Abstract
Amyloid Beta (Aβ) and hyperphosphorylated tau are hallmark lesions of Alzheimer disease (AD). However, the loss of synapses and dysfunctions of neurotransmission are more directly tied to disease severity. The role of these lesions in the pathoetiological progression of the disease remains contested. Biochemical, cellular, molecular and pathological studies provided several lines of evidence and improved our understanding of how amyloid beta and hyperphosphorylated tau accumulation may directly harm synapses and alter neurotransmission. In-vitro evidence suggests that amyloid beta and hyperphosphorylated tau have both direct and indirect cytotoxic effects that affect neurotransmission, axonal transport, signaling cascades, organelle function, and immune response in ways that lead to synaptic loss and dysfunctions in neurotransmitter release. Observations in preclinical models and autopsy studies support these findings, suggesting that while the pathoetiology of positive lesions remains elusive, their removal may reduce disease severity and progression. The purpose of this article is to highlight the need for further investigation of the role of tau in disease progression and its interactions with Aβ and neurotransmitters alike.
Keywords: Amyloid, tau, synapse, neurotransmitters
Introduction
Alzheimer disease (AD) is a progressive neurodegenerative disorder marked by the accumulation of beta-amyloid (Aβ) plaques and hyperphosphorylated tau tangles. Although these positive lesions receive a great deal of attention, the loss of neuronal synapses is the best correlate of cognitive decline in patients with AD [1–7]. Deterioration of synapses begins at the level of dendritic spines [8–10]. As such, a great deal of focus has been placed on attempts to understand the mechanisms underlying the structural and functional plasticity of dendritic spines and synapses in AD [11].
Alterations of neurons, synapses, and neurotransmission are common observations in AD and are therefore hypothesized to play a major role in its progression [11–12]. From these studies come the observation that phosphorylated tau has been mislocalized to the postsynaptic compartment [11]. It is important to note, however, that synaptic and dendritic spine pathology is common amongst several neurodegenerative diseases and may represent the same pathogenic mechanisms across them all [11]. In the following review, we will focus on findings that are primarily unique to AD and will highlight significant areas of overlap with other neurodegenerative diseases that may be known.
Neurotransmitter abnormalities in Alzheimer’s disease
Acetylcholine
In-depth coverage of neurotransmitter changes in AD has previously been reported [13–14]. Death of cholinergic neurons was amongst the first findings in AD pathology. Given their known physiological roles, combined with the symptomology of AD, loss of cholinergic neurons was originally postulated as the cause of the disease and was dubbed “the cholinergic hypothesis” [15]. Evidence for this hypothesis came from several sources. Neuronal loss in areas with high cholinergic neuron populations, such as the nucleus Basalis of Meynert (nBM), the hippocampus and cortical areas, is well documented and consistent [16–20]. Further cholinergic system dysfunctions include a decrease in acetylcholine (ACh) synthesis, decreased choline acetyltransferase (ChAT) activity, reduced choline uptake, [21] and altered levels of acetylcholine receptors (AChRs) [22]. Additionally, severe deficits of presynaptic cholinergic markers had been noted in the cerebral cortex of patients with early-onset AD since the late 1970’s [16, 23–25]. Even in the earliest stages of AD as well as its common precursor mild cognitive impairment (MCI) decreased trophic responses in nBM neurons are consistently observed [26]. To date, cholinergic deficits are still the strongest neurochemical correlate of dementia severity in AD [1, 27–30].
However, recent studies no longer support that cholinergic depletion is solely responsible for causing AD. PET-labeled acetylcholinesterase (AChE) ligands show only mild loss of activity in patients with MCI and early stage AD [20, 31] and autopsies show no change in ChAT activity in a number of brain regions studied in these groups [20, 32] nor in the number of ChAT-positive cells in MCI patients [33]. Further support comes from the lack of efficacy of acetylcholinesterase inhibitors (AChEIs) in clinical trials [34, 35] and the finding that cholinergic depletion does not result in severe memory deficits in rats [30. 36]. Finally, basal forebrain cholinergic cell degeneration is common in many neurodegenerative disorders, including Parkinson’s disease [37–39], Down-syndrome (DS), progressive supranuclear palsy, Creutzfeldt-Jakob disease [40], Korsakoff’s syndrome [41–43], and traumatic brain injury [44–45]. This led Craig, Hong, and McDonald to propose that although ACh depletion is not the cause of AD, it leads to a reduction in the ability of the brain to compensate for secondary insults. In their model, this depletion would reduce the ability of the brain to compensate for the accumulation of risk factors that occur with increasing frequency during the aging process [46]. Support for this model can be found in literature on rodents, nonhuman, and human primate models [46]. Detailed information about cholinergic dysfunctions in AD can be found elsewhere [47].
NMDA
Similar to the cholinergic deficits observed in AD, several lines of evidence suggest a role for the presynaptic N-methyl-D-aspartate receptor (NMDAR) localization in reshaping synaptic transmission by regulating presynaptic glutamate release [48]. A combination of increased presynaptic glutamate release and decreased reuptake is believed to result in an excess of extracellular glutamate, which in turn leads to a tonic activation of these receptors [49]. This view is further supported by a study by Bell et al., which showed that MCI subjects displayed a paradoxical elevation in glutamatergic presynaptic bouton density, similar to that observed in the cholinergic system, which then depletes and drops with disease progression [50]. These findings further demonstrated that dystrophic neurite generation and reduced presynaptic bouton densities detrimentally influenced neurotransmission and cognitive function in later stages of AD [50]. More information on NDMA-based synaptic dysfunction is given in a review article by Mota and colleagues [51].
GABA
Disruption of neurotransmission in AD is not limited to excitatory pathways. Several studies indicate dysfunctions in GABAergic signaling, including results from autopsy, neuroimaging, and CNS GABA marker studies [52]. Normally, GABA is released by neurons in response to glutamate excitotoxicity [53–54] and while chronic growth factor deprivation causes GABA transmission to change from an inhibitory to an excitatory stimulus [55], the significance of this transition is not entirely clear [56].
The A class receptors (GABAARs) are crucially involved in synaptic plasticity and help to ensure that NMDARs are activated mainly during high-frequency synaptic transmission [57]. Additionally, GABAAR agonists protect neurons in culture against the neurotoxicity of Aβ and of various glutamate receptor agonists [58–60]. Because of this, stimulating GABAergic transmission has been attempted to treat behavioral and psychological dysfunctions in AD [52, 61], but these efforts are limited due to issues of tolerance after long-term use and side effects [52; 62]. Detailed information on GABA’s role in AD was given by Paula-Lima and colleagues [63].
Serotonin
Similar to GABA, loss of serotonergic activity also appears to correlate with behavioral and psychological dysfunctions, most notably the presence of neuropsychiatric symptoms (NPS) [64], which, combined with several recent reports that suggest selective and early involvement of the dorsal raphe nucleus (DRN), in the pathogenesis of AD, led Simic et al. [64] to propose a novel pathogenic scheme of AD progression. The role of serotonin receptors in AD gained prominence when observation suggested the density of 5-HT positive neurons significantly decline in AD brains [65] and that areas of the brain concerned with learning and memory show high concentrations of these receptors [66–67]. Further support came from imaging studies [68–71] and autopsies [72–78] that showed reduced serotonin levels, receptors, reuptake sites, and metabolites in the frontal and temporal areas [79]. However, the interaction of serotonin in the nervous system is complicated because 5-HT receptors co-localize on glutamatergic, cholinergic and GABAergic neurons, suggesting the serotoninergic system may regulate a variety of other neurotransmitter systems [67]. Detailed information on alteration to serotonin in AD was given in articles by Rodriguez and colleagues [80].
Dopamine
Unlike serotonin, dopamine’s involvement in AD is substantially more limited, but is still informative as it also relates to NPS. While autopsy studies show that the nigrostriatal system remains relatively intact [81], the mesolimbic system is selectively affected [82]. Neuroimaging studies confirm relative sparing of striatal dopamine metabolism and transporters. Additionally, several studies have reported that dysfunction within corticostriatal dopaminergic circuits is associated with NPS in AD. Based on the available data, greater overall behavioral disturbance is associated with lower striatal D2 receptors while higher striatal D2 receptors is associated with delusions and wandering behavior and lower dopamine transporters are associated with apathy [79].
Norepinephrine
Indeed, nearly every major neurotransmitter system is affected to some degree in AD. Perhaps the best example of a system that has been overlooked is the noradrenergic system. Although much of the histopathology of AD was documented since Alzheimer’s first case in 1906, it was not until over 100 years later that the Braak and Del Tredici [83] determined that the locus coeruleus (LC), not the entorhinal hippocampal cortex, is the first area of the brain to exhibit AD pathology. This area is the primary source of norepinephrine (NE), a neurotransmitter that has been implicated in systems of learning, attention, and wakefulness [84]. Additionally, recent data from basic research in animal models of AD indicate that loss of NE incites a neurotoxic proinflammatory condition, reduces Aβ clearance, and negatively impacts cognition [85]. Remarkably, restoration of NE reverses these effects and slows neurodegeneration in animal models, raising the possibility that treatments which increase NE transmission may have the potential to delay or reverse AD-related pathology [85].
Amyloid Beta
Physiological roles of Amyloid Precursor Protein
Now that we have some understanding of the negative lesions of AD (i.e. synaptic loss and dysfunctions of neurotransmission), we will turn our attention to the positive lesions, Aβ and hyperphosphorylated tau. Understanding the physiological roles of their precursor proteins has the potential to lead us to a greater understanding of how their aberrant products may influence the pathology of AD. Full-length amyloid precursor protein (APP) has been shown to promote synaptic activity, synapse formation, and dendritic spine formation, which suggests APP has a pivotal role in learning and memory [86]. This leads to some debate as to whether the deficiency of the functional protein or accumulation of the abnormal form is more important for the progression of the disease. However, a great deal of evidence exists implicating that aberrant proteins have pathological consequences of their own, as we shall discuss below.
Amyloid-based pathology
Aβ is created by the abnormal cleavage of APP by β- and γ-secretases [87–89]. Suspicion of its consequences may date back as far as Alzheimer’s first report [90], but strong support for the pathogenic role first arose from genetic findings in familial AD (FAD) cases, which displayed unusually high Aβ levels and identical clinical manifestations to the sporadic form, but had an earlier onset [89, 91]. A significant portion of these cases carried either APP or presenilin (PS) mutations, suggesting that dysregulation of their normal functions may lead to Aβ formation [89, 91]. These mutations were in turn the basis for several important transgenic mouse models that have been used to study AD and to evaluate potential therapeutic interventions [92–94]. These findings led to the formulation of the “amyloid hypothesis” [95], which proposed that beta amyloid accumulation is responsible for AD-related pathology, including the initiation of neurofibrillary tangles (NFTs), and eventual neuronal death [96]. However, more recent findings suggest soluble Aβ oligomers disrupt glutamatergic synaptic function, which in turn leads to the characteristic cognitive deficits [97–103]. More information on Aβ pathology is available elsewhere [104–105].
Aβ-induced memory impairment
Compelling evidence of Aβ being a causative factor in AD comes from studies in which synthetic Aβ microinjection into the brains of animal models led to impaired working memory in a manner consistent with the memory deficits seen in AD patients [106–108]. A series of studies utilizing synthetic, natural, and human AD-derived Aβ have indicated that soluble Aβ oligomers are both necessary and sufficient to disrupt normal learning and memory function [109–111]. At the two extremes of aggregation, monomers and fibrils appear to act in vivo both as sources and sinks of certain metastable conformations that disrupt synaptic plasticity [112].
Synaptic damage due to beta amyloid accumulations at the synaptic cleft
However, one difficulty with the original amyloid hypothesis is that the temporal patterns of amyloid deposits do not correlate well with the cognitive deficits in affected patients [113]. Furthermore, synaptic plasticity is impaired before Aβ deposits are detected in mouse models of AD [98, 114]. This may be explained by the existence of intraneuronal accumulations of Aβ that have been reported in transgenic mouse models of AD as well as in humans with AD and DS [115–116]. These accumulations correlate with the onset of synaptic and behavioral abnormalities in transgenic models [117–120] and marked intraneuronal accumulation was associated with early ultrastructural pathology, especially within distal processes and synaptic compartments [121].
Indeed, soluble oligomers of Aβ have a direct synaptotoxic effect at nanomolar concentrations [122]. In a mouse model of AD, the vicinity of amyloid plaques was characterized by highly dysmorphic neurites and spine turnover [123–124] causing a net loss of spines. Continuous overproduction of Aβ at dendrites or axons acts locally to reduce the number and plasticity of synapses [125–126]. In hippocampal culture, soluble Aβ produced abnormalities in spine composition, shape, and abundance that strongly support the hypothesis that soluble Aβ initiates toxic mechanisms in AD brains that account for synaptic damage [127]. Continued exposure to Aβ caused abnormal spine morphology and a significant decrease in spine density [127]. In a direct investigation of the acute effects of extracellular and intracellular Aβ42 peptides on synaptic transmission, Moreno et al. [128] noticed inhibition of synaptic transmission by nanomolar concentrations of intracellular oligomeric Aβ42, but not extracellular oligomeric Aβ42 or oligomeric Aβ40. Thus, local dendritic and axonal abnormalities associated with amyloid deposits lead to loss of synapses and the destruction of dendrites and axons in AD [124, 129]. Parihar and Brewer [130] have provided detailed on the synaptotoxic effects of Aβ in AD.
Additionally, while numerous reports indicate that Aβ causes impaired synaptic plasticity, there are paradoxical lines of evidence from both in vitro and in vivo studies that have shown that increased synaptic activity may induce Aβ secretion [102, 131–132]. This suggests that although excessive production of Aβ is synaptotoxic, lower concentrations may serve as a physiological molecule that regulates normal synaptic plasticity and memory [105]. In the following sections we will discuss how Aβ may mediate synaptic damage. A summary schematic is provided in figure 1.
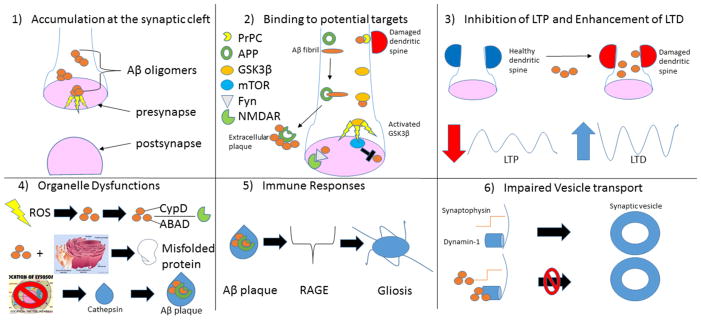
Figure 1.
Amyloid beta’s many sites of neuronal damage. 1) Soluble intracellular oligomers of Aβ are cytotoxic [122,128]. 2) Interaction with PrPC leads to dendritic spine damage [139] (top right). Aβ fibril binds to APP during formation of extracellular plaques [144] (middle left). Aβ inappropriately activates GSK3β [105] (middle right). Aβ synergizes with Fyn to disrupt synaptic receptors [104] (bottom left). mTOR may inhibit Aβ [105] (bottom right). 3) Mounting evidence suggests Aβ-mediated destruction of spines and synapses leads to LTP impairment and LTD enhancement [105]. 4) Mitochondrial distress may lead to production of ROS that may accelerate Aβ formation. Aβ may then affect NMDAR activity by binding to intracellular mitochondrial CypD or mitochondrial ABAD [130] (Top). Aβ-mediated cytotoxicity may be particularly damaging to the endoplasmic reticulum, affecting its production and modification of crucial proteins [234] (middle). Identification of cathepsins in Aβ plaques suggest protease dysfunctions from lysosomes may play a critical role in advancement of pathology [235] (bottom). 5) RAGE may play a prominent role in the increased microglial activation and proinflammatory markers commonly associated with senile plaques [160]. 6) Aβ interacts with synaptophysin in presynaptic terminals of the hippocampus [256] to impair neurotransmission (top). Aβ also interferes with dynamin-1 allowing synaptic vesicles to reenter the synaptic pool [257,258] (bottom).
Signaling Partners of Aβ
Although there is no consensus on the precise molecular pathways involved for Aβ oligomer-induced synaptic deficits a number of intracellular signaling pathways have been implicated [104]. Attempts to identify signaling partners, both those that directly bind to Aβ and those affected by its presence, offer great insights into how Aβ-induced synaptotoxicity may occur. We therefore turn our attention to some of the most promising candidates of such interactions described herein.
Cellular prion protein (PrPC)
We start with Cellular prion protein (PrPC) because the loss of synaptic markers in APP/ Presenilin 1 (PS1) mice is fully dependent on it [133] and treatment of aged APP/PS1 mice with anti-PrPC antibodies allows a recovery of depleted synaptic density in the dentate gyrus [134]. In vitro studies have described dendritic spine loss after acute Aβ oligomer exposure and imaging of spines continuously over 6h shows that Aβ oligomer treatment of dissociated hippocampal neurons induces a 10–15% loss of spines. On the other hand, PRioN Protein knockout (Prnp−/−) cultures are fully protected [135]. Further support that PrPC mediates Aβ oligomer effects on spine composition, morphology and density comes from the observation that PrPC colocalizes to synapses and is present in the postsynaptic density [135–136] similar to Aβ [127, 137–138]. See [139] for more information on PrPC’s role in AD.
Amyloid Precursor Protein (APP)
Next, we consider APP because a direct interaction between Aβ fibrils and the ectodomain of APP has been demonstrated using a variety of experimental conditions [140–142]. In contrast with fibrillar Aβ, monomeric Aβ peptide does not bind APP [140–142], suggesting that conversion of monomeric Aβ into toxic amyloid fibrils is required for binding to APP. The potential pathologic relevance of these observations is suggested by reports showing accumulation of APP at the proximity of fibrillar neuritic Aβ plaques in AD brains [143]. It is also likely that toxic Aβ assemblies other than fibrils could also bind APP including dimers, oligomers, or protofibrils. Bignante and colleagues [144] have provided detailed information on APP’s normal functioning in humans.
Mammalian target of rapamycin (mTOR)
Mammalian target of rapamycin (mTOR) is an evolutionarily conserved serine/threonine protein kinase that plays an essential role in the control of mRNA translation and cell growth [145–147]. While it may be best known for its association with cancer biology, numerous reports have firmly established a pivotal role of mTOR in central nervous system function, including its effects on the consolidation of long-term memory [148–157]. Decreased mTOR signaling has been reported in the brains of an APP-PS1 AD transgenic mouse model [158], which is consistent with subsequent findings that rapamycin, a specific inhibitor of mTORC1, exacerbates neurotoxicity of Aβ [159]. More information on mTOR’s role in AD was given in a review article by Ma and Klann [105].
Receptor for Advanced Glycation End products (RAGE)
Mounting evidence suggests that the damage caused by Aβ includes immune-mediated responses, which may lead to additional neuronal injury [160]. This will be discussed in greater detail in section 2.1.6 “Aβ-induced immune responses”. Receptor for advanced glycation end products (RAGE), is an immunoglobulin-like cell surface receptor that is important for Aβ-mediated cellular perturbation relevant to the pathogenesis of AD [160]. The interaction of RAGE with Aβ in neurons, microglia, and vascular cells accelerates and amplifies deleterious effects on neuronal and synaptic function in vitro [160–163] and in animal models [164–168] including cognitive deficits [165]. Blockade of RAGE significantly attenuates neuronal and synaptic injury [164–166].
Fyn
In addition to immune-response reactions, Aβ may interfere with the function of synaptic receptors by synergizing with Fyn, which induces aberrant increases in neuronal activity, triggering inhibitory mechanisms that limit network overexcitation but that may also diminish the capacity for synaptic plasticity [104]. Fyn increases NMDA receptor-mediated currents, modulates release of calcium from intracellular stores, and enhances synaptic transmission [169–172]. Overexpression of Fyn and Aβ in FYN/hAPP–J9 double-transgenic mice results in severe neuronal and cognitive impairments similar to those otherwise seen only in hAPP mice with much higher levels of Aβ production (hAPP-J20 mice) [173–176]. Conversely, ablation of Fyn prevents several aspects of Aβ-induced neurotoxicity [97, 174].
Glycogen synthase kinase-3β (GSK3β)
Dysregulation of Glycogen synthase kinase-3 (GSK3β) activity has been observed in both sporadic and FAD and increasing evidence suggests that the relationship between GSK3β and Aβ is bidirectional [105]. For example, high GSK3β activity may interfere with normal APP processing via modulation of the function of secretases, leading to Aβ accumulation [177–179]. Suppression of GSK3β activity by either pharmacological inhibitors or genetic manipulations results in abolishment of Aβ production and reducing plaque pathology in the brains of AD transgenic mice [178, 180]. Conversely, Aβ can activate GSK3β through dephosporylation, which is linked to downregulation of the PI3K/Akt signaling pathway [181–186]. GSK3β also has been proposed to play an important role in synaptic plasticity [184, 186] and evidence is accumulating to suggest the therapeutic potential of GSK3β inhibition for AD-related synaptic dysfunction based on rescue of long term potentiation (LTP) in the hippocampus of an AD transgenic mouse by two structurally distinct GSK3β antagonists [187] and pretreatment of rat brain slices with a specific GSK3β inhibitor [181].
Aβ effects on Long-term potentiation and depression (LTP/LTD)
A plethora of studies have examined the effects of Aβ on LTP in vitro and in vivo. In most cases, LTP can be blocked by either direct exogenous Aβ application (at a concentration of 100 nM or above for synthetic Aβ), or in AD transgenic mouse models in which an abnormally high level of Aβ is present [188]. Of note, multiple lines of evidence suggest that there is an early, pre-plaque phase when learning and memory deficits are not detected in AD transgenic mice, but LTP is already impaired [117, 187, 189]. These observations are in line with the hypothesis that soluble Aβ oligomers are synaptotoxic [190, 191].
Compared to LTP, few studies have been conducted to examine the effects of Aβ on long-term depression (LTD) [192–194]. It was reported that LTD in vivo induced by low-frequency stimulation (LFS) can be facilitated by injection of different forms of synthetic Aβ [195, 196]. Additional studies on LTD in vitro have confirmed that LFS-induced LTD is enhanced by Aβ from various sources, including synthetic, cell culture, and human AD brains extracts [111, 197]. It should also be noted that LTD induced by stronger LFS protocol is not altered by Aβ [198–199].
Significant parallels were found between Aβ-induced synaptic changes and LTD suggesting that Aβ affects synaptic function and dendritic spine morphology through LTD-related signaling mechanisms [200]. Overexpression of Aβ resulted in decreased spine density and postsynaptic AMPA receptor number. Notably, expression of a mutant form of AMPA receptor that resists LTD-driven endocytosis blocked the morphological effects and synaptic depression induced by Aβ therefore implicating the endocytosis of AMPA receptors as a major mechanism through which Aβ causes synaptic dysfunction [200]. Aβ’s effects on LTP/LTD may therefore occur through interaction with calcium channel activity [201–202] or glutamate receptor dependent signaling pathways [125, 197, and 200].
Aβ effects on organelle function
Mitochondria
The properties of mitochondrial membranes are essential to their ability to transport metabolites and maintain the necessary conditions for oxidative phosphorylation. Mitochondrial dysfunction is a common finding in AD postmortem brains [203–208] and Aβ treated cell [209–218], suggesting a role for loss of energy production and generation of radical oxygen species (ROS) in the progression of the disease. Voltage-dependent anion channel proteins (VDACs) are ubiquitously located in the mitochondrial outer membrane and are thought to be the primary means for metabolite diffusion between the mitochondria and the cytosol [219–222]. Aβ interacts with VDAC1, blocking the mitochondrial permeability transition pore complex (MTP) pores, which stops transport of mitochondrial proteins from the nucleus leading to defective oxidative phosphorylation, which may in turn induce an increase in free radical production [223].
Additionally, Aβ has been suspected of affecting NMDAR activity by binding to intracellular mitochondrial cyclophilin D (CypD) or mitochondrial Aβ alcohol dehydrogenase (ABAD), as well as other targets [130]. Synaptic terminals from aged rats were shown to be more sensitive to Aβ toxicity, evidencing an age-related decline in mitochondrial activity in both resting and depolarized conditions. In addition, ultrastructural changes including increased mitochondrial size and a significant reduction of synaptic vesicle contents were also observed in presynaptic nerve endings from rat hippocampi exposed to Aβ at different ages [224].
It is likely that in pathological situations, unusually high levels of ROS are produced constantly, overwhelming the ability of the endogenous antioxidants, which then results in impairments in synaptic function. For example, AD mutant mice with decreased mitochondrial superoxide dismutase (SOD-2) expression exhibit elevated levels of brain Aβ and accelerated cognitive dysfunction [225–226]. Conversely, it has been shown that overexpression of SOD-2 in two different AD mouse models is capable of reducing brain Aβ deposition and preventing memory deficits [227–228].
More recently, it was demonstrated using both pharmacological and genetic approaches that AD-associated synaptic plasticity impairments can be prevented and reversed by targeting ROS derived from mitochondrial, but not NADPH oxidase [229]. An interesting implication that arises from these studies is that ROS play different roles in synaptic plasticity, depending either on their subcellular localization or on the age of the animals being studied [105, 230–232].
Alternatively, Aβ has been shown to interact with dynamin-related protein 1 (Drp1), which leads to increased mitochondrial fission and decreased fusion that ultimately results in increased mitochondrial fragmentation [233]. Mitochondrial fragmentation may lead to accelerated ROS production and we can therefore see that Aβ has multiple effects on mitochondria that may occur simultaneously, leading to synaptic damage and neuronal loss.
The Endoplasmic Reticulum and Golgi
Likewise, microarray analyses demonstrated that exposure of SN56.B5.G4 cells to oligomeric β-amyloid (1–42) affected the expression level of a number of genes that were not influenced by non-specific oxidative stress, suggesting that Aβ displays a particular toxicity for cholinergic neurons [234]. Many of the gene products affected were present in the endoplasmic reticulum (ER), Golgi apparatus or were otherwise involved in protein modification and degradation (chaperones, ATF6) indicating a possible role for ER-mediated stress in Aβ-mediated toxicity [234].
Lysosome
Finally, new insight into the broad dysfunction of the lysosomal system in AD was obtained following the identification of cathepsins in Aβ plaques. These findings combined with our understanding of APP cleavage to bring proteases to the forefront of investigation in neurodegenerative diseases [235].
Aβ-induced immune responses
Under ideal circumstances, organelle dysfunctions like those discussed above may elicit immune responses that prevent damaged neurons from having deleterious effects on others. However, immune responses may overreact and exacerbate local cellular damage. Microglia play a critical role in Aβ-mediated neuronal dysfunction and death in the pathogenesis of AD [236–242] and many inflammatory mediators detected in AD brains are of microglial origin [236, 242–247]. Increased microglial activation, microglial association with senile plaques, and elevated levels of proinflammatory mediators (i.e. cytokines, chemokines and free radicals) have all been observed in the AD brain and AD mouse models and evidence indicates that these processes contribute to neuronal damage [236, 248–249]. Observations of brains from AD patients and AD mouse models show that activated microglia and astrocytes accumulate to the greatest extent in the proximity of amyloid plaques [236]. Furthermore, studies carried out with an in vitro cell culture model show that direct administration of Aβ to multiple cell types can induce cellular stress, and this effect is probably increased in the presence of activated microglia [236, 240]. Information Aβ-mediated responses was given by Yan and colleagues [250].
Aβ effects on synaptic vesicle transport
Although we have now seen how Aβ may damage neurons through several direct and indirect process, arguably its most fundamental effect is the disruption of neurotransmission. Endogenous Aβ peptides appear to have a crucial role in activity dependent regulation of synaptic vesicle release [251]. Deleterious effects of Aβ oligomers were shown to be present on multiple steps of synaptic vesicle trafficking [252] and acute treatment of cultured rat hippocampal neurons with Aβ oligomers decreased vesicle endocytosis and regeneration, which led to weakened synaptic transmission [252]. Furthermore, these effects were prevented by an antibody against Aβ [252]. Electron microscopy (EM) studies demonstrate that oligomeric Aβ is localized within the synaptic compartment [121] or that it is bound to the extracellular surface of the spine suggesting that oligomeric Aβ may interact directly at the synapse to cause dysfunction and spine collapse [253].
Presynaptic proteins such as SNAP-25, synaptophysin, and synaptotagmin were reduced in brains of patients with AD [254] and in the hippocampus of Tg2576 mice one month after injection of Aβ into the third ventricle [255]. Russell et al. [256] demonstrated a time-dependent interaction of Aβ with synaptophysin in presynaptic terminals of hippocampal neurons and electrophysiology recordings in hippocampal brain slices confirmed that Aβ affects baseline neurotransmission. Additionally, Aβ oligomers can alter dynamin-1, a neuron-specific GTPase that pinches off synaptic vesicles, allowing them to re-enter the synaptic vesicle pool [257–258]. For more information on how Aβ affects synaptic vesicle trafficking, see [51].
Aβ-induced Neurotransmitter Modulation
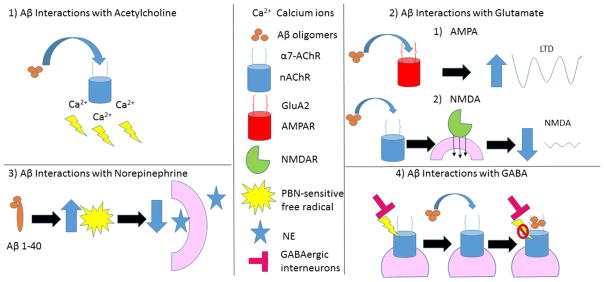
In addition to synaptic changes, Aβ is suspected of altering the actions of several major neurotransmitter systems. A summary of Aβ’s proposed effects is depicted in figure 2.
Figure 2.
Summary of amyloid beta’s interaction with neurotransmitters at neuronal synapses. 1) Aβ may lead to an overactivation of nAChRs and excessive Ca2+ influx that impairs the signaling cascades of memory formation [16, 25]. 2) Aβ oligomers are believed to interact with the GluA2 subunit of AMPAR [274] leading to increased LTD (top). Aβ promotes endocytosis of NMDARs in cortical neurons and produces a rapid and persistent depression of NMDA-evoked currents via activation of nAChRs [138, 280, 281] (bottom). 3) Aβ1–40 allows a PBN-sensitive free radical to deleteriously effect evoked NE release [285]. Aβ inhibits nicotinic currents from GABAergic interneurons by directly blocking the postsynaptic a7-nAChR channels [263].
Acetylcholine
Physiological levels of Aβ (within the picomolar range) can activate presynaptic nicotinic acetylcholine receptors (nAChR) and evoke changes in Ca2+ levels in rat hippocampus and neocortex while toxic levels (within the nanomolar range) inhibit both the presynaptic nAChR and presynaptic Ca2+ levels [259]. Binding of Aβ to nAChR can modulate presynaptic glutamate-mediated synaptic transmission or glutamate release, which suggests Aβ42 activates a signal transduction mechanism that results in synaptic transmission and memory consolidation via nAChR [260].
However, it is unknown if these effects are due to a direct physical interaction of Aβ peptide and nAChRs [130]. Immunohistochemical (IHC) studies on human sporadic AD brains show that Aβ42 and nAChR are both present in neuritic plaques and co-localize in individual cortical neurons [261], but Aβ may regulate nAChR function through binding of membrane lipids [262]. Aβ42 acting through nAChRs, can elicit ERK/MAPK activation in hippocampal cultures [263–264] via Ca2+ influx [265–266]. ERK may then phosphorylate CREB and Elk-1 [267], which help initiate transcription of memory associated genes that contain their respective regulatory elements [268]. Therefore, Aβ may lead to an overactivation of nAChRs and excessive Ca2+ influx that impairs the signaling cascades of memory formation [16, 25]. For more information on ACh and Aβ, see [130 and 269].
Glutamate
There is an abundant literature on the interactions of glutamatergic neurons and Aβ [51, 63, 104, 130, 270–272]. In brief, Aβ promotes glutamatergic excitotoxicity and disrupts glutamatergic synapses and plasticity providing an explanation for the cognitive deficits seen in AD [190]. Although Aβ greatly reduces levels of AMPA and NMDA receptors at the neuronal plasma membrane through endocytosis [127, 138, 272], it is unclear how this loss is induced [273].
AMPA
Aβ oligomers are believed to interact with complexes containing the glutamate alpha-2 (GluA2) subunit of AMPA receptors (AMPAR) based on co-immunoprecipitation and photoactivated amino acid cross-linking studies [274] as well as the observation that pharmacological inhibition and removal of surface AMPARs reduce Aβ binding to neurons [274–276]. Similarly, a mouse model of AD showed that the synaptic removal of AMPAR plays a key role in the Aβ-induced synaptic dysfunction [277]. D’Amelio et al., [277] demonstrated an increase of Aβ-dependent synaptotoxicity leading to a synaptic activation of mitochondrial-dependent protease, caspase-3, which activates calcineurin. Calcineurin then dephosphorylates GluR1 subunit of AMPARs triggering their removal from post-synaptic sites. This reduction of AMPAR mediated currents leads to an increase of LTD, which correlates with dendritic spine degeneration and memory loss [271].
NMDA
The binding of Aβ oligomers to synapses may involve the assembly of a multi-protein receptor complex created by interaction with NMDAR [278]. Once bound to synaptic membranes, Aβ instigates synaptic failure by down-regulating plasticity-related receptors, which leads to abnormal trafficking or degradation [63]. Although Aβ selectively enhances NMDAR-mediated currents and synaptic transmission at physiological levels [258, 279] at higher concentrations it promotes endocytosis of NMDARs in cortical neurons and produces a rapid and persistent depression of NMDA-evoked currents via activation of nAChRs [138, 280–281]. Activation of NMDARs and mGluRs, in turn, promotes the amyloidogenic processing of APP, increasing Aβ levels in vitro [282–283]. Thus Aβ instigates a positive feedback cycle, in which Aβ induces glutamate receptor overactivation, leading to the generation of more Aβ [63]. Aβ can also promote cytotoxicity through increased Ca2+ influx and elevated levels of potentially toxic ROS in an NMDAR dependent manner [258, 284]. Collectively, these findings suggest that NMDARs are specifically required for pathogenic Aβ binding to dendrites and that binding induces the endocytosis of NMDAR and other plasticity-related membrane receptors, culminating in synapse failure and elimination [63].
Norepinephrine
In contrast, very little work has examined the effects of Aβ on NE. We were only able to find one such study [285]. In it, they examined the effects of Aβ1–40 on the release of [3H] NE from rat brain cortical slices and synaptosomes, respectively. The results show that 3nM Aβ1–40 decreased electrically stimulated [3H]NE release approximately 50%, and increased [Ca2]i by 78%. Complete prevention of both effects was observed when the free radical scavenger 1mM N-tert-butyl-α-phenylnitrone (PBN) was present or used as pretreatment. This led them to conclude that Aβ1–40 allows a PBN-sensitive free radical to deleteriously effect both evoked [3H]NE release and [Ca2+]i regulation in rat cortical slices and synaptosomes [285].
GABA
Also like NE, little work has been done on Aβ’s interaction with GABA. This is likely because soluble Aβ oligomers do not bind to GABAergic neurons [127, 286] and, as previously mentioned, the significance of the transition of GABAergic neurons from inhibitory to excitatory in AD is not yet understood. GABA is often known as the major inhibitory neurotransmitter in the brain and Aβ has been observed to inhibit whole-cell and single-channel nicotinic currents from rat GABAergic interneurons by directly blocking the postsynaptic a7-nAChR channels [241]. For more information of GABA’s interaction with Aβ, see [104 and 271].
Tau
Physiological role of Tau proteins
Tau proteins play an important role in the assembly of tubulin monomers into microtubules and in stabilizing microtubules to other cytoskeletal elements in neurons [287]. Microtubule formation is a dynamic process of assembly and disassembly that is crucial for axonal transport. Disruption of this process by abnormal phosphorylation of tau may therefore lead to synaptic transmission dysfunction and neuronal death.
Tau-based pathology
Observations from molecular analyses suggest that the aggregation of tau filaments inside of neurons may be caused by abnormal phosphorylation. This, combined with the observation that a direct correlation between the progressive involvement of the neocortical areas and the increasing severity of dementia, suggests that pathological tau proteins may be a reliable marker of the neurodegenerative process [287]. The conformation and hydrophobicity of tau oligomers play a critical role in the initiation and spread of tau pathology in the naïve host in a manner reminiscent of sporadic AD. These oligomers were shown to be potent inhibitors of LTP in hippocampal brain slices and to disrupt memory in wild type mice [288]. Furthermore, intracerebral injection of brain extracts containing tau aggregates in transgenic animals can induce tau pathology and tau oligomers directly extracted from the cerebral cortex of an AD brain can propagate abnormal tau conformation of endogenous murine tau after prolonged incubation [288]. These findings highlight how abnormal phosphorylation of tau can lead to impairment of neuronal functioning and spread in a prion-like fashion.
Synaptic damage due to phosphorylated tau accumulations
Transgenic mouse models of AD have implicated tau as a major mediator of Aβ toxicity at the postsynaptic compartment and dendritic spines [11]. Crossing of APP transgenic mice with tau transgenic mice, promoted NFT pathology [289–291] and immunization of APP/Psn/tau triple transgenic (3xTg-AD) mice with antibodies against Aβ reduced the levels of hyperphosphorylated tau [292]. Consistent with previous work in cultured neurons [293], removing endogenous tau can prevent Aβ-induced behavioral deficits in an AD mouse model expressing human APP and block excitotoxin-induced neuronal dysfunction in both transgenic and nontransgenic mice [294]. Synaptic physiological data also indicate that tau reduction corrects several abnormalities in multiple hippocampal subregions of hAPPJ20 mice [295]. Together, these studies support the notion that tau phosphorylation abnormalities contribute to disease pathogenesis [100] via promoting the accumulation of the toxic Aβ peptides [11].
It may seem confusing as to how tau, a normally presynaptic protein, could end up influencing Aβ in the postsynaptic compartment, but a study by [296] indicated that phosphorylated tau could accumulate in dendritic spines where it may affect the synaptic trafficking and/or anchoring of glutamate receptors, which in turn affects postsynaptic function; thus revealing a critical role for tau phosphorylation in causing mislocalization of tau and subsequent synaptic impairment, thereby establishing dendritic spines as pathogenic targets of tau action. Conversely, Aβ could enhance the production of hyperphosphorylated tau by activating a number of kinases, including cyclin-dependent kinase-5 (CDK5) [297–298], Fyn kinase [174, 299], GSK3β [300], and MARK [301–303]. Ittner et al. [304] showed that the interaction of tau with Fyn leads to the targeting of Fyn to dendritic spines, where Fyn can phosphorylate NMDA receptor subunit 2 (GluR2), resulting in stabilization of the interaction between GluR2 and post synaptic density-95 (PSD-95), which leads to enhanced excitotoxicity. PSD-95 also interacts with tau, further supporting a dendritic role of tau outside of its axonal function [304]. Importantly, the toxic effects of APP/Aβ were attenuated by interfering with GluR2/PSD-95 interaction with a cell-permeable peptide [304], supporting that dendritic tau-mediated Fyn recruitment and GluR2/PSD-95 interaction confer Aβ toxicity at the postsynapse. Thus, a “tau hypothesis” has been postulated which states: Aβ triggers the phosphorylation of tau, causing tau to dissociate from the microtubules and accumulate at the dendritic compartments. Phosphorylated tau then interacts with Fyn, localizing Fyn to dendritic spines. Fyn then sensitizes the NMDA receptors, which renders neurons more vulnerable to the toxicity of Aβ in the postsynaptic compartment (see figure 3) [305].
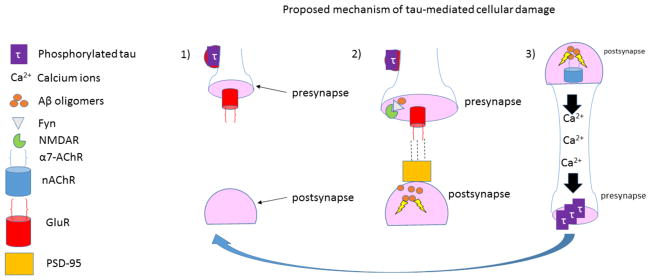
Figure 3.
Tau at the synapse. 1) Phosphorylated tau accumulates in dendritic spines where it may affect the synaptic trafficking and anchoring of GluR [296]. 2) Dendritic tau-mediated Fyn recruitment and GluR2/PSD-95 interaction confer Aβ toxicity at the postsynapse. 3) Upon activation by Aβ, a7 AChR mediates a rise in intracellular Ca2+, which may activate kinases that phosphorylate tau [328, 329]. Thus, Aβ and hyperphosphorylated tau may promote each other’s role in the pathogenesis of Alzheimer’s disease.
Effects of phosphorylated tau on mitochondria
Similar to Aβ, phosphorylated tau has been shown to interact with VDAC1 in AD postmortem brains and in 3xTg-AD mice [223]. This interaction may interrupt axonal transport to the mitochondria resulting in synaptic damage [306]. Likewise, phosphorylated tau also interacts with Drp1[307], as did Aβ, highlighting the dual site of interaction for these lesions within the mitochondria and further supporting the notion that phosphorylated tau-mediated disruption of axonal transport to the mitochondria may lead to synaptic damage.
Factors affecting tau accumulation
In addition to the factors we have already discussed, SRPK2, and S6K1 have been implicated as phosphorylating tau leading to its toxic accumulation [308–309]. Factors specifically involved in the “tau hypothesis” mentioned above include PAR-1/MARK and its activator, LKB1 [11] as well as ALOX5 [310], and PS1 [311]. Conversely, magnesium has been suggested to reduce tau accumulations, which is believed to occur via increased inhibitory phosphorylation of GSK-3β [312].
mTOR
mTOR’s interactions with Aβ were previously discussed, but upregulation of mTOR has also been reported in tangle-bearing neurons of postmortem AD brains [313]. This finding is in agreement with a recent report showing that mTOR signaling is enhanced in the 3xTg-AD mouse model [314]. However, studies using other mouse models have not found the same results [187; 315] suggesting that mTOR’s response to excess Aβ is driven by PS or tau mutations. Observations on mTOR’s role in AD remain unclear as studies often contain several differences in methodology including the mouse model used, the age of the mice, and the experimental conditions making it difficult to draw interpretations across experiments [187, 314, and 315]. However, mTOR’s involvement connects Aβ and tau to work on the insulin-PI3K-Akt pathway in AD, as this pathway is a well-established upstream regulator of mTOR [182; 183; 316–319]. More information on phosphorylated tau’s interactions with mTOR was given in a review article by Ma and Klann [105].
Neurotransmitter abnormalities due to phosphorylated tau accumulations at the synaptic cleft
Far fewer investigations appear to have examined the role of tau on neurotransmitter dysfunction than Aβ. The only substantial literature appears to deal with tau’s potential interaction with the cholinergic system.
Acetylcholine
Patients with MCI and AD have been shown to have tau pathology in cholinergic basal forebrain neurons [30, 320–323]. Single cell gene expression profiling of human cholinergic basal forebrain neurons revealed a shift in the ratio of three-tandem repeat to four-tandem repeat tau within the nBM and CA1 hippocampal neurons that is exclusive to the progression of AD compared to normal aging [324]. Furthermore, observations of pretangles and tangles in the basal forebrain prior to entorhinal/perirhinal cortex pathology suggest that abnormalities in cortical cholinergic neurons and tau formations within the basal forebrain cholinergic system occur early in life and increase in frequency in old age and AD [30, 325–326]. Additional findings from a tau transgenic mouse model, are consistent with observations in humans and suggest tau pathology may participate in cholinergic degeneration [327].
Conversely, nAChR activation results in a significant increase in tau phosphorylation, whereas muscarinic acetylcholine receptor (mAChR) activation may prevent this [328–329]; for reviews see [269, 330]. Nicotine was also found to induce tau phosphorylation at sites that were also hyperphosphorylated in AD [331]. Support for a nicotine-induced phosphorylation of tau model also comes from a study in which five months of nicotine administration to one-month-old 3xTg-AD mice did not change soluble Aβ levels but increased phosphorylation and aggregation of tau. This appears to be mediated by p38-MAP kinase [331]. Detailed information on phosphorylated tau’s interaction with Ach was given in a review article by Schlieb and Arendt [47].
The nicotine-induced phosphorylation of tau model is assumed to work through the a7 AChR [329]. Upon activation by either nicotine or Aβ, a7 AChR has been shown to mediate high Ca2+ permeability, which leads to dramatic increases in intracellular Ca2+ levels [265]. This rise in intracellular Ca2+ activates Ca2+-dependent kinases, which may phosphorylate tau [328–329]. Therefore, accumulation of intraneuronal Aβ may occur prior to plaque and neurofibrilar tangle formation and initiate tau pathology through the a7 AChR. This view is supported by evidence that a selective decrease in Aβ-42 markedly delays the progression of tau pathology [332]. Conversely, a7 nicotinic agonists predictably increase phosphorylation of tau protein [328], yet simultaneously attenuate Aβ-mediated toxicity [333]. Hence, in vitro and in vivo evidence suggests that a7 AChR may operate as a link between Aβ and tau pathology (figure 3) [334].
Amyloid Beta and hyperphosphorylated tau-independent mechanisms of synaptic damage
Although the intent of this review is to discuss amyloid and tau-mediated synaptic and neurotransmitter dysfunctions, it is important to acknowledge several other major factors that are associated with these dysfunctions as well. Some factors, like PS1, are known for their interactions with Aβ and tau, but have independent synaptotoxic mechanisms as well. Similar alterations in ACh and NMDA receptor-mediated components of synaptic plasticity are evident in 3×TgAD mice with PS1, amyloid precursor protein and tau mutations, suggesting that the adverse effects of mutant PS1 on synaptic plasticity can occur in the absence or presence of amyloid and tau pathologies [335]. Likewise, Aβ-independent mechanisms of apolipoprotein E4 (APOE4) include differential effects of apoE on cerebral energy metabolism, neuroinflammation, neurovascular function, neurogenesis, and synaptic plasticity. ApoE is believed to effect synaptic plasticity via low-density lipoprotein receptor-related protein 1 (LRP1), syndecan, and LRP8/ApoER2 [336].
Other factors, such as nitric oxide (NO) and estrogen may reduce synaptic damage. At the early stages of the disease in AD mouse models, evidence of abnormal synaptic function presents as increased LTD revealed only when homeostasis is challenged; suggesting compensatory mechanisms are recruited to maintain a functionally normal hippocampal circuit. Chakraborty et al. [337] concluded that recruitment of NO, which acts presynaptically to boost vesicle release and glutamatergic transmission, serves a compensatory role to boost synaptic transmission and plasticity during early-stage AD. However, NO’s dual role in neuroprotection and neurodegeneration may convert to maladaptive functions as the disease progresses [313]. Additionally, estrogen replacement in postmenopausal women appears to decrease the risk of developing AD [338] as estrogens promote axonal and dendritic plasticity in the limbic neurons of male and female brains [339–343]. In female mice, ovariectomy severely impairs the reactive hippocampal synaptogenesis that follows entorhinal damage, but this effect was reversed by estrogen replacement [344]. Postmenopausal estrogen deficiency may thus suppress the potential for neuroplasticity [345].
Conclusion
Throughout this review, we focused on how Aβ and phosphorylated tau could influence the accumulation of one another, impair synaptic integrity, and alter neurotransmission. From this we have seen that the potential interplay between these two lesions can be at times synergistic, antagonistic, and almost always complicated. Following our review of the literature a few points are readily noticeable. First, there is considerably more literature on Aβ’s involvement than tau’s. We identified 169 articles specific to Aβ versus 26 for tau and only 16 explicitly looked at both. However, whether that is because Aβ is truly more responsible for a wider variety of issue or if this is simply because it has received more scrutiny given its historical precedent is not entirely clear. We say this because although it is reasonable for works before the early 2000s to have focused primarily on Aβ of the aforementioned articles on Aβ only 20 of them are from before 2000. Therefore the vast majority of work that we have discussed herein comes from after that time, yet shows little attention to tau; often making it unclear if observations noted for Aβ are absent from similar manipulations of tau or more likely that they simply have not been investigated. Given the relatively recent reassertion of tau’s importance from Braak and Del Tredici [83] observation that its accumulation in the LC is the first positive lesion of AD and Brier et al.’s [346] conclusion from PET imaging that tau may be a more informative predictor of a person’s cognitive decline and potential response to treatment than Aβ, investigations on the significance of tau, both at the cellular and clinical level, are imperative in order to achieve a fuller understanding of AD.
Likewise, while investigations of cholinergic and glutamatergic systems have provided great insights and offer potential targets for novel therapies [56] more work needs to be done on the GABAergic and noradrenergic systems. Understanding how the GABAergic system is affected is important for two reasons. First, because it is the major inhibitory system in healthy individuals, but we know that it can shift to an excitatory system in the presence of AD; the full consequences of which are unknown [56]. Second, as novel therapeutics that rely on restoring excitatory systems undergo clinical trials these drugs may face hurdles from side effects that are induced by a lack of inhibitory feedback from GABA thereby rendering otherwise successful treatments as impractical; similar to the current problem of using GABAergic agonists to treat NPS as previously discussed [52]. NE, on the other hand, has received surprisingly little attention given that the destruction of its largest source of cells, the LC, is known to be the first to show AD pathology [83] and its physiological function is believed to be involved in both memory and attention [84]; two of the hallmark aspects of AD pathology.
For these reasons, it is our recommendation that investigations of the interaction between tau and NE receive a renewed sense of priority. Although neither the formation of phosphorylated tau nor loss of NE has been observed to result in the most severe of pathologies related to AD, their unique position as the earliest events in disease progression provides reason to investigate these processes with greater scrutiny as an understanding of how these early events occur may lead to greater insights into how the disease may be delayed, prevented, or even cured.
References
- 1.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer disease: correlation with cognitive severity. Annals of neurology. 1990;27(5):457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 2.Scheff SW, DeKosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer disease. Neurobiology of aging. 1990;11(1):29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- 3.Scheff SW, Price DA. Synapse loss in the temporal lobe in Alzheimer disease. Annals of neurology. 1993;33(2):190–199. doi: 10.1002/ana.410330209. [DOI] [PubMed] [Google Scholar]
- 4.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68(18):1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 5.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 6.Masliah E, Mallory M, Hansen L, Richard D, Alford M, Terry R. Synaptic and neuritic alterations during the progression of Alzheimer disease. Neuroscience letters. 1994;174(1):67–72. doi: 10.1016/0304-3940(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 7.Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, Frosch MP, Albert MS, Hyman BT, Irizarry MC. Early Aβ accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62(6):925–931. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- 8.Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 9.Carlisle HJ, Kennedy MB. Spine architecture and synaptic plasticity. Trends Neurosci. 2005;28:182–187. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- 11.Yu W, Lu B. Synapses and dendritic spines as pathogenic targets in Alzheimer’s disease. Neural plasticity. 2012;2012 doi: 10.1155/2012/247150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spires-Jones T, Knafo S. Spines, plasticity, and cognition in Alzheimer’s model mice. Neural plasticity. 2011;2012 doi: 10.1155/2012/319836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai MK, Ramírez MJ, Tsang SW, Francis PT. Alzheimer’s disease as a neurotransmitter disease. Neurobiology of Alzheimer’s Disease. 2007:245–281. [Google Scholar]
- 14.Heii A, Yosuke I, Kenji K, Takashi M, Reiji I. Neurotransmitter changes in early-and late-onset Alzheimer-type dementia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1992;16(6):883–890. doi: 10.1016/0278-5846(92)90106-o. [DOI] [PubMed] [Google Scholar]
- 15.Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 16.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 17.Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J. 1978;2(6150):1457–1459. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Annals of neurology. 1981;10(2):122–126. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- 19.Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215(4537):1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 20.Davis KL, Mohs RC, Marin D, Purohit DP, Perl DP, Lantz M, Austin G, Haroutunian V. Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA. 1999;281:1401–6. doi: 10.1001/jama.281.15.1401. [DOI] [PubMed] [Google Scholar]
- 21.Slotkin TA, Seidler FJ, Crain BJ, Bell JM, Bissette G, Nemeroff CB. Regulatory changes in presynaptic cholinergic function assessed in rapid autopsy material from patients with Alzheimer disease: implications for etiology and therapy. Proc Natl Acad Sci U S A. 1990;87:2452–2455. doi: 10.1073/pnas.87.7.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, Yan J, Zhou P, Li J, Gao H, Xia Y, Wang Q. Neurotransmitter receptors and cognitive dysfunction in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol. 2012;97:1–13. doi: 10.1016/j.pneurobio.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowen DM, Smith CB, White P, Davison AN. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976;99:459–96. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- 24.Perry EK, Gibson PH, Blessed G, Perry RH, Tomlinson BE. Neurotransmitter enzyme abnormalities in senile dementia. Choline acetyltransferase and glutamic acid decarboxylase activities in necropsy brain tissue. J Neurol Sci. 1977;34:247–65. doi: 10.1016/0022-510x(77)90073-9. [DOI] [PubMed] [Google Scholar]
- 25.Perry EK, Perry RH, Blessed G, Tomlinson BE. Necropsy evidence of central cholinergic deficits in senile dementia. Lancet. 1977;1:189. doi: 10.1016/s0140-6736(77)91780-9. [DOI] [PubMed] [Google Scholar]
- 26.Mufson EJ, Counts SE, Fahnestock M, Ginsberg SD. Cholinotrophic molecular substrates of mild cognitive impairment in the elderly. Curr Alzheimer Res. 2007;4:340–350. doi: 10.2174/156720507781788855. [DOI] [PubMed] [Google Scholar]
- 27.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 28.Bierer LM, Haroutunian V, Gabriel S, Knott PJ, Carlin LS, Purohit DP, Perl DP, Schmeidler J, Kanof P, Davis KL. Neurochemical correlates of dementia severity in Alzheimer’s disease: relative importance of the cholinergic deficits. J Neurochem. 1995;64:749–760. doi: 10.1046/j.1471-4159.1995.64020749.x. [DOI] [PubMed] [Google Scholar]
- 29.DeKosky ST, Harbaugh RE, Schmitt FA, Bakay RA, Chui HC, Knopman DS, Reeder TM, Shetter AG, Senter HJ, Markesbery WR. Cortical biopsyin Alzheimer’s disease: diagnostic accuracy and neurochemical, neuropathological, and cognitive correlations Intraventricular Bethanecol Study Group. Ann Neurol. 1992;32:625–632. doi: 10.1002/ana.410320505. [DOI] [PubMed] [Google Scholar]
- 30.Mesulam M. The cholinergic lesion of Alzheimer’s disease: pivotal factor or side show? Learning & Memory. 2004;11(1):43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- 31.Shinotoh H, Namba H, Fukushi K, Nagatsuka S, Tanaka N, Aotsuka A, Ota T, Tanada S, Irie T. Progressive loss of cortical acetylcholinesterase activity in association with cognitive decline in Alzheimer’s disease: a positron emission tomography study. Ann Neurol. 2000;48:194–200. [PubMed] [Google Scholar]
- 32.Tiraboschi P, Hansen LA, Alford M, Masliah E, Thal LJ, Corey-Bloom J. The decline in synapses and cholinergic activity is asynchronous in Alzheimer’s disease. Neurology. 2000;55:1278–83. doi: 10.1212/wnl.55.9.1278. [DOI] [PubMed] [Google Scholar]
- 33.Gilmor ML, Erickson JD, Varoqui H, Hersh LB, Bennett DA, Cochran EJ, Mufson EJ, Levey AI. Preservation of nucleus basalis neurons containing choline acetyltransferase and the vesicular acetylcholine transporter in the elderly with mild cognitive impairment and early Alzheimer’s disease. J Comp Neurol. 1999;411:693–704. [PubMed] [Google Scholar]
- 34.Mohs RC, Doody RS, Morris JC, Ieni JR, Rogers SL, Perdomo CA, Pratt RD. “312” Study Group, A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology. 2001;57:481–488. doi: 10.1212/wnl.57.3.481. [DOI] [PubMed] [Google Scholar]
- 35.Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, Wetterholm AL, Zhang R, Haglund A, Subbiah P. Donepezil Nordic Study Group, A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489–495. doi: 10.1212/wnl.57.3.489. [DOI] [PubMed] [Google Scholar]
- 36.Parent MB, Baxter MG. Septohippocampal acetylcholine: involved in but not necessary for learning and memory? Learn Mem. 2004;11:9–20. doi: 10.1101/lm.69104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arendt T, Bigl V, Arendt A, Tennstedt A. Loss of neurons in the nucleus basalis of Meynert in Alzheimer’s disease, paralysis agitans and Korsakoff’s disease. Acta Neuropathol (Berl) 1983;61:101–8. doi: 10.1007/BF00697388. [DOI] [PubMed] [Google Scholar]
- 38.Jellinger KA. Morphological substrates of mental dysfunctions in Lewy body disease: an update. J Neural Transm Suppl. 2000;59:185–212. doi: 10.1007/978-3-7091-6781-6_21. [DOI] [PubMed] [Google Scholar]
- 39.Whitehouse PJ, Hedreen JC, White CL, 3rd, Price DL. Basal forebrain neurons in the dementia of Parkinson disease. Ann Neurol. 1983;13:243–8. doi: 10.1002/ana.410130304. [DOI] [PubMed] [Google Scholar]
- 40.Arendt T, Bigl V, Arendt A. Neuron loss in the nucleus basalis of Meynert in Creutzfeldt–Jakob disease. Acta Neuropathol (Berl) 1984;65:85–8. doi: 10.1007/BF00689832. [DOI] [PubMed] [Google Scholar]
- 41.Arendt T. Impairment in memory function and neurodegenerative changes in the cholinergic basal forebrain system induced by chronic intake of ethanol. J Neural Transm Suppl. 1994;44:173–87. doi: 10.1007/978-3-7091-9350-1_13. [DOI] [PubMed] [Google Scholar]
- 42.Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, Mathis CA, Moore RY, DeKosky ST. Cortical cholinergic function is more severely affected in Parkinsonian dementia than in Alzheimer’s disease: an in vivo positron tomography study. Arch Neurol. 2003;60:1745–8. doi: 10.1001/archneur.60.12.1745. [DOI] [PubMed] [Google Scholar]
- 43.Terry AV, Jr, Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther. 2003;306:821–7. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- 44.Salmond CH, Chatfield DA, Menon DK, Pickard JD, Sahakian BJ. Cognitive sequelae of head injury: involvement of basal forebrain and associated structures. Brain. 2005;128(Pt 1):189–200. doi: 10.1093/brain/awh352. [DOI] [PubMed] [Google Scholar]
- 45.Donat CK, Walter B, Kayser T, Deuther-Conrad W, Schliebs R, Nieber K, Bauer R, Härtig W, Brust P. Effects of lateral fluid percussion injury on cholinergic markers in the newborn piglet brain. Int J Dev Neurosci. 2010;28:31–8. doi: 10.1016/j.ijdevneu.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Craig LA, Hong NS, McDonald RJ. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neuroscience & Biobehavioral Reviews. 2011;35(6):1397–1409. doi: 10.1016/j.neubiorev.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behavioural brain research. 2011;221(2):555–563. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 48.Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot BD. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. J Neurosci. 2007;27:9835–9845. doi: 10.1523/JNEUROSCI.5494-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Revett TJ, Baker GB, Jhamandas J, Kar S. Glutamate system, amyloid ss peptides and tau protein: functional interrelationships and relevance to Alzheimer disease pathology. J Psychiatry Neurosci. 2013;38:6–23. doi: 10.1503/jpn.110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bell KF, Bennett DA, Cuello AC. Paradoxical upregulation of glutamatergic presynaptic boutons during mild cognitive impairment. J Neurosci. 2007;27:10810–10817. doi: 10.1523/JNEUROSCI.3269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mota SI, Ferreira IL, Rego AC. Dysfunctional synapse in Alzheimer’s disease–A focus on NMDA receptors. Neuropharmacology. 2014;76:16–26. doi: 10.1016/j.neuropharm.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 52.Lanctôt KL, Herrmann N, Mazzotta P, Khan LR, Ingber N. GABAergic function in Alzheimer’s disease: evidence for dysfunction and potential as a therapeutic target for the treatment of behavioural and psychological symptoms of dementia. Can J Psychiatry. 2004;49:439–453. doi: 10.1177/070674370404900705. [DOI] [PubMed] [Google Scholar]
- 53.Saransaari P, Oja SS. Taurine release from the developing and ageing hippocampus: stimulation by agonists of ionotropic glutamate receptors. Mech Ageing Dev. 1997;99:219–232. doi: 10.1016/s0047-6374(97)00101-2. [DOI] [PubMed] [Google Scholar]
- 54.Vignes M. Regulation of spontaneous inhibitory synaptic transmission by endogenous glutamate via non-NMDA receptors in cultured rat hippocampal neurons. Neuropharmacology. 2001;40:737–748. doi: 10.1016/s0028-3908(00)00213-6. [DOI] [PubMed] [Google Scholar]
- 55.Lagostena L, Rosato-Siri M, D’Onofrio M, Brandi R, Arisi I, Capsoni S, Franzot J, Cattaneo A, Cherubini E. In the adult hippocampus, chronic nerve growth factor deprivation shifts GABAergic signaling from the hyperpolarizing to the depolarizing direction. J Neurosci. 2010;30:885–893. doi: 10.1523/JNEUROSCI.3326-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology. 2014;76:27–50. doi: 10.1016/j.neuropharm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Collingridge GL, Isaac JTR, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 58.Paula-Lima AC, Louzada PR, De Mello FG, Ferreira ST. Neuroprotection against Abeta and glutamate toxicity by melatonin: are GABA receptors involved? Neurotox Res. 2003;5:323–327. doi: 10.1007/BF03033152. [DOI] [PubMed] [Google Scholar]
- 59.Paula-Lima AC, De Felice FG, Brito-Moreira J, Ferreira ST. Activation of GABA(A) receptors by taurine and muscimol blocks the neurotoxicity of beta-amyloid in rat hippocampal and cortical neurons. Neuropharmacology. 2005;49:1140–1148. doi: 10.1016/j.neuropharm.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 60.Louzada PR, Paula Lima AC, Mendonca-Silva DL, No€el F, De Mello FG, Ferreira ST. Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer’s disease and other neurological disorders. FASEB J. 2004;18:511–518. doi: 10.1096/fj.03-0739com. [DOI] [PubMed] [Google Scholar]
- 61.Möhler H. The rise of a new GABA pharmacology. Neuropharmacology. 2011;60:1042–1049. doi: 10.1016/j.neuropharm.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 62.Wallace SJ. Newer antiepileptic drugs: advantages and disadvantages. Brain Dev. 2001;23:277–283. doi: 10.1016/s0387-7604(01)00230-3. [DOI] [PubMed] [Google Scholar]
- 63.Paula-Lima AC, Brito-Moreira J, Ferreira ST. Deregulation of excitatory neurotransmission underlying synapse failure in Alzheimer’s disease. Journal of neurochemistry. 2013;126(2):191–202. doi: 10.1111/jnc.12304. [DOI] [PubMed] [Google Scholar]
- 64.Simic G, Stanic G, Mladinov M, Jovanov-Milosevic N, Kostovic I, Hof PR. Does Alzheimer’s disease begin in the brainstem? Neuropathology and applied neurobiology. 2009;35(6):532–554. doi: 10.1111/j.1365-2990.2009.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reynolds GP, Mason SL, Meldrum A, De Keczer S, Parnes H, Eglen RM, Wong EH. 5-Hydroxytryptamine (5-HT)4 receptors in post mortem human brain tissue: distribution, pharmacology and effects of neurodegenerative diseases. Br J Pharmacol. 1995;114:993–998. doi: 10.1111/j.1476-5381.1995.tb13303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cifariello A, Pompili A, Gasbarri A. 5-HT(7) receptors in the modulation of cognitive processes. Behav Brain Res. 2008;195:171–179. doi: 10.1016/j.bbr.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 67.King MV, Marsden CA, Fone KC. A role for the 5-HT(1A), 5-HT4 and 5-HT6 receptors in learning and memory. Trends Pharmacol Sci. 2008;29:482–492. doi: 10.1016/j.tips.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 68.Zweig RM, Ross CA, Hedreen JC, Steele C, Cardillo JE, Whitehouse PJ, Folstein MF, Price DL. The neuropathology of aminergic nuclei in Alzheimer’s disease. Ann Neurol. 1988;24:233–242. doi: 10.1002/ana.410240210. [DOI] [PubMed] [Google Scholar]
- 69.Baker GB, Reynolds GP. Biogenic amines and their metabolites in Alzheimer’s disease: noradrenaline, 5-hydroxytryptamine and 5-hydroxyindole-3-acetic acid depleted in hippocampus but not in substantia innominata. Neurosci Lett. 1989;100:335–339. doi: 10.1016/0304-3940(89)90709-x. [DOI] [PubMed] [Google Scholar]
- 70.Nazarali AJ, Reynolds GP. Monoamine neurotransmitters and their metabolites in brain regions in Alzheimer’s disease: a postmortem study. Cell Mol Neurobiol. 1992;12:581–587. doi: 10.1007/BF00711237. [DOI] [PubMed] [Google Scholar]
- 71.Palmer AM, Francis PT, Benton JS, Sims NR, Mann DM, Neary D, Snowden JS, Bowen DM. Presynaptic serotonergic dysfunction in patients with Alzheimer’s disease. J Neurochem. 1987;48:8–15. doi: 10.1111/j.1471-4159.1987.tb13120.x. [DOI] [PubMed] [Google Scholar]
- 72.Palmer AM, Stratmann GC, Procter AW, Bowen DM. Possible neuro-transmitter basis of behavioral changes in Alzheimer’s disease. Ann Neurol. 1988;23:616–620. doi: 10.1002/ana.410230616. [DOI] [PubMed] [Google Scholar]
- 73.D’Amato RJ, Zweig RM, Whitehouse PJ, Wenk GL, Singer HS, Mayeux R, Price DL, Snyder SH. Aminergic systems in Alzheimer’s disease and Parkinson’s disease. Ann Neurol. 1987;22:229–236. doi: 10.1002/ana.410220207. [DOI] [PubMed] [Google Scholar]
- 74.Bowen DM, Allen SJ, Benton JS, Goodhardt MJ, Haan EA, Palmer AM, Sims NR, Smith CC, Spillane JA, Esiri MM, Neary D, Snowdon JS, Wilcock GK, Davison AN. Biochemical assessment of serotonergic and cholin-ergic dysfunction and cerebral atrophy in Alzheimer’s disease. J Neurochem. 1983;41:266–272. doi: 10.1111/j.1471-4159.1983.tb11838.x. [DOI] [PubMed] [Google Scholar]
- 75.Cross AJ, Crow TJ, Johnson JA, Joseph MH, Perry EK, Perry RH, Blessed G, Tomlinson BE. Monoamine metabolism in senile dementia of Alzheimertype. J Neurol Sci. 1983;60:383–392. doi: 10.1016/0022-510x(83)90149-1. [DOI] [PubMed] [Google Scholar]
- 76.Volicer L, Langlais PJ, Matson WR, Mark KA, Gamache PH. Serotoninergic system in dementia of the Alzheimer type. Abnormal forms of5-hydroxytryptophan and serotonin in cerebrospinal fluid. Arch Neurol. 1985;42:1158–1161. doi: 10.1001/archneur.1985.04060110040013. [DOI] [PubMed] [Google Scholar]
- 77.Sparks DL. Aging and Alzheimer’s disease Altered cortical serotonergic binding. Arch Neurol. 1989;46:138–140. doi: 10.1001/archneur.1989.00520380038010. [DOI] [PubMed] [Google Scholar]
- 78.Bowen DM, White P, Spillane JA, Goodhardt MJ, Curzon G, Iwangoff P, Meier-Ruge W, Davison AN. Accelerated ageing or selective neuronal loss asan important cause of dementia? Lancet. 1979;1:11–14. doi: 10.1016/s0140-6736(79)90454-9. [DOI] [PubMed] [Google Scholar]
- 79.Hirao K, Pontone GM, Smith GS. Molecular imaging of neuropsychiatric symptoms in Alzheimer’s and Parkinson’s disease. Neuroscience & Biobehavioral Reviews. 2015;49:157–170. doi: 10.1016/j.neubiorev.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodríguez JJ, Noristani HN, Verkhratsky A. The serotonergic system in ageing and Alzheimer’s disease. Progress in neurobiology. 2012;99(1):15–41. doi: 10.1016/j.pneurobio.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 81.Palmer AM. Neurochemical studies of Alzheimer’s disease. Neurodegeneration. 1996;5:381–391. doi: 10.1006/neur.1996.0051. [DOI] [PubMed] [Google Scholar]
- 82.Trillo L, Das D, Hsieh W, Medina B, Moghadam S, Lin B, Dang V, Sanchez MM, De Miguel Z, Ashford JW, Salehi A. Ascending monoaminergic systems alterations in Alzheimer’s disease. Translating basic science into clinical care. Neurosci Biobehav Rev. 2013;37:1363–1379. doi: 10.1016/j.neubiorev.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 83.Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta neuropathologica. 2011;121(2):171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 84.Berridge CW. Noradrenergic modulation of arousal. Brain research reviews. 2008;58(1):1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chalermpalanupap T, Kinkead B, Hu WT, Kummer MP, Hammerschmidt T, Heneka MT, Weinshenker D, Levey AI. Targeting norepinephrine in mild cognitive impairment and Alzheimer’s disease. Alzheimers Res Ther. 2013;5(2):21. doi: 10.1186/alzrt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoe HS, Lee HK, Pak DT. The upside of APP at synapses. CNS neuroscience & therapeutics. 2012;18(1):47–56. doi: 10.1111/j.1755-5949.2010.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Esch F, Keim P, Beattie E, Blacher R, Culwell A, Oltersdorf T, McClure D, Ward P. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990;248:1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- 88.Kang J, Lemaire H-G, Unterbeck A, Salbaum JM, Masters CL, Grzeschik K-H, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 89.Selkoe DJ. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol. 1998;8:447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- 90.Alzheimer Alois. Über eine eigenartige Erkrankung der Hirnrinde [About a peculiar disease of the cerebral cortex] Allgemeine Zeitschrift fur Psychiatrie und Psychisch-Gerichtlich Medizin. 1907;64(1–2):146–148. (German) [Google Scholar]
- 91.Chapman PF, Falinska AM, Knevett SG, Ramsay MF. Genes, models and Alzheimer’s disease. Trends Genet. 2001;17:254–261. doi: 10.1016/s0168-9525(01)02285-5. [DOI] [PubMed] [Google Scholar]
- 92.Ashe KH. Learning and memory in transgenic mice modeling Alzheimer’s disease. Learn Mem. 2001;8:301–308. doi: 10.1101/lm.43701. [DOI] [PubMed] [Google Scholar]
- 93.Janus C, Westaway D. Transgenic mouse models of Alzheimer’s disease. Physiol Behav. 2001;73:873–886. doi: 10.1016/s0031-9384(01)00524-8. [DOI] [PubMed] [Google Scholar]
- 94.Zahs KR, Ashe KH. ‘Too much good news’ - are Alzheimer mouse models trying to tell us how to prevent, not cure, Alzheimer’s disease? Trends Neurosci. 2010;33:381–389. doi: 10.1016/j.tins.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 95.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 96.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 97.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- 100.Hardy JA, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 101.Klein WL. Abeta toxicity in Alzheimer’s disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 102.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 103.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 104.Venkitaramani DV, Chin J, Netzer WJ, Gouras GK, Lesne S, Malinow R, Lombroso PJ. β-amyloid modulation of synaptic transmission and plasticity. The Journal of Neuroscience. 2007;27(44):11832–11837. doi: 10.1523/JNEUROSCI.3478-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ma T, Klann E. Amyloid β: linking synaptic plasticity failure to memory disruption in Alzheimer’s disease. Journal of neurochemistry. 2012;120(s1):140–148. doi: 10.1111/j.1471-4159.2011.07506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cleary J, Hittner JM, Semotuk M, Mantyh P, O’Hare E. Beta-amyloid(1–40) effects on behavior and memory. Brain Res. 1995;682:69–74. doi: 10.1016/0006-8993(95)00323-i. [DOI] [PubMed] [Google Scholar]
- 107.McDonald M, Dahl E, Overmier J, Mantyh P, Cleary J. Effects of an exogenous beta-amyloid peptide on retention for spatial learning. Behav Neural Biol. 1994;62:60–67. doi: 10.1016/s0163-1047(05)80059-7. [DOI] [PubMed] [Google Scholar]
- 108.Stéphan A, Laroche S, Davis S. Generation of aggregated beta-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J Neurosci. 2001;21:5703–5714. doi: 10.1523/JNEUROSCI.21-15-05703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 110.Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 111.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klyubin I, Cullen WK, Hu NW, Rowan MJ. Alzheimer’s disease Aβ assemblies mediating rapid disruption of synaptic plasticity and memory. Molecular brain. 2012;5(1):1. doi: 10.1186/1756-6606-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 114.Larson J, Lynch G, Games D, Seubert P. Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res. 1999;840:23–35. doi: 10.1016/s0006-8993(99)01698-4. [DOI] [PubMed] [Google Scholar]
- 115.Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Abeta accumulation and origin of plaques in Alzheimer’s disease. Neurobiol Aging. 2005;26:1235–1244. doi: 10.1016/j.neurobiolaging.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 116.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 117.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003;24:1063–70. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 118.Echeverria V, Ducatenzeiler A, Alhonen L, Janne J, Grant SM, Wandosell F, Muro A, Baralle F, Li H, Duff K, Szyf M, Cuello AC. Rat transgenic models with a phenotype of intracellular Abeta accumulation in hippocampus and cortex. J Alzheimers Dis. 2004;6:209–219. doi: 10.3233/jad-2004-6301. [DOI] [PubMed] [Google Scholar]
- 119.Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 120.Knobloch M, Konietzko U, Krebs DC, Nitsch RM. Intracellular Abeta and cognitive deficits precede beta-amyloid deposition in transgenic arc Abeta mice. Neurobiol Aging. 2007;28:1297–1306. doi: 10.1016/j.neurobiolaging.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 121.Takahashi RH, Almeida CG, Kearney PF, Yu F, Lin MT, Milner TA, Gouras GK. Oligomerization of Alzheimer’s β-amyloid within processes and synapses of cultured neurons and brain. J Neurosci. 2004;24:3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 123.Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- 125.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010;13:190–196. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moreno H, Yu E, Pigino G, Hernandez AI, Kim N, Moreira JE, Sugimori M, Llinas RR. Synaptic transmission block by presynaptic injection of oligomeric amyloid beta. Proc Natl Acad Sci U S A. 2009;106:5901–5906. doi: 10.1073/pnas.0900944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Parihar MS, Brewer GJ. Amyloid-β as a modulator of synaptic plasticity. Journal of Alzheimer’s Disease. 2010;22(3):741–763. doi: 10.3233/JAD-2010-101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cirrito JR, Kang J-E, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 133.Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Laurén J, Gimbel ZA, Strittmatter SM. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci. 2010;30:6367–74. doi: 10.1523/JNEUROSCI.0395-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chung E, Ji Y, Sun Y, Kascsak RJ, Kascsak RB, Mehta PD, Strittmatter SM, Wisniewski T. Anti-PrPC monoclonal antibody infusion as a novel treatment for cognitive deficits in an Alzheimer’s disease model mouse. BMC Neurosci. 2010;11:130. doi: 10.1186/1471-2202-11-130. http://dx.doi.org/10.1186/1471-2202-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Um JW, Nygaard HB, Heiss JK, Kostylev MA, Stagi M, Vortmeyer A, Wisniewski T, Gunther EC, Strittmatter SM. Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat Neurosci. 2012;15:1227–35. doi: 10.1038/nn.3178. http://dx.doi.org/10.1038/nn.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Collins MO, Husi H, Yu L, Brandon JM, Anderson CN, Blackstock WP, Choudhary JS, Grant SG. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J Neurochem. 2006;97(Suppl 1):16–23. doi: 10.1111/j.1471-4159.2005.03507.x. http://dx.doi.org/10.1111/j.1471-4159.2005.03507.x. [DOI] [PubMed] [Google Scholar]
- 137.Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–32. doi: 10.1038/nature07761. http://dx.doi.org/10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 139.Um JW, Strittmatter SM. Amyloid-β induced signaling by cellular prion protein and Fyn kinase in Alzheimer disease. Prion. 2013;7(1):37–41. doi: 10.4161/pri.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lorenzo A, Yuan M, Zhang Z, Paganetti PA, Sturchler-Pierrat C, Staufenbiel M, Mautino J, Vigo FS, Sommer B, Yankner BA. Amyloid beta interacts with the amyloid precursor protein: a potential toxic mechanism in Alzheimer’s disease. Nat Neurosci. 2000;3:460–464. doi: 10.1038/74833. [DOI] [PubMed] [Google Scholar]
- 141.Melchor JP, Van Nostrand WE. Fibrillar amyloid beta-protein mediates the pathologic accumulation of its secreted precursor in human cerebrovascular smooth muscle cells. J Biol Chem. 2000;275:9782–9791. doi: 10.1074/jbc.275.13.9782. [DOI] [PubMed] [Google Scholar]
- 142.Wagner MR, Keane DM, Melchor JP, Auspaker KR, Van Nostrand WE. Fibrillar amyloid beta-protein binds protease nexin-2/amyloid beta-protein precursor: stimulation of its inhibition of coagulation factor XIa. Biochemistry. 2000;39:7420–7427. doi: 10.1021/bi0002840. [DOI] [PubMed] [Google Scholar]
- 143.Rozemuller AJ, Roos RA, Bots GT, Kamphorst W, Eikelenboom P, Van Nostrand WE. Distribution of beta/A4 protein and amyloid precursor protein in hereditary cerebral hemorrhage with amyloidosis-Dutch type and Alzheimer’s disease. Am J Pathol. 1993;142:1449–1457. [PMC free article] [PubMed] [Google Scholar]
- 144.Bignante EA, Heredia F, Morfini G, Lorenzo A. Amyloid β precursor protein as a molecular target for amyloid β–induced neuronal degeneration in Alzheimer’s disease. Neurobiology of aging. 2013;34(11):2525–2537. doi: 10.1016/j.neurobiolaging.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene. 2006;25:6423–6435. doi: 10.1038/sj.onc.1209887. [DOI] [PubMed] [Google Scholar]
- 146.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 147.Yang Q, Guan K-L. Expanding mTOR signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 148.Hoeffer CA, Cowansage KK, Arnold EC, Banko JL, Moerke NJ, Rodriguez R, Schmidt EK, Klosi E, Chorev M, Lloyd RE, Pierre P, Wagner G, LeDoux JE, Klann E. Inhibition of the interactions between eukaryotic initiation factors 4E and 4G impairs long-term associative memory consolidation but not reconsolidation. Proc Natl Acad Sci U S A. 2011;108:3383–3388. doi: 10.1073/pnas.1013063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Richter JD, Klann E. Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev. 2009;23:1–11. doi: 10.1101/gad.1735809. [DOI] [PubMed] [Google Scholar]
- 150.Antion MD, Merhav M, Hoeffer CA, Reis G, Kozma SC, Thomas G, Schuman EM, Rosenblum K, Klann E. Removal of S6K1 and S6K2 leads to divergent alterations in learning, memory, and synaptic plasticity. Learn Mem. 2008;15:29–38. doi: 10.1101/lm.661908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Banko JL, Merhav M, Stern E, Sonenberg N, Rosenblum K, Klann E. Behavioral alterations in mice lacking the translation repressor 4E-BP2. Neurobiol Learn Mem. 2007;87:248–256. doi: 10.1016/j.nlm.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 152.Banko JL, Poulin F, Hou L, DeMaria CT, Sonenberg N, Klann E. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J Neurosci. 2005;25(42):9581–9590. doi: 10.1523/JNEUROSCI.2423-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blundell J, Kouser M, Powell CM. Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem. 2008;90:28–35. doi: 10.1016/j.nlm.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hoeffer CA, Klann E. mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gafford GM, Parsons RG, Helmstetter FJ. Consolidation and reconsolidation of contextual fear memory requires mammalian target of rapamycin-dependent translation in the dorsal hippocampus. Neuroscience. 2011;182:98–104. doi: 10.1016/j.neuroscience.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stoica L, Zhu PJ, Huang W, Zhou H, Kozma SC, Costa-Mattioli M. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc Natl Acad Sci U S A. 2011;108:3791–3796. doi: 10.1073/pnas.1014715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lafay-Chebassier C, Paccalin M, Page G, Barc-Pain S, Perault-Pochat MC, Gil R, Pradier L, Hugon J. mTOR/p70S6k signaling alteration by Abeta exposure as well as in APP-PS1 transgenic models and in patients with Alzheimer’s disease. J Neurochem. 2005;94:215–225. doi: 10.1111/j.1471-4159.2005.03187.x. [DOI] [PubMed] [Google Scholar]
- 159.Lafay-Chebassier C, Pérault-Pochat MC, Page G, Bilan AR, Damjanac M, Pain S, Houeto J-L, Gil R, Hugon J. The immunosuppressant rapamycin exacerbates neurotoxicity of Abeta peptide. J Neurosci Res. 2006;84:1323–1334. doi: 10.1002/jnr.21039. [DOI] [PubMed] [Google Scholar]
- 160.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stem D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 161.Du Yan S, Zhu H, Fu J, Yan SF, Roher A, Tourtellotte WW, Rajavashisth T, Chen X, Godman GC, Stem D, Schmidt AM. Amyloid-beta peptide-receptor for advanced glycation end product interaction elicits neuronal expression of macrophage-colony stimulating factor: a proinflammatory pathway in Alzheimer disease. Proc Natl Acad Sci USA. 1997;94:5296–5301. doi: 10.1073/pnas.94.10.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hadding A, Kaltschmidt B, Kaltschmidt C. Overexpression of receptor of advanced glycation end products hypersensitizes cells for amyloid beta peptide-induced cell death. Biochim Biophys Acta. 2004;1691:67–72. doi: 10.1016/j.bbamcr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 163.Onyango IG, Tuttle JB, Bennett JP., Jr Altered intracellular signaling and reduced viability of Alzheimer’s disease neuronal cybrids is reproduced by beta-amyloid peptide acting through receptor for advanced glycation end products (RAGE) Mol Cell Neurosci. 2005;29:333–343. doi: 10.1016/j.mcn.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 164.Origlia N, Capsoni S, Cattaneo A, Fang F, Arancio O, Yan SD, Domenici L. Abeta-dependent Inhibition of LTP in different intracortical circuits of the visual cortex: the role of RAGE. J Alzheimers Dis. 2009;17:59–68. doi: 10.3233/JAD-2009-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, Puzzo D, Liu S, Hegde A, Yan SF, Stem A, Luddy JS, Lue LF, Walker DG, Roher A, Buttini M, Mucke L, Li W, Schmidt AM, Kindy M, Hyslop PA, Stern DM, Du Yan SS. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. Embo J. 2004;23:4096–4105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Origlia N, Righi M, Capsoni S, Cattaneo A, Fang F, Stern DM, Chen JX, Schmidt AM, Arancio O, Yan SD, Domenici L. Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-beta-mediated cortical synaptic dysfunction. J Neurosci. 2008;25:3521–3530. doi: 10.1523/JNEUROSCI.0204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Origlia N, Bonadonna C, Rosellini A, Leznik E, Arancio O, Yan SS, Domenici L. Microglial Receptor for Advanced Glycation End Product-Dependent Signal Pathway Drives {beta}-Amyloid-Induced Synaptic Depression and Long-Term Depression Impairment in Entorhinal Cortex. J Neurosci. 2010;30:11414–11425. doi: 10.1523/JNEUROSCI.2127-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nature medicine. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 169.Kojima N, Ishibashi H, Obata K, Kandel ER. Higher seizure susceptibility and enhanced tyrosine phosphorylation on N-methyl-d-aspartate receptor subunit 2B in fyn transgenic mice. Learn Mem. 1998;5:429–445. [PMC free article] [PubMed] [Google Scholar]
- 170.Lu YF, Kojima N, Tomizawa K, Moriwaki A, Matsushita M, Obata K, Matsui H. Enhanced synaptic transmission and reduced threshold for LTP induction in fyn-transgenic mice. Eur J Neurosci. 1999;11:75–82. doi: 10.1046/j.1460-9568.1999.00407.x. [DOI] [PubMed] [Google Scholar]
- 171.Cui J, Matkovich SJ, deSouza N, Li S, Rosemblit N, Marks AR. Regulation of the type 1 inositol 1,4,5-trisphosphate receptor by phosphorylation at tyrosine 353. J Biol Chem. 2004;279:16311–16316. doi: 10.1074/jbc.M400206200. [DOI] [PubMed] [Google Scholar]
- 172.Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 173.Palop JJ, Jones B, Kekonius L, Chin J, Yu G-Q, Raber J, Masliah E, Mucke L. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc Natl Acad Sci USA. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chin J, Palop JJ, Yu G-Q, Kojima N, Masliah E, Mucke L. Fyn kinase modulates synaptotoxicity, but not aberrant sprouting, in human amyloid precursor protein transgenic mice. J Neurosci. 2004;24:4692–4697. doi: 10.1523/JNEUROSCI.0277-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chin J, Palop JJ, Puoliväli J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E, Mucke L. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2005;25:9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Palop JJ, Chin J, Bien-Ly N, Massaro C, Yeung BZ, Yu G-Q, Mucke L. Vulnerability of dentate granule cells to disruption of Arc expression in human amyloid precursor protein transgenic mice. J Neurosci. 2005;25:9686–9693. doi: 10.1523/JNEUROSCI.2829-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Phiel CJ, Wilson CA, Lee VM-Y, Klein PS. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 179.Sun X, Sato S, Murayama O, Murayama M, Park J, Yamaguchi H, Takashima A. Lithium inhibits amyloid secretion in COS7 cells transfected with amyloid precursor protein C100. Neurosci Lett. 2002;321:61–64. doi: 10.1016/s0304-3940(01)02583-6. [DOI] [PubMed] [Google Scholar]
- 180.Su Y, Ryder J, Li B, Wu X, Fox N, Solenberg P, Brune K, Paul S, Zhou Y, Liu F, Ni B. Lithium, a common drug for bipolar disorder treatment, regulates amyloid-beta precursor protein processing. Biochemistry. 2004;43:6899–6908. doi: 10.1021/bi035627j. [DOI] [PubMed] [Google Scholar]
- 181.Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo S-C, Bru-Mercier G, Dickinson B, Scullion S, Sheng M, Collingridge G, Cho K. Aβ(1–42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3β. Nat Neurosci. 2011;14:545–547. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- 182.Lee H-K, Kumar P, Fu Q, Rosen KM, Querfurth HW. The insulin/Akt signaling pathway is targeted by intracellular beta-amyloid. Mol Biol Cell. 2009;20:1533–1544. doi: 10.1091/mbc.E08-07-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Magrané J, Rosen KM, Smith RC, Walsh K, Gouras GK, Querfurth HW. Intraneuronal beta-amyloid expression downregulates the Akt survival pathway and blunts the stress response. J Neurosci. 2005;25(47):10960–10969. doi: 10.1523/JNEUROSCI.1723-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Peineau S, Bradley C, Taghibiglou C, Doherty A, Bortolotto ZA, Wang YT, Collingridge GL. The role of GSK-3 in synaptic plasticity. Br J Pharmacol. 2008;153:S428–437. doi: 10.1038/bjp.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Terwel D, Muyllaert D, Dewachter I, Borghgraef P, Croes S, Devijver H, Leuven FV. Amyloid activates GSK-3beta to aggravate neuronal tauopathy in bigenic mice. Am J Pathol. 2008;172:786–798. doi: 10.2353/ajpath.2008.070904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron 2007. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 187.Ma T, Hoeffer CA, Capetillo-Zarate E, Yu F, Wong H, Lin MT, Tampellini D, Klann E, Blitzer RD, Gouras GK. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e12845. doi: 10.1371/journal.pone.0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rowan MJ, Klyubin I, Wang Q, Anwyl R. Synaptic plasticity disruption by amyloid beta protein: modulation by potential Alzheimer’s disease modifying therapies. Biochem Soc Trans. 2005;33:563–567. doi: 10.1042/BST0330563. [DOI] [PubMed] [Google Scholar]
- 189.Jacobsen JS, Wu C-C, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 191.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 192.Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. doi: 10.1016/0166-2236(93)90081-v. [DOI] [PubMed] [Google Scholar]
- 193.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 194.Stanton PK. LTD, LTP, and the sliding threshold for long-term synaptic plasticity. Hippocampus. 1996;6:35–42. doi: 10.1002/(SICI)1098-1063(1996)6:1<35::AID-HIPO7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 195.Cheng L, Yin WJ, Zhang JF, Qi JS. Amyloid beta-protein fragments 25–35 and 31–35 potentiate long-term depression in hippocampal CA1 region of rats in vivo. Synapse. 2009;63:206–214. doi: 10.1002/syn.20599. [DOI] [PubMed] [Google Scholar]
- 196.Kim J-H, Anwyl R, Suh Y-H, Djamgoz MBA, Rowan MJ. Use-dependent effects of amyloidogenic fragments of (beta)-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. J Neurosci. 2001;21:1327–1333. doi: 10.1523/JNEUROSCI.21-04-01327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Raymond CR, Ireland DR, Abraham WC. NMDA receptor regulation by amyloid-beta does not account for its inhibition of LTP in rat hippocampus. Brain Res. 2003;968:263–272. doi: 10.1016/s0006-8993(03)02269-8. [DOI] [PubMed] [Google Scholar]
- 199.Wang H-W, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, Viola KL, Klein WL, Stine WB, Krafft GA, Trommer BL. Soluble oligomers of beta amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924:133–140. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- 200.Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52(5):831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Arispe N, Rojas E, Pollard HB. Alzheimer disease amyloid β protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(2):567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT. Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418(6895):291. doi: 10.1038/418291a. article 291. [DOI] [PubMed] [Google Scholar]
- 203.Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- 204.Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 1990;40:1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- 207.Maurer I, Zierz S, Möller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 208.Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer’s. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- 209.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Calkins MJ, Reddy PH. Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer’s disease neurons. Biochim Biophys Acta. 2011;1812:507–513. doi: 10.1016/j.bbadis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2011;20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimers Dis. 2010;20:609–631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci USA. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang X, Perry G, Smith MA, Zhu X. Amyloid-beta-derived diffusible ligands cause impaired axonal transport of mitochondria in neurons. Neurodegener Dis. 2010;7:56–59. doi: 10.1159/000283484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Diana A, Simic G, Sinforiani E, Orru N, Pichiri G, Bono G. Mitochondria morphology and DNA content upon sublethal exposure to beta-amyloid (1-42) peptide. Coll Antropol. 2008;32:51–58. [PubMed] [Google Scholar]
- 217.Schmidt C, Lepsverdize E, Chi SL, Das AM, Pizzo SV, Dityatev A, Schachner M. Amyloid precursor protein and amyloid beta-peptide bind to ATP synthase and regulate its activity at the surface of neural cells. Mol Psychiatry. 2008;13:953–969. doi: 10.1038/sj.mp.4002077. [DOI] [PubMed] [Google Scholar]
- 218.Matsumoto K, Akao Y, Yi H, Shamoto-Nagai M, Maruyama W, Naoi M. Overexpression of amyloid precursor protein induces susceptibility to oxidative stress in human neuroblastoma SH-SY5Y cells. J Neural Transm. 2006;113:125–135. doi: 10.1007/s00702-005-0318-0. [DOI] [PubMed] [Google Scholar]
- 219.Hodge T, Colombini M. Regulation of metabolite flux through voltage-gating of VDAC channels. J Membr Biol. 1997;157:271–279. doi: 10.1007/s002329900235. [DOI] [PubMed] [Google Scholar]
- 220.Xu X, Colombini M. Autodirected insertion: preinserted VDAC channels greatly shorten the delay to the insertion of new channels. Biophys J. 1997;72:2129–2136. doi: 10.1016/S0006-3495(97)78855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Rostovtseva T, Colombini M. VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. Biophys J. 1997;72:1954–1962. doi: 10.1016/S0006-3495(97)78841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Colombini M. VDAC structure, selectivity, and dynamics. Biochim Biophys Acta. 2012;1818:1457–1465. doi: 10.1016/j.bbamem.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Manczak M, Reddy PH. Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer’s disease. Human molecular genetics. 2012:dds360. doi: 10.1093/hmg/dds360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Quiroz-Baez R, Flores-Dominguez D, Arias C. Synaptic aging is associated with mitochondrial dysfunction, reduced antioxidant contents and increased vulnerability to amyloid-beta toxicity. Curr Alzheimer Res. 2013;10:324–331. doi: 10.2174/1567205011310030012. [DOI] [PubMed] [Google Scholar]
- 225.Esposito L, Raber J, Kekonius L, Yan F, Yu G-Q, Bien-Ly N, Puoliväli J, Scearce-Levie K, Masliah E, Mucke L. Reduction in mitochondrial superoxide dismutase modulates Alzheimer’s disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci. 2006;26:5167–5179. doi: 10.1523/JNEUROSCI.0482-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, Takahashi RH, Carlson GA, Beal MF, Lin MT, Gouras GK. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J Neurochem. 2004;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- 227.Dumont M, Wille E, Stack C, Calingasan NY, Beal MF, Lin MT. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer’s disease. FASEB J. 2009;23:2459–2466. doi: 10.1096/fj.09-132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Massaad CA, Washington TM, Pautler RG, Klann E. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106:13576–13581. doi: 10.1073/pnas.0902714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ma T, Hoeffer CA, Wong H, Massaad CA, Zhou P, Iadecola C, Murphy MP, Pautler RG, Klann E. Amyloid β-induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial superoxide. J Neurosci. 2011;31:5589–5595. doi: 10.1523/JNEUROSCI.6566-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hu D, Cao P, Thiels E, Chu CT, Wu G-Y, Oury TD, Klann E. Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase. Neurobiol Learn Mem. 2007;87:372–384. doi: 10.1016/j.nlm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2006;26:3933–3941. doi: 10.1523/JNEUROSCI.5566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kamsler A, Segal M. Paradoxical actions of hydrogen peroxide on long-term potentiation in transgenic superoxide dismutase-1 mice. J Neurosci. 2003;23:10359–10367. doi: 10.1523/JNEUROSCI.23-32-10359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Human molecular genetics. 2011;20(13):2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Heinitz K, Beck M, Schliebs R, Perez-Polo JR. Cholinotoxicity mediated by soluble oligomers of β-amyloid(1–42) differs from cholinotoxic effects of oxidative stress as revealed by gene expression analysis. J Neurochem. 2006;98:1930–45. doi: 10.1111/j.1471-4159.2006.04015.x. [DOI] [PubMed] [Google Scholar]
- 235.Nixon RA, Cataldo AM. Lysosomal system pathways: genes to neurodegeneration in Alzheimer’s disease. Journal of Alzheimer’s Disease. 2006;9(3 Supplement):277–289. doi: 10.3233/jad-2006-9s331. [DOI] [PubMed] [Google Scholar]
- 236.Fang F, Lue LF, Yan S, Xu H, Luddy JS, Chen D, Walker DG, Stern DM, Schmidt AM, Chen JX, Yan SS. RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. FASEB J. 2010;24:1043–1055. doi: 10.1096/fj.09-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Harper SJ, Wilkie N. MAPKs: new targets for neurodegeneration. Expert Opin Ther Targets. 2003;7:187–200. doi: 10.1517/14728222.7.2.187. [DOI] [PubMed] [Google Scholar]
- 238.Van Eldik LJ, Thompson WL, Ranaivo HR, Behanna HA, Watterson DM. Glia proinflammatory cytokine upregulation as a therapeutic target for neurodegenerative diseases: function-based and target-based discovery approaches. Int Rev Neurobiol. 2007;82:277–296. doi: 10.1016/S0074-7742(07)82015-0. [DOI] [PubMed] [Google Scholar]
- 239.Munoz L, Ranaivo HR, Roy SM, Hu W, Craft JM, McNamara LK, Chico LW, Van Eldik LJ, Watterson DM. A novel p38 alpha MAPK inhibitor suppresses brain proinflammatory cytokine up-regulation and attenuates synaptic dysfunction and behavioral deficits in an Alzheimer’s disease mouse model. J Neuroinflammation. 2007;4:21. doi: 10.1186/1742-2094-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lue LF, Walker DG, Rogers J. Modeling microglial activation in Alzheimer’s disease with human postmortem microglial cultures. Neurobiology of aging. 2001;22:945–956. doi: 10.1016/s0197-4580(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 241.Johnson GV, Bailey CD. The p38 MAP kinase signaling pathway in Alzheimer’s disease. Exp Neurol. 2003;183:263–268. doi: 10.1016/s0014-4886(03)00268-1. [DOI] [PubMed] [Google Scholar]
- 242.Hensley K, Floyd RA, Zheng NY, Nael R, Robinson KA, Nguyen X, Pye QN, Stewart CA, Geddes J, Markesbery WR, Patel E, Johnson GV, Bing G. p38 kinase is activated in the Alzheimer’s disease brain. J Neurochem. 1999;72:2053–2058. doi: 10.1046/j.1471-4159.1999.0722053.x. [DOI] [PubMed] [Google Scholar]
- 243.Dudal S, Krzywkowski P, Paquette J, Morissette C, Lacombe D, Tremblay P, Gervais F. Inflammation occurs early during the Abeta deposition process in TgCRND8 mice. Neurobiology of aging. 2004;25:861–871. doi: 10.1016/j.neurobiolaging.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 244.Britschgi M, Wyss-Coray T. Immune cells may fend off Alzheimer disease. Nat Med. 2007;13:408–409. doi: 10.1038/nm0407-408. [DOI] [PubMed] [Google Scholar]
- 245.Fan R, Xu F, Previti ML, Davis J, Grande AM, Robinson JK, Van Nostrand WE. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci. 2007;27:3057–3063. doi: 10.1523/JNEUROSCI.4371-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Walker DG, Lue LF. Investigations with cultured human microglia on pathogenic mechanisms of Alzheimer’s disease and other neurodegenerative diseases. Journal of neuroscience research. 2005;81:412–425. doi: 10.1002/jnr.20484. [DOI] [PubMed] [Google Scholar]
- 247.Morgan D, Gordon MN, Tan J, Wilcock D, Rojiani AM. Dynamic complexity of the microglial activation response in transgenic models of amyloid deposition: implications for Alzheimer therapeutics. J Neuropathol Exp Neurol. 2005;64:743–753. doi: 10.1097/01.jnen.0000178444.33972.e0. [DOI] [PubMed] [Google Scholar]
- 248.Griffin WS, Liu L, Li Y, Mrak RE, Barger SW. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J Neuroinflammation. 2006;3:5. doi: 10.1186/1742-2094-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.McGeer PL, Rogers J, McGeer EG. Inflammation, anti-inflammatory agents and Alzheimer disease: the last 12 years. J Alzheimers Dis. 2006;9:271–276. doi: 10.3233/jad-2006-9s330. [DOI] [PubMed] [Google Scholar]
- 250.Yan SS, Chen D, Yan S, Guo L, Chen JX. RAGE is a key cellular target for Aβ-induced perturbation in Alzheimer’s disease. Frontiers in bioscience (Scholar edition) 2012;4:240. doi: 10.2741/265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12:1567–1576. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- 252.Park J, Jang M, Chang S. Deleterious effects of soluble amyloid-beta oligomers on multiple steps of synaptic vesicle trafficking. Neurobiol Dis. 2013;55:129–139. doi: 10.1016/j.nbd.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 253.Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009;106:4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W, Jr, Kaye J, Manczak M. Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis. 2005;7:103–117. doi: 10.3233/jad-2005-7203. [DOI] [PubMed] [Google Scholar]
- 255.Chauhan NB, Siegel GJ. Reversal of amyloid beta toxicity in Alzheimer’s disease model Tg2576 by intraventricular antiamyloid beta antibody. J Neurosci Res. 2002;69:10–23. doi: 10.1002/jnr.10286. [DOI] [PubMed] [Google Scholar]
- 256.Russell CL, Semerdjieva S, Empson RM, Austen BM, Beesley PW, Alifragis P. Amyloid-beta acts as a regulator of neurotransmitter release disrupting the interaction between synaptophysin and VAMP2. PLoS One. 2012;7:e43201. doi: 10.1371/journal.pone.0043201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kelly BL, Vassar R, Ferreira A. Beta-amyloid-induced dynamin 1 depletion in hippocampal neurons. A potential mechanism for early cognitive decline in Alzheimer disease. J Biol Chem. 2005;280:31746–31753. doi: 10.1074/jbc.M503259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kelly BL, Ferreira A. beta-Amyloid-induced dynamin 1 degradation is mediated by N-methyl-D aspartate receptors in hippocampal neurons. J Biol Chem. 2006;281:28079–28089. doi: 10.1074/jbc.M605081200. [DOI] [PubMed] [Google Scholar]
- 259.Dougherty JJ, Wu J, Nichols RA. Beta-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex. J Neurosci. 2003;23:6740–6747. doi: 10.1523/JNEUROSCI.23-17-06740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Garcia-Osta A, Alberini CM. Amyloid beta mediates memory formation. Learn Mem. 2009;16:267–272. doi: 10.1101/lm.1310209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wang HY, Lee DH, D’Andrea MR, Peterson PA, Shank RP, Reitz AB. beta-Amyloid(1-42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. J Biol Chem. 2000;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 262.Small DH, Maksel D, Kerr ML, Ng J, Hou X, Chu C, Mehrani H, Unabia S, Azari MF, Loiacono R, Aguilar MI, Chebib M. The beta-amyloid protein of Alzheimer’s disease binds to membrane lipids but does not bind to the alpha7 nicotinic acetylcholine receptor. J Neurochem. 2007;101:1527–1538. doi: 10.1111/j.1471-4159.2006.04444.x. [DOI] [PubMed] [Google Scholar]
- 263.Pettit DL, Shao Z, Yakel JL. beta-Amyloid(1-42) peptide directly modulates nicotinic receptors in the rat hippocampal slice. J Neurosci. 2001;21(1–5):RC120. doi: 10.1523/JNEUROSCI.21-01-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Liu Q, Kawai H, Berg DK. beta -Amyloid peptide blocks the response of alpha 7-containing nicotinic receptors on hippocampal neurons. Proc Natl Acad Sci U S A. 2001;98:4734–4739. doi: 10.1073/pnas.081553598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Dineley KT, Bell KA, Bui D, Sweatt JD. beta -Amyloid peptide activates alpha 7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Biol Chem. 2002;277:25056–25061. doi: 10.1074/jbc.M200066200. [DOI] [PubMed] [Google Scholar]
- 266.Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 268.Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Buckingham SD, Jones AK, Brown LA, Sattelle DB. Nicotinic acetylcholine receptor signaling: roles in Alzheimer’s disease and amyloid neuroprotection. Pharmacol Rev. 2009;61:39–61. doi: 10.1124/pr.108.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hu NW, Ondrejcak T, Rowan MJ. Glutamate receptors in preclinical research on Alzheimer’s disease: update on recent advances. Pharmacology Biochemistry and Behavior. 2012;100(4):855–862. doi: 10.1016/j.pbb.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 271.D’Amelio M, Rossini PM. Brain excitability and connectivity of neuronal assemblies in Alzheimer’s disease: from animal models to human findings. Progress in neurobiology. 2012;99(1):42–60. doi: 10.1016/j.pneurobio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 272.Roselli F, Tirard M, Lu J, Hutzler P, Lamberti P, Livrea P, Morabito M, Almeida OF. Soluble beta-amyloid1-40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses. J Neurosci. 2005;25:11061–11070. doi: 10.1523/JNEUROSCI.3034-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Gu Z, Liu W, Yan Z. {beta}-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J Biol Chem. 2009;284:10639–10649. doi: 10.1074/jbc.M806508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zhao W-Q, Santini F, Breese R, Ross D, Zhang XD, Stone DJ, Ferrer M, Townsend M, Wolfe AL, Seager MA, Kinney GG, Shughrue PJ, Ray WJ. Inhibition of calcineurin mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption. J Biol Chem. 2010;285:7619–7632. doi: 10.1074/jbc.M109.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Liu S-J, Gasperini R, Foa L, Small DH. Amyloid-beta decreases cell-surface AMPA receptors by increasing intracellular calcium and phosphorylation of GluR2. J Alzheimers Dis. 2010;21:655–666. doi: 10.3233/JAD-2010-091654. [DOI] [PubMed] [Google Scholar]
- 276.Small DH. Dysregulation of Ca2+ homeostasis in Alzheimer’s disease: role in acetylcholinesterase production and AMPA receptor internalization. Neurodegenerative Diseases. 2012;10(1–4):76–79. doi: 10.1159/000333126. [DOI] [PubMed] [Google Scholar]
- 277.D’Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, Diamantini A, De Zio D, Carrara P, Battistini L, Moreno S, Bacci A, Ammassari-Teule M, Marie H, Cecconi F. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nature Neuroscience. 2011;14:69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- 278.Ferreira ST, Klein WL. The Ab oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol Learn Mem. 2011;96:529–543. doi: 10.1016/j.nlm.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Domingues A, Almeida S, Da Cruz e Silva EF, Oliveira CR, Rego AC. Toxicity of beta-amyloid in HEK293 cells expressing NR1/NR2A or NR1/NR2B N-methyl-D-aspartate receptor subunits. Neurochem Int. 2007;50:872–880. doi: 10.1016/j.neuint.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 280.Coan EJ, Irving AJ, Collingridge GL. Low-frequency activation of the NMDA receptor system can prevent the induction of LTP. Neurosci Lett. 1989;105:205–210. doi: 10.1016/0304-3940(89)90038-4. [DOI] [PubMed] [Google Scholar]
- 281.Zorumski CF, Izumi Y. Modulation of LTP induction by NMDA receptor activation and nitric oxide release. Prog Brain Res. 1998;118:173–182. doi: 10.1016/s0079-6123(08)63207-0. [DOI] [PubMed] [Google Scholar]
- 282.Lesné S, Ali C, Gabriel C, Croci N, MacKenzie ET, Glabe CG, Plotkine M, Marchand-Verrecchia C, Vivien D, Buisson A. NMDA receptor activation inhibits alpha-secretase and promotes neuronal amyloid-beta production. J Neurosci. 2005;25:9367–9377. doi: 10.1523/JNEUROSCI.0849-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kim SH, Fraser PE, Westaway D, St George-Hyslop PH, Ehrlich ME, Gandy S. Group II metabotropic glutamate receptor stimulation triggers production and release of Alzheimer’s amyloid(beta)42 from isolated intact nerve terminals. J Neurosci. 2010;30:3870–3875. doi: 10.1523/JNEUROSCI.4717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 285.Li M, Smith CP. β-Amyloid 1–40 inhibits electrically stimulated release of [3 H] norepinephrine and enhances the internal calcium response to low potassium in rat cortex: Prevention with a free radical scavenger. Brain research bulletin. 1996;39(5):299–303. doi: 10.1016/0361-9230(96)00031-7. [DOI] [PubMed] [Google Scholar]
- 286.Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Buée L, Bussiere T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Research Reviews. 2000;33(1):95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 288.Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, Kiritoshi T, Neugebauer V, Jackson GR, Kayed R. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Scientific reports. 2012:2. doi: 10.1038/srep00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293(5534):1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 290.Götz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Aβ42 fibrils. Science. 2001;293(5534):1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 291.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 292.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Aβ immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43(3):321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 293.Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to β-amyloid-induced neurotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(9):6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316(5825):750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 295.Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, Wu T, Hamto P, Devidze N, Yu GQ, Palop JJ, Noebels JL, Mucke L. Amyloid-β/Fyn–induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. The Journal of neuroscience. 2011;31(2):700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, Ashe KH, Liao D. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68(6):1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402(6762):615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 298.Cruz JC, Kim D, Moy LY, Dobbin MM, Sun X, Bronson RT, Tsai LH. p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid β in vivo. The Journal of Neuroscience. 2006;26(41):10536–10541. doi: 10.1523/JNEUROSCI.3133-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zhang C, Qiu HE, Krafft GA, Klein WL. Aβ peptide enhances focal adhesion kinase/Fyn association in a rat CNS nerve cell line. Neuroscience letters. 1996;211(3):187–190. doi: 10.1016/0304-3940(96)12761-0. [DOI] [PubMed] [Google Scholar]
- 300.Baum L, Hansen L, Masliah E, Saitoh T. Glycogen synthase kinase 3 alteration in Alzheimer disease is related to neurofibrillary tangle formation. Molecular and chemical neuropathology. 1996;29(2–3):253–261. doi: 10.1007/BF02815006. [DOI] [PubMed] [Google Scholar]
- 301.Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule- associated proteins and trigger microtubule disruption. Cell. 1997;89(2):297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 302.Nishimura I, Yang Y, Lu B. PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell. 2004;116(5):671–682. doi: 10.1016/s0092-8674(04)00170-9. [DOI] [PubMed] [Google Scholar]
- 303.Chin JY, Knowles RB, Schneider A, Drewes G, Mandelkow EM, Hyman BT. Microtubule-affinity regulating kinase (MARK) is tightly associated with neurofibrillary tangles in Alzheimer brain: a fluorescence resonance energy transfer study. Journal of Neuropathology and Experimental Neurology. 2000;59(11):966–971. doi: 10.1093/jnen/59.11.966. [DOI] [PubMed] [Google Scholar]
- 304.Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wölfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Götz J. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell. 2010;142(3):387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 305.Ittner LM, Götz J. Amyloid-β and tau—a toxic pas de deux in Alzheimer’s disease. Nature Reviews Neuroscience. 2011;12(2):65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 306.Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2011;20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Manczak M, Reddy PH. Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage. Human molecular genetics. 2012;21(11):2538–2547. doi: 10.1093/hmg/dds072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Hong Y, Chan CB, Kwon IS, Li X, Song M, Lee HP, Liu X, Sompol P, Jin P, Lee HG, Yu SP, Ye K. SRPK2 phosphorylates tau and mediates the cognitive defects in Alzheimer’s disease. The Journal of Neuroscience. 2012;32(48):17262–17272. doi: 10.1523/JNEUROSCI.3300-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Caccamo A, Branca C, Talboom JS, Shaw DM, Turner D, Ma L, Messina A, Huang Z, Wu J, Oddo S. Reducing ribosomal protein S6 kinase 1 expression improves spatial memory and synaptic plasticity in a mouse model of Alzheimer’s disease. The Journal of Neuroscience. 2015;35(41):14042–14056. doi: 10.1523/JNEUROSCI.2781-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Joshi YB, Giannopoulos PF, Chu J, Sperow M, Kirby LG, Abood ME, Praticò D. Absence of ALOX5 gene prevents stress-induced memory deficits, synaptic dysfunction and tauopathy in a mouse model of Alzheimer’s disease. Human molecular genetics. 2014:ddu412. doi: 10.1093/hmg/ddu412. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 311.Peethumnongsin E, Yang L, Kallhoff-Muñoz V, Hu L, Takashima A, Pautler RG, Zheng H. Convergence of presenilin-and tau-mediated pathways on axonal trafficking and neuronal function. The Journal of Neuroscience. 2010;30(40):13409–13418. doi: 10.1523/JNEUROSCI.1964-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Xu ZP, Li L, Bao J, Wang ZH, Zeng J, Liu EJ, Li XG, Huang RX, Gao D, Li MZ, Zhang Y, Liu GP, Wang JZ. Magnesium protects cognitive functions and synaptic plasticity in streptozotocin-induced sporadic Alzheimer’s model. PloS one. 2014;9(9):e108645. doi: 10.1371/journal.pone.0108645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.An W-L, Cowburn RF, Li L, Braak H, Alafuzoff I, Iqbal K, Iqbal I-G, Winblad B, Pei J-J. Upregulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer’s disease. Am J Pathol. 2003;163:591–607. doi: 10.1016/S0002-9440(10)63687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A. 2009;106:1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Townsend M, Mehta T, Selkoe DJ. Soluble Abeta inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem. 2007;282:33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- 318.Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 319.Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, Masliah E, Dillin A. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139:1157–1169. doi: 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Counts SE, Mufson EJ. The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J Neuropathol Exp Neurol. 2005;64:263–72. doi: 10.1093/jnen/64.4.263. [DOI] [PubMed] [Google Scholar]
- 321.Mufson EJ, Counts SE, Ginsberg SD. Gene expression profiles of cholinergic nucleus basalis neurons in Alzheimer’s disease. Neurochem Res. 2002;27:1035–48. doi: 10.1023/a:1020952704398. [DOI] [PubMed] [Google Scholar]
- 322.Forman MS, Mufson EJ, Leurgans S, Pratico D, Joyce S, Leight S, Lee VM, Trojanowski JQ. Cortical biochemistry in MCI and Alzheimer disease: lack of correlation with clinical diagnosis. Neurology. 2007;68:757–63. doi: 10.1212/01.wnl.0000256373.39415.b1. [DOI] [PubMed] [Google Scholar]
- 323.Ginsberg SD, Che S, Counts SE, Mufson EJ. Shift in the ratio of three-repeat tau and four-repeat tau mRNAs in individual cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. J Neurochem. 2006;96:1401–8. doi: 10.1111/j.1471-4159.2005.03641.x. [DOI] [PubMed] [Google Scholar]
- 324.Ginsberg SD, Che S, Counts SE, Mufson EJ. Single cell gene expression profiling in Alzheimer’s disease. NeuroRx. 2006;3:302–18. doi: 10.1016/j.nurx.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Geula C, Nagykery N, Nicholas A, Wu CK. Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:309–18. doi: 10.1097/NEN.0b013e31816a1df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sassin I, Schultz C, Thal DR, Rüb U, Arai K, Braak E, Braak H. Evolution of Alzheimer’s disease-related cytoskeletal changes in the basal nucleus of Meynert. Acta Neuropathol. 2000;100:259–69. doi: 10.1007/s004019900178. [DOI] [PubMed] [Google Scholar]
- 327.Belarbi K, Schindowski K, Burnouf S, Caillierez R, Grosjean ME, Demeyer D, Hamdane M, Sergeant N, Blum D, Buée L. Early Tau pathology involving the septohippocampal pathway in a Tau transgenic model: relevance to Alzheimer’s disease. Curr Alzheimer Res. 2009;6:152–7. doi: 10.2174/156720509787602843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Hellstrom-Lindahl E, Moore H, Nordberg A. Increased levels of tau protein in SH-SY5Y cells after treatment with cholinesterase inhibitors and nicotinic agonists. J Neurochem. 2000;74:777–84. doi: 10.1046/j.1471-4159.2000.740777.x. [DOI] [PubMed] [Google Scholar]
- 329.Wang HY, Li W, Benedetti NJ, Lee DH. α7 nicotinic acetylcholine receptors mediate β-amyloid peptide-induced tau protein phosphorylation. J BiolChem. 2003;278:31547–53. doi: 10.1074/jbc.M212532200. [DOI] [PubMed] [Google Scholar]
- 330.Bencherif M, Lippiello PM. Alpha7 neuronal nicotinic receptors: the missing link to understanding Alzheimer’s etiopathology? Med Hypotheses. 2010;74:281–5. doi: 10.1016/j.mehy.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 331.Oddo S, Caccamo A, Green KN, Liang K, Tran L, Chen Y, Leslie FM, LaFerla FM. Chronic nicotine administration exacerbates tau pathology in a transgenic model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2005;102:3046–51. doi: 10.1073/pnas.0408500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Oddo S, Caccamo A, Tseng B, Cheng D, Vasilevko V, Cribbs DH, LaFerla FM. Blocking Abeta42 accumulation delays the onset and progression of tau pathology via the C terminus of heat shock protein70-interacting protein: a mechanistic link between Abeta and tau pathology. J Neurosci. 2008;28:12163–12175. doi: 10.1523/JNEUROSCI.2464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Kihara T, Shimohama S, Sawada H, Honda K, Nakamizo T, Shibasaki H, Kume T, Akaike A. alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity. J Biol Chem. 2001;276:13541–13546. doi: 10.1074/jbc.M008035200. [DOI] [PubMed] [Google Scholar]
- 334.Barrantes FJ, Borroni V, Vallés S. Neuronal nicotinic acetylcholine receptor–cholesterol crosstalk in Alzheimer’s disease. FEBS letters. 2010;584(9):1856–1863. doi: 10.1016/j.febslet.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 335.Wang Y, Greig NH, Yu QS, Mattson MP. Presenilin-1 mutation impairs cholinergic modulation of synaptic plasticity and suppresses NMDA currents in hippocampus slices. Neurobiology of aging. 2009;30(7):1061–1068. doi: 10.1016/j.neurobiolaging.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Kim J, Yoon H, Basak J, Kim J. Apolipoprotein E in synaptic plasticity and Alzheimer’s disease: potential cellular and molecular mechanisms. Mol Cells. 2014;37(11):767–776. doi: 10.14348/molcells.2014.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Chakroborty S, Kim J, Schneider C, West AR, Stutzmann GE. Nitric oxide signaling is recruited as a compensatory mechanism for sustaining synaptic plasticity in Alzheimer’s disease mice. The Journal of Neuroscience. 2015;35(17):6893–6902. doi: 10.1523/JNEUROSCI.4002-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease The Baltimore Longitudinal Study of Aging. Neurology. 1997;48(6):1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 339.Ferreira A, Caceres A. Estrogen-enhanced neurite growth: evidence for a selective induction of Tau and stable microtubules. The Journal of neuroscience. 1991;11(2):392–400. doi: 10.1523/JNEUROSCI.11-02-00392.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lorenzo A, Diaz H, Carrer H, Caceres A. Amygdala neurons in vitro: neurite growth and effects of estradiol. J Neurosci Res. 1992;33:418–435. doi: 10.1002/jnr.490330308. [DOI] [PubMed] [Google Scholar]
- 341.Woolley CS, Wenzel HJ, Schwartzkroin PA. Estradiol increases the frequency of multiple synapse boutons in the hippocampal CA1 region of the adult female rat. Journal of Comparative Neurology. 1996;373(1):108–117. doi: 10.1002/(SICI)1096-9861(19960909)373:1<108::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 342.McEwen BS, Alves SE, Bulloch K, Weiland NG. Ovarian steroids and the brain implications for cognition and aging. Neurology. 1997;48(5 Suppl 7):8S–15S. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- 343.Teter B, Harris-White ME, Frautschy SA, Cole GM. Role of apolipoprotein E and estrogen in mossy fiber sprouting in hippocampal slice cultures. Neuroscience. 1999;91(3):1009–1016. doi: 10.1016/s0306-4522(98)00630-7. [DOI] [PubMed] [Google Scholar]
- 344.Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: implications for Alzheimer’s disease. The Journal of neuroscience. 1998;18(9):3180–3185. doi: 10.1523/JNEUROSCI.18-09-03180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Mesulam MM. Neuroplasticity failure in Alzheimer’s disease: bridging the gap between plaques and tangles. Neuron. 1999;24(3):521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 346.Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, Owen C, Aldea P, Su Y, Hassenstab J, Cairns NJ, Holtzman DM, Fagan AM, Morris JC, Benzinger TL, Ances BM. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Science translational medicine. 2016;8(338):338ra66–338ra6. doi: 10.1126/scitranslmed.aaf2362. [DOI] [PMC free article] [PubMed] [Google Scholar]