.3 | 50.5–54.0 | 690 | 17.0 | 13.4–20.9 |
| Black | 1769 | 61.9 | 57.9–65.6 | 988 | 71.1 | 65.2–76.2 | 612 | 56.9 | 50.4–62.8 | 93 | 15.0 | 8.1–24.0 |
| Penile SCCg | 6248 | 47.4 | 45.7–49.2 | 2184 | 66.6 | 63.4–69.6 | 2251 | 41.2 | 38.3–44.0 | 1320 | 26.7 | 23.3–30.3 |
| Age at diagnosis, yf | ||||||||||||
| <40 | 131 | 65.3 | 55.5–73.4 | 51 | 87.5 | 71.0–94.9 | 43 | 56.7 | 40.0–70.3 | NR | NR | NR |
| 40–49 | 560 | 56.6 | 51.6–61.2 | 177 | 80.2 | 72.0–86.3 | 205 | 51.1 | 43.0–58.6 | 140 | 38.7 | 28.6–48.6 |
| 50–59 | 1487 | 51.2 | 48.1–54.2 | 458 | 71.9 | 66.2–76.9 | 601 | 47.1 | 42.4–51.7 | 330 | 29.8 | 23.6–36.3 |
| ≥60 | 4076 | 45.3 | 43.1–47.4 | 1500 | 63.3 | 59.3–67.1 | 1403 | 39.9 | 36.4–43.3 | 826 | 24.3 | 20.2–28.6 |
| Race | ||||||||||||
| White | 5317 | 48.4 | 46.4–50.3 | 1933 | 67.2 | 63.8–70.3 | 1925 | 41.9 | 38.9–44.9 | 1055 | 26.6 | 22.8–30.8 |
| Black | 528 | 34.7 | 28.5–41.0 | 110 | 57.8 | 37.9–73.4 | 219 | 31.1 | 22.5–40.1 | 160 | 22.0 | 13.4–31.9 |
| Anal SCCg | 26,026 | 65.9 | 65.0–66.9 | 13,215 | 77.6 | 76.2–79.0 | 7841 | 58.5 | 56.6–60.2 | 2521 | 31.7 | 29.0–34.5 |
| Age at diagnosis, yf | ||||||||||||
| <40 | 1111 | 66.1 | 62.8–69.1 | 585 | 76.4 | 72.3–80.0 | 331 | 58.3 | 52.1–64.0 | 104 | 30.4 | 20.8–40.5 |
| 40–49 | 5072 | 72.6 | 71.2–74.0 | 2582 | 82.6 | 80.8–84.3 | 1612 | 65.3 | 62.5–68.0 | 428 | 41.6 | 36.1–46.9 |
| 50–59 | 7703 | 72.5 | 71.2–73.8 | 3923 | 82.5 | 80.8–84.0 | 2339 | 66.8 | 64.3–69.2 | 802 | 38.9 | 34.6–43.2 |
| ≥60 | 12,165 | 64.5 | 63.2–65.7 | 6135 | 77.1 | 75.3–78.7 | 3559 | 56.7 | 54.3–59.0 | 1187 | 29.6 | 26.1–33.2 |
| Race | ||||||||||||
| White | 22,472 | 66.5 | 65.5–67.6 | 11,537 | 78.1 | 76.6–79.5 | 6670 | 58.7 | 56.7–60.5 | 2147 | 32.4 | 29.5–35.3 |
| Black | 2971 | 61.8 | 57.9–65.4 | 1412 | 73.3 | 67.3–78.4 | 1001 | 56.9 | 50.2–63.1 | 319 | 28.8 | 19.6–38.6 |
| Sex | ||||||||||||
| Women | 16,541 | 69.3 | 68.1–70.4 | 8260 | 80.2 | 78.5–81.7 | 4976 | 63.4 | 61.2–65.5 | 1763 | 36.8 | 33.5–40.0 |
| Men | 9485 | 59.8 | 57.9–61.7 | 4955 | 73.1 | 70.3–75.7 | 2865 | 49.1 | 45.6–52.5 | 758 | 18.6 | 14.5–23.1 |
| Rectal SCCg | 4145 | 56.2 | 54.0–58.3 | 1796 | 70.4 | 67.0–73.6 | 976 | 53.7 | 49.2–58.0 | 558 | 13.3 | 10.0–17.1 |
| Age at diagnosis, yf | ||||||||||||
| <40 | 136 | 65.9 | 56.9–73.5 | 60 | 83.8 | 70.6–91.4 | 28 | 50.5 | 30.1–67.8 | NR | NR | |
| 40–49 | 617 | 62.2 | 57.5–66.4 | 268 | 78.0 | 71.5–83.2 | 164 | 48.8 | 38.6–58.3 | 83 | 34.3 | 22.9–46.1 |
| 50–59 | 1139 | 65.0 | 61.5–68.3 | 483 | 76.3 | 71.1–80.8 | 296 | 67.4 | 60.2–73.6 | 156 | 14.1 | 7.8–22.2 |
| ≥60 | 2254 | 53.7 | 50.9–56.5 | 985 | 68.8 | 64.3–72.8 | 488 | 53.0 | 46.8–58.9 | 300 | 10.5 | 6.5–15.7 |
| Race | ||||||||||||
| White | 3539 | 57.2 | 54.9–59.5 | 1558 | 70.6 | 67.0–74.0 | 827 | 55.8 | 51.0–60.4 | 461 | 14.5 | 10.7–18.8 |
| Black | 505 | 46.6 | 39.7–53.3 | 202 | 63.8 | 52.2–73.3 | 129 | 41.6 | 27.2–55.4 | 89 | 13.6 | 6.2–24.0 |
| Sex | ||||||||||||
| Women | 2812 | 61.2 | 58.5–63.7 | 1255 | 73.4 | 69.3–76.9 | 686 | 58.0 | 52.8–62.9 | 332 | 17.6 | 12.8–23.0 |
| Men | 1333 | 45.5 | 41.6–49.3 | 541 | 63.4 | 56.4–69.6 | 290 | 40.1 | 31.7–48.3 | 226 | 6.9 | 3.6–11.6 |
| Oropharyngeal SCCg | 80,151 | 51.2 | 50.7–51.8 | 12,738 | 59.4 | 58.1–60.7 | 50,877 | 54.5 | 53.7–55.3 | 13,162 | 30.8 | 29.6–32.0 |
| Age at diagnosis, yf | ||||||||||||
| <40 | 1603 | 73.4 | 70.9–75.8 | 255 | 81.3 | 75.1–86.2 | 1050 | 77.0 | 73.9–79.7 | 233 | 48.4 | 40.6–55.8 |
| 40–49 | 11,714 | 69.1 | 68.1–70.0 | 1481 | 77.5 | 74.9–79.9 | 8054 | 72.9 | 71.7–74.0 | 1738 | 43.4 | 40.6–46.2 |
| 50–59 | 28,053 | 64.2 | 63.5–64.9 | 3668 | 71.4 | 69.5–73.1 | 18,821 | 68.9 | 68.0–69.7 | 4550 | 40.3 | 38.5–42.1 |
| ≥60 | 38,911 | 49.1 | 48.4–49.8 | 7354 | 55.5 | 53.9–57.1 | 22,979 | 53.3 | 52.4–54.2 | 6642 | 30.0 | 28.5–31.5 |
| Race | ||||||||||||
| White | 69,377 | 53.5 | 52.9–54.1 | 11,136 | 60.5 | 59.1–61.9 | 44,597 | 56.9 | 56.1–57.7 | 10,822 | 33.2 | 31.9–34.5 |
| Black | 8971 | 32.4 | 30.6–34.1 | 1287 | 46.1 | 41.6–50.4 | 5235 | 35.0 | 32.5–37.4 | 2056 | 16.6 | 13.7–19.7 |
| Sex | ||||||||||||
| Women | 16,694 | 49.8 | 48.8–50.8 | 3676 | 60.0 | 57.8–62.1 | 6919 | 51.1 | 49.7–52.5 | 2572 | 29.9 | 27.6–32.2 |
| Men | 63,457 | 51.7 | 50.9–52.4 | 9062 | 59.1 | 57.4–60.8 | 41,261 | 55.6 | 54.6–56.5 | 10,590 | 30.9 | 29.5–32.4 |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; NA, not applicable (statistics could not be calculated); NR, not reported (because there were <25 patients); SCC, squamous cell carcinoma.
Data were compiled from 27 population-based cancer registries that participate in the National Program of Cancer Registries, meet the data-quality standards for inclusion in the US Cancer Statistics, and meet the criteria for inclusion in the survival data set, which covers approximately 59% of the US population.
Localized cancers are the those identified only in the part of the body where they started.
Regional cancers are those that have spread to adjacent organs or structures or regional lymph nodes.
Distant cancers are those that have metastasized.
Age was standardized to the International Cancer Survival Standard 2 (ages ≥15 years).
The numbers may not add up to the total for each cancer site, because multiple cancers may occur in each site.
Age was standardized to the International Cancer Survival Standard 1 (ages ≥15 years).
For many HPV-associated cancers, 5-year age-standardized relative survival decreased with increasing age at diagnosis; however, we observed slightly increased survival for cancers diagnosed among those ages 40 to 49 years compared with those aged <40 years at the time of diagnosis for vaginal SCCs (66.6% and 62.7%, respectively), vulvar SCCs (85.9% and 84.5%, respectively), and anal SCCs (72.6% and 66.1%, respectively). Survival consistently decreased with increasing age for cervical carcinomas, penile SCCs, and OPSCCs (Table 2). The difference in 5-year relative survival was at least 10% higher for those aged <40 years at the time of diagnosis compared with those aged ≥60 years at the time of diagnosis for all HPV-associated cancers, with the exception of anal SCCs, in which the difference was <2% (Table 2). A greater percentage of cases were diagnosed at older ages for most cancer types, except cervical cancer (73% were diagnosed at age <60 years), anal cancer (53% were diagnosed at aged <60 years), and oropharyngeal cancer (51% were diagnosed at age <60 years).
Most of our study population was white (83%). More than 85% of each of the HPV-associated cancers were diagnosed among whites, with the exception of cervical carcinomas and vaginal SCCs, in which smaller proportions were diagnosed among whites (77.2% and 80.9%, respectively) (Table 1). Five-year age-standardized relative survival was consistently higher for whites compared with blacks for all HPV-associated cancers, with higher survival observed for cervical carcinomas (64.8% and 57%, respectively), penile SCCs (48.4% and 34.7%, respectively), anal SCCs (69.3% and 59.8%, respectively), rectal SCCs (61.2% and 45.5%, respectively), and OPSCCs (53.5% and 32.4%, respectively) (Table 2). The lowest survival and the greatest differences by race were observed for blacks compared with whites for OPSCCs and penile SCCs (32.4% vs 53.5% and 34.7% vs 48.4%, respectively) (Table 2).
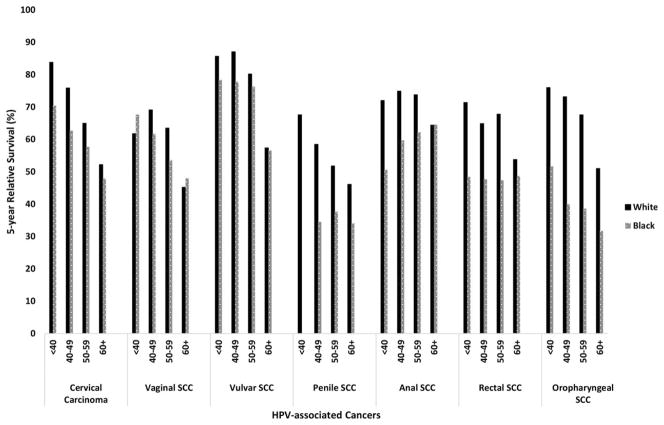
When examining survival by age at diagnosis and race, the largest differences in survival between whites and blacks were observed for patients with OPSCCs ages 40 to 49 years (73.2% and. 40%, respectively), 50 to 59 years (67.6% and 38.6%, respectively), and <40 years (76% and 51.7%, respectively) at the time of diagnosis. In addition, large differences in survival between whites and blacks were observed for men ages 40 to 49 years with penile SCCs (58.5% and 34.5%, respectively) (Fig. 1).
Figure 1.
Age-standardized 5-year relative survival is illustrated for human papillomavirus (HPV)-associated cancers by race and age at diagnosis (data from the National Program of Cancer Registries, 2001–2011). Cervical carcinomas were age standardized to the International Cancer Survival Standard 2 (ages ≥15 years). Vaginal, vulvar, penile, anal, rectal, and oropharyngeal squamous cell carcinomas (SCCs) were age standardized to the International Cancer Survival Standard 1 (ages ≥15 years). Data from the National Program of Cancer Registries (2001–2011) were compiled from 27 population-based cancer registries that participate in the National Program of Cancer Registries, meet the data-quality standards for inclusion in US Cancer Statistics, and meet the criteria for inclusion in the survival data set, which covers approximately 59% of the US population. Note that statistics could not be displayed for blacks aged <40 years for penile SCC because there were <25 patients.
For cancers that occurred among both men and women, more than one-half of anal and rectal SCCs occurred among women compared with only 20% of OPSCCs (Table 1). Compared with men, women had higher 5-year relative survival for anal SCCs (69.3% and 59.8%, respectively) and rectal SCCs (61.2% and 45.5%, respectively) and slightly decreased survival for OPSCCs (Table 2). The greatest differences in survival by sex were observed for rectal SCCs.
More than one-third of all HPV-associated cancers were diagnosed at localized stage, with the exception of OPSCCs, in which 16% were diagnosed at localized stage (Table 1). The 5-year relative survival rate was higher for all HPV-associated cancers diagnosed at localized stage and decreased for cancers diagnosed at regional and distant stages. The 5-year age-standardized relative survival rate ranged from 59.4% to 86% for cancers diagnosed in localized stage, from 41.2% to 58.5% for cancers diagnosed in regional stage, and from 13.3% to 31.7% for cancers diagnosed in distant stages. The shortest survival was observed for rectal SCCs diagnosed at distant stage (13.3%). The greatest differences in survival between cancers diagnosed in localized and distant stages were observed for vulvar SCCs (79% and 16.5%, respectively) and OPSCCs (59.4% and 30.8%, respectively) (Table 2). When examining 5-year relative survival by population characteristics and disease stage at diagnosis, the poorest survival was observed for men who had rectal SCCs and for patients aged ≥60 years who had rectal and vulvar SCCs diagnosed at distant stage (6.9%, 10.5%, and 10.9%, respectively) (Table 1).
DISCUSSION
This study is the largest population-based study of survival for HPV-associated cancers in the United States, covering 59% of the population. Previous studies covered a smaller number of the US population, ranging from <100 to approximately 50,000 cases, whereas the current analysis included 220,211 cases.22–30 Furthermore, this study is the first comprehensive survival analysis for all HPV-associated cancers that includes 7 cancer sites. We report the highest 5-year age-standardized relative survival for cervical carcinomas, vulvar, and anal SCCs and the lowest for penile SCCs and OPSCCs. Our overall findings include higher survival rates for whites compared with blacks, with the largest differences observed among those with OPSCCs, as well as higher survival rates for those who had cancers diagnosed at localized stage compared with those diagnosed at regional or distant stages. In addition, although survival usually decreased with age, we observed slightly higher 5-year age-standardized relative survival rates for those diagnosed between ages 40 and 49 years with vaginal SCC, vulvar SCC, and anal SCC compared with those diagnosed at age ≤40 years with these cancers, a finding that merits further investigation (eg, whether there were differences by sex or human immuno-deficiency virus status for anal SCCs).
More than two-thirds of cases in our study were cervical carcinomas or HPV-associated OPSCCs. For patients with cervical carcinoma, the 5-year relative survival rate was high, which may be because of screening and early detection (almost one-half of cervical carcinomas in our study were detected at localized stage). For patients with OPSCC, the survival rate was lower compared with that for patients with other HPV-associated cancers, which is concerning given the recent increase in OPSCCs as well as the lack of routinely recommended, population-based screening tests.31 Similar to previous findings, we report that most HPV-associated OPSCCs were observed among whites and men, and about one-half occurred in patients aged ≤60 years.12,22,23,32,33 These findings, combined with the observation that survival was consistently lower among patients with OPSCC, is troubling. Furthermore, similar to the report by Goodman and colleagues, we observed that a large proportion of HPV-associated OPSCCs were diagnosed at regional stage; >60% of HPV-associated OPSCCs, compared with <40% of other HPV-associated cancers, were diagnosed at regional stage.7 The few studies that have examined whether there is a difference in survival by HPV status among patients with OPSCC have indicated that patients with HPV-positive OPSCC survive longer than those with HPV-negative OPSCC.7,32 We could not examine survival by HPV status, because our classification of HPV-associated cancers was based on histologic type and not on actual assessments of individual tumor status for the presence of HPV DNA.
For other rare HPV-associated cancers, we observed the highest survival for anal SCC and the lowest for penile SCC. The published evidence about survival from these cancers is limited and includes smaller populations as well as various definitions of HPV-associated cancers. In a study of HPV-associated anal cancers that included rectal SCCs in the United States, Joseph et al reported that more than one-half of these cancers were diagnosed in localized stage, similar to our findings. Those authors also observed higher survival among women compared with men and among whites compared with blacks.30 Relatively little is known about the epidemiology of penile SCCs, and recent survival studies for penile cancers are lacking.34 A small study representing 12% of the US population and with data from 1973 through 1998 reported that the average duration of disease-specific survival was <5 years and decreased for localized, regional, and distant tumors, which is similar to our findings of decreased survival with advanced stages of cancer at diagnosis.35
Several factors highlight the importance of primary prevention of HPV-associated cancers. First, the incidence of HPV-associated cancers increased during 2004 through 2012 in the United States, indicating an increased burden from HPV infection.5 Second, the proportion of OPSCCs in all age groups with HPV-positive status increased from 21% before the 1990s to >70% today8,15,31,36; and, if increasing trends continue, then OPSCC will become the most common HPV-associated cancer by 2020.31 Third, our study demonstrated that survival from HPV-associated cancers is poor compared with survival from all sites combined (67%) and compared with other cancers, such as breast cancers (90%) and prostate cancers (99%).37 In the absence of routine screening recommendations for any HPV-associated cancers, with the exception of cervical carcinomas, primary prevention through HPV vaccine is essential, especially for OPSCC, in which there is an increasing trend in incidence. HPV vaccines are approved and recommended for use among both boys and girls.38 Beyond primary prevention, assessing tumor HPV status is essential for OPSCC management and treatment, because some studies have noted differential responses to therapy in HPV-positive versus HPV-negative head and neck cancers.23,31,39–42
There are a few limitations to our study. The main limitation includes the classification of HPV-associated cancers, which was based on histologic criteria and not on actual assessment of individual tumor status for the presence of HPV DNA. In addition, we were not able to examine survival for any other racial/ethnic groups, because current life tables are only available for whites and blacks. Although, to our knowledge, this is the largest study to date of survival for HPV-associated cancers among blacks, the 95% CIs were wider for blacks than for whites, indicating that comparisons should be made with caution. Finally, treatment data were not available; therefore, we were not able to account for treatment differences in this analysis.
Although it is not a limitation, it is important to note that relative survival is not disease-specific and is a net survival measure representing the likelihood that a patient will not die from causes associated specifically with the cancer under study. Furthermore, patients with cancer can die from comorbidities unrelated to their cancer diagnosis. A recent study concluded that, of all deaths that occurred in patients diagnosed with breast, prostate, colon, and rectal cancers at localized stage, a small proportion were because of cancer compared with other comorbidities, and the proportions of death from cancer increased with increasing stages of cancer at diagnosis.43
On the basis of the largest population-based study of survival for HPV-associated cancers in the United States, we observed large disparities in relative survival for HPV-associated cancers by sex, race, and age, especially by race and age for penile and oropharyngeal SCCs and by sex for rectal SCCs. HPV vaccination and improved access to screening and treatment, especially among groups that experience higher incidence and lower survival, may reduce disparities in survival from HPV-associated cancers.
Acknowledgments
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
AUTHOR CONTRIBUTIONS
Hilda Razzaghi: Contributed to the conception, design, analysis, and interpretation of data; wrote the initial draft; approved the final version submitted for publication; and agreed to be accountable for all aspects of the work. Mona Saraiya: Contributed to the conception, design, and interpretation of data; contributed to revising the article critically for important intellectual content; approved the final version submitted for publication; and agreed to be accountable for all aspects of the work. Trevor D. Thompson: Contributed to the conception, design, analysis and interpretation of data; contributed to drafting and revising the article critically for important intellectual content; approved the final version submitted for publication; and agreed to be accountable for all aspects of the work. S. Jane Henley: Contributed to the conception, design, analysis, and interpretation of data; contributed to drafting and revising the article critically for important intellectual content; approved the final version submitted for publication; agreed to be accountable for all aspects of the work. Laura Viens: Contributed to study conception and design, revised the article critically for important intellectual content, approved the final version submitted for publication, and agreed to be accountable for all aspects of the work. Reda Wilson: Contributed to the conception, design, and interpretation of data; revised the article critically for important intellectual content; approved the final version submitted for publication; and agreed to be accountable for all aspects of the work.
References
- 1.Satterwhite C, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer (IARC) Volume 90: Human Papillomaviruses. Lyon, France: IARC Press/World Health Organization; 2007. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Google Scholar]
- 3.Shiels MS, Kreimer AR, Coghill AE, Darragh TM, Devesa SS. Anal cancer incidence in the United States, 1977–2011: distinct patterns by histology and behavior. Cancer Epidemiol Biomarkers Prev. 2015;24:1548–1556. doi: 10.1158/1055-9965.EPI-15-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines [serial online] J Natl Cancer Inst. 2015;107:djv086. doi: 10.1093/jnci/djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viens L, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661–666. doi: 10.15585/mmwr.mm6526a1. [DOI] [PubMed] [Google Scholar]
- 6.Wright JD, Chen L, Tergas AI, et al. Population-level trends in relative survival for cervical cancer. Am J Obstet Gynecol. 2015;213:670e1–670.e17. doi: 10.1016/j.ajog.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodman MT, Saraiya M, Thompson TD, et al. Human papillomavirus genotype and oropharynx cancer survival in the United States of America. Eur J Cancer. 2015;51:2759–2767. doi: 10.1016/j.ejca.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein AP, Saha S, Yu M, Kimple RJ, Lambert PF. Prevalence of human papillomavirus in oropharyngeal squamous cell carcinoma in the United States across time. Chem Res Toxicol. 2014;27:462–469. doi: 10.1021/tx500034c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh SD, Henley SJ, Ryerson AB. Surveillance for cancer incidence and mortality—United States, 2013. MMWR Surveill Summ. 2017;66:1–36. doi: 10.15585/mmwr.ss6604a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson R, Ryerson AB, Zhang K, Dong X. Relative survival analysis using the Centers for Disease Control and Prevention’s National Program of Cancer Registries Surveillance System Data, 2000–2007. J Registry Manag. 2014;41:72–76. [PMC free article] [PubMed] [Google Scholar]
- 11.US Cancer Statistics Working Group. 1999–2013 Cancer Incidence and Mortality Data. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control, and Prevention and National Cancer Institute; 2016. [Accessed December, 2016]. United States Cancer Statistics. Available at: https://nccd.cdc.gov/uscs/ [Google Scholar]
- 12.Steinau M, Saraiya M, Goodman MT, et al. Human papillomavirus prevalence in oropharyngeal cancer before vaccine introduction. United States Emerg Infect Dis. 2014;20:822–828. doi: 10.3201/eid2005.131311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. International Classification of Diseases for Oncology. 3. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 14.Edge S, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A, editors. American Joint Commitee on Cancer (AJCC) Cancer Staging Manual. 7. Chicago, IL: Springer; 2010. [Google Scholar]
- 15.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 16.Watson M, Saraiya M, Ahmed F, et al. Using population-based cancer registry data to assess the burden of human papillomavirus-associated cancers in the United States: overview of methods. Cancer. 2008;113(10 suppl):2841–2854. doi: 10.1002/cncr.23758. [DOI] [PubMed] [Google Scholar]
- 17.Viens L, Henley J, Saraiya M. Defining the HPV-associated cancer burden in the United States. J Lower Genital Tract Dis. 2016;20:S10–S32. [Google Scholar]
- 18.Coghill AE, Shiels MS, Rycroft RK, et al. Rectal squamous cell carcinoma in immunosuppressed populations: is this a distinct entity from anal cancer? AIDS. 2016;30:105–112. doi: 10.1097/QAD.0000000000000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickman PW, Adami HO. Interpreting trends in cancer patient survival. J Intern Med. 2006;260:103–117. doi: 10.1111/j.1365-2796.2006.01677.x. [DOI] [PubMed] [Google Scholar]
- 20.Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40:2307–2316. doi: 10.1016/j.ejca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Surveillance Research Program, National Cancer Institute. SEER*Stat software. Version 8.3.2. Bethesda, MD: National Institutes of Health; National Cancer Institute; Surveillance, Epidemiology, and End Results Program; 2016. [Google Scholar]
- 22.Chernock RD, Zhang Q, El-Mofty SK, Thorstad WL, Lewis JS., Jr Human papillomavirus-related squamous cell carcinoma of the oropharynx: a comparative study in whites and African Americans. Arch Otolaryngol Head Neck Surg. 2011;137:163–169. doi: 10.1001/archoto.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 24.Sethi S, Al-Fehmi R, Francheschi S, et al. Characteristics and survival of head and neck cancer by HPV status: a cancer registry-based study. Int J Cancer. 2012;131:1179–1186. doi: 10.1002/ijc.26500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posner MR, Lorch JH, Goloubeva O, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22:1071–1077. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith EM, Rubenstein LM, Hoffman H, Haugen TH, Turek LP. Human papillomavirus, p16 and p53 expression associated with survival of head and neck cancer [serial online] Infect Agent Cancer. 2010;5:4. doi: 10.1186/1750-9378-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin BM, Wang H, D’Souza G, et al. Long-term prognosis and risk factors among patients with HPV-associated oropharyngeal squamous cell carcinoma. Cancer. 2013;119:3462–3471. doi: 10.1002/cncr.28250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan HM, Gabbidon K, Saxena A, Abdool-Ghany F, Dodge HM, 3rd, Lenzmeier T. Disparities in cervical cancer characteristics and survival between white Hispanics and white non-Hispanic women. J Womens Health (Larchmt) 2016;25:1052–1058. doi: 10.1089/jwh.2015.5585. [DOI] [PubMed] [Google Scholar]
- 29.Wright J, Chen L, Tergas AI, et al. Population-level trends in relative survival for cervical cancer. Am J Obstet Gynecol. 2015;213:670e1–670.e7. doi: 10.1016/j.ajog.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joseph DA, Miller JW, Wu X, et al. Understanding the burden of human papillomavirus-associated anal cancers in the US. Cancer. 2008;113(10 suppl):2892–2900. doi: 10.1002/cncr.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 2012;48:1191–1201. doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998–2003. Cancer. 2008;113(10 suppl):2883–2891. doi: 10.1002/cncr.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rippentrop J, Joslyn S, Konety B. Squamous cell carcinoma of the penis: evaluation of data from the Surveillance, Epidemiology, and End Results program. Cancer. 2004;101:1357–1363. doi: 10.1002/cncr.20519. [DOI] [PubMed] [Google Scholar]
- 36.Simard E, Ward EM, Siegel ER, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62:118–128. doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 37.Henley SJ, Singh SD, King J, Wilson RJ, O’Neil ME, Ryerson AB. Invasive cancer incidence and survival—United States, 2013. MMWR Morb Mortal Wkly Rep. 2017;66:69–75. doi: 10.15585/mmwr.mm6603a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63(RR-05):1–30. [PubMed] [Google Scholar]
- 39.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805–813. doi: 10.1002/1097-0142(20010815)92:4<805::aid-cncr1386>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck cancers—major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 43.Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–2314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]