Abstract
PURPOSE:
To compare the efficacy and safety of endoscopic cyclophotocoagulation (ECP) versus trabeculectomy with mitomycin C (trab) in combination with cataract surgery.
MATERIALS AND METHODS:
We evaluated the 6-month results of patients undergoing phacoemulsification (phaco) with either ECP or trab. The primary outcome was mean intraocular pressure (IOP) at 6 months; secondary outcomes were change in glaucoma medications, visual acuity, intraocular inflammation, and postoperative complications. Complete success was a target IOP of <21 mmHg and >6 mmHg without glaucoma medications. Qualified success was target IOP achieved through glaucoma medications.
RESULTS:
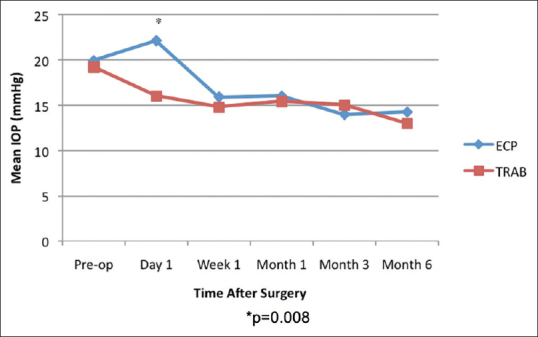
We evaluated 53 eyes of 53 patients; 24 (45.3%) eyes were treated with ECP-phaco and 29 (54.7%) with trab-phaco. At 6 months, there was no significant difference in mean IOP of the two groups (ECP-phaco 14.2 ± 3.6 mmHg; trab-phaco 13.0 ± 2.5 mmHg; P = 0.240). Six (25.0%) ECP-phaco eyes and 20 (69.0%) trab-phaco eyes achieved complete success (P = 0.002). Qualified success was achieved in 18 (75.0%) ECP-phaco eyes and 9 (31.0%) trab-phaco eyes (P = 0.002). The mean reduction of medication from baseline was significant (ECP-phaco 1.2 ± 1.1; trab-phaco 2.1 ± 1.5; P = 0.020). ECP-phaco resulted in more IOP spikes on the 1st postoperative day (P = 0.040) and more anterior cellular reaction at 1 week and 1 month compared to trab-phaco (P < 0.05). The rate of postoperative complications was not significantly different between groups.
CONCLUSION:
At 6 months, ECP-phaco demonstrated similar improvements in IOP and visual acuity compared to trab-phaco. However, ECP-phaco patients had higher incidences of immediate postoperative IOP spikes and anterior chamber inflammation as well as requiring additional medications postoperatively.
Keywords: Endoscopic cyclophotocoagulation, glaucoma, trabeculectomy
Introduction
Endoscopic cyclophotocoagulation (ECP) is a procedure that lowers intraocular pressure (IOP) by reducing aqueous production through ab interno direct visualization and coagulation of ciliary processes using a diode laser. Historically, transscleral and endoscopic cyclodestructive procedures have been used in cases with glaucoma refractory to other forms of surgical or medical intervention.[1,2] Recently, there has been a trend to use ECP in medically controlled and uncontrolled glaucoma, often in combination with phacoemulsification (ECP-phaco). Significant reductions in IOP and glaucoma medications from baseline have been reported using this procedure.[3,4,5]
The ECP Collaborative Study Group[6] analyzed outcomes of the procedure and identified the following complications: early IOP spike, hemorrhage, serous choroidal effusion, intraocular lens (IOL) displacement, more than two lines of visual loss, no light perception (NLP) vision, retinal detachment, choroidal hemorrhage, cataract, and hypotony/phthisis.
Trabeculectomy with mitomycin C is routinely performed as a treatment for glaucoma to lower the IOP. Combining trabeculectomy with phacoemulsification (trab-phaco) has been used to lower IOP in patients with glaucoma and visually significant cataract.[7,8]
Frequently reported complications of trabeculectomy include intraoperative anterior chamber bleeding, conjunctival buttonhole, shallow anterior chamber, encapsulated bleb, ptosis, serous choroidal detachment, hyphema, suprachoroidal hemorrhage, and endophthalmitis.[9,10]
Studies have suggested that ECP is safer than trabeculectomy;[4] however, evidence comparing ECP-phaco to trab-phaco is scarce.[4,5] The objective of this study was to compare the efficacy and safety of the two procedures.
Materials and Methods
Our retrospective cohort study considered all consecutive patients undergoing combined ECP and phacoemulsification cataract extraction by 3 glaucoma surgeons between August 2008 and February 2010 at the Royal Alexandra Hospital in Edmonton, Alberta, Canada. Controls were age-matched patients undergoing combined trabeculectomy and phacoemulsification cataract extraction during the same period [Table 1]. Selection for each procedure was dependent on the surgeon's assessment of the relevant indicators. Trab-phaco was preferred when the patient had a diagnosis of primary open angle glaucoma (POAG) or another glaucoma with a healthy conjunctiva. Indications for ECP-phaco included not only POAG but also other types of glaucoma, where the conjunctiva was thin or plateau iris was present. All three glaucoma surgeons were experienced in both ECP-phaco and trab-phaco. Protocol was approved by the University of Alberta Health Research Ethics Board.
Table 1.
Baseline demographic and ocular characteristics of patients undergoing combined cataract extraction and glaucoma surgery
Phacoemulsification cataract extraction was performed as follows: After administration of either topical or peribulbar anesthesia, a 3.2 mm keratome was used to create a temporal clear corneal incision. Viscoelastic was injected to maintain the anterior chamber. A capsulorrhexis was created using utrata forceps or a bent-needle cystotome. Hydrodissection was performed using a saline solution, and phacoemulsification of the nuclear material was completed using an Infiniti system (Alcon, Fort Worth, Texas). Residual cortex was aspirated using a Simcoe or irrigation/aspiration handpiece. A posterior chamber IOL was inserted in the bag after inflating it with viscoelastic.
For patients undergoing ECP, viscoelastic (Healon®, Abbot Medical Optics Inc.,) was injected into the sulcus and the straight ECP probe was inserted through the main incision. In some cases, side-port incisions were created during cataract surgery. The ciliary processes were visualized, and ECP was performed with the end point being whitening and shrinking of ciliary tissue. Treatment was conducted between 180° and 270°, depending on the IOP lowering effect desired. Residual viscoelastic was aspirated and the wound was sealed with either a 10/0 nylon suture or by hydration of the incisional edges.
Patients who underwent trabeculectomy had a superior fornix-based conjunctival flap and application of mitomycin C (0.2–0.3 mg/ml) between 1 and 2 min, depending on the surgeon's preference. A scleral flap was fashioned, and a sclerostomy was performed using a Descemet's punch. A peripheral iridectomy was performed according to the surgeon's discretion. The flap was closed with 10/0 nylon sutures and the conjunctiva was sutured with either interrupted 10/0 nylon sutures or a single continuous vicryl 9/0 suture to achieve a watertight closure. In a few cases, the surgeon used the superior site for both the trabeculectomy and the phacoemulsification procedure.
All patients received postoperative steroid and antibiotic drops; ECP-phaco patients also received nonsteroidal anti-inflammatory drops. Postoperative glaucoma medication was instituted based on the surgeon's discretion.
Data were entered into customized data collection sheets, which included demographics, previous treatments, clinical findings at presentation, surgical indications, postoperative findings, and surgical complications. If a patient underwent bilateral procedures, only one eye was selected using a computer-generated randomization tool.
The primary outcome measure was mean IOP at 6 months. The secondary outcome was the proportion of eyes achieving “complete success,” “qualified success,” or “failure.” Complete success was defined as an IOP of <21 mmHg and ≥6 mmHg without the use of glaucoma medication. Qualified success was defined as an IOP of <21 mmHg and ≥6 mmHg, with concomitant use of glaucoma medication. Failure was defined as an IOP >21 mmHg or <6 mmHg, the need for additional glaucoma surgery (excluding bleb needling), or development of NLP vision.
Other secondary outcomes included mean difference in IOP between baseline and 6 months, presence of a postoperative IOP spike (≥6 mmHg from baseline IOP), change in mean number of glaucoma medications, change in visual acuity (Snellen visual acuity converted to logMAR), number of additional surgical procedures, for example, bleb needling, postoperative inflammation, and the proportion of surgical complications in early (<30 days) and late (≥30 days) postoperative periods.
SAS (version 9.2, SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses. Normal data were analyzed using t-tests, and Wilcoxon rank-sum was utilized for nonnormal data. Categorical variables were analyzed using Chi-square test or Fisher's exact test, when possible.
Results
Thirty-four eyes underwent ECP, while 33 eyes were treated with trabeculectomy. Phacoemulsification was performed in all eyes. Fourteen patients were lost to follow-up, presumably because they attended eye clinics that were closer to their home, and the final study group consisted of 24 (45.3%) ECP-phaco eyes (cases) and 29 (54.7%) trab-phaco eyes (controls).
There were no statistically significant differences in baseline characteristics between the two groups [Table 1]. ECP-phaco eyes were treated with a mean power of 372 ± 75.2 mW (range 250–550) over a mean ciliary body arc of 269° ±61.5° (range 140–360).
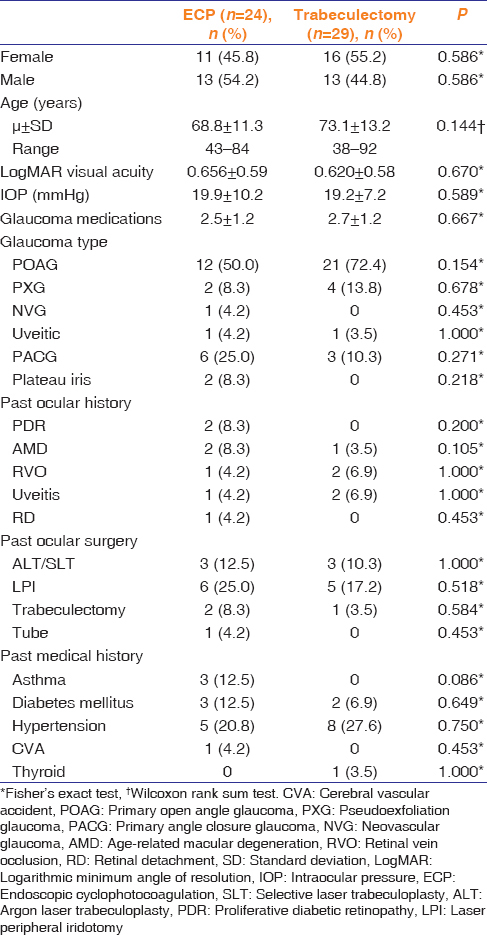
On the 1st postoperative day, ECP-phaco eyes had a significantly higher IOP (22.1 ± 7.8 mmHg) than trab-phaco eyes (16.0 ± 12.3 mmHg; P = 0.008). However, the IOP was not statistically significant at other points in time [Figure 1]. Significantly more ECP-phaco eyes (n = 12; 50.0%) developed an IOP spike (≥6 mmHg from baseline IOP) on the 1st postoperative day compared to trab-phaco eyes (n = 6; 20.7%; P = 0.040). At 6 months, there was no significant difference between the mean IOP in ECP-phaco eyes (14.2 ± 3.6 mmHg) and trab-phaco eyes (13.0 ± 2.5 mmHg; P = 0.240).
Figure 1.
Mean intraocular pressure trend from baseline to 6 months
More eyes achieved complete success after trab-phaco (n = 20; 69.0%) than with ECP-phaco (n = 6; 25.0%; P = 0.002); [Table 2]. Correspondingly, more eyes achieved qualified success in the ECP-phaco group (n = 18; 75.0%) compared to the trab-phaco group (n = 9; 31.0%; P = 0.002). The mean reduction in glaucoma medication from baseline was significantly greater in the trab-phaco group at 6 months (P = 0.02). More glaucoma medication was used in the ECP-phaco group than in the trab-phaco group from week 1 through 6 months (P < 0.005); [Figure 2].
Table 2.
Primary and secondary outcomes by group after 6 months of follow-up in comparison to baseline values
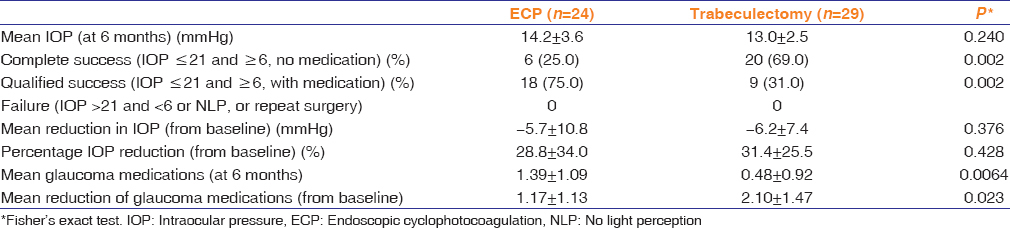
Figure 2.
Mean trend of glaucoma medication from baseline to 6 months
Figure 3 demonstrates the anterior chamber cellular reaction over the 6-month period. The ECP-phaco group had more anterior chamber cells at 1 week and 1 month (P < 0.05), but by the third and 6th month, there was no difference.
Figure 3.
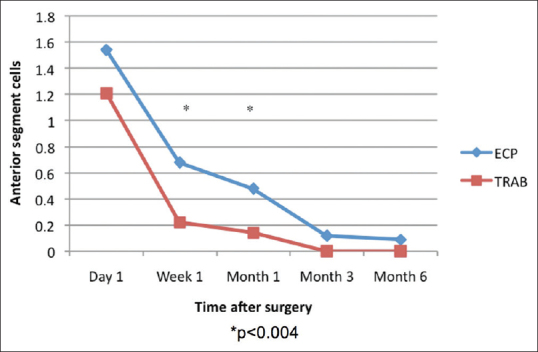
Mean anterior chamber cells from baseline to 6 months
At week 1, the trab-phaco group had significantly reduced visual acuity (P = 0.03). However, both groups demonstrated improved visual acuity at 6 months. ECP-phaco eyes improved by 0.24 ± 0.50 logMAR units (from approximately 20/90 to 20/50) and trab-phaco eyes by 0.33 ± 0.48 logMAR units (from approximately 20/80 to 20/35), although these values were not statistically significant (P = 0.388).
Intraoperative complications in the ECP-phaco group included posterior capsular rupture with vitreous loss requiring anterior vitrectomy (n = 2), while one eye had massive hyphema during the ECP-phaco procedure (from disruption of a ciliary process), which resulted in poor visualization and prevented the application of further laser. There were no intraoperative complications in the trab-phaco group.
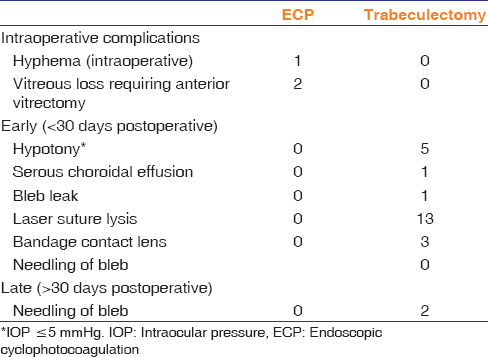
Table 3 summarizes the postoperative complications and additional procedures. In the trab-phaco group, the five patients who developed hypotony within the 1st week had normal IOPs by the 1st month visit. One patient experienced a bleb leak, which resolved by 1 month.
Table 3.
Postoperative complications and additional procedures during the 6-month period
Discussion
There was no significant difference in mean IOP between eyes treated with ECP-phaco or trab-phaco; however, ECP-phaco eyes required more medication (P = 0.004). Both procedures demonstrated a significant difference in the mean reduction of IOP from baseline to 6 months; the ECP-phaco resulting in a 28.8% ±34.0% and trab-phaco in a 31.4% ±25.5% reduction (P = 0.428). Gayton found similar results of IOP lowering of 28.8% ± 22.8% for ECP-phaco and 31.9% ±29.0% for trab-phaco.[4] Gayton treated between 240° and 270° compared to our wider range of 140° and 360°. In addition, studies have demonstrated improved IOP lowering when ECP-phaco is compared to phaco alone. Francis reported an IOP reduction of 13.6% ± 15.1% with ECP-phaco and 5.1% ± 10.4% with phaco alone,[11] while Siegel noted a reduction of 12.6% ± 1.4% with ECP-phaco and 7.1% ± 5.9% with phaco alone, after 36-month follow-up.[12] These results indicate that ECP-phaco is an efficacious method for lowering IOP in combined cataract and glaucoma surgery. The main advantage of ECP is that it preserves the conjunctiva, leaving room for a future trabeculectomy or glaucoma drainage device. ECP is also repeatable and fairly easy to perform compared to trabeculectomy.[3]
When evaluating success, more eyes achieved complete success in the trab-phaco group (69.0%) compared to ECP-phaco (25.0%; P = 0.002) at 6 months. Consequently, more ECP-phaco cases (75.0%) achieved qualified success compared to trab-phaco cases (31.0%; P = 0.002). These findings indicate that IOP control after ECP-phaco requires more medication compared to trab-phaco. Lima reported 90.8% eyes achieving qualified success (IOP <21 and >5 mmHg with or without medication) and 55.7% achieving complete success (without medication) in eyes that underwent ECP-phaco,[13] which is higher compared to our study. Gayton reported 30% of the ECP-phaco group were controlled (IOP <19 mmHg) without medication and 65% were controlled with medication.[4] His results were comparable to our study.
At 6 months, both procedures demonstrated a reduction in the number of glaucoma medications from baseline, although trab-phaco patients required significantly less medication than ECP-phaco patients (P = 0.02). Lima found a significant decrease from a baseline of 1.44 ± 0.97 to 0.37 ± 0.74 (P < 0.001) in ECP-phaco patients.[13] This reduction of glaucoma medication was also noted in other studies.[3,4,11,12] The results highlight that ECP-phaco reduces the number of glaucoma medications; however, trab-phaco patients require even less medication for glaucoma control.
In both groups, visual acuity improved at 6 months from baseline. Vision was significantly worse compared to baseline in the trab-phaco group at 1 week; however, there was no significant difference between the two groups at 6 months. Lima also found a significant improvement in the logMAR visual acuity (P = 0.01) in patients treated with ECP-phaco alone.[13] Thus, both procedures appear to be similar in terms of visual acuity.
On the 1st postoperative day, ECP-phaco eyes had a significantly higher IOP (P = 0.008) and were more likely to have an IOP spike (P = 0.04). This is likely caused by retained viscoelastic, which is injected into the sulcus to aid in the visualization of the ciliary processes during the procedure. In addition, more postoperative inflammation is expected in the ECP-phaco procedure due to the direct damage of ciliary processes, which may also contribute to the IOP spike. Lima reported an immediate postoperative IOP spike in 14.4%,[13] Berke in 14.5%,[3] and Traynor in 7.1% of eyes that underwent ECP.[5] Berke advocates meticulous removal of viscoelastic agents, use of oral carbonic anhydrous inhibitors (acetazolamide 500 mg), and topical antiglaucoma medication during the immediate postoperative period to prevent this spike.[3] Patient selection for ECP-phaco is also critical as those with advanced glaucoma (cup-disc ratio >0.9 and/or visual field (VF) defect within 10° of fixation, and/or mean deviation worse than −12 dB on humphrey VF 24-2)[14] are more suited for trab-phaco in order to avoid the IOP spike which can compromise an already vulnerable optic nerve.
The ECP-phaco group had significantly more anterior chamber inflammation at 1 week and 1 month postoperatively (P < 0.03). At 6 months, there was no significant difference between the two groups. Gayton found quieter eyes with ECP-phaco compared to trab-phaco during the early postoperative period.[4] Lima found postoperative fibrin exudates in the anterior chamber in 7.06% of ECP-phaco cases.[13] Traynor reported transient fibrin in 16.4% of eyes that had undergone ECP-phaco.[5] ECP-phaco is associated with more intraocular inflammation because as the laser is applied to the ciliary processes, one would expect more inflammatory cytokines and cells to be released. This inflammation can be managed with frequent use of topical steroids.
Other postoperative complications in the ECP-phaco group were hyphema, which was noted in one patient and cleared within a week. Lima recorded cystoid macular edema, CME (4.34%), transitory hypotony (2.17%), and iris bombé (1.08%).[13] CME was also noted in Berke[3] (1%) and Siegel[12] (3%). In our study, there were no cases of CME in either group. However, this was from clinical evaluation as no optical coherence tomography was done on the macula for any of the patients. In the trab-phaco group, five patients developed early hypotony, with one case of choroidal effusion. All complications were resolved within 1 month. None of the eyes needed additional glaucoma surgeries or had loss of light perception vision.
Limitations of this study relate to its retrospective nature. There was missing data from patient charts, and a number of patients were lost to follow-up. Furthermore, the follow-up period of 6 months is relatively short given the chronic nature of glaucoma. Given the ethnic differences in glaucoma, with African Americans and Hispanics at higher risk, a future study should also explore ethnic differences in response to treatment. The patients were not randomized into the groups, as those with healthy conjunctiva underwent trab-phaco, while thinner conjunctiva underwent ECP-phaco. Additional limitations include the lack of ECP power standardization as well as the lack of a standard phaco-trabeculectomy procedure (this was done largely as a two-site procedure but in some cases done through the same superior wound).
Overall, while ECP-phaco produced similar improvements in IOP and visual acuity as trab-phaco at 6 months, ECP-phaco was associated with fewer cases of complete success, with many patients requiring additional medications. In addition, patients in the ECP-phaco group experienced higher immediate IOP spikes and anterior chamber inflammatory reactions. In comparison, trab-phaco patients experienced higher levels of complete success, without the need of postoperative medications. A prospective randomized controlled trial with a longer follow-up period could help to better delineate the indications for ECP-phaco as opposed to trab-phaco.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Dr. Marianne Edwards and Dr. Michael Dorey contributed patients for this study. Alice Tieszen and Georgie Jarvis assisted with access to patient records and research ethics approval, respectively.
References
- 1.Chen J, Cohn RA, Lin SC, Cortes AE, Alvarado JA. Endoscopic photocoagulation of the ciliary body for treatment of refractory glaucomas. Am J Ophthalmol. 1997;124:787–96. doi: 10.1016/s0002-9394(14)71696-4. [DOI] [PubMed] [Google Scholar]
- 2.Murthy GJ, Murthy PR, Murthy KR, Kulkarni VV, Murthy KR. A study of the efficacy of endoscopic cyclophotocoagulation for the treatment of refractory glaucomas. Indian J Ophthalmol. 2009;57:127–32. doi: 10.4103/0301-4738.45502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berke SJ. Endolaser cyclophotocoagulation in glaucoma management. Tech Ophthalmol. 2006;4:74–81. [Google Scholar]
- 4.Gayton JL, Van Der Karr M, Sanders V. Combined cataract and glaucoma surgery: Trabeculectomy versus endoscopic laser cycloablation. J Cataract Refract Surg. 1999;25:1214–9. doi: 10.1016/s0886-3350(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 5.Traynor MP, Khaimi MA, Sarkisian SR. [Last accessed on 2010 Apr 19]. Available from: http://www.ascrs2009.abstractsnet.com/handouts/000357_ECP2.pp .
- 6.Noecker RJ. Paper Presented at: The ASCRS Symposium on Cataract, IOL and Refractive Surgery; Complications of Endoscopic Cyclophotocoagulation: ECP Collaborative Study Group; San Diego CA. 2007. [Google Scholar]
- 7.Lai JS, Tham CC, Chan JC, Lam DS. Phacotrabeculectomy in treatment of primary angle-closure glaucoma and primary open-angle glaucoma. Jpn J Ophthalmol. 2004;48:408–11. doi: 10.1007/s10384-003-0075-2. [DOI] [PubMed] [Google Scholar]
- 8.Vass C, Menapace R. Surgical strategies in patients with combined cataract and glaucoma. Curr Opin Ophthalmol. 2004;15:61–6. doi: 10.1097/00055735-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Jampel HD, Musch DC, Gillespie BW, Lichter PR, Wright MM, Guire KE, et al. Perioperative complications of trabeculectomy in the collaborative initial glaucoma treatment study (CIGTS) Am J Ophthalmol. 2005;140:16–22. doi: 10.1016/j.ajo.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Kuang TM, Lin YC, Liu CJ, Hsu WM, Chou CK. Early and late endophthalmitis following trabeculectomy in a Chinese population. Eur J Ophthalmol. 2008;18:66–70. doi: 10.1177/112067210801800111. [DOI] [PubMed] [Google Scholar]
- 11.Francis BA, Berke SJ, Dustin L, Noecker R. Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg. 2014;40:1313–21. doi: 10.1016/j.jcrs.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Siegel MJ, Boling WS, Faridi OS, Gupta CK, Kim C, Boling RC, et al. Combined endoscopic cyclophotocoagulation and phacoemulsification versus phacoemulsification alone in the treatment of mild to moderate glaucoma. Clin Exp Ophthalmol. 2015;43:531–9. doi: 10.1111/ceo.12510. [DOI] [PubMed] [Google Scholar]
- 13.Lima FE, Carvalho DM, Avila MP. Phacoemulsification and endoscopic cyclophotocoagulation as primary surgical procedure in coexisting cataract and glaucoma. Arq Bras Oftalmol. 2010;73:419–22. doi: 10.1590/s0004-27492010000500006. [DOI] [PubMed] [Google Scholar]
- 14.Canadian Ophthalmological Society Glaucoma Clinical Practice Guideline Expert Committee, Canadian Ophthalmological Society. Canadian ophthalmological society evidence.based clinical practice guidelines for the management of glaucoma in the adult eye. Can J Ophthalmol. 2009;44(Suppl 1):S7–93. doi: 10.3129/cjo44s1. [DOI] [PubMed] [Google Scholar]


