Abstract
Purpose
Adenosine triphosphate is capable of relaxing and contracting urethral smooth muscle. The mechanisms responsible for the relaxing effects of adenosine triphosphate have been well studied but those involved in the contractile response are still unclear. We investigated the contributions of interstitial cells of Cajal and smooth muscle cells to nerve mediated, adenosine triphosphate dependent contractions of urethral smooth muscle.
Materials and Methods
Tension recordings were made from strips of rabbit urethral smooth muscle. Recordings were made of membrane potential and ionic currents from freshly isolated smooth muscle cells and interstitial cells of Cajal using the patch clamp technique.
Results
Stimulating intramural nerves in urethral smooth muscle yielded contractions that were inhibited by the broad spectrum P2 receptor inhibitor pyri-doxal-phosphate-6-azophenyl-2′,4′-disulfonate and the P2X receptor agonist α,β-methylene adenosine triphosphate but not by the P2Y receptor antagonist MRS2500. When studied under voltage clamp at a holding potential of −60 mV, interstitial cells of Cajal showed spontaneous transient inward currents that were increased in frequency by adenosine triphosphate but not by α,β-methylene adenosine triphosphate. In contrast, smooth muscle cells were quiescent but responded to adenosine triphosphate and α,β-methylene adenosine triphosphate by producing a single transient inward current. Currents evoked by adenosine triphosphate in smooth muscle cells were inhibited by α,β-methylene adenosine triphosphate, pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate and suramin, and by a decrease in extracellular Na+ from 130 to 13 mM.
Conclusions
Stimulating purinergic nerves in rabbit urethral smooth muscle induces contractions via the activation of P2X receptors on smooth muscle cells.
Keywords: urethra, muscle, smooth, muscle contraction, purinergic P2X receptor, rabbits
The smooth muscle layer of the urethra generates spontaneous myogenic tone that has an important role in maintaining urinary continence.1 The level of tone is modulated by ATP and its derivatives adenosine and ADP, although the precise mechanisms underlying their effects are unclear. Several groups suggested that ATP is an inhibitory neurotransmitter in the urethra since EFS of intramural nerves in strips of urethra or bladder neck smooth muscle produced relaxation that was mimicked by ATP and inhibited by purinergic receptor antagonists.2–5 However, contractile responses to ATP or related compounds in USM were also described.5–8
The exact nature of the response appears to depend at least in part on the degree of tissue tone at the point of ATP application. For example, Callahan and Creed found that when urethral tone was increased by applying noradrenaline, ATP produced relaxation but when it was applied at resting tone, contractions were observed.6 Similar effects were reported by Bradley et al, who found that ATP induced contractions when applied at resting tone, but relaxation when tone was increased by applying arginine vasopressin.8
There is broad consensus that the inhibitory responses to ATP in the urethra result from P2Y receptor activation.2,4,5 However, contractile effects appear to be more complex. Deplanne et al noted that applying the P2X receptor agonist α,β-methylene ATP, which also causes rapid desensitization of certain P2X receptors, produced tonic contractions in all regions of the rabbit urethra but did not inhibit NANC EFS induced contraction.7 Hernández et al also reported ATP induced contractions in pig bladder neck smooth muscle but again noted that this response was not inhibited by α,β-methylene ATP.5 However, Hashitani and Edwards noted that stimulating transmural nerves in guinea pig USM evoked excitatory junction potentials and triggered slow waves that were abolished by α,β-methylene ATP.9 Bradley et al suggested that the contractile effects of ATP in rabbit USM were mediated by P2Y receptors since they were inhibited by the selective P2Y receptor antagonist MRS2500 and mimicked by the P2Y receptor agonist 2-MeSADP.8 Suramin sensitive, NANC neurogenic contractions were also reported in this study but the exact receptor subtype involved was not investigated.
This issue is further complicated by the fact that 2 cell types, SMCs and ICCs, which are thought to act as putative pacemaker cells in the urethra,10 responded differently to ATP.8 For example, ATP evokes a large, single transient inward current in SMCs but increases the frequency of STICs in ICCs.
Thus, we 1) investigated the purinergic receptor subtype involved in NANC neurogenic contractions of rabbit USM, 2) investigated ATP responses and receptor subtypes in freshly isolated urethral SMCs and ICCs, and 3) equated ATP responses found at the single cell level to the whole tissue.
MATERIALS AND METHODS
Cell Isolation
Male and female New Zealand White rabbits 16 to 20 weeks old were humanely sacrificed by lethal intravenous injection of pentobarbitone. The most proximal 1.5 cm of the urethra was removed and placed in Krebs solution. Individual ICCs and SMCs were isolated enzymatically, as described previously.10 All procedures were done with the approval of the institutional animal care and use committee.
Tension Recordings
Strips of circular and longitudinally oriented smooth muscle (8 × 1 × 1 mm) were removed from the urethra, placed in water jacketed organ baths maintained at 37C and bathed with Krebs solution bubbled with 95% O2-5% CO2 containing atropine (1 μM), phentolamine (1 μM) and No-ARG (100 μM). Strips were adjusted to a tension of 2 to 4 mN and allowed to equilibrate for 50 minutes before experimentation. Contractions were measured using the multichannel Myobath system and data were acquired using Data-Trax 2 (World Precision Instruments, Sara-sota, Florida). Transmural nerve stimulation was applied via 2 platinum electrode wires 5 mm long and 2.5 mm apart by a MultiStim™ system D330 stimulator, which delivered 0.3-milllisecond pulses of 20 V (nominal) at a frequency of 4 Hz for 30-second durations.
Patch Clamp Recordings
Currents were recorded using the perforated patch configuration of the whole cell patch clamp technique.11 The membrane was perforated using the antibiotic amphotericin B (600 μg/ml). Recordings were made as described previously by Bradley et al.8
Solutions and Drugs
Hanks solution was composed of 130 mM Na+, 5.8 mM K+, 135 mM Cl−, 4.16 mM HCO3−, 0.34 mM HPO32−, 0.44 mM H2PO4−, 1.8 mM Ca2+, 0.9 mM Mg2+, 0.4 mM SO42−, 10 mM dextrose, 2.9 mM sucrose and 10 mM HEPES, pH adjusted to 7.4 with NaOH. Ca2+-free Hanks solution for cell dispersal was composed of 125 mM NaCl, 5.36 mM KCl, 10 mM glucose, 2.9 mM sucrose, 15.5 mM NaHCO3, 0.44 mM KH2PO4, 0.33 mM Na2HPO4, and 10 mM HEPES, pH adjusted to 7.4 with NaOH. Krebs’ solution was composed of 120 mM NaCl, 5.9 mM KCl, 1.2 mM NaHCO3, 5.5 mM glucose, 12.5 mM CaCl2 and 6 mM MgCl2, pH maintained at 7.4 by bubbling with 95% O2-5% CO2. Perforated patch solution was composed of 133 mM CsCl, 1.0 mM MgCl2, 0.5 mM ethylene glycol tetraacetic acid and 10 mM HEPES, pH adjusted to 7.2 with CsOH.
Drugs used were α,β-methylene ATP, apyrase, ATP, suramin, phentolamine, atropine (Sigma®), No-ARG (Calbiochem®), 2-MeSADP, MRS2500 (Tocris Bioscience, Bristol, United Kingdom) and PPADS (Ascent Scientific, Cambridge, Massachusetts).
Statistics
Summary data are shown as the mean ± SEM. Except where stated, statistical differences in experiments were compared using Student’s paired t test with p < 0.05 considered significant. Values are reported according to the number of cells per experimental series obtained from a minimum of 2 animals.
RESULTS
Tension Recording
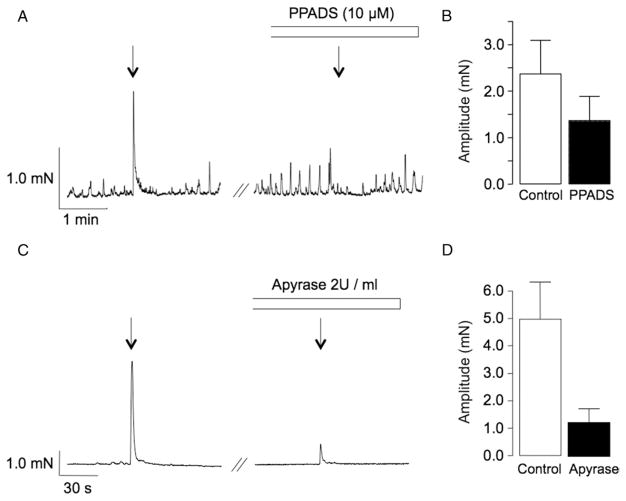
Bradley et al reported that EFS of intramural nerves in strips of rabbit USM in the presence of atropine, phentolamine and No-ARG produced transient contractions that were significantly attenuated by tetrodotoxin and suramin.8 To investigate whether these contractions were mediated by ATP we examined the effect of the broad-spectrum P2 receptor antagonist PPADS and that of apyrase, an enzyme that catalyzes ATP hydrolysis. PPADS significantly decreased the mean amplitude of EFS evoked contractions from 2.4 ± 0.7 to 1.4 ± 0.5 mN in 10 preparations (p < 0.05, fig. 1, A and B). Apyrase (2 U/ml) also significantly inhibited neurogenic contractions (fig 1, C and D). Mean contraction amplitude was decreased from 5 ± 1.4 to 1.3 ± 0.5 mN in 6 preparations (p < 0.05).
Figure 1.
Effect of PPADS and apyrase on rabbit USM contraction induced by 0.3-millisecond pulse duration, 4 Hz frequency, 30-second (s) EFS. Transient smooth muscle strip contraction was attenuated by 10 μM purinergic receptor inhibitor PPADS (A) and ATP diphosphohydrolase enzyme apyrase, which catalyzes ATP hydrolysis (C). Note summary data on these effects (B and D).
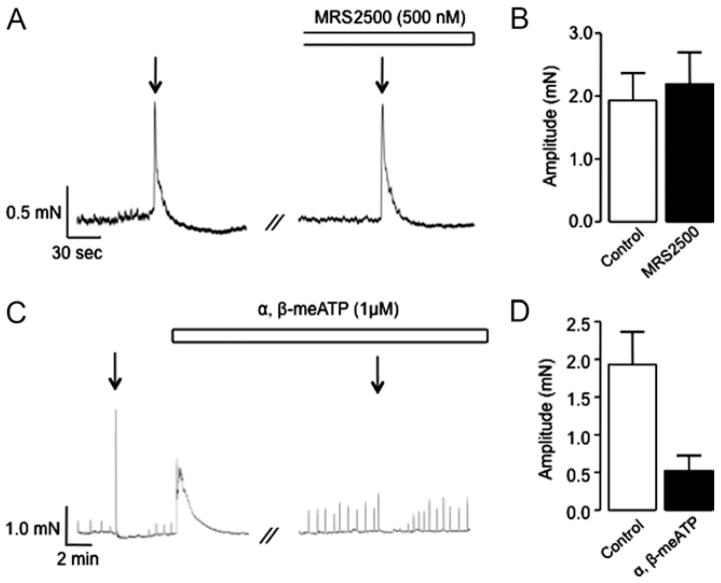
We next investigated the effects of the selective P2Y1 receptor antagonist MRS2500, which was previously shown to inhibit ATP induced USM contraction,8 and α,β-methylene ATP, which desensitizes P2X1 and P2X3 receptors.12 The mean amplitude of EFS induced contractions was unaffected by MRS2500 compared to those in controls in 10 preparations (2.2 ± 0.5 vs 1.9 ± 0.4 mN, p > 0.05, fig. 2, A and B). However, α,β-methylene ATP (1 μM) produced a robust contraction in all muscle strips (mean amplitude 2.6 ± 0.7 mN) and significantly decreased the amplitude of the EFS induced contraction from 1.9 ± 0.4 to 0.5 ± 0.2 mN in 10 preparations (p < 0.05, fig. 2, C and D). Together these data suggest that NANC neurogenic contractions of rabbit USM are mediated by P2X receptors. This is in contrast to contractions produced by exogenous application of ATP in the same preparation, which depend on P2Y receptor activation.8
Figure 2.
Effect of MRS2500 and α,β-methylene ATP on EFS induced rabbit USM contraction. Representative trace (A) and summary chart (B) show that EFS evoked contractions were not inhibited by P2Y receptor antagonist MRS2500. Representative trace (C) and summary chart (D) show that α,β-methylene ATP evoked contraction and inhibited EFS induced contraction.
Voltage Clamp Experiments
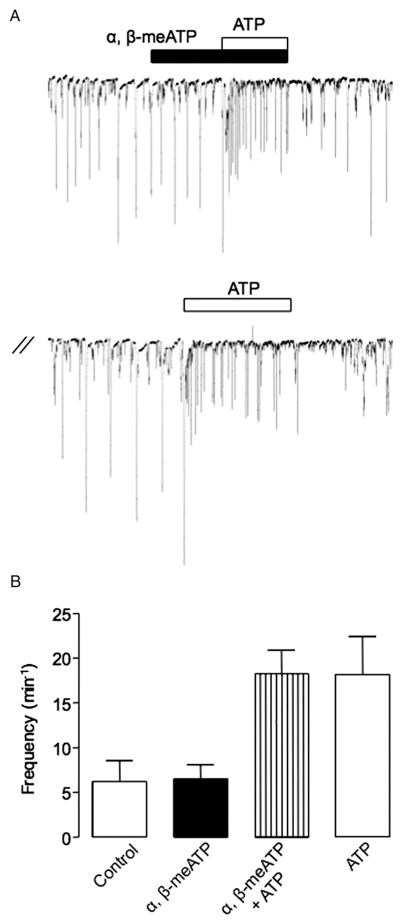
We investigated the cellular basis of the described P2X mediated contraction by examining the effects of ATP on freshly isolated urethral ICCs and SMCs. In contrast to the effect of ATP, the frequency of STICs recorded from an ICC voltage clamped at −60 mV was not affected by applying α,β-methylene ATP (fig. 3, A). In 7 cells the mean frequency of STICs under control conditions was 6.2 ± 2.3 minute−1 vs 6.5 ± 1.7 in the presence of α,β-methylene ATP (p > 0.05). In contrast, ATP significantly increased STIC frequency to 18.4 ± 2.6 minute−1 in the presence of α,β-methylene ATP and to 18.2 ± 4.2 minute−1 after washout (p < 0.05). These data show that α,β-methylene ATP did not affect STICs in urethral ICCs or the ATP response in these cells. This suggests that the ATP response in urethral ICCs is not mediated via P2X receptors and, thus, it is unlikely to contribute to the P2X mediated contraction.
Figure 3.
Effect of α,β-methylene ATP and ATP on STICs in rabbit USM isolated ICCs. One μM α,β-methylene ATP did not affect STIC frequency in ICCs held at −60 mV or inhibit increased STIC frequency induced by subsequent application of 10 μM ATP (A). Note summary data on these effects (B).
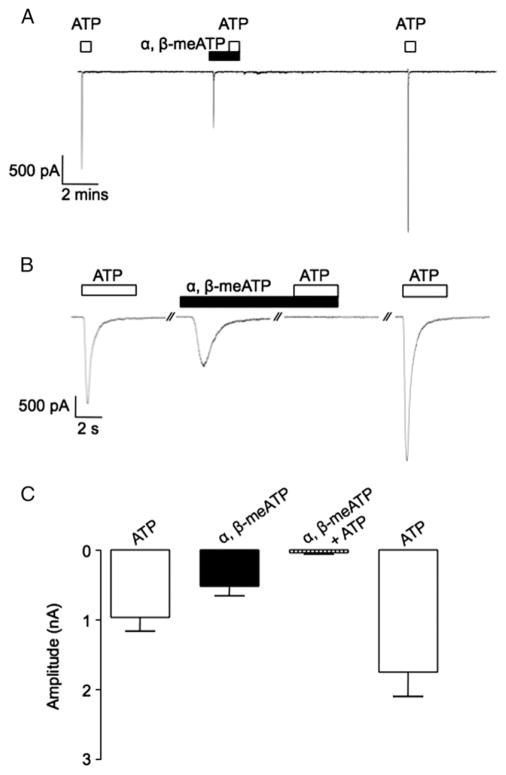
In contrast to the effects on ICCs, ATP and α,β-methylene ATP evoked a robust transient inward current in urethral SMCs (fig. 4). The ATP current was completely and reversibly abolished by α,β-methylene ATP. Figure 4, A shows a representative trace of this effect. Figure 4, B shows each current on an expanded time scale. Figure 4, C shows the mean peak amplitude of current evoked by ATP, α,β-methylene ATP and ATP in the continued presence of α,β-methylene ATP. The mean amplitude of the α,β-methylene ATP evoked current was −490 ± 120 pA vs −902 ± 180 for ATP. In the presence of α,β-methylene ATP the ATP current was significantly decreased to −58 ± 8 pA in 7 preparations (p < 0.05). These data indicate that the ATP evoked current in urethral SMCs is due to P2X receptor activation.
Figure 4.
Effect of α,β-methylene ATP and ATP on inward currents in freshly isolated rabbit USM SMCs. Continuous recording reveals that α,β-methylene ATP and ATP induced transient inward currents in SMCs held at −60 mV (A). Same currents on expanded time scale (B). s, seconds. Note summary plot of mean current amplitudes evoked by α,β-methylene ATP, ATP and ATP in presence of α,β-methylene ATP (C).
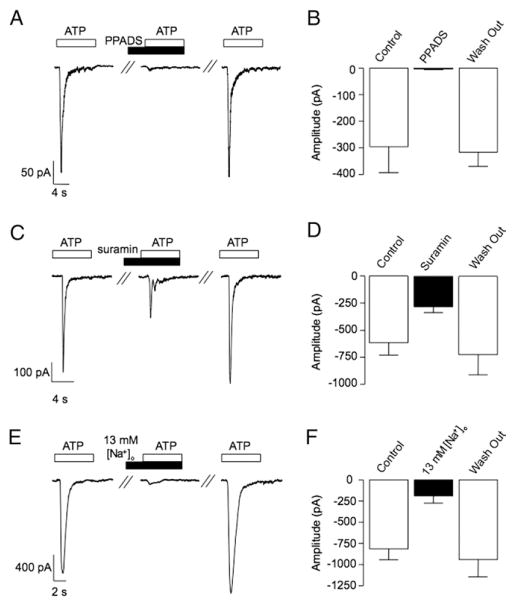
We then investigated the sensitivity of the ATP evoked current in SMCs to the purinergic receptor antagonists PPADS and suramin. Figure 5, A shows representative current traces evoked by ATP (10 μM) before, during and after washout of PPADS (10 μM). The ATP current was greatly attenuated by PPADS. Figure 5, B shows that the ATP evoked current significantly decreased from −297 ± 97 to −3 ± 2 pA in 7 preparations (p < 0.05). Suramin (100 μM) also had inhibitory effects on the ATP evoked current (fig 5, C). It significantly decreased the mean peak current amplitude in 13 cells from −613 ± 115 pA under control conditions to −281 ± 50 pA in the presence of the drug (p <0.05, fig. 5, D).
Figure 5.
Effect of PPADS, suramin and 13 mM [Na+]o on inward currents evoked by ATP in rabbit urethral SMCs. Representative trace (A) and summary plot (B) demonstrate that ATP evoked currents in urethral SMCs were significantly decreased by PPADS. Representative trace (C) and summary plot (D) show that ATP evoked currents were significantly decreased by suramin. Representative trace shows that decreasing [Na+]o from 130 to 13 mM greatly decreased ATP evoked current amplitude (E). Note summary plot of latter effect (F). s, seconds.
The inhibitory effects of α,β-methylene ATP, suramin and PPADS on the ATP current in urethral SMCs were consistent with the activation of a P2X receptor current. Since P2X receptors are cation selective ligand gated ion channels, we examined the effect of decreasing [Na+]o from 130 to 13 mM to test swhether the ATP evoked current was decreased, as would be predicted by P2X receptor activation. Figure 5, E and F show that this was the case. In 12 cells the mean amplitude of the ATP evoked current was significantly decreased from −819 ± 130 pA under control conditions to −193 ± 83 pA in 13 mM [Na+]o (p < 0.05).
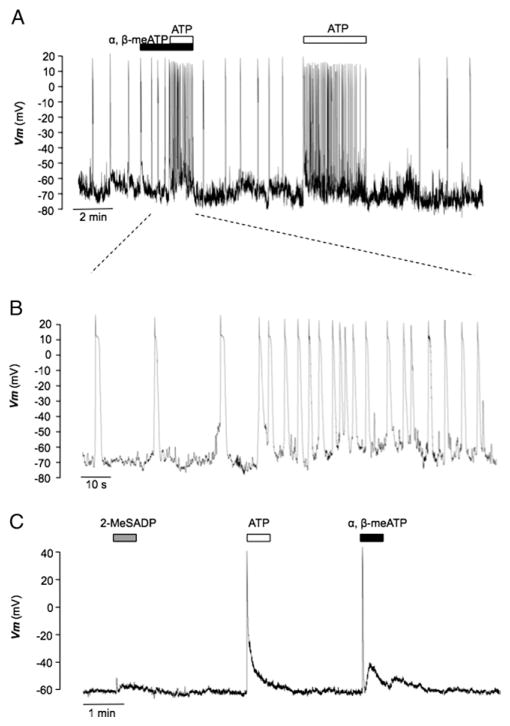
We then examined the effects of ATP and α,β-methylene ATP on isolated ICCs and SMCs under current clamp conditions. Figure 6, A shows a trace from a urethral ICC that demonstrated STDs, as described previously.10 When challenged with α,β-methylene ATP (1 μM), no discernible response was observed. However, when ATP (10 μM) was added in the continued presence of α,β-methylene ATP, STD frequency dramatically increased. After washout STD frequency returned to control levels. When ATP was reapplied in the absence of α,β-methylene ATP, a similar response was observed. These effects were representative of 4 experiments in which ATP increased STD frequency from 2.2 ± 1.3 to 8.8 ± 2.4 minute−1 (p < 0.05). Mean STD frequency was not affected by α,β-methylene ATP compared to that under control conditions in 5 preparations (2.22 ± 0.5 vs 2.72 ± 0.9 minute−1, p > 0.05). On the other hand, isolated urethral SMCs were quiescent but responded to ATP and α,β-methylene ATP in similar fashion by evoking transient depolarization of membrane potential (fig. 6, C). The mean amplitude of depolarization in response to ATP was 79.7 ± 5.7 mV in 10 preparations, which was not significantly different from the 77.6 ± 7.4 mV in 5 produced by α,β-methylene ATP (unpaired t test p > 0.05). However, application of the P2Y receptor agonist 2-MeSADP produced only a small response (fig. 6, C). The mean amplitude of depolarization in 4 cells was 4.1 ± 7.4 mV, indicating that P2Y receptor activation is unlikely to explain ATP induced depolarization in urethral SMCs.
Figure 6.
Effect of purinergic agonists on membrane potential recordings of isolated urethral ICCs and SMCs. Current clamp recording reveals that 1 μM α,β-methylene ATP did not affect STD frequency or inhibit increased STD frequency induced by subsequent 10 μM ATP (A). Same recording on expanded time scale (B). ATP and α,β-methylene ATP transiently depolarized urethral SMCs but 2-MeSADP had little effect (C).
DISCUSSION
USM level of tone is influenced by various factors, including the principal excitatory neurotransmitter noradrenaline and the main inhibitory neurotransmitter nitric oxide.13 However, there is now increasing awareness that ATP and its derivatives, ADP and adenosine, also modulate urethral tone and, thus, are likely to contribute to continence and/or voiding mechanisms. Bladder purinergic innervation has been well studied and ATP is known to be the neurotransmitter responsible for the atropine resistant neurogenic contraction.14 This effect, which is caused by the activation of P2X receptors on detrusor SMCs,15,16 was suggested to be up-regulated under certain pathophysiological conditions, such as idiopathic bladder.17 Consequently P2X receptors have been mooted as potential targets for overactive bladder treatment.17,18
In contrast to the bladder, the effects of ATP on the urethra are less clear. For example, ATP is capable of relaxing and contracting USM strips.5–8 The net balance of the response appears to depend on the degree of tissue tone at the point of ATP stimulation with higher tone favoring the inhibitory response. The issue is complicated further by the finding that P2Y receptors are implicated in each type of response.4,5,8 However, other groups have alluded to the potential involvement of P2X receptors in the contractile response to ATP in the urethra. For example, Deplanne et al found that α,β-methylene ATP contracted all regions of rabbit urethra7 and Teixeira et al noted that α,β-methylene ATP also contracted strips of rat USM.19 Hashitani and Edwards reported that EFS of intramural nerves in guinea pig USM yielded excitatory junction potentials and slow waves, which were inhibited by α,β-methylene ATP.9 Bradley et al described a transient inward current in urethral SMCs that was activated by ATP but not mimicked by the P2Y agonist 2-MeSADP.8
We investigated this current further. We examined whether it might contribute to suramin sensitive contractions evoked by the stimulation of nerves also reported by Bradley et al.8 NANC neurogenic contractions of rabbit USM were mediated by P2X receptor activation as they were inhibited by α,β-methylene ATP and PPADS but not by the P2Y receptor antagonist MRS2500. Also, α,β-methylene ATP evoked robust USM contractions. Since P2X receptor dependent currents were only found in SMCs, these data suggest that SMCs and not ICCs are the target of neurally released ATP in USM. Therefore, our results highlight a novel regulatory mechanism in USM that may contribute to the maintenance of urinary continence. However, these results also call into question the precise physiological role of the ATP response in urethral ICCs, which does not appear to be involved in purinergic neurotransmission. In this regard it is interesting to compare the effects of ATP on ICCs in the GI tract.
Intramuscular ICCs in the mouse and rat stomach are known to participate in nitrergic and cholinergic neurotransmission.20–22 Several studies indicate that urethral ICCs may have a similar role. For example, Lyons23 and García-Pascual24 et al reported that ICCs form close associations with nerves. Studies of isolated urethral ICCs revealed that spontaneous activity in these cells is modulated by noradrenaline, nitric oxide and ATP.8,25,26 Pacemaker activity in intestinal ICCs is also modulated by ATP27 but available evidence indicates that ICCs in the GI tract are not involved in purinergic neurotransmission. For example, Burns et al reported that purinergic inhibitory junction potentials activated by low frequency nerve stimulation were preserved or even slightly enhanced in the gastric fundus region of W/Wv mice, which lack ICCs.20 Sergeant et al observed that the expression of P2Y receptors, which mediate these inhibitory junction potentials, were up-regulated in the W/Wv mutant, suggesting that they were not located on ICCs.28 Others reported that ATP is released from nonneuronal sources in the GI tract. For example, Katsuragi et al noted that ATP is released from smooth muscle during spontaneous contractions29 while Jessen and Burn-stock reported that it could be released from enteric glial cells.30 Thus, ICCs in the GI tract could possibly be regulated by ATP from various sources and, therefore, it may have important modulatory roles besides direct involvement with neurotransmission. If such ATP release mechanisms exist in USM, as is likely, it is possible that despite a lack of involvement in purinergic neurotransmission pacemaker activity in urethral ICCs may be modulated by other pathways.
CONCLUSIONS
Our results reveal that EFS of intramural nerves in strips of rabbit USM in the presence of atropine and phentolamine generates transient contractions. These contractions were inhibited by apyrase, PPADS and α,β-methylene ATP but not by MRS2500, suggesting that they were due to P2X receptor activation. ATP increased STIC and STD frequency in freshly dispersed urethral ICCs but these effects were not mimicked or blocked by α,β-methylene ATP. This indicates that ATP effects in ICCs are not mediated by P2X receptors and, thus, they are unlikely to contribute to P2X dependent nerve mediated contractions. On the other hand, urethral SMC responded to ATP and α,β-methylene ATP by producing a large transient inward current under voltage clamp at −60 mV and a transient depolarization under current clamp conditions. In contrast, the P2Y agonist 2-MeSADP had no effect on isolated SMCs, consistent with previous observations.8 ATP evoked currents in SMCs were inhibited by α,β-methylene ATP and PPADS, and by decreasing [Na+]o from 130 to 13 mM, suggesting that they were caused by P2X receptor activation. In conclusion, our results suggest that ATP release from USM nerves produces contractions by activating P2X receptors located on SMCs.
Acknowledgments
Supported by National Institutes of Health RO1 DK68565, Health Research Board RP/2006/127 and the Council of Directors.
Abbreviations and Acronyms
- 2-MeSADP
2 methylthio ADP
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- EFS
electric field stimulation
- GI
gastrointestinal
- ICC
interstitial cell of Cajal
- NANC
nonadrenergic, noncholinergic
- No-ARG
NG-nitro-L-arginine
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate
- SMC
smooth muscle cell
- STD
spontaneous transient depolarization
- STIC
spontaneous transient inward current
- USM
urethral smooth muscle
Footnotes
Study received institutional animal care and use committee approval.
References
- 1.Bridgewater M, MacNeil HF, Brading AF. Regulation of tone in pig urethral smooth muscle. J Urol. 1993;150:223. doi: 10.1016/s0022-5347(17)35451-4. [DOI] [PubMed] [Google Scholar]
- 2.Ohnishi N, Park YC, Kurita T. Role of ATP and related purine compounds on urethral relaxation in male rabbits. Int J Urol. 1997;4:191. doi: 10.1111/j.1442-2042.1997.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 3.Pinna C, Puglisi L, Burnstock G. ATP and vasoactive intestinal polypeptide relaxant responses in hamster isolated proximal urethra. Br J Pharmacol. 1998;124:1069. doi: 10.1038/sj.bjp.0701908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinna C, Glass R, Knight GE. Purine- and pyrimidine-induced responses and P2Y receptor characterization in the hamster proximal urethra. Br J Pharmacol. 2005;144:510. doi: 10.1038/sj.bjp.0706047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernández M, Knight GE, Wildman SS. Role of ATP and related purines in inhibitory neurotransmission to the pig urinary bladder neck. Br J Pharmacol. 2009;157:1463. doi: 10.1111/j.1476-5381.2009.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callahan SM, Creed KE. Electrical and mechanical activity of the isolated lower urinary tract of the guinea-pig. Br J Pharmac. 1981;74:353. doi: 10.1111/j.1476-5381.1981.tb09978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deplanne V, Palea S, Angel I. The adrenergic, cholinergic and NANC nerve-mediated contractions of the female rabbit bladder neck and proximal, medial and distal urethra. Br J Pharmacol. 1998;123:1517. doi: 10.1038/sj.bjp.0701757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley E, Kadima S, Drumm B. Novel excitatory effects of adenosine triphosphate on contractile and pacemaker activity in rabbit urethral smooth muscle. J Urol. 2010;183:801. doi: 10.1016/j.juro.2009.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashitani H, Edwards FR. Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. J Physiol. 1999;514:459. doi: 10.1111/j.1469-7793.1999.459ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sergeant GP, Hollywood MA, McCloskey KD. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;15:359. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rae J, Cooper K, Gates P. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991;37:15. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- 12.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 13.Andersson KE. Neurotransmission and drug effects in urethral smooth muscle. Scand J Urol Nephrol Suppl. 2001;207:26. doi: 10.1080/003655901750174854. [DOI] [PubMed] [Google Scholar]
- 14.Burnstock G, Dumsday B, Smythe A. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972;44:451. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue R, Brading AF. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol. 1990;100:619. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vial C, Evans RJ. P2X receptor expression in mouse urinary bladder and the requirement of P2X(1) receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br J Pharmacol. 2000;131:1489. doi: 10.1038/sj.bjp.0703720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford AP, Gever JR, Nunn PA. Purinoceptors as therapeutic targets for lower urinary tract dysfunction. Br J Pharmacol, suppl. 2006;147:S132. doi: 10.1038/sj.bjp.0706637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rapp DE, Lyon MB, Bales GT. A role for the P2X receptor in urinary tract physiology and in the pathophysiology of urinary dysfunction. Eur Urol. 2005;48:303. doi: 10.1016/j.eururo.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Teixeira CE, Liming J, Fernanda BM. Comparative pharmacological analysis of Rho-kinase inhibitors and identification of molecular components of Ca2+ sensitization in the rat lower urinary tract. Biochem Pharmacol. 2007;74:647. doi: 10.1016/j.bcp.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns AJ, Lomax AE, Torihashi S. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci USA. 1996;93:12008. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward SM, Beckett EA, Wang X. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beckett EA, Horiguchi K, Khoyi M. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl(d) mice. J Physiol. 2002;543:871. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyons AD, Gardiner TA, McCloskey KD. Kit-positive interstitial cells in the rabbit urethra: structural relationships with nerves and smooth muscle. BJU Int. 2007;99:687. doi: 10.1111/j.1464-410X.2006.06617.x. [DOI] [PubMed] [Google Scholar]
- 24.García-Pascual A, Sancho M, Costa G. Interstitial cells of Cajal in the urethra are cGMP-mediated targets of nitrergic neurotransmission. Am J Physiol Renal Physiol. 2008;295:F971. doi: 10.1152/ajprenal.90301.2008. [DOI] [PubMed] [Google Scholar]
- 25.Sergeant GP, Thornbury KD, McHale NG. Characterization of norepinephrine-evoked inward currents in interstitial cells isolated from the rabbit urethra. Am J Physiol Cell Physiol. 2002;283:C885. doi: 10.1152/ajpcell.00085.2002. [DOI] [PubMed] [Google Scholar]
- 26.Sergeant GP, Johnston L, McHale NG. Activation of the cGMP/PKG pathway inhibits electrical activity in rabbit urethral interstitial cells of Cajal by reducing the spatial spread of Ca2+ waves. J Physiol. 2006;574:167. doi: 10.1113/jphysiol.2006.108621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuzono S, Nakayama S, Imaizumi Y. Purinergic modulation of pacemaker Ca2+ activity in interstitial cells of Cajal. Neuropharmacology. 2005;48:264. doi: 10.1016/j.neuropharm.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Sergeant GP, Large RJ, Beckett EA. Microarray comparison of normal and W/Wv mice in the gastric fundus indicates a supersensitive phenotype. Physiol Genomics. 2002;11:1. doi: 10.1152/physiolgenomics.00052.2002. [DOI] [PubMed] [Google Scholar]
- 29.Katsuragi T, Matsuo K, Sato C. Non-neuronal release of ATP and inositol 1,4,5-trisphosphate accumulation evoked by P2- and M-receptor stimulation in guinea pig ileal segments. J Pharmacol Exp Ther. 1996;277:747. [PubMed] [Google Scholar]
- 30.Jessen KR, Burnstock G. The enteric nervous system in tissue culture: a new mammalian model for study of complex nervous networks. In: Kalsner S, editor. Trends in Autonomic Pharmacology. II. Baltimore: Urban and Schwartzenberg; 1982. pp. 95–115. [Google Scholar]