Abstract
Background
Because the survival rate for patients experiencing late complications after pancreaticoduodenectomy (PD) is increasing, late complications should receive as much attention as early complications do.
Methods
Between April 2007 and August 2016, 133 patients underwent PD at our institution. We analyzed their cases to determine the predictors of late cholangitis after PD.
Results
Of the 133 patients, 28 (21.1%) were diagnosed with postoperative cholangitis. A multivariate analysis showed that abnormal postoperative values of alkaline phosphatase were independently associated with postoperative cholangitis (odds ratio, 3.81; 95% confidence interval, 1.519–9.553; P = 0.004). The optimal cut-off value for postoperative alkaline phosphatase calculated from the receiver operating characteristic curve was 410 IU/L (sensitivity, 76.2%; specificity, 67.9%; area under the curve, 0.73). A univariate analysis to identify risk factors showed that pneumobilia was significantly related to a postoperative alkaline phosphatase value ≥ 410 IU/L (P = 0.041).
Conclusion
This study suggests that an alkaline phosphatase level ≥ 410 IU/L is a predictor of late postoperative cholangitis. In addition, pneumobilia is also related to the postoperative alkaline phosphatase level. Therefore, alkaline phosphatase levels should be carefully monitored in patients with postoperative pneumobilia in the late postoperative course.
Keywords: Pancreaticoduodenectomy, Cholangitis, Late complication, Alkaline phosphatase
Background
The survival rate for patients undergoing pancreaticoduodenectomy (PD) for peripancreatic carcinoma is increasing due to improvements in operative techniques, perioperative management, and early detection. Thus, late complications after PD should receive as much attention as early complications do. Reported early complications after PD include pancreatic fistula, delayed gastric emptying, infectious complications, and biliary complications [1, 2]. Several studies focused on late complications after PD have been reported. However, very few have focused on late biliary complications, especially postoperative cholangitis.
Most patients with late postoperative cholangitis are managed with conservative therapy. Because severe cholangitis is a critical condition, it is often difficult to manage. Moreover, severe and recurrent cholangitis may prevent recovery after surgery. However, the mechanism underlying postoperative cholangitis has not been clarified yet.
The aim of this retrospective study was to determine the predictors of late cholangitis after PD.
Methods
Between April 2007 and August 2016, 133 patients underwent PD at our institution. Preoperative demographic and clinical data and details related to the surgical procedure and postoperative course were collected retrospectively. We analyzed these data to determine the predictors of cholangitis after PD. The Clinical Ethics Committee of our hospital approved this study, and informed consent was waived.
If patients were diagnosed with a biliary abnormality such as liver dysfunction, jaundice, cholangitis, and/or bile duct dilatation due to a periampullary tumor, preoperative biliary drainage was performed. The method of biliary drainage (i.e., endoscopic nasobiliary drainage [ENBD], endoscopic retrograde biliary drainage [ERBD], or percutaneous transhepatic biliary drainage [PTBD]) was chosen by a gastroenterologist in accordance with local policy.
All operations were performed by experienced pancreatic surgeons. Lymph nodes around the head of the pancreas, the common hepatic artery, and the hepatoduodenal ligament were dissected during pancreatectomy. After resection, reconstructions were developed according to the modified Child method or Traverso method. After resection, anastomoses were constructed to a single jejunal loop, which was brought through the transverse mesocolon in a retrocolic manner. First, a pancreaticojejunal anastomosis was performed in an end-to-side fashion. After pancreaticojejunal anastomosis, hepaticojejunostomy was performed with an end-to-side single layer of interrupted sutures using 4–0 absorbable suture materials. In general, we did not use biliary stenting. In cases of a small bile duct, an internal drainage tube was placed in the anastomosis. Finally, the operation was completed with an end-to-side duodenojejunostomy or gastrojejunostomy 40 cm downstream from the hepaticojejunostomy.
Perioperative management was standardized. All patients received broad-spectrum antibiotics for 1 day. No prophylactic somatostatin or octreotide was used. The nasogastric tube was removed on the first postoperative day when discharge was less than 500 mL. Total parenteral nutrition was used only in patients who could not tolerate a diet after postoperative day 5. Peripancreatic drains were removed if there was no evidence of leakage. If there was evidence of leakage or suspicion of infective complications (fever, leukocytosis, or purulent drain fluid), peripancreatic drains were left in situ, and a contrast-enhanced computed tomography (CT) scan was performed to determine if there was any intra-abdominal collection.
Patients underwent follow-up consisting of laboratory tests and ultrasonography or CT every 3 months during the first 3 years postoperatively. After 3 years, they were followed at 6-month intervals. Postoperative alkaline phosphatase concentrations greater than the normal range (104–338 IU/L) were regarded as abnormal. Patients who were followed with no evidence of cholangitis within 1 year after surgery were excluded to avoid future migrations.
Cholangitis was defined based on systemic inflammation, cholestasis, and imaging findings, in accordance with the updated Tokyo Guidelines (TG13) [3]. Our study included patients with a suspected diagnosis based on the TG13 diagnostic criteria for acute cholangitis. Symptoms occurring in the first month after surgery were excluded to avoid confusion caused by contamination due to an inflammatory response related to surgical stress. All the patients who were diagnosed as having cholangitis were hospitalized, and antibiotic treatments were started promptly.
Pancreatic fistula was defined according to the guidelines of the International Study Group on Pancreatic Fistula (ISGPF) [4]. Grades B and C were considered to be clinically relevant in this study. Bile leakage was defined according to the guidelines of the International Study Group of Liver Surgery (ISGLS) [5] as a drain bilirubin concentration of at least three times of the serum bilirubin concentration. Delayed gastric emptying was defined by the guidelines of the International Study Group of Pancreatic Surgery (ISGPS) [6]. Patients with all grades (grades A, B, or C) of delayed gastric emptying were enrolled in this study.
Continuous data are expressed as the mean ± standard deviation (SD). The chi-squared test or Fisher’s exact test was used to compare categorical data, and Student’s t test or the Mann-Whitney U test was used for continuous data, as appropriate. A logistic regression analysis was performed for a multivariate analysis to determine the independent risk factors. A P value < 0.05 was considered to be statistically significant. Analyses were performed using SPSS 19.0 software (SPSS Japan Inc., Tokyo, Japan).
Results
One hundred and thirty-three consecutive patients underwent PD between April 2007 and August 2016, consisting of 77 men and 56 women with an average age of 67.2 years (range, 44–85). The average total bilirubin value of all patients before biliary drainage was 4.94 ± 5.49 mg/dL. Overall, 93 (69.9%) patients underwent preoperative biliary drainage. The diagnoses of the patients included pancreatic carcinoma (n = 49, 36.8%), cholangiocarcinoma (n = 49, 36.8%), ampullary carcinoma (n = 13, 9.8%), intraductal papillary mucinous neoplasm (n = 6, 4.5%), neuroendocrine tumor (n = 5, 3.8%), and others (n = 11, 8.3%). The types of operations performed were as follows: 20 (15.0%) PD, 41 (30.8%) subtotal stomach-preserving PD (SSPPD), and 72 (54.1%) pylorus-preserving PD (PPPD). The average operation time and blood loss for all patients were 454.3 ± 99.8 min and 990.6 ± 701.3 mL, respectively. Transfusions were performed in 33 (24.8%) patients. The characteristics of all patients are listed in Table 1.
Table 1.
Characteristics of patients who underwent pancreaticoduodenectomy
| Characteristic | n = 133 |
|---|---|
| Age (years)a | 67.2 ± 9.4 |
| Sex | |
| Male | 77 |
| Female | 56 |
| Diagnosis | |
| Pancreatic carcinoma | 49 |
| Cholangiocarcinoma | 49 |
| Ampullary carcinoma | 13 |
| Intraductal papillary mucinous neoplasm | 6 |
| Neuroendocrine tumor | 5 |
| Others | 11 |
| Total bilirubin (mg/dL)a | 4.9 ± 5.5 |
| Preoperative biliary drainage | |
| Yes | 93 |
| No | 40 |
| Operation | |
| PD | 20 |
| SSPPD | 41 |
| PPPD | 72 |
| Operation time (min)a | 454.3 ± 99.8 |
| Blood loss (mL)a | 990.6 ± 701.3 |
| Transfusion | |
| Yes | 33 |
| No | 100 |
PD pancreaticoduodenectomy, SSPPD subtotal stomach-preserving pancreaticoduodenectomy, PPPD pylorus-preserving pancreaticoduodenectomy
aData are presented as mean ± standard deviation or n (%)
Of the 133 patients, 28 (21.1%) were diagnosed with postoperative cholangitis. The median duration to postoperative cholangitis onset was 275 (range, 30–3037) days after surgery. The median follow-up duration was 861 (range, 74–3000) days after surgery. Postoperative cholangitis occurred within the first year after surgery in 15 patients (53.6%) and within 2 years after surgery in 23 patients (82.1%) (Fig. 1). Cholangitis occurred more than 1000 days postoperatively in the remaining 5 patients (17.9%). The frequency of postoperative cholangitis was 1.8 ± 1.3 (range, 1–5) times. Postoperative cholangitis occurred more than twice in 11 (39.3%) patients.
Fig. 1.
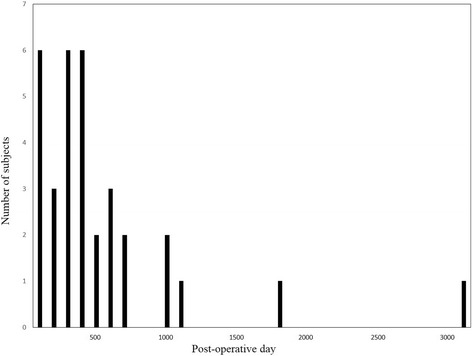
The time of postoperative cholangitis after surgery. Postoperative cholangitis occurred within the first year after pancreaticoduodenectomy in 15 patients (53.6%) and within 2 years after surgery in 23 patients (82.1%). Cholangitis occurred more than 1000 days postoperatively in the remaining 5 patients (17.9%)
Table 2 shows the patient characteristics. The preoperative, perioperative, and postoperative characteristics were compared between the postoperative cholangitis group and no-cholangitis group. In the univariate analysis, there was no significant difference in age, sex, diagnosis, presence of diabetes mellitus, jaundice, preoperative biliary drainage, type of operation, operation time, blood loss, whether a transfusion was required, pancreatic fistula, bile leakage, delayed gastric emptying, or pneumobilia between the two groups. Significant differences were found in the rate of patients with a body mass index ≥ 24 kg/m2 (P = 0.039) and with an abnormal postoperative value of alkaline phosphatase (P = 0.003). The multivariate analysis showed that an abnormal postoperative value of alkaline phosphatase was independently associated with postoperative cholangitis (odds ratio, 3.81; 95% confidence interval, 1.519–9.553; P = 0.004).
Table 2.
Analyses of risk factors for late postoperative cholangitis
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Late postoperative cholangitis | Odds ratio | 95% CI | P value | ||||
| (+) | (−) | ||||||
| n = 28 | n = 105 | P value | |||||
| Age (years) | ≥ 70 | 11 | 46 | 0.667 | |||
| < 70 | 17 | 59 | |||||
| Sex | Male | 17 | 60 | 0.734 | |||
| Female | 11 | 45 | |||||
| Body mass index (kg/m2) | ≥ 24 | 15 | 34 | 0.039 | 2.147 | 0.891–5.174 | 0.089 |
| < 24 | 13 | 71 | |||||
| Diagnosis | Benign | 1 | 8 | 0.684 | |||
| Malignancy | 27 | 97 | |||||
| Diabetes mellitus | Yes | 9 | 23 | 0.260 | |||
| No | 19 | 82 | |||||
| Jaundice (≥ 2.0 mg/dL) | Yes | 16 | 58 | 0.857 | |||
| No | 12 | 47 | |||||
| Preoperative biliary drainage | Yes | 16 | 77 | 0.097 | |||
| No | 12 | 28 | |||||
| Type of operation | PD/SSPPD | 9 | 52 | 0.101 | |||
| PPPD | 19 | 53 | |||||
| Operation time (min) | ≥ 420 | 16 | 64 | 0.715 | |||
| < 420 | 12 | 41 | |||||
| Blood loss (mL) | ≥ 800 | 16 | 57 | 0.787 | |||
| < 800 | 12 | 48 | |||||
| Transfusion | Yes | 7 | 26 | 0.979 | |||
| No | 21 | 79 | |||||
| Pancreatic fistule grade B/C | Yes | 6 | 13 | 0.224 | |||
| No | 22 | 92 | |||||
| Bile leakage | Yes | 1 | 1 | 0.378 | |||
| No | 27 | 104 | |||||
| Delayed gastric emptying | Yes | 0 | 9 | 0.203 | |||
| No | 28 | 96 | |||||
| Surgical site infection | Yes | 5 | 17 | 0.833 | |||
| No | 23 | 88 | |||||
| Pneumobilia | Yes | 15 | 41 | 0.167 | |||
| No | 13 | 64 | |||||
| Alkaline phosphatase levela | Abnormal | 20 | 42 | 0.003 | 3.81 | 1.519–9.553 | 0.004 |
| Normal | 8 | 63 | |||||
PD pancreaticoduodenectomy, SSPPD subtotal stomach-preserving pancreaticoduodenectomy, PPPD pylorus-preserving pancreaticoduodenectomy
aNormal range of alkaline phosphatase level, 104–338 IU/L
Regarding the factors associated with the incidence of postoperative cholangitis, a receiver operating characteristic (ROC) curve was constructed to evaluate the optimal cut-off point for the postoperative value of alkaline phosphatase. The optimal value calculated by the ROC curve was 410 IU/L (sensitivity, 76.2%; specificity, 67.9%). The area under the curve (AUC) was 0.73 (Fig. 2).
Fig. 2.
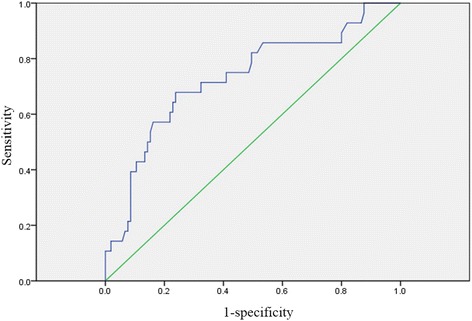
The ROC curve for alkaline phosphatase values and postoperative cholangitis in patients who underwent pancreaticoduodenectomy. The ROC curve was constructed to evaluate the optimal cut-off point for the postoperative value of alkaline phosphatase as a predictive factor associated with the incidence of postoperative cholangitis
A univariate analysis was performed to identify risk factors for the presence of a postoperative alkaline phosphatase value ≥ 410 IU/L. Pneumobilia was significantly related to a postoperative alkaline phosphatase value ≥ 410 IU/L (P = 0.041) (Table 3).
Table 3.
Relationship between alkaline phosphatase value and other factors
| Alkaline phosphatase | ||||
|---|---|---|---|---|
| ≥ 410 IU/L | < 410 IU/L | |||
| n = 44 | n = 89 | P value | ||
| Age (years) | ≥ 70 | 22 | 35 | 0.242 |
| < 70 | 22 | 54 | ||
| Sex | Male | 27 | 50 | 0.569 |
| Female | 17 | 39 | ||
| Body mass index (kg/m2) | ≥ 24 | 17 | 32 | 0.763 |
| < 24 | 27 | 57 | ||
| Diagnosis | Benign | 2 | 7 | 0.717 |
| Malignancy | 42 | 82 | ||
| Diabetes mellitus | Yes | 13 | 19 | 0.298 |
| No | 31 | 70 | ||
| Jaundice (≥ 2.0 mg/dL) | Yes | 26 | 48 | 0.573 |
| No | 18 | 41 | ||
| Preoperative biliary drainage | Yes | 32 | 61 | 0.620 |
| No | 12 | 28 | ||
| Type of operation | PD/SSPPD | 20 | 41 | 0.947 |
| PPPD | 24 | 48 | ||
| Operation time (min) | ≥ 420 | 31 | 49 | 0.088 |
| < 420 | 13 | 40 | ||
| Blood loss (mL) | ≥ 800 | 29 | 44 | 0.073 |
| < 800 | 15 | 45 | ||
| Transfusion | Yes | 13 | 20 | 0.374 |
| No | 31 | 69 | ||
| Pancreatic fistule grade B/C | Yes | 8 | 11 | 0.367 |
| No | 36 | 78 | ||
| Bile leakage | Yes | 2 | 0 | 0.108 |
| No | 42 | 89 | ||
| Delayed gastric emptying | Yes | 2 | 7 | 0.717 |
| No | 42 | 82 | ||
| Pneumobilia | Yes | 24 | 32 | 0.041 |
| No | 20 | 57 | ||
PD pancreaticoduodenectomy, SSPPD subtotal stomach-preserving pancreaticoduodenectomy, PPPD pylorus-preserving pancreaticoduodenectomy
Discussion
Postoperative cholangitis is likely to occur when the sphincter of Oddi is resected with a barrier function of reflux. As an early complication, the incidence of postoperative cholangitis was 1.0–16.6% of patients [7–10]. However, it might be difficult to diagnose cholangitis accurately in the early postoperative course because of contamination from an inflammatory response related to surgical stress. On the other hand, late biliary complications, such as postoperative cholangitis and biliary stricture, have been reported. Of these, postoperative cholangitis is a rarely encountered complication that may require emergent hospital readmission [11]. The reported long-term outcomes of biliary-enteric anastomoses were as follows: the incidence of postoperative cholangitis was 10.9% in the choledochoduodenostomy group and 6.4% in the hepaticojejunostomy group [12]. Late cholangitis after PD has reportedly occurred in 6.7–14.4% [7, 13, 14] of patients. These results are slightly lower than our result that 28 (21.1%) of 133 patients were diagnosed with postoperative cholangitis.
It is well known that postoperative cholangitis is caused by a biliary obstruction, such as biliary stricture, bile stasis, or stone. In addition, other reasons for postoperative cholangitis are as follows: intestinal obstruction, afferent limb syndrome, and stasis due to jejunal peristaltic failure [15]. Biliary stricture, defined as the need for endoscopic, percutaneous, or surgical intervention [14, 16], may cause postoperative cholangitis following bile stasis. Bile stasis is considered to be associated with bacterial growth in the bile juice [17]. Duconseil et al. found that 47% of 17 patients with biliary stricture developed postoperative cholangitis [16]. In our study, 2 patients with biliary stricture were also diagnosed with cholangitis. In addition, 7 (25%) patients with postoperative cholangitis were found to have biliary dilatation and anastomotic stenosis. However, follow-up CT evaluation revealed that there was no evidence of biliary obstruction after conservative therapy. Parra-Membrives et al. found even when postoperative cholangitis is caused by a true biliary stricture, about 40% of patients have a recurrent episode without a proven biliary stricture [14]. Thus, it was suggested that they had retained activity in the biliary tree. Therefore, there is a limitation to determining biliary obstruction without cholangitis by using CT. It seems reasonable to suppose that detecting bile stasis due to anastomotic stenosis before cholangitis is useful. In this study, as a predictive aid, results of the multivariate analysis showed that an abnormal postoperative value of alkaline phosphatase was independently associated with postoperative cholangitis. Additionally, pneumobilia was significantly related to a postoperative alkaline phosphatase value ≥ 410 IU/L. Another factor that causes postoperative cholangitis is bile stasis due to afferent limb syndrome; 50% of patients with afferent limb syndrome were reported to present with obstructive jaundice or cholangitis [15]. Despite bile stasis, proven afferent limb obstruction may be detected, because it was considered to be responsible for the reconstruction method chosen [18]. The duration to cholangitis onset after PD was fascinating. In this study, the median duration to postoperative cholangitis onset was 275 (range, 30–3037) days after surgery, and postoperative cholangitis occurred at a rate of approximately 50% within a year and of 80% within 2 years, respectively. Our results for the duration to cholangitis onset were similar to those previously reported for biliary stricture. The median reported duration to biliary stricture after PD was reported to be 13 months (range, 1 month to 9 years) [19] and 205 days (range, 12–1380 days) [16], respectively. Hence, it was assumed that postoperative cholangitis was associated with biliary stricture.
A previous study showed that preoperative biliary drainage with surgery for cancer of the head of the pancreas significantly increased the rate of postoperative cholangitis [20]. On the other hand, another study showed that preoperative biliary drainage was not associated with postoperative cholangitis. However, it has been suggested that the incidence of postoperative cholangitis was significantly higher in patients with bile duct carcinoma and was significantly associated with hospitalization and intensive care unit stay [13]. The efficacy of preoperative biliary drainage for postoperative cholangitis remains controversial. Few studies have reported on late postoperative cholangitis. In our investigation of postoperative cholangitis, the multivariate analysis showed that an abnormal postoperative value of alkaline phosphatase was independently associated with postoperative cholangitis. Additionally, we focused on the impact of the alkaline phosphatase cut-off value demonstrated by the ROC curve, which we expect to be a predictor of postoperative cholangitis. Moreover, pneumobilia was significantly related to a postoperative alkaline phosphatase value ≥ 410 IU/L. Chan et al. reported that pneumobilia was detected in 52% of patients with recurrent pyogenic cholangitis [21]. We compared differences between the postoperative cholangitis group and no-cholangitis group in this study, and there was not a significant difference in pneumobilia between the two groups. However, pneumobilia was significantly related to a postoperative alkaline phosphatase value ≥ 410 IU/L. Therefore, a strict follow-up that includes measurement of alkaline phosphatase levels should be provided, especially to patients with pneumobilia during the postoperative course.
Generally speaking, with the exception of postoperative day 1, patients received no antimicrobial therapy because unnecessary antimicrobial therapy could induce drug-resident bacteria. Cammann et al. reported that intraoperative bile culture as a prophylaxis for postoperative cholangitis was useful because it can be altered by antimicrobial prophylaxis [13]. Another study reported that a short course of postoperative antimicrobial therapy reduced the occurrence of infectious complications after PD [22]. Therefore, antimicrobial therapy was provided to patients at the time that cholangitis was diagnosed because little has been reported on antimicrobial prophylaxis for postoperative cholangitis. Both of the previous studies investigated complications in the early postoperative course. Prophylactic antibiotics for late postoperative cholangitis is still incompletely understood. In this study, 11 (39.3%) of 28 patients with postoperative cholangitis experienced recurrent cholangitis. Long-term exposure to bile juice due to biliary stasis, reflux, or infection may arise from cholangiocarcinoma [23]. An experimental study demonstrated that exposure to digestive enzymes and bacteria caused epithelial hyperplasia in rats [24]. Moreover, Tocchi et al. reported the long-term outcomes for patients undergoing biliary-enteric anastomosis [12]. The incidence of cholangiocarcinoma was 7.6% after choledochoduodenostomy and 1.9% after hepaticojejunostomy. Reflux of digestive fluid and bacteria by recurrent cholangitis is considered a risk factor for carcinogenesis of the choledochal epithelium. Although most patients with postoperative cholangitis improve with conservative therapy, patients with frequent recurrence should be considered for improvement measures.
As noted, the mechanism underlying postoperative cholangitis is still unclear. How to prevent these complications should be considered. Few studies have focused on preoperative biliary drainage. A previous report showed that preoperative biliary drainage was associated with a rate of complications that was significantly higher than that in the early-surgery group [20]. In particular, the biliary drainage group had a higher incidence of postoperative cholangitis (26%) than did the early-surgery group (2%). On the other hand, Sahora et al. reported that there was no significant difference in the overall postoperative morbidity and mortality between the two groups [25]. However, the number of positive bile cultures was significantly higher in the preoperative biliary drainage group than in the non-preoperative biliary drainage group. Thus, taken together, the results obtained so far are controversial. In addition, regarding postoperative biliary drainage, the incidence of postoperative cholangitis was significantly higher in patients with external stents (25%) than in patients with no stents (3.8%). The limitation of this study was the small number of patients and its retrospective nature. Furthermore, Hiyoshi et al. reported that hepaticoplasty to widen the small bile duct was useful for preventing postoperative cholangitis [26]. Since late postoperative cholangitis may occur suddenly, it would be helpful to predict its occurrence.
Our study has several limitations. First, a small number of patients were included to investigate postoperative cholangitis. Second, it is occasionally difficult to diagnose cholangitis after PD, so there may have been bias because of contamination of postoperative conditions. Third, the follow-up duration for patients without cholangitis was not sufficient to exclude its occurrence entirely. This is supported by our result showing that approximately 20% of patients with postoperative cholangitis developed it more than 2 years after PD. Thus, patients who might have developed postoperative cholangitis in the future may have been included in the group of patients without postoperative cholangitis. The final limitation of this study is the inability to assess for antibioprophylaxis and management of postoperative cholangitis. Therefore, a randomized controlled trial is required to confirm a specific postoperative management.
Conclusion
A postoperative alkaline phosphatase value ≥ 410 IU/L was useful for predicting the development of late postoperative cholangitis. Additionally, pneumobilia was related to the postoperative alkaline phosphatase value. Therefore, careful follow-up is needed in the late postoperative course.
Acknowledgements
Not applicable.
Funding
This work was not supported by any sources of funding.
Availability of data and materials
The authors presented all necessary information about the study in the manuscript and do not wish to share the data.
Abbreviations
- AUC
Area under the curve
- ISGPF
International Study Group on Pancreatic Fistula
- PD
Pancreaticoduodenectomy
- PPPD
Pylorus-preserving pancreaticoduodenectomy
- ROC
Receiver operating characteristic
- SSPPD
Subtotal stomach-preserving pancreaticoduodenectomy
- TG13
Tokyo Guidelines
Authors’ contributions
YI and YA designed methods and carried out the instructions, analyzed the data, interpreted the results, and drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Clinical Ethics Committee of Saiseikai Yokohamashi Tobu Hospital approved this study. Informed consent was not obtained because this was a retrospective study and person’s information was not included.
Consent for publication
Written informed consent for publication was obtained from the parents of the patients.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yasuhiro Ito, Email: yasuito@ca3.so-net.ne.jp.
Yuta Abe, Phone: +81 3 3353 1211, Email: abey3666@gmail.com.
Minoru Kitago, Email: dragonpegasus427@gmail.com.
Osamu Itano, Email: laplivertiger@gmail.com.
Yuko Kitagawa, Email: kitagawa@a3.keio.jp.
References
- 1.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiriyama S, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Pitt HA, Gouma DJ, Garden OJ, Buchler MW, Yokoe M, et al. TG13 guidelines for diagnosis and severity grading of acute cholangitis (with videos) J Hepatobiliary Pancreat Sci. 2013;20:24–34. doi: 10.1007/s00534-012-0561-3. [DOI] [PubMed] [Google Scholar]
- 4.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi K, Tanaka M, Chijiiwa K, Nagakawa T, Imamura M, Takada T. Early and late complications of pylorus-preserving pancreatoduodenectomy in Japan 1998. J Hepato-Biliary-Pancreat Surg. 1999;6:303–311. doi: 10.1007/s005340050122. [DOI] [PubMed] [Google Scholar]
- 8.Braga M, Capretti G, Pecorelli N, Balzano G, Doglioni C, Ariotti R, Di Carlo V. A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg. 2011;254:702–707. doi: 10.1097/SLA.0b013e31823598fb. [DOI] [PubMed] [Google Scholar]
- 9.Imai H, Osada S, Tanahashi T, Sasaki Y, Tanaka Y, Okumura N, Matsuhashi N, Nonaka K, Nagase M, Takahashi T, et al. Retrospective evaluation of the clinical necessity of external biliary drainage after pancreaticoduodenectomy. Hepato-Gastroenterology. 2013;60:2119–2124. doi: 10.5754/hge13555. [DOI] [PubMed] [Google Scholar]
- 10.Malgras B, Duron S, Gaujoux S, Dokmak S, Aussilhou B, Rebours V, Palazzo M, Belghiti J, Sauvanet A. Early biliary complications following pancreaticoduodenectomy: prevalence and risk factors. HPB (Oxford) 2016;18:367–374. doi: 10.1016/j.hpb.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong ZV, Ferrone CR, Thayer SP, Wargo JA, Sahora K, Seefeld KJ, Warshaw AL, Lillemoe KD, Hutter MM, Fernandez-Del Castillo C. Understanding hospital readmissions after pancreaticoduodenectomy: can we prevent them?: a 10-year contemporary experience with 1,173 patients at the Massachusetts General Hospital. J Gastrointest Surg. 2014;18:137–144. doi: 10.1007/s11605-013-2336-9. [DOI] [PubMed] [Google Scholar]
- 12.Tocchi A, Mazzoni G, Liotta G, Lepre L, Cassini D, Miccini M. Late development of bile duct cancer in patients who had biliary-enteric drainage for benign disease: a follow-up study of more than 1,000 patients. Ann Surg. 2001;234:210–214. doi: 10.1097/00000658-200108000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cammann S, Timrott K, Vonberg RP, Vondran FW, Schrem H, Suerbaum S, Klempnauer J, Bektas H, Kleine M. Cholangitis in the postoperative course after biliodigestive anastomosis. Langenbeck’s Arch Surg. 2016;401:715–724. doi: 10.1007/s00423-016-1450-z. [DOI] [PubMed] [Google Scholar]
- 14.Parra-Membrives P, Martinez-Baena D, Sanchez-Sanchez F. Late biliary complications after pancreaticoduodenectomy. Am Surg. 2016;82:456–461. [PubMed] [Google Scholar]
- 15.Pannala R, Brandabur JJ, Gan SI, Gluck M, Irani S, Patterson DJ, Ross AS, Dorer R, Traverso LW, Picozzi VJ, Kozarek RA. Afferent limb syndrome and delayed GI problems after pancreaticoduodenectomy for pancreatic cancer: single-center, 14-year experience. Gastrointest Endosc. 2011;74:295–302. doi: 10.1016/j.gie.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Duconseil P, Turrini O, Ewald J, Berdah SV, Moutardier V, Delpero JR. Biliary complications after pancreaticoduodenectomy: skinny bile ducts are surgeons’ enemies. World J Surg. 2014;38:2946–2951. doi: 10.1007/s00268-014-2698-5. [DOI] [PubMed] [Google Scholar]
- 17.Chuang JH, Lee SY, Chen WJ, Hsieh CS, Chang NK, Lo SK. Changes in bacterial concentration in the liver correlate with that in the hepaticojejunostomy after bile duct reconstruction: implication in the pathogenesis of postoperative cholangitis. World J Surg. 2001;25:1512–1518. doi: 10.1007/s00268-001-0162-9. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto N. Hepatobiliary imaging after pancreaticoduodenectomy—a comparative study on Billroth I and Billroth II reconstruction. Hepato-Gastroenterology. 2005;52:1023–1025. [PubMed] [Google Scholar]
- 19.House MG. Cameron JL, Schulick RD, Campbell KA, Sauter PK, Coleman J, Lillemoe KD, Yeo CJ. Incidence and outcome of biliary strictures after pancreaticoduodenectomy. Ann Surg. 2006;243:571–576. doi: 10.1097/01.sla.0000216285.07069.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, Gerritsen JJ, Greve JW, Gerhards MF, de Hingh IH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129–137. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 21.Chan FL, Man SW, Leong LL, Fan ST. Evaluation of recurrent pyogenic cholangitis with CT: analysis of 50 patients. Radiology. 1989;170:165–169. doi: 10.1148/radiology.170.1.2909092. [DOI] [PubMed] [Google Scholar]
- 22.Sourrouille I, Gaujoux S, Lacave G, Bert F, Dokmak S, Belghiti J, Paugam-Burtz C, Sauvanet A. Five days of postoperative antimicrobial therapy decreases infectious complications following pancreaticoduodenectomy in patients at risk for bile contamination. HPB (Oxford) 2013;15:473–480. doi: 10.1111/hpb.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hakamada K, Sasaki M, Endoh M, Itoh T, Morita T, Konn M. Late development of bile duct cancer after sphincteroplasty: a ten- to twenty-two-year follow-up study. Surgery. 1997;121:488–492. doi: 10.1016/S0039-6060(97)90101-X. [DOI] [PubMed] [Google Scholar]
- 24.Kurumado K, Nagai T, Kondo Y, Abe H. Long-term observations on morphological changes of choledochal epithelium after choledochoenterostomy in rats. Dig Dis Sci. 1994;39:809–820. doi: 10.1007/BF02087428. [DOI] [PubMed] [Google Scholar]
- 25.Sahora K, Morales-Oyarvide V, Ferrone C, Fong ZV, Warshaw AL, Lillemoe KD, Fernandez-del Castillo C. Preoperative biliary drainage does not increase major complications in pancreaticoduodenectomy: a large single center experience from the Massachusetts General Hospital. J Hepatobiliary Pancreat Sci. 2016;23:181–187. doi: 10.1002/jhbp.322. [DOI] [PubMed] [Google Scholar]
- 26.Hiyoshi M, Wada T, Tsuchimochi Y, Hamada T, Yano K, Imamura N, Fujii Y, Nanashima A. Hepaticoplasty prevents cholangitis after pancreaticoduodenectomy in patients with small bile ducts. Int J Surg. 2016;35:7–12. doi: 10.1016/j.ijsu.2016.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors presented all necessary information about the study in the manuscript and do not wish to share the data.


