Abstract
Background
On account of deterioration of chronic heart failure (CHF) and extensive exploration of Chinese herbal injections (CHIs), we performed a network meta-analysis to investigate the efficacy of CHIs (Huangqi injection, Shenfu injection, Shengmai injection, Shenmai injection, Shenqi Fuzheng injection, Yiqifumai injection) on the basis of western medicine (WM) treatment in CHF.
Methods
Literature search was conducted in Embase, the Cochrane Library, Pubmed, Chinese Biological Medicine Database, China National Knowledge Infrastructure, Wanfang Database, Chinese Scientific Journal Database from inception to June 12nd 2017, and study selection was abided by a prior eligible criteria.
Results
Ultimately, a total of 113 randomized controlled trials (RCTs) were enrolled. The clinical data of the effective clinical rate, left ventricular ejection fraction, cardiac output and others outcomes was estimated by Stata software and Winbugs software. Risk of bias was assessed by Cochrane Collaboration’s tools. Integrating the each outcome’s results, a combination of Shengmai injection/Shenmai injection and WM obtain a first rank in most outcomes, particularly primary outcomes.
Conclusions
In conclusion, on the basis of WM, Shengmai injection or Shenmai injection may be a perforable treatment in CHF. In terms of insufficient of this study, more high quality RCTs needed to implement to support our conclusions.
Electronic supplementary material
The online version of this article (10.1186/s12906-018-2090-3) contains supplementary material, which is available to authorized users.
Keywords: Network meta-analysis (NMA), Chronic heart failure (CHF), Chinese herbal injection (CHI)
Background
Chronic heart failure (CHF) refers to a pathologic condition that cardiac output is absolute or relative reduce and cannot meet the whole body tissue metabolism under the normal venous return, then result in decreasing the myocardial contractile force and ventricular compliance, ultimately dyspnea, edema, feeble and so on. It was estimated that five-year survival rate of CHF was lower as malignant tumor and CHF was a main reason of disability and death on a global scale [1–3]. Impaired cardiac function of CHF patients may lessen their ability of daily living and render them a heavy economic pressure [1, 4]. At present, the primary aims of alleviating CHF symptoms are to inhibit myocardial remodeling, and perfect cardiac function [5]. Therefore, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), digoxin, and diuretics are become standard western medicine (WM) treatment in CHF [6], while it cannot obtain a desired effect own to poor compliance, lower heart rate of patients and others questions [5]. In consideration of its limitations, the application of Chinese herbal injections (CHIs) could be promoted. Currently, a combination between CHIs and WM treatment has already been a supportive measure in treatment of CHF in China. In accordance with traditional Chinese medicine (TCM) theories, CHF pertain to “heart impediment (xin bi)”, “palpitation”, “edema” and so forth, which caused by heart and then affect others organs. The clinical principle is to strengthen the body resistance to eliminate pathogenic factors [2]. Due to the relative low recognition of CHIs in CHF, this study selected six CHIs commonly used in CHF treatment, all of them were authorized by China Food and Drug Administration (CFDA), namely Huangqi injection (HQI), Shenfu injection (SFI), Shengmai injection (SI), Shenmai injection (SMI), Shenqi Fuzheng injection (SQFZI), Yiqifumai injection (YQFMI), to explore and rank their efficacy in CHF by the approach of network meta-analysis (NMA). Compared with conventional pairwise meta-analysis, NMA can sort the interventions via indirect comparison [7]. At the same time, the clinical trials compared those six CHIs head to head was lack. Thus, an attempt to conduct a NMA was necessarily. The goal of this study was to provide evidence-based hierarchies of the comparative efficacy and more insights for selection of CHF treatment.
Methods
The study was congrunt with The Prisma Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions [8]. And the Prisma check list was displayed in Additional file 1.
Eligibility criteria and study selection
A study was considered eligible if it suited for these criteria: 1) randomized controlled trial (RCT); 2) patients enrolled were diagnosed as CHF according to “Guidelines on the Diagnosis and Treatment of Heart Failure” conducted by The Chinese medical association cardiovascular epidemiology branch in 2014 [9] or “Clinical Guideline of New Drugs for Traditional Chinese Medicine” released by CFDA in 2002 [10]. Both of them contained both western diagnostics, the latter included TCM diagnostics as well; 3) patients receive WM treatment (e.g. cardiotonic, diuretic, ACEIs, β-blocker and so forth), meanwhile patients needed relevant therapy if they had complications during therapeutic process. On the basis of it, the treatment group received one of the included CHIs, the control group received another or just adopted WM. Besides, the dosages of CHIs were reported; 4) RCTs tested the clinical effective rate. The clinical effective rate calculated by this formula: (number of remarkable recovery patients + number of basic recovery patients) / total number of patients * 100%. Cardiac function classification was conformed to the standard issued by New York Heart Association (NYHA) in the United States. Clinical symptoms disappeared and cardiac function improved 2 levels at least was deemed as the class of remarkable recovery, clinical symptoms relieved and cardiac function increase 1 level was classified into the part of basic recovery, clinical symptoms and cardiac function was unaltered or worse belonged to deterioration. Besides, the incidence of left ventricular ejection fraction (LVEF), cardiac output (CO), stroke volume (SV), 6-min walk test (6MWT), brain natriuretic peptide (BNP), left ventricular end-diastolic dimension (LVEDD), left ventricular end- systolic dimension (LVESD), adverse drug reactions/adverse drug events (ADRs/ADEs) were also evaluated. The clinical effective rate and LVEF were regarded as dominating outcomes of the study, because the clinical effective rate can inflect the efficacy directly and LVEF was a main indicator for CHF. And others were counted as secondary outcomes. A study was excluded when it met these following criteria: 1) the study without full text; 2) duplicated reports; 3) RCTs with incomplete or inaccurate data; 4) RCTs with wrong sequence generation method. For example, sequence generated by odd or even date of birth, some rules based on date (or day) of admission and so forth; 5) patients received physiotherapy, acupuncture and moxibustion therapy, and Chinese materia medica preparation.
A comprehensive literatures searching was carried out in seven database including Embase, the Cochrane Library, Pubmed, Chinese Biological Medicine Database (CBM), China National Knowledge Infrastructure (CNKI), Wanfang Database, Chinese Scientific Journal Database (VIP) from their inception up to June 12nd 2017. In addition, there was no restriction on language. The method that incorporated the medical subject headings (MeSH) term and the free text was applied in searching process, and it would vary from different databases. Each searching item included three parts of terms that chronic heart failure, CHIs, and randomization. Detailed searching strategies were illustrated in Additional file 2.
After literatures duplicate checking, the rest literatures were firstly screened by titles and abstracts, reviews, irrelevant literatures and animals’ experiments reports were excluded. Literatures passed the initial filtration were read full text in order to sort out the eligible RCTs. Two reviewers undertook literature selection respectively, any divergences resolved by discussion or the third reviewer.
Data extraction and quality assessment
Information from the eligible RCTs was extracted based on a custom-made form. The data consisted of the following items: 1) basic information of the eligibility: the first author, nationality, publication year, study desgin; 2) basic characteristics of patients: sample size, gender composition, average age, course of disease, primary diseases, cardiac function classification; 3) detail of RCTs’ intervention; 4) outcomes results and RCTs’ quality assessment.
The quality analysis was assessed with the Cochrane Collaboration’s tools (version 5.1.0 the Nordic Chchrane Center, the Cochrane Collaboration, 2012 Copenhagen, Denmark) by two reviewers independently. The tool comprised following these 7 items: 1) the method of randomization; 2) the concealment of random allocation; 3) the blinding method for patients and clinicians; 4) the blinding method for assessor; 5) the integrality of outcomes data; 6) the condition of selective reporting; 7) others bias. Each item was rated as “high risk”, “low risk” and “unclear”. And any difference between two reviewers settled by discussion or the third reviewer.
It is not necessary for this meta-analysis to obtain an ethical approval, because this study was the procedure that just gathered the clinical data in each RCT without any leak of patients’ information.
Statistical analysis
NMA was performed with Stata software (version 12, Stata Corporation, College Station, Texas, U.S.) and Winbugs (version 1.4, MRC Biostatistics Unit, Cambridge, UK) software by using Mantel-Haenszel random-effects model. In Winbugs software, the number of iteration was set as 50,000, the first 20,000 was used for annealing algorithm in order to eliminate the impact of initial value. For binary outcomes, the pooled results were calculated as odds ratios (ORs). For continuous outcomes, mean differences (MD) were used. Both types of outcomes were presented with their 95% credible intervals (95% CIs) as well. Besides, the network graph showed indirect comparative relationship between different interventions was described. The node area of each intervention on behalf of its number of patients, and the thickness between different interventions represented the number of relative RCTs [11]. To rank various CHIs in treatment in CHF, the surface under the cumulative ranking curve (SUCRA) was utilized, which expressed each intervention’s efficacy with percentages. A larger area of SCUAR indicated that corresponding intervention was more preferable in certain outcomes [12]. After that, the funnel plots were depicted to reflect publication bias. Due to non-close loops in this NMA, the assumption of consistency between direct and indirect evidence was not utilized.
Results
Literature selection
A total of 9968 literatures were identified in initial search (Fig. 1). After removing duplicates, there were 4852 remained. By screened titles and abstracts, 1491 literatures were excluded because they were irrelevant literatures, reviews and animals’ experiments reports. 3361 literatures were eligible and then examined respectively, among which 3248 were further excluded, for the following reasons: 1) the RCT’s intervention or diseases missed eligibility criteria (n = 2694); 2) the therapeutic effect standard missed eligibility criteria (n = 256);3) the RCT with wrong randomization (n = 68); 4) the RCT did not divide patients in two groups (n = 68); 5) case reports (n = 40); 6) the RCT without full-text (n = 104); 7) the RCT with duplicated data (n = 18). As results, 113 RCTs that evaluated CHIs combined with WM for CHF were eligible in the NMA, and all of then carried out in China between 2001 and 2017. Meanwhile, 6 types of CHIs were identified, including HQI (12 RCTs), SFI (39 RCTs), SI (31 RCTs), SMI (13 RCTs), SQFZI (12 RCTs) and YQFMI (6 RCTs).
Fig. 1.
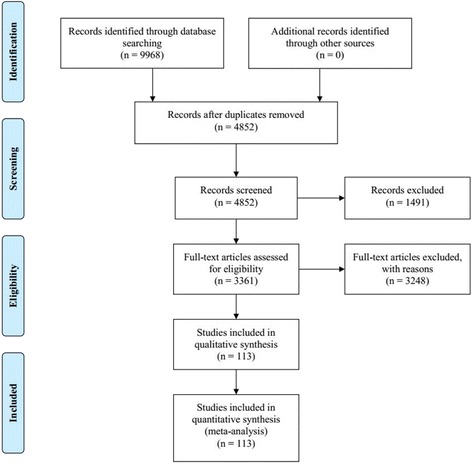
Flow chart of the search for eligible RCTs
Study characteristics and quality evaluation
One hundered thirteen [3, 13–124] RCTs with 9525 patients were accorded with the eligible criteria, among which 4852 patients in the treatment groups and 4673 patients in the control groups. Among patients, the male patients were about 55% of total, and majority of patients were middle aged and elderly people. The intervention of the control groups were WM treatment, for instance, ACEIs, β-blocker, cardiotonic, diuretic. In the meantime, the treatment groups received one of the identified CHIs on the basis of the control groups. HQI, SFI, SI, SMI was a kind of injection that clinicians injected them with 5%–10% dextrose solution or 0.9% normal saline, the specific dosage were determined by clinicians. SQFZI was a kind of already made injection with menstruum. And YQFMI was a powder-injection, clinicians injected them with 5%–10% dextrose solution or 0.9% normal saline as well. All of identified CHIs were injected once a day via mainline. Characteristics of included RCTs can be found in Additional file 3. And the compared connections among each intervention for each outcome were displayed in Fig. 2.
Fig. 2.
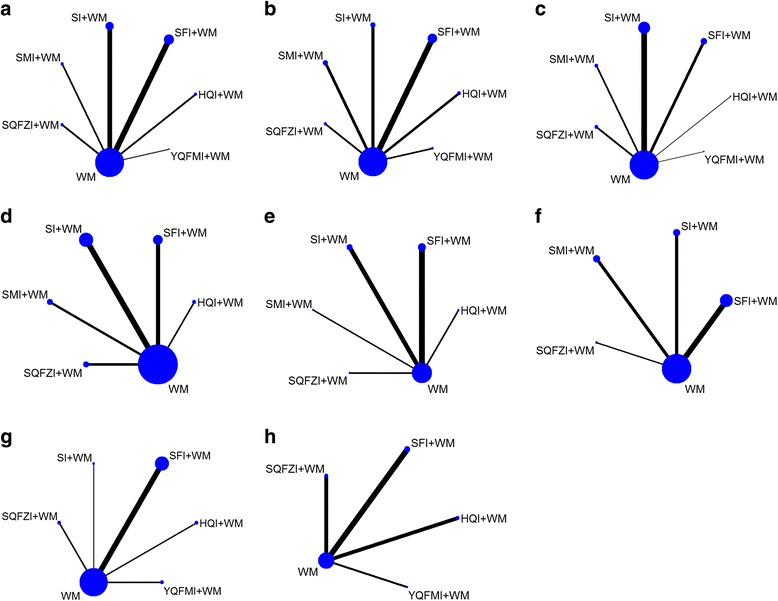
Network graph of the clinical effective rate, LVEF, CO, SV, 6MWT, BNP, LVEDD and LVESD. Note: a: the clinical effective rate; b: LVEF; c: CO; d: SV; e: 6MWT; f: BNP; g: LVEDD; h: LVESD
For the eligible RCTs, 19 RCTs [15, 26, 32, 34, 37, 56, 57, 61, 64, 75, 81, 88, 96, 101, 103, 104, 107, 110, 118] used the random number table method or sortation randomization method to generate groups and 1 RCTs [88] utilized double blind method. Thus all of them were assessed as low risk. The rest RCTs were evaluated as high risk due to insufficient information. Besides, none of the included RCTs assessed had incomplete data, so the attrition bias was appraised as low risk. As for the part of reporting bias and others bias, the included RCTs did not provide relevant contents about selective report and mention any factors leading to high risk. Therefore these two items were evaluated as unclear risk. The graphical summary was depicted in Fig. 3.
Fig. 3.
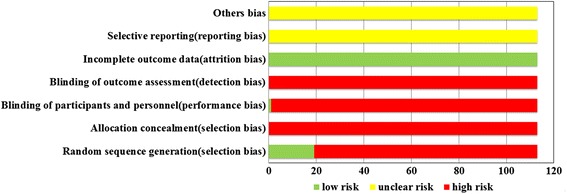
Risk of bias graph
Outcomes
The clinical effective rate
The clinical effective rate was deemed as the primary outcomes, as shown in the right upper part of Table 1 [3, 13–124], HQI + WM (OR = 0.28, 95% CIs: 0.19–0.41),SFI + WM (OR = 0.29, 95% CIs: 0.24–0.35), SI + WM (OR = 0.28, 95% CIs: 0.22–0.35), SMI + WM (OR = 0.25, 95% CIs: 0.17–0.36), SQFZI+WM (OR = 0.28, 95% CIs: 0.19–0.39), YQFMI+WM (OR = 0.42, 95% CIs: 0.25–0.70), these six interventions with 95% CIs between 0 and 1 possessed the obvious strengthen in increasing clinical effective rate.
Table 1.
Odds ratios/mean difference (95%CIs) of the clinical effective rate (right upper part) and LVEF (left lower part)
| the clinical effective rate | |||||||
|---|---|---|---|---|---|---|---|
| LVEF | HQI + WM | 0.98(0.62,1.49) | 1.03(0.62,1.59) | 1.15(0.65,1.96) | 1.00(0.60,1.76) | 0.67(0.36,1.28) | 0.28(0.19,0.41) |
| 0.13(−7.46,7.87) | SFI + WM | 1.05(0.76,1.41) | 1.17(0.79,1.83) | 1.05(0.72,1.60) | 0.69(0.42,1.21) | 0.29(0.24,0.35) | |
| −4.31(−11.77,4.08) | − 4.50(−8.43,1.48) | SI + WM | 1.12(0.73,1.75) | 1.00(0.67,1.58) | 0.66(0.39,1.16) | 0.28(0.22,0.35) | |
| −3.08(−12.25,5.93) | −3.26(−9.21,3.26) | 1.03(− 5.62,7.12) | SMI + WM | 0.88(0.52,1.51) | 0.59(0.31,1.10) | 0.25(0.17,0.36) | |
| −2.60(−12.30,7.90) | − 2.69(− 10.87,5.50) | 1.63(−6.79,9.48) | 0.38(−8.25,9.34) | SQFZI + WM | 0.66(0.36,1.23) | 0.28(0.19,0.39) | |
| −3.11(−12.39,6.93) | − 3.17(− 10.26,4.48) | 1.26(−6.88,8.28) | 0.25(−8.18,8.53) | −0.50(− 10.09,9.22) | YQFMI + WM | 0.42(0.25,0.70) | |
| 4.27(− 2.61,11.46) | 4.05(1.00,7.59) | 8.61(4.22,10.99) | 7.29(1.97,12.70) | 6.81(−0.59,14.06) | 7.26(0.42,13.64) | WM | |
Note: The result underlined meant it had statistical significant
In the Table 5 and Fig. 4, ranking analysis suggested that SMI + WM was the optimal combination, SI + WM was the second and the third was SQFZI+WM.
Table 5.
Ranking probability for all treatments on the clinical effective rate, LVEF, CO, SV, 6MWT, BNP, LVEDD and LVESD
| Treatment | Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|
| the clinical effective rate | LVEF | CO | SV | 6MWT | BNP | LVEDD | LVESD | |
| HQI + WM | 0.615 | 0.391 | 0.370 | 0.384 | 0.507 | __ | 0.795 | 0.583 |
| SFI + WM | 0.559 | 0.359 | 0.462 | 0.481 | 0.596 | 0.020 | 0.503 | 0.533 |
| SI + WM | 0.649 | 0.783 | 0.872 | 0.747 | 0.776 | 0.392 | 0.499 | __ |
| SMI + WM | 0.806 | 0.670 | 0.695 | 0.550 | 0.378 | 0.575 | __ | __ |
| SQFZI+WM | 0.635 | 0.613 | 0.530 | 0.697 | 0.460 | 0.849 | 0.361 | 0.501 |
| YQFMI+WM | 0.236 | 0.650 | 0.449 | __ | __ | __ | 0.563 | 0.619 |
| WM | 0.00 | 0.034 | 0.122 | 0.141 | 0.284 | 0.664 | 0.279 | 0.263 |
Fig. 4.
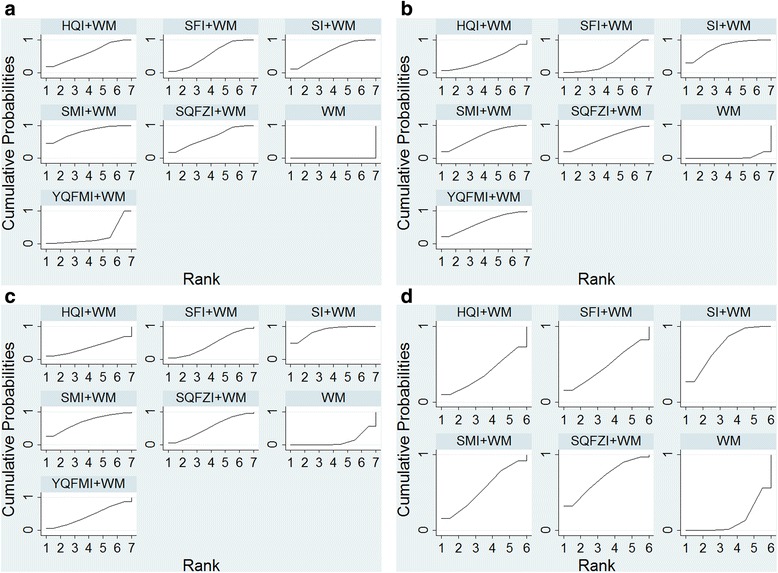
Plot of the surface under the cumulative ranking curves for all treatments on the clinical effective rate, LVEF, CO and SV. Note: a: the clinical effective rate; b: LVEF; c: CO; d: SV
LVEF
As the other dominating outcomes, LVEF (%) was estimated in 57 RCTs [3, 13–15, 17, 19, 20, 23, 24, 32, 33, 36, 38, 39, 42, 45–49, 53, 55, 56, 58, 60, 61, 64, 65, 72, 75, 82, 84, 88, 91–93, 95–97, 99–103, 105, 106, 109–111, 114, 116, 117, 119–123]. According to Table 1, if the 95% CIs was more than 0, the result was significant. Four of them were noticeably better than WM treatment for LVEF, as SFI + WM (MD = 4.05, 95% CIs: 1.00–7.59), SI + WM (MD = 8.61, 95% CIs: 4.22–10.99), SMI + WM (MD = 7.29, 95% CIs: 1.97–12.70), YQFMI+WM (MD = 7.26, 95% CIs: 0.42–13.64) were outstanding among them compared with WM.
Results of ranking analysis manifested that SI + WM was efficacious in LVEF. Another beneficial treatments were SMI + WM and YQFMI+WM (Table 5 and Fig. 4).
Co
CO (L/min) was tested in 22 RCTs [20, 30, 31, 34, 45, 61, 68, 73, 75, 76, 81–83, 87, 92, 97, 99, 102, 111, 112, 117, 119] involved seven interventions. Based on Table 2, only SI + WM (MD = 1.29, 95% CIs: 0.74–1.72) had excellent performance in improving CO.
Table 2.
Mean difference (95%CIs) of CO (right upper part) and SV (left lower part)
| CO | |||||||
|---|---|---|---|---|---|---|---|
| SV | HQI + WM | −0.22(−2.09,1.59) | −0.97(− 2.61,0.80) | −0.64(− 2.68,1.31) | −0.36(− 2.21,1.53) | −0.20(− 2.14,1.82) | 0.32(− 1.29,1.99) |
| −1.92(− 21.24,16.33) | SFI + WM | − 0.73(− 1.69,0.34) | −0.46(− 1.89,1.03) | −0.12(− 1.36,1.13) | 0.03(−1.38,1.49) | 0.55(− 0.29,1.43) | |
| − 6.07(− 21.13,9.09) | −4.32(− 17.07,9.69) | SI + WM | 0.29(− 1.02,1.51) | 0.61(− 0.46,1.58) | 0.77(− 0.54,1.98) | 1.29(0.74,1.72) | |
| −3.05(− 20.33,14.23) | −1.10(− 17.34,15.64) | 2.99(− 8.76,14.46) | SMI + WM | 0.32(− 1.15,1.77) | 0.46(− 1.15,2.13) | 0.99(− 0.17,2.16) | |
| − 5.74(− 23.58,11.31) | −3.84(− 20.16,12.65) | 0.25(− 11.63,12.08) | − 2.51(− 17.27,11.40) | SQFZI + WM | 0.14(− 1.31,1.65) | 0.68(− 0.21,1.57) | |
| __ | __ | __ | __ | __ | YQFMI + WM | 0.52(− 0.64,1.68) | |
| 3.28(− 10.90,17.25) | 5.12(− 6.59,18.01) | 9.35(3.75,14.90) | 6.46(−3.94,16.34) | 9.04(− 1.36,19.54) | __ | WM | |
Note: The result underlined meant it had statistical significant
The SUCRA mentioned above was also affirmed, SI + WM was the best choice, and the following two were SMI + WM, and SQFZI+WM (Table 5 and Fig. 4).
SV
SV (ml) was reported in 20 RCTs [20, 23, 30, 36, 38, 45, 61, 68, 73, 75, 81, 82, 87, 92, 97, 99, 102, 111, 112, 117] involved six interventions. In terms of Table 2, only SI + WM (MD = 9.35, 95% CIs: 3.75–14.90) was remarkable among them.
Base on its SUCRA, SI + WM was the optimum, SQFZI+WM was the second and SMI + WM was the third (Table 5 and Fig. 4).
6MWT
The potency of lengthening the distance of 6MWT (m) was assessed, and six interventions with 10 RCTs [24, 32, 39, 46, 51, 74, 85, 92, 106, 109] had data in contrast with WM, shown in Table 3. While the results showed no significant difference in most cases.
Table 3.
Mean difference (95%CIs) of 6MWT (right upper part) and BNP (left lower part)
| 6MWT | |||||||
|---|---|---|---|---|---|---|---|
| BNP | HQI + WM | − 5.40(− 71.99,70.70) | −29.10(− 115.00,66.32) | 16.53(− 82.29,163.00) | 7.97(−134.30,150.90) | __ | 17.38(− 52.09,84.04) |
| __ | SFI + WM | −22.05(− 91.18,37.25) | 25.48(− 84.97,155.60) | 15.06(− 137.40,161.20) | __ | 22.42(−9.55,53.97) | |
| __ | 54.65(−19.88,134.30) | SI + WM | 50.83(− 75.61,178.60) | 36.18(− 109.80,204.00) | __ | 45.03(− 12.06,101,10) | |
| __ | 80.17(16.67,147.5) | 24.53(−58.64,110.20) | SMI + WM | −16.56(− 213.70,188.00) | __ | − 1.23(− 133.50,100.10) | |
| __ | 110.00(35.08,186.40) | 53.69(− 41.32,151.3) | 27.54(− 44.17,108.30) | SQFZI + WM | __ | __ | |
| __ | __ | __ | __ | __ | YQFMI + WM | 7.12(−141.80,149.50) | |
| __ | 87.77(32.61,129.90) | 34.25(−44.31,100.20) | 8.33(−52.43,63.95) | −20.41(− 87.19,35.51) | __ | WM | |
Note: The result underlined meant it had statistical significant
The ranking analysis indicated that SI + WM was the favorable intervention (Table 5).
BNP
In terms of BNP (pg/ml), five treatments with 21 RCTs [3, 33, 36, 38, 44, 46, 47, 53, 58, 64, 65, 69, 72, 88, 94–97, 104, 107, 109] were compared with WM in Table 3. SFI + WM vs SMI + WM (MD = 80.17, 95% CIs: 16.67–147.5), SFI + WM vs SQFZI+WM (MD = 110.00, 95% CIs: 35.08–186.40), SFI + WM vs WM (MD = 87.77, 95% CIs: 32.61–129.90) had statistically significance.
Based on ranking analysis, SQFZI+WM attained the first-rank (Table 5).
LVEDD & LVESD
The efficiency of decreasing LVEDD (mm) and LVESD (mm) was estimated as well. These two indexes were tested in 22 RCTs [13, 19, 20, 28, 30, 33, 36, 38, 39, 45, 53, 55, 58, 61, 65, 72, 107, 109, 111, 119, 121, 122] and 8 RCTs [13, 20, 30, 55, 61, 107, 109, 119] respectively. According to Table 4, it appeared that there was no significant difference between each comparison.
Table 4.
Mean difference (95%CIs) of LVEDD (right upper part) and LVESD (left lower part)
| LVEDD | |||||||
|---|---|---|---|---|---|---|---|
| LVESD | HQI + WM | −6.39(− 20.58,8.88) | −5.90(− 22.48,11.36) | __ | − 7.40(− 21.99,8.39) | −5.02(− 20.71,10.80) | − 7.87(− 21.64,7.22) |
| −1.36(− 17.43,15.51) | SFI + WM | 0.32(−9.47,10.49) | __ | −1.04(− 6.88,5.10) | 1.08(− 8.40,10.10) | −1.49(− 5.58,2.45) | |
| __ | __ | SI + WM | __ | −1.43(− 11.31,8.84) | 0.68(− 12.36,13.41) | −1.88(− 10.71,7.12) | |
| __ | __ | __ | SMI + WM | __ | __ | __ | |
| −2.21(−17.01,13.77) | −0.72(− 10.35,8.84) | __ | __ | SQFZI + WM | 2.22(−7.61,11.31) | − 0.37(−5.41,3.66) | |
| 0.97(− 20.42,23.54) | 2.30(− 15.51,17.43) | __ | __ | 3.02(− 14.80,20.13) | YQFMI + WM | −2.68(−10.91,6.07) | |
| − 4.03(− 18.02,10.96) | −2.57(− 10.73,5.24) | __ | __ | − 1.82(− 7.68,3.52) | −4.80(− 21.19,12.13) | WM | |
The ranking analysis suggested that HQI + WM and SQFZI+WM was the optimum for these two indexes (Table 5).
ADRs/ADEs
Among 113 RCTs, a total of 36 [22, 23, 26, 30, 32, 33, 36, 39, 40, 43, 45, 48, 49, 51, 54, 64, 65, 67, 71–74, 77–81, 88, 93, 97–99, 103, 107, 108, 119, 120] RCTs (HQI (2 RCTs), SFI (13 RCTs), SI (13 RCTs), SMI (4 RCTs), SQFZI (2 RCTs), YQFMI (2 RCTs)) did not appear ADRs/ADEs during the trials. Another 72 RCTs (HQI (10 RCTs), SFI (25 RCTs), SI (17 RCTs), SMI (6 RCTs), SQFZI (10 RCTs), YQFMI (4 RCTs)) did not mention the situation of ADRs/ADEs. In others RCTs, one of the SFI treatment group occurred 2 cases of mild elevation of blood pressure and 2 cases of slight dry cough, and the corresponding control group occurred 3 cases of slight dry cough and 2 cases of headache [37]. Besides, one of the SI treatment group occurred 2 cases of mild anaphylaxis. There were 3 RCTs with SMI treatment group appeared ADRs [96, 100, 104]. One RCT occurred 1 case of pruritus in the treatment group and 6 cases mild headache in the control group. Another occurred 3 cases of mild gum bleeding in the control group. Another occurred 2 cases of stomach upset in the treatment and control group respectively. All of the symptom were alleviated after corresponding treatment and did not influence the RCTs.
Funnel plot characteristics
A comparison-adjusted funnel plot for the clinical effective rate was displayed in Fig. 5. The funnel plot was general symmetrical in visual. Thus we concluded that the obvious publication bias did not exist.
Fig. 5.
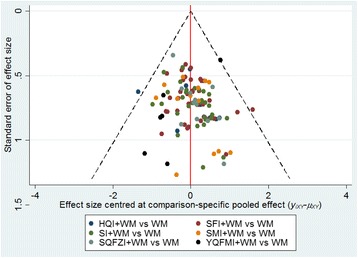
Funnel plot of the clinical effective rate
Discussion
The impairment of CHF has been a global public health issue [125], with the utilization of a conjunction between CHIs and WM in its treatment, the efficacy of CHF has been promoted, meanwhile, more and more relevant RCTs and pairwise meta-analysis were carried out. But almost RCTs concerned about the efficacy between a kind of CHI plus WM and WM, many CHIs have not been compared head to head. Thus, researchers could merely figure out the efficacy of a CHI based on these RCTs via pairwise meta-analysis. While NMA can address this void, the efficacy of CHIs can be obtained at a time based on indirect comparison. By comparing with WM, the efficacy of CHIs for CHF and their rank can be demonstrated. we conducted a NMA in order to appraise the efficacy and safety of seven interventions: HQI + WM, SFI + WM, SI + WM, SMI + WM, SQFZI+WM, YQFMI+WM and WM.
This study made an extensive literature review and evaluation. The clinical data derived from 113 RCTs in the aspects of the clinical effective rate, LVEF, CO, SV, 6MWT, BNP, as well as the value of LVEDD and LVESD. CO, SV, LVEDD and LVESD was regarded as a supplement of cardiac condition, while the consequence of LVEDD and LVESD was no significant difference in most cases, these two outcomes’ results were merely deemed as a reference. Besides, 6MWT was vital indicator of patients’ recovery, and its importance was emphasized in the guide [9], though the amount of relevant RCTs in this study was small and its statistical power was low, we just treat it as a secondary outcome. In addition, the measurement of BNP was highlighted in guide as an exclusion for CHF [126]. Therefore, we viewed it as a secondary index as well. In terms of the primary outcomes, SI + WM and SMI + WM exhibited superior performance. What more, these two interventions did a noteworthy effect on CO and SV. And SI + WM also obtained a first-rank with respect to 6MWT. Overall, on the basis of receiving WM, CHF patients received SI or SMI may be more efficacious. Both of them were approved by CFDA on the market of CHF. SI was derived from Shengmai San which has been widely used for cardiovascular diseases since 1186 in China [127]. It was mainly made from the extractive of Panax ginseng, Radix Ophiopogonis and Schisandra chinensis, and had a function as replenishing qi-yin deficiency. Pharmacological researches have confirmed that SI had features in perfecting cardiac function and alleviating heart failure, enhancing myocardial contractility and cardiac pumping [128]. Under the guideline of TCM, SI was employed in CHF treatment routinely with its preferable curative effect, and several pairwise meta-analysis manifested that a conjunctive between SI and WM owned a superior capability on increasing the effective rate and LVEF [128–130]. As for SMI, it stemmed from Shenmai Yin which was prescribed by Simiao Sun in the Tang Dynasty [131], and its ingredients did not contain Schisandra chinensis compared with SI, but it also had a superior capacity in nourishing yin and benefiting qi. Upon pharmacological researches, the effect of SMI on promoting myocardial contractility and antiarrhythmic action has been verified [132]. Besides, several pairwise meta-analysis demonstrated that SMI plus WM exhibited a better performance in improving the effective rate, LVEF, CO, SV and decreasing BNP than WM [133–135].
Apart from efficacy, the safety of interventions was the other crucial element that must be considered in clinical trials. In this study, the occurrence rate of ADRs/ADEs was small, but about 64% of the research did not report the ADRs/ADEs. Hence, we could not draw a certain conclusion on it. As suggested in previous study, anaphylaxis was the main ADRs/ADEs of CHIs, and it would appear within 30 min at first time [136–139]. Hence, it is crucial for clinicians to monitor the ADRs/ADEs after using CHIs. Meanwhile, it is necessary to reported exactly if ADRs/ADEs occurred [136].
Upon the design and contents, three merits enhanced the creditable of this study. Firstly, this study made a comprehensive literature search and a contrast for six CHIs which have been already adopted in CHF treatment. Besides, this study expressed the efficacy of CHIs objectively due to the relevant large number of eligible RCTs. Furthermore, a strict eligibility criterion was formulated before implementing NMA. The consistency of the intervention and the curative standard lowered the clinical heterogeneity. What’s more, it was significant that the outcomes demonstrated cardiac condition in multiaspect. According to corresponding conclusions, this study provided several clinical suggestions for treatment in CHF.
Limitation
Nevertheless, there was still insufficient in this study. Frist, the enrolled patients in RCTs were merely Chinese, which may lead to a bias on whether non-Chinese use eligible CHIs effectively or not. Although CHIs was mostly adopted in China, clinicians also can not only recruit Chinese. Next, just ten of included RCTs reported 6MWT in this study. While it is 6MWT, and readmission rate that associate with CHF patients closely and influence patients’ survival quality. Thus, these aspects should be paid more emphasis when RCTs are designed. In addition, the methodological quality was general, and most included RCTs did not mention the details of randomization and allocation concealment, which may generate an overestimate for eligible CHIs. It should be note that clinicians utilize low risk randomization and concealment method as possible. Based on the limitations, the RCTs conducted in the future should be perfected in relevant areas.
Conclusion
To sum up, this study found that a combination between SI/SMI and WM exerted a positive effect on improving efficacy of CHF. However, the strength of evidence needed be promoted by more high quality RCTs. Moreover, safety of SI and SMI should be cautious monitoring in trials.
Additional files
PRISMA checklist for network meta-analysis. This file contained items about PRISMA checklist for network meta-analysis and corresponding pages of this study. (DOC 66 kb)
Search strategy. This file contained the search strategy of traditional Chinese medicine injections and English database. (DOC 39 kb)
Characteristics of included randomized controlled trials. This file contained the information about included randomized controlled trials. (DOC 38 kb)
Acknowledgments
Funding
National Natural Science Foundation of China (No. 81473547; No.81673829).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- 6MWT
6-min walk test
- 95% CIs
95% credible intervals
- ACEIs
Angiotensin-converting enzyme inhibitors
- ADRs/ADEs
Dverse drug reactions/adverse drug events
- ARBs
Angiotensin II receptor blockers
- BNP
brain Natriuretic peptide
- CBM
Chinese Biological Medicine Database
- CFDA
China Food and Drug Administration
- CHF
Chronic heart failure
- CHIs
Chinese herbal injections
- CNKI
China National Knowledge Infrastructure
- CO
Cardiac output
- HQI
Huangqi injection
- LVEDD
Left ventricular end-diastolic dimension
- LVEF
Left ventricular ejection fraction
- LVESD
Left ventricular end-systolic dimension
- MD
Mean differences
- MeSH
Medical subject headings
- NMA
Network meta-analysis
- NYHA
New York Heart Association
- ORs
Odds ratios
- RCTs
Randomized controlled trials
- SFI
Shenfu injection
- SI
Shengmai injection
- SMI
Shenmai injection
- SQFZI
Shenqi Fuzheng injection
- SUCRA
Surface under the cumulative ranking curve
- SV
Stroke volume
- TCM
Traditional Chinese medicine
- VIP
Chinese Scientific Journal Database
- WM
Western medicine
- YQFMI
Yiqifumai injection
Authors’ contributions
Conception and design of the network meta-analysis: JRW, KHW. Performance of the network meta-analysis: KHW. Quality assessment of the network meta-analysis: MWN, KHW, JRW. Analysis of study data: DZ, XJD. Writing of the paper: KHW, DZ. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The ethical approval was not necessary in current meta-analysis because our NMA just gathered the RCTs from literature search, this procedure was without deal with any patients’ personal data and harm to any patient.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests in any aspects.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12906-018-2090-3) contains supplementary material, which is available to authorized users.
Contributor Information
Kai-Huan Wang, Email: wkhlinda@163.com.
Jia-Rui Wu, Email: exogamy@163.com.
Dan Zhang, Email: 2426394372@qq.com.
Xiao-Jiao Duan, Email: duanxiaojiaodgh@163.com.
Meng-Wei Ni, Email: nmh15764346161@163.com.
References
- 1.Wang E-W, Jia X-S, Ruan C-W, et al. miR-487b mitigates chronic heart failure through inhibition of the IL-33/ST2 signaling pathway. Oncotarget. 2017;8:51688–51702. doi: 10.18632/oncotarget.18393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Z-Q, Mao J-Y, Wang X-L, et al. Application and evaluation of Chinese medicine in treatment of chronic heart failure. Chin J Integ Med. 2013;33:1701. [PubMed] [Google Scholar]
- 3.Wu D-H. Effects of the shenfu injection on the level of serum cerebral sodium peptide in patients with chronic heart failure. Zhejiang J Integ Tradit Chin West Med. 2013;23:837–838. [Google Scholar]
- 4.Vetrovsky T, Siranec M, Parenica J, et al. Effect of a 6-month pedometer-based walking intervention on functional capacity in patients with chronic heart failure with reduced (HFrEF) and with preserved (HFpEF) ejection fraction: study protocol for two multicenter randomized controlled trials. J Transl Med. 2017;15:153. doi: 10.1186/s12967-017-1257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y-Z, Zhuang Y, Gu Y, Chen Q-Q, Wang Z-X. Progress in the evolution of chronic heart failure syndrome. Guiding J Tradit Chin Med Pharmacol. 2016;22:62–68. [Google Scholar]
- 6.Sane R, Aklujkar A, Patil A, et al. Effect of heart failure reversal treatment as add-on therapy in patients with chronic heart failure: a randomized open-label study. Indian Heart J. 2017;69:299. doi: 10.1016/j.ihj.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 8.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 9.Cardiology Branch of the Chinese Medical Association Guidelines for the diagnosis and treatment of heart failure in China 2014. Chin J Cardiol. 2014;42:98–122. [Google Scholar]
- 10.Zheng Y-Y. Clinical guideline of new drugs for traditional Chinese medicine. Beijing: Chin Med Sci and Tech Press; 2013. p. 77–85.
- 11.Zhang D, Wu J-R, Liu S, Zhang X-M, Zhang B. Network meta-analysis of Chinese herbal injections combined with the chemotherapy for the treatment of pancreatic cancer. Med. 2017;96:e7005. doi: 10.1097/MD.0000000000007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang L, Guo R. Comparison of the efficacy among multiple chemotherapeutic interventions combined with radiation therapy for patients with cervix cancer after surgery: a network meta-analysis. Oncotarget. 2017;8:49515–49533. doi: 10.18632/oncotarget.17259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing G-P, Nie X-M. Effects of astragalus injection on hemodynamics in patients with congestive heart failure. Med ind inf. 2006;3:67–69. [Google Scholar]
- 14.Gu X, Shang S-Z, Guo R. 68 cases of congestive heart failure assisted by astragalus injection. Her Med. 2003;22:556–557. [Google Scholar]
- 15.Zhang Z-B. Clinical observation of astragalus injection combined with conventional western medicine for senile patients of chronic congestive heart failure. J New Chin Med. 2017;49:25–28. [Google Scholar]
- 16.Luo X-C. Clinical observation of 30 cases of congestive heart failure treated by huangqi injection. J Emerg Syndromes Tradit Chin Med. 2003;12:35. [Google Scholar]
- 17.Feng L-Y, Hao W. Clinical observation of 34 cases of congestive heart failure treated with astragalus injection. Chin J Integr Med Cardio/ Cerebrovasc Dis. 2008;6:1362–1363. [Google Scholar]
- 18.Gao J-H. Clinical observation of astragalus injection for treating congestive heart failure. Acad J Guangdong Coll Pharm. 2005;21:610–611. [Google Scholar]
- 19.Yan F-Y, Lin H-Z. 80 cases of chronic congestive heart failure treated with astragalus injection. Jiangxi J Tradit Chin Med. 2009;40:27–28. [Google Scholar]
- 20.Lin Y, Huang C-L, Zhu C-L. Clinical study on astragalus injection in treating chronic congestive heart failure. Chin Pharm. 2009;18:22–23. [Google Scholar]
- 21.Zhang C-M, Duan Q-F, Bian X-J. Clinical observation of astragalus injection in the treatment of chronic heart failure. Med Front. 2013;17:19–20. [Google Scholar]
- 22.Yuan G-P. Evaluation of clinical efficacy of astragalus injection for treatment of chronic heart failure. Chin J Clin Pharmacol Ther. 2003;8:710–711. [Google Scholar]
- 23.Wang X-X. Treatment of chronic heart failure by astragalus injection. Chin Foreign Health Abstr. 2015;14:53–54. [Google Scholar]
- 24.Jia B-Q, Xu L. Effect of astragalus injection on chronic heart failure. Chin J Clin Res. 2013;26:654–656. [Google Scholar]
- 25.Wang X-G. Effects of shenfu injection on chronic heart failure induced by different etiology and the effect of b-type natriuretic peptide. Speci health. 2014;4:598. [Google Scholar]
- 26.Wang H, Hu Y-H, Song Q-Q, Qiu Z-L, Bo R-Q. The impact of shenfu injection on the immune function in patients with chronic heart failure and heart kidney yang deficiency syndrome. Chin J Integr Med Cardio/ Cerebrovasc Dis. 2016;14:1441–1446. [Google Scholar]
- 27.Zhou K, Lin S-Y, Yang WM. Effects of injection on NT-proBNP in heart failure patients with coronary heart disease. J Emerg Syndromes Tradit Chin Med. 2013;22:1625–1626. [Google Scholar]
- 28.Xu L-L, Guan C-Y, Wang C-Y, Chen J, Zhang L-L. Effects of shenfu injection on the nt-probnp and life quality of chronic heart failure patients. J Emerg Syndromes Tradit Chin Med. 2015;24:918–920. [Google Scholar]
- 29.Cui Y, et al. Effect of shenfu injection on cardiac function and blood index in patients with chronic heart failure. Hebei med. 2016;22:1623–1625. [Google Scholar]
- 30.Wu H-Q. Study about efficacy of shenfu injection on cardiac function and bone marrow stem cell mobilization in cardiac failure. Guang’anmen hospital. 2008;
- 31.Chen G-Q, Wu H-Q, Chen G-L, Dong L-M, Wang Z-M. Effect of shenfu injection on the serum NT-proBNP level of patients with heart failure. J Emerg Syndromes Tradit Chin Med. 2014;24:134–135. [Google Scholar]
- 32.Ren Q, Wang Q, Lin P. Clinical observation of the effect of shenfu injection on chronic heart failure. Zhejiang J Integr Tradit Chin West Med. 2015;25:350–352. [Google Scholar]
- 33.Cheng G, et al. Observation of clinical efficacy of shenfu injection in patients with congestive heart failure. Shaanxi Med J. 2014;43:482–484. [Google Scholar]
- 34.Li L-N. Observation of clinical efficacy of shenfu injection in treating congestive heart failure. J Emerg Syndr Tradit Chin Med. 2016;25:2375–2377. [Google Scholar]
- 35.Mao X-Z. Clinical effect of shenfu injection on patients with chronic heart failure. J Baotou Med Coll. 2016;32:92–93. [Google Scholar]
- 36.Wu H-J, Duan H-W. Clinical study of shenfu injection for heart failure of coronary heart disease. Chin J Integr Med Cardio/ Cerebrovasc Dis. 2009;7:505–507. [Google Scholar]
- 37.Li C-Y. Observation of the effect of shenfu injection on chronic heart failure in elderly patients. Academic Conference of Gansu Province Medical Association; 2012.
- 38.Lv H-Y. Effects of shenfu injection on treatment of chronic heart failure and its effect on plasma BNP. Liaoning J Tradit Chin Med. 2014;41:726–727. [Google Scholar]
- 39.Yang Z-Y, Dong J-Y, Miao L-H. Clinical observation of shenfu injection in elderly patients with chronic heart failure. J Emerg Syndr Tradit Chin Med. 2010;19:2058–2059. [Google Scholar]
- 40.Cao Z-Q. Observation of efficacy of shenfu injection in treating congestive heart failure in elderly patients. Chin Foreign Med Treat. 2014;30:118–119. [Google Scholar]
- 41.Bao G-H, Yu L-H. 30 cases of chronic congestive heart failure were treated with shenfu injection. Chin Med Mod Dis Educ China. 2011;9:27. [Google Scholar]
- 42.Zhang A-P, Song G-P, Cai J-S. Clinical observation of 40 cases of chronic congestive heart failure treated with shenfu injection. J Emerg Syndromes Tradit Chin Med. 2011;20:1140–1141. [Google Scholar]
- 43.Liu Y. Clinical observation of 40 cases of chronic congestive heart failure treated with shenfu injection. J Emerg Syndromes Tradit Chin Med. 2008;16:51–52. [Google Scholar]
- 44.Ma L-P. Clinical study on 98 cases of chronic congestive heart failure treated with shenfu injection. World Health Dig. 2013;9:117. [Google Scholar]
- 45.Yao J, Lu X-R. Clinical observation on the treatment of chronic congestive heart failure with shenfu injection. J Med Theory Pract. 2010;23:287–288. [Google Scholar]
- 46.Ran X, Chen L, Wu W. Clinical observation of shenfu injection for chronic congestive heart failure. J New Chin Med. 2012;44:15–17. [Google Scholar]
- 47.Ding L. Observation of the effect of shenfu injection on chronic heart failure. Clin J Tradit Chin Med. 2013;25:482–483. [Google Scholar]
- 48.Zhu G-Y. Clinical observation of shenfu injection in the treatment of chronic heart failure. Hubei University of Traditional Chinese Medicine. 2013;
- 49.Qiu Q. Clinical observation of shenfu injection in treating chronic heart failure. Hunan J Tradit Chin Med. 2013;29:33–35. [Google Scholar]
- 50.Wang C-L. Clinical observation of 100 cases of chronic heart failure treated with shenfu injection. J Emerg Syndromes Tradit Chin Med. 2012;29:958–959. [Google Scholar]
- 51.Liu D-H, Chen G-Y, Chen W-X. Clinical observation of the curative effect of shenfu injection on chronic heart failure. The fourth Traditional Chinese Medicine continued education peak conference. 2011;
- 52.Qiu W-W. Clinical observation of the effectiveness of shenfu injection on chronic heart failure. J Emerg Syndromes Tradit Chin Med. 2010;19:420–421. [Google Scholar]
- 53.Qin Y, Zhou Q, Chen Y, Li J-P, Lv X-F. Clinical observation on the treatment of chronic heart failure with shenfu injection. J Emerg Syndromes Tradit Chin Med. 2015;24:161–162. [Google Scholar]
- 54.Fan T-B, Yang Z-X. Clinical observation on the treatment of chronic heart failure with shenfu injection. J Emerg Syndromes Tradit Chin Med. 2012;21:1851–1852. [Google Scholar]
- 55.Fu R. Clinical observation on the treatment of chronic heart failure with shenfu injection. J Chin Tradit Chin Med Inf. 2011;3:245–246. [Google Scholar]
- 56.Jin Z-C, Jin Z-X, Han L. Study on clinical efficacy of shenfu injection in the treatment of chronic heart failure. Pharmacol Clin Chin Mater Med. 2015;31:159–161. [Google Scholar]
- 57.Gao F. Effect of shenfu injection on inflammatory cytokines in patients with chronic heart failure. J Emerg Syndromes Tradit Chin Med. 2012;21:1646–1647. [Google Scholar]
- 58.Wang J, Zhang J, Ran G-Y. Clinical effect of shenfu injection on chronic heart failure and its impact on serum hs-CRP and IL-6. J Hubei Univ Chin Med. 2015;17:10–12. [Google Scholar]
- 59.Jiang L-X, Luo P. 96 cases of heart failure in treatment with shenfu injection. Shaanxi J Tradit Chin Med. 2011;32:1285–1286. [Google Scholar]
- 60.Zhang Z-F, Lv H-G. Observation on the curative effect of shenfu injection on chronic heart failure in elderly patients. People’s Mil Surg. 2009;52:360–361. [Google Scholar]
- 61.Liu D-Q, Zheng Z-M, Zhang K. Treatment of chronic congestive heart failure with shenfu injection. Jiangxi J Med Pharm. 2005;40:529–530. [Google Scholar]
- 62.Yu H-B, et al. Changes of nitric oxide synthase, adrenal medulla lignin, interleukin-10 in heart failure patients and the intervention effect of shenfu injection. Chin J Gerontol. 2014;34:7085–7086. [Google Scholar]
- 63.Wang Y-X. Clinical observation on the short-term treatment of chronic congestive heart failure in elderly patients with shengmai injection. Chin Tradit Pat Med. 2010;32:713–714. [Google Scholar]
- 64.Wang G-T, He Y-Q, Yang P. Effects of shengmai injection on cardiac function and plasma brain natriuretic peptide in patients with congestive heart failure. Chin J Integr Med. 2010;30:551–553. [Google Scholar]
- 65.Zhou J. Clinical effect of shengmai injection on plasma BNP in patients with congestive heart failure. J Emerg Syndromes Tradit Chin Med. 2009;18:732–733. [Google Scholar]
- 66.Pan J-J. Therapeutic effect of shengmai injection on chronic heart failure. China Contemp Med. 2014;20:70–71. [Google Scholar]
- 67.Wang Z-Y, Hu X-Y, Liu F-Z. Clinical observation of shengmai injection for congestive heart failure. Lishizhen Med Mater Med Res. 2004;15:353. [Google Scholar]
- 68.Zhao Q-F, Dai J, Bai X-L. Clinical efficacy of shengmai injection as adjuvant therapy for elderly patients with chronic congestive heart failure: an analysis of 31 cases. Hunan J Tradit Chin Med. 2015;31:23–25. [Google Scholar]
- 69.Wu C-Z, Hu M. Clinical observation of 36 cases of chronic congestive heart failure in elderly patients. Yunnan J Tradit Chin Med Mater Med. 2011;32:30. [Google Scholar]
- 70.Yang Z-Z, Zhang Z. Clinical observation of 43 cases of chronic congestive heart failure assisted by shengmai injection. Chin Med Her. 2010;7:80–81. [Google Scholar]
- 71.Cheng F-Y. Clinial treatment of 45 cases of chronic congestive heart failure with shengmai injection. Chin J Integr Med Cardio/ Cerebrovasc Dis. 2007;5:1239–1240. [Google Scholar]
- 72.Ni Y-M. 43 cases of chronic heart failure in treatment with shengmai injection. Her Med. 2012;31:171–172. [Google Scholar]
- 73.Xu J. 50 cases of chronic heart failure in treatment with shengmai injection. J M Udanjiang M Ed Coll. 2004;25:32–33. [Google Scholar]
- 74.Wen Y. Shengmai injection combined with conventional drug in the treatment of chronic heart failure for 20 cases. Chin Med Mod Dis Educ China. 2016;14:139–141. [Google Scholar]
- 75.Wen Y. Efficacy of shengmai injection in treatment of 64 cases with chronic heart failure. J Chengdu Univ Tradit Chin Med. 2013;36:76–78. [Google Scholar]
- 76.Kong LG, Zhu KW. Clinical observation of 30 cases of congestive heart failure in treatment of congestive heart failure. J Clin Emerg Call. 2004;5:26–27. [Google Scholar]
- 77.Tang E-W, Zeng Y-R. Observation of 33 cases of congestive heart failure treated by shengmai injection. Youjiang Med J. 2011;29:10–11. [Google Scholar]
- 78.Li W-D, Zhang T-S. Therapeutic effect of shengmai injection on congestive heart failure. Chin Commun Doct. 2002;18:35. [Google Scholar]
- 79.Chen Z-G. Therapeutic effect of shengmai injection on chronic heart failure in elderly patients. For All Health. 2016;10:28. [Google Scholar]
- 80.Luo X-Y. Therapeutic effect of shengmai injection on chronic heart failure in elderly patients. Med Inf. 2014;27:271. [Google Scholar]
- 81.Zhai Y-M. Clinical research of pulse-activating injection on 30 cases of chronic congestive heart failure. J Henan Univ Chin Med. 2009;24:59–61. [Google Scholar]
- 82.He D-Y, Wang P, Liu Q. Clinial treatment of 20 cases of chronic heart failure with shengmai injection. Henan Tradi Chin Med. 2006;26:71–72. [Google Scholar]
- 83.Chen K-H, Xu Z-Q, Liu QS. Analysis of 36 cases of chronic heart failure treated by shengmai injection. Zhejiang J Integr Tradit Chin West Med. 2008;18:155–156. [Google Scholar]
- 84.Wu X. Clinical observation of 52 cases of chronic heart failure treated by shengmai injection. For All Health. 2013;7:56. [Google Scholar]
- 85.Lu F. Clinical observation of 68 cases of chronic heart failure treated by shengmai injection. J Emerg Syndromes Tradit Chin Med. 2012;21:1695. [Google Scholar]
- 86.Liu S-H. Clinical observation of 120 cases of chronic heart failure treated by shengmai injection. National academic seminar on Wang qingren thought. 2008;
- 87.Zou X, Shi S-Q, Han Y. Clinical observation of the treatment of chronic heart failure by shengmai injection. Clin J Tradit Chin Med. 2011;23:777–778. [Google Scholar]
- 88.Kong W-W. Study on shengmai injeetion in patients with chronic heart failure of type qi and yin deficiency. Guangdong University Traditional Chinese Medicine; 2010.
- 89.Ni L-M, Ding C-Y. Curative effect observation on the treatment of congestive heart failure. Zhejiang J Tradit Chin Med. 2007;42:668. [Google Scholar]
- 90.Wang R-L, Zhou X-Y, Tian X-Z. 80 cases of chronic heart failure in treated with shengmai injection. Med Inf. 2007;20:326–327. [Google Scholar]
- 91.Li S-G. Clinical study on effects of shengmai injection with chronic heart failure treatment by monitoring the level of plasma NT-probnp. Fujian College of Traditional Chinese Medicine. 2009;
- 92.Zhang Y-T. Clinical effect of Chinese and western medicine on 103 cases of chronic congestive heart failure. Xinjiang J Tradit Chin Med. 2015;33:42–43. [Google Scholar]
- 93.Shi G-R. Treatment of chronic heart failure by Chinese and western medicine. Liaoning University of Traditional Chinese Medicine. 2009;
- 94.Pan Q-H, Chu S-X, Zheng Z-X, Jiang L-Q, Chen J-S. Effect of shenmai injection on the efficacy and plasma BNP of chronic congestive heart failure. Zhejiang J Tradit Chin Med. 2014;49:422. [Google Scholar]
- 95.Huang S-E, Huang Q, Yao Q, Pei D-A. Influence of shenmai injection on chronic contractive heart failure patients’ heart function and BNP. J Zhejiang Univ Tradit Chin Med. 2011;35:718–719. [Google Scholar]
- 96.Hou X-L, Hong J-K, Xiao X-Y, Chen S-X. Effect of shenmai injection on cardiac function and brain natriuretic peptide in patients with chronic heart failure. Mod J Integ Tradit Chin West Med. 2014;23:2904–2905. [Google Scholar]
- 97.Wang J-L, Zhou W-J, Shi G-P. Clinical observation of 51 cases of senile heart failure assisted by shenmai injection. Suzhou Univ J Med Sci. 2010;30:1289–1290. [Google Scholar]
- 98.Liu XR. Clinical study of combined western medicine with shenmai injection to treat chronic heart failure. World Health Dig Med Period. 2011;8:103. [Google Scholar]
- 99.Qu F. Clinical observation of shenmai injection to treat congestive heart failure. J Emerg Syndromes Tradit Chin Med. 2006;15:1102. [Google Scholar]
- 100.Wu QG. Effect of shenmai injection on chronic congestive heart failure. Mod J Integr Tradit Chin West Med. 2011;20:3961–3962. [Google Scholar]
- 101.Tian J-H, Zhang R-H. Clinical efficacy of shenmai injection in chronic heart failure. Chin Med Mod Dis Educ China. 2010;8:98–99. [Google Scholar]
- 102.Cui H-S. Analysis of 42 cases of chronic heart failure treated by shenmai injection. Neimonggu J Tradit Chin Med. 2008;27:65–66. [Google Scholar]
- 103.Hu C-L. Effective observation on shenmai injection for chronic heart failure of 132 cases. Med J West China. 2011;23:2162–2163. [Google Scholar]
- 104.Ye J-F. Effect of shenmai injection on the treatment of chronic heart failure and its effect on plasma cerebral natriuretic peptide. Mod Pract Med. 2015;27:201–202. [Google Scholar]
- 105.Wang J. Therapeutic effective observation on 22 cases of congestive heart-failure treated with combined Chinese traditional and western therapy. Hunan J T Radit Chin Med. 2002;18:7–8. [Google Scholar]
- 106.Guo H-J, Tao Q-X, Li S-L. Chinese and western medicine combined treatment of 58 cases of chronic heart failure. Jilin J Tradit Chin Med. 2012;32:681–683. [Google Scholar]
- 107.Wu Y. Effect of shenqi fuzheng injection on the left ventricle of the heart and BNP on patients with chronic heart failure. Pract J Cardiac Cereb Pneum Vasc Dis. 2014;22:45–46. [Google Scholar]
- 108.Su H-M. Observation of the quality of chronic ischemic slow heart failure in the injection of shenqi fuzheng injection. Chin Commun Doct. 2009;11:163–164. [Google Scholar]
- 109.Wang L-W. Observation of shenqi fuzheng injection on cardiac function, BNP and myocardial troponin in patients with chronic congestive heart failure. Mod J Integ Tradit Chin West Med. 2014;23:1766–1768. [Google Scholar]
- 110.Wang L, Ye B-H, Wang D-X, Li BT. Effects of shenqi fuzheng injection on plasma n terminal pro-brain natriuretic peptide in patients with chronic heart failure. Pract J Cardiac Cereb Pneum Vasc Dis. 2011;19:1774–1775. [Google Scholar]
- 111.Liu P, Xu X-Y, Li N. Effect of shenqi fuzheng injection on peripheral blood ANGII, ET-1 levels and clinical curative effect in patients with congestive heart failure. Chin J Biochem Pharm. 2015;35:81–83. [Google Scholar]
- 112.Liang S-X. Observation of 43 cases of chronic congestive heart failure with elderly patients in treating with shenqi fuzheng injection. J New Chin Med. 2009;47:74–75. [Google Scholar]
- 113.Chen F-J, Chen L-C. Clinical curative effect and safety of shenqi fuzheng injection in the treatment of elderly patients with chronic heart failure. Chin J Biochem Pharm. 2017;37:137–139. [Google Scholar]
- 114.Yang S-J. Observation of curative effect of shenqi fuzheng injection on chronic congestive heart failure. World Latest Med Inf. 2015;15:166–167. [Google Scholar]
- 115.He F-T. Observation of curative effect of shenqi fuzheng injection on congestive heart failure. J Emerg Syndromes Tradit Chin Med. 2003;12:399–340. [Google Scholar]
- 116.Yun M-L. Clinic observation of 55 cases with chronic heart failure treated by shenqi fuzheng injection. Nat Sci J Hainan Univ. 2006;24:371–373. [Google Scholar]
- 117.Wu Z-G, Feng F-J, Xie W-B. Clinical effect of shenqi fuzheng injection on the treatment of chronic heart failure. Res Integrated Tradit West Med. 2015;7:236–237. [Google Scholar]
- 118.Mao H-Y. Clinical observation of chinese and western medicine in treating chronic congestive heart failure. J Pract Tradit Chin Med. 2014;30:120–121. [Google Scholar]
- 119.Wang H-P, Wu Z-G. Clinical observation of digoxin tablet combined with yiqifumai in treatment of chronic heart failure. Drugs & Clin. 2014;29:532–535. [Google Scholar]
- 120.Zhao Y-B. Clinical observation on treating chronic heart failure by yiqifumai injection. Mod Med J China. 2015;17:72–73. [Google Scholar]
- 121.Xue L-X, Wang H-L, Lei X, Feng L. Effects of yiqifumai injection on cardiac function and plasma BNP in chronic heart failure. Chin J Integr Med Cardio/ Cerebrovasc Dis. 2014;12:279–280. [Google Scholar]
- 122.Ren H. Clinical observation on treating chronic heart failure by yiqifumai injection (freeze-dried) combined with trimetazidine. The World Clin Med. 2016;10:4–5. [Google Scholar]
- 123.Yang C-L, Liu Z-H. Clinical study on the treatment of chronic heart failure in elderly patients with coronary heart disease by yiqifumai injection (freeze-dried) Pract Geriatr. 2014;28:607–608. [Google Scholar]
- 124.Zhao X-F, Liu J-Y, Zhang M-L. Clinical efficacy of injection therapy for chronic heart failure with yiqifumai injection (freeze-dried) Shanxi Med J. 2015;44:1533–1535. [Google Scholar]
- 125.Punchik B, et al. Can home care for homebound patients with chronic heart failure reduce hospitalizations and costs? PLoS One. 2017;12:e0182148. doi: 10.1371/journal.pone.0182148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cui W. Highlights of european society of cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Chin J Cardiovasc Med. 2012;16:324–326. [Google Scholar]
- 127.Chen C-Y, Lu L-Y, Chen P, et al. Shengmai injection, a traditional Chinese patent medicine, for intradialytic hypotension: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2013;2013:1-14. [DOI] [PMC free article] [PubMed]
- 128.Chen J, Luo M-X, Zheng Q, Zhou Y-C. Effect of shengmai injection on patients with chronic heart failure: a meta-analysis. Chin J Inf Tradit Chin Med. 2011;18:25–29. [Google Scholar]
- 129.Hu Z-Z, Tang L-M, Lin Y. Meta-analysis of the efficacy of shengmai injection in treating congestive heart failure. Zhejiang J Integr Tradit Chin West Med. 2016;26:32–37. [Google Scholar]
- 130.Yuan Y, Mao J-Y, Tang E, Hou Y-Z, Wang X-L. Treatment of chronic heart failure with western drugs combining shengmai injection: a systematic review on randomized controlled trials. Chin J Evid Based Cardiovasc Med. 2014;6:519–524. [Google Scholar]
- 131.Shi L, Xie Y, Liao X, Luo Y. Shenmai injection as an adjuvant treatment for chronic cor pulmonale heart failure: a systematic review and meta-analysis of randomized controlled trials. BMC Complement Altern Med. 2015;15:1. doi: 10.1186/s12906-015-0939-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Duan Y-P, Meng C-L, Li P. Pharmacological action and clinical application of shenmai injection. Chin Med Abstr. 2007;23:698–700. [Google Scholar]
- 133.Chen H-D, Xie Y-M, Wang L-X, Wu J-B. Systematic review of efficacy and safety of shenmai injection for chronic heart failure. China J Chin Mater Med. 2014;39:3650–3662. [PubMed] [Google Scholar]
- 134.Hou Y-Z, Mao J-Y, Wang X-L, Liu C-X, Zhang C. Shenmai injection in heart failure patients: a systematic review and meta-analysis. Chin J Evid Base Med. 2010;10:939–945. [Google Scholar]
- 135.Duan P. Systematic review of efficacy and safety of shenmai injection in treatment of heart failure. J Taishan Med Coll. 2014;35:1063–1064. [Google Scholar]
- 136.Yan W-L, Tong X-T, Yang S-Q. The causes and countermeasures of adverse reactions of traditional Chinese medicine injection. Mod Tradit Chin Med. 2016;36:56–58. [Google Scholar]
- 137.Liu J-G, Liu Y-L, Zhang H-M, Li Y-L. Exploration on the adverse reactions of chinese herbal injection and the preventive measures. World J Integr Tradit West Med. 2017;12:81–84. [Google Scholar]
- 138.Hao C, Liu F-Q. Progress on cause of adverse reaction of traditional Chinese medicine. J Pharm Res. 2017;36:369–372. [Google Scholar]
- 139.Zhang X-X. A review on adverse reaction from TCM injection. Clin J Chin Med. 2017;9:141–142. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist for network meta-analysis. This file contained items about PRISMA checklist for network meta-analysis and corresponding pages of this study. (DOC 66 kb)
Search strategy. This file contained the search strategy of traditional Chinese medicine injections and English database. (DOC 39 kb)
Characteristics of included randomized controlled trials. This file contained the information about included randomized controlled trials. (DOC 38 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


