Abstract
Background & objectives:
Northeast (NE) India is one of the high endemic regions for malaria with a preponderance of Plasmodium falciparum, resulting in high morbidity and mortality. The P. falciparum parasite of this region showed high polymorphism in drug-resistant molecular biomarkers. However, there is a paucity of information related to merozoite surface protein 1 (msp-1) and glutamate-rich protein (glurp) which have been extensively studied in various parts of the world. The present study was, therefore, aimed at investigating the genetic diversity of P. falciparum based on msp-1 and glurp in Arunachal Pradesh, a State in NE India.
Methods:
Two hundred and forty nine patients with fever were screened for malaria, of whom 75 were positive for P. falciparum. Blood samples were collected from each microscopically confirmed patient. The DNA was extracted; nested polymerase chain reaction and sequencing were performed to study the genetic diversity of msp-1 (block 2) and glurp.
Results:
The block 2 of msp-1 gene was found to be highly polymorphic, and overall allelic distribution showed that RO33 was the dominant allele (63%), followed by MAD20 (29%) and K1 (8%) alleles. However, an extensive diversity (9 alleles and 4 genotypes) and 6-10 repeat regions exclusively of R2 type were observed in glurp.
Interpretation & conclusions:
The P. falciparum population of NE India was diverse which might be responsible for higher plasticity leading to the survival of the parasite and in turn to the higher endemicity of falciparum malaria of this region.
Keywords: Genetic diversity, glutamate-rich protein, merozoite surface protein 1, North East India, Plasmodium falciparum
Malaria, one of the major vector-borne public health problems, is endemic throughout tropics. Although, in recent years, significant progress has been made to reduce malaria morbidity and mortality1, the estimated global burden of malaria is still immense with 212 million cases, resulting 429,000 deaths annually1. There are several immunogenic antigens which are stage specific and have been targeted and characterized with respect to their use in vaccine development against Plasmodium falciparum with varying success2. However, several studies have shown high genetic polymorphisms as well as antigenic variation in P. falciparum vaccine candidates3. Hence, proper mapping of the genetic diversity of the malaria parasite is of utmost importance not only for the development of effective malaria vaccine but also for future vaccine trials.
The polymorphic regions of merozoite surface proteins-1 (msp-1) and glutamate-rich protein (glurp) have been the targeted markers for parasite genotyping in antimalarial drug trials to distinguish between recrudescence and reinfection4. The msp-1 (block 2), the most abundant surface protein, is a leading vaccine candidate gene, as it is believed to play an important role in parasite invasion5. It is divided into 17 blocks that are further categorized as variables, conserved or semi-conserved blocks6,7. The block 2 region consists of three allele families (K1, MAD20 and RO33). Alleles in K1 and MAD20 have antigenically unique, tripeptide repeats5. Though the tripeptide repeats observed in the other two families were absent in RO33, but outside block 2, this allele was similar to the MAD20 type8. Fragment size in the three block allele families is commonly used as a molecular marker for malaria transmission dynamics as also for host immunity in P. falciparum malaria9,10,11,12.
The glurp is an exoantigen of P. falciparum on which Phase I vaccine trials have been completed13. It is expressed in both the pre-erythrocytic and erythrocytic stages of the parasite life cycle and also on the surface of newly released merozoites14. Moreover, glurp has been found to inhibit the in vitro growth of P. falciparum with or without the cooperation of monocytes, indicating its important role in controlling parasitaemia15. It contains three repeat regions: the N-terminal non-repeat region (RO), a central repeat region (R1) and an immunodominant C-terminal repeat region (R2). The glurp is highly polymorphic, and this polymorphism mainly involves variations in the number of repeats of certain genomic sequences that affect the size of the gene and its protein product. The effect of malaria control interventions could not be assessed due to the lack of information regarding the genetic diversity of P. falciparum from NE India.
NE India comprises eight States, namely, Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, Sikkim and Tripura. Although NE India represents only four per cent of the entire population of the country, it contributes to 8-12 per cent of the malaria cases in the country16. This indicates the high prevalence of malaria in the region. The region has an inimitable topography favourable for the spread of malaria, and its meagre socio-economic situation increases the transmission risk17,18,19. The earlier study conducted from NE India was on genetic polymorphism of only one antigenic marker i.e., glurp which was carried out in Darang district of Assam20. However, the present study was conducted in Arunachal Pradesh to investigate the genetic diversity of P. falciparum parasite in the region.
Material & Methods
During July-September 2013, a total of 249 fever cases were screened for malaria by microscopical examination using Giemsa staining at primary health centre of Miao area of Changlang district, Arunachal Pradesh, India. Of these, 75 were found to be positive for P. falciparum. Before treatment, 2 ml of blood was drawn from each microscopically confirmed patient in cryoprotectant vials and stored in liquid nitrogen and transported to the laboratory at Regional Medical Research Centre (RMRC), Dibrugarh, Assam, India.
Cases were selected based on the following criteria: greater than one year of age, devoid of severe malnutrition, not having any other illness and temperature >37.5°C. The study was approved by the Ethical Committee of the RMRC for NE Region (Indian Council of Medical Research), Dibrugarh, Assam, India. Written informed consents were obtained from all patients.
Preparation of DNA, PCR amplification and sequencing: Genomic DNA was extracted from blood samples using QIAamp DNA blood kit (Qiagen, CA, USA). In the final step of elution instead of 150 μl, 40 μl of elution buffer was used and stored at −80°C for further use. The allelic variation in polymorphic region (555 bp) of block 2 of msp-1 was amplified using amplification conditions and primers as described by Bharti et al21. The R2 region (1063 bp) of glurp was determined by nested PCR using the primers GLPF: 5’-TGCAAGTGTTGATCCTGAAGT- 3’ and GLPR: 5’-AATGTAGGTACCACGGGTTC- 3’(designed by WCMC-Q, Doha, Qatar). The primary PCR was performed in 50 μl in platinum PCR SuperMix (Invitrogen, USA) with 0.4 μM each forward and reverse primers (GLPF and GLNF) and 3 μl extracted genomic DNA. The PCR reaction was initiated at 95°C for 5 min and followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 53.5°C for 2 min, extension at 72°C for 2 min and final extension of 10 min at 72°C. Primary PCR product was diluted (1:10) and further used for nested PCR using the primers GLNF: 5’-ATGTATCTGAAGTTGTTGAAGA-3’ and GLNR: 5’- GTTTGTGATGGTACTTCTTCA-3’. The nested PCR was performed with annealing at 48.5°C for 35 cycles. Other nested PCR conditions were the same as those described for the primary PCR. The PCR products were resolved on 1.5 per cent agarose gel. A positive control (3D7-DNA) and a negative control (nuclease free water) were included in each amplification reaction. The nested PCR products were purified using High Pure PCR Product Purification Kit (Roche, Penzberg, Germany). Purified products were sequenced using the Sanger method (Genewiz INC, NJ, USA) with both respective forward and reverse primers.
Analysis of sequencing data: Obtained DNA sequences (msp-1 495 and glurp 972) were edited manually in BioEdit22 and aligned using ClustalW23. A total of 75 sequences were obtained. Multiple sequence alignment (MSA) was performed to identify intraspecific variation, if any, amongst the sequences. The homology search was done using BLASTn programme with default parameters (https://blast.ncbi.nlm.nih.gov/Blast.cgi) for msp-1 and glurp genes. All nucleotide sequences were translated online using Expasy portal (https://www.expasy.org/) and obtained amino acid sequences were compared with available sequences in the NCBI database using ClustalW. The number of repeat regions of glurp in our isolates was determined based on the well-conserved amino acid repeat pattern DKNEKGQHEIVEVEEILPE of glurp R2 repeat region. The alleles of glurp were determined on the basis of the presence of GQ/VE/VQ on the 6th and 7th positions of the amino acid repeat region.
Results
Allelic polymorphisms of merozoite surface protein-1 (msp-1) (block 2): The polymorphic msp-1 (block 2) was studied in the P. falciparum- infected patients for allelic diversity. In these patients, in the block 2 of msp-1, the nucleotide and the deduced amino acid sequence were found to be highly polymorphic. The nucleotide changes were non-synonymous; hence, the deduced amino acid variations corresponded to one or the other alleles. The samples comprised three alleles, namely, R033, MAD20 and K1. The allelic frequency in the studied population was 47 (63%), 22 (29%) and 6 (8%) for R033, MAD20 and K1, respectively. The samples were composed of two variants in R033 allele whereas MAD20 had only one variant and the remaining K1 allele was found to be composed of three variants. Further analysis showed that the R033 allelic sequences were identical and showed 80-100 per cent similarity with reported R033 isolates of Iran, Sudan, Brazil and Tanzania, whereas the MAD20 and K1 allelic sequences showed similarity with that from Tanzania and Thailand, respectively.
Allelic diversity of glutamate-rich protein (glurp) R2 repeat region: Of the 75 samples, four genotypes were observed based on the sequence length variation of the R2 repeat region of glurp. Genotype 1 consisted of six amino acid repeat sequence units (AAUs), whereas genotypes 2, 3 and 4 had 7, 8 and 10 AAUs, respectively. From the total of 75 samples, 43 (57.3%) samples were genotype 1 and 14 (18.7%), 11 (14.7%) and 7 (9.3%) were genotypes 2, 3 and 4, respectively. The changes at positions sixth and seventh (GQ/VE/VQ) yielded a total of nine alleles across the 75 samples of our study (Figure). The more conserved AAU of glurp R2 i.e. DKNEKGQHEIVEVEEILPE was present in 33 (44.0%) samples. However, at positions sixth and seventh, GQ was replaced by either VE or VQ in the remaining 42 (56.0%) samples. The repeat VQ was found in 31 (42.67%) samples and VE was present in 10 (10.33%) samples.
Figure.
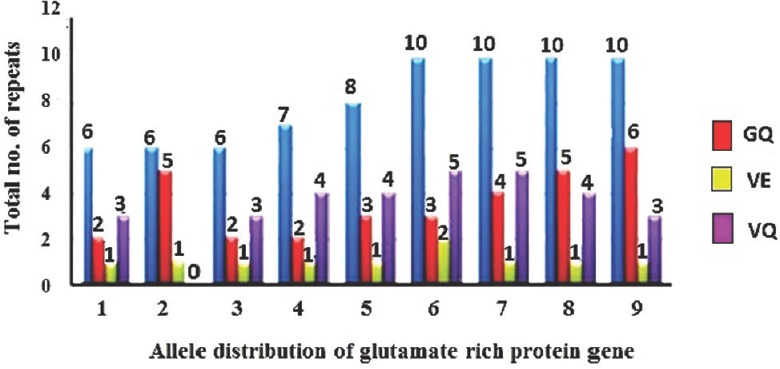
Distribution of repeat regions amongst the alleles. Alleles were identified based on the presence of GQ/VE/VQ on the 6th and 7th positions of the amino acid repeat region DKNEKGQHEIVEVEEILPE.
Discussion
The present study indicated considerable genetic polymorphism in msp-1 (block 2) and glurp sequences of P. falciparum from a malaria endemic region of NE India. Earlier reports suggested that genetic diversity and assortment of Plasmodium species were accountable for the natural success of the malaria parasite24. Genetic polymorphism in the malaria parasite helps it to evade the human immune response as well as to develop resistance to antimalarial drugs. Highly polymorphic vaccine candidate antigens such as msp-1 elicit variant-specific immunity. Single-nucleotide replacement leads to amino acid diversity in malaria parasite antigens. Based on amino acid substitution, MSP-1 has been defined into 17 blocks. Most variations seen in malarial antigens have been due to genetic recombination.
Earlier studies carried on block 2 of msp-1 from India have reported varying patterns of diversity25,26. Our study revealed that R033 alleles were the most dominant (63%), followed by MAD20 (29%) and K1 (8%), which was in agreement with the previous report26. In the present study, two variants of the dominant allele R033 were observed. Previous reports have suggested that the differences in the number of alleles for each gene associated with P. falciparum correlate with the degree of endemicity of a particular area27,28. The severity of malaria and the association between the distributions of allelic families have been investigated, and studies have yielded a variety of results. The RO33 allelic family has been frequently reported in asymptomatic malaria cases and K1 family in severe cases29.
Low numbers of alleles have been reported from areas of low endemicity30 whereas higher numbers of alleles have been reported from highly endemic areas of Africa and Asia31,32. Findings of the present study revealed diverse nature of P. falciparum from the malaria endemic region of Arunachal Pradesh with respect to the presence of all the three allelic families of msp1, which corroborated with previous findings from India26,33. These alleles are highly variable amongst the three groups but less variable within the group. The hypervariability in malaria antigens are mainly due to genetic recombination as it occurs in several orders of magnitude more frequently than mutation34.
The glurp is considered to have an important role in the induction of protective immunity against P. falciparum malaria35. However, only a few studies36 have been conducted on conserved amino acid sequences of R2 repeat region of glurp from field isolate of P. falciparum, and only one study has reported the polymorphism and amino acid repeat order of glurp in Assam, NE India20. Hence, the present study was an attempt to understand the overall population diversity of P. falciparum, and arrangement of amino acid sequence pair repeat in R2 region of P. falciparum glurp gene. Our data showed diversity in our populations, which was consistent with the findings reported earlier from high endemicity area of Africa and Asia20,37 This finding, however, were in contrast with low endemic regions of Central South America, where frequencies of glurp allele varied from two to four38. In the present study, nine alleles and four genotypes were observed in the glurp gene and presence of a high number of repeats ranging from six to 10 signified a high level of diversity in the parasite population and malaria endemicity in this region.
In conclusion, the P. falciparum population of NE India was genetically diverse based on the two antigenic markers signifying this part of India as a malaria endemic zone. The information generated through this study will help in designing malaria vaccines in future.
Acknowledgment
The authors duly acknowledge Qatar National Research Foundation for funding the study under National Priority Research Programme. The authors thank Dr S. Bhattacharjee, Chief Medical Officer, Changlang District, Arunachal Pradesh, for his support during the study. Technical assistance provided by Sarvshri B.K. Goswami, P. Borah, P Borgohain and M. Dutta is also acknowledged.
Footnotes
Conflicts of Interest: None.
References
- 1.World Malaria Report. 2016. [accessed on August 21, 2017]. Available from: http://apps.who.int/iris/bitstream/10665/252038/1/9789241511711-eng.pdf .
- 2.Richards JS, Beeson JG. The future for blood-stage vaccines against malaria. Immunol Cell Biol. 2009;87:377–90. doi: 10.1038/icb.2009.27. [DOI] [PubMed] [Google Scholar]
- 3.Takala SL, Plowe VC. Genetic diversity and malaria vaccine design, testing, and efficacy: Preventing and overcoming “vaccine resistant malaria”. Parasite Immunol. 2009;31:560–73. doi: 10.1111/j.1365-3024.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Methods and techniques for clinical trials on antimalarial drug efficacy: Genotyping to identify parasite population. Informal consultation organized by the Medicines for Malaria Venture and cosponsored by the World Health Organization, May 29-31, 2007, Amsterdam, The Netherlands. 2007. [accessed on August 21, 2017]. Available from: https://apps.who.int/iris/bitstream/10665/43824/1/9789241596305_eng.pdf .
- 5.Holder AA, Blackman MJ, Burghaus PA, Chappel JA, Ling IT, McCallum-Deighton N, et al. A malaria merozoite surface protein (MSP1)-structure, processing and function. Mem Inst Oswaldo Cruz. 1992;87(Suppl 3):37–42. doi: 10.1590/s0074-02761992000700004. [DOI] [PubMed] [Google Scholar]
- 6.Tanabe K, Mackay M, Goman M, Scaife JG. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987;195:273–87. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- 7.Miller LH, Roberts T, Shahabuddin M, McCutchan TF. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1) Mol Biochem Parasitol. 1993;59:1–14. doi: 10.1016/0166-6851(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 8.Hughes AL. Positive selection and interallelic recombination at the merozoite surface antigen-1 (MSA-1) locus of Plasmodium falciparum. Mol Biol Evol. 1992;9:381–93. doi: 10.1093/oxfordjournals.molbev.a040730. [DOI] [PubMed] [Google Scholar]
- 9.Ariey F, Chalvet W, Hommel D, Peneau C, Hulin A, Mercereau-Puijalon O, et al. Plasmodium falciparum parasites in French Guiana: Limited genetic diversity and high selfing rate. Am J Trop Med Hyg. 1999;61:978–85. doi: 10.4269/ajtmh.1999.61.978. [DOI] [PubMed] [Google Scholar]
- 10.Da Silveira LA, Dorta ML, Kimura EA, Katzin AM, Kawamoto F, Tanabe K, et al. Allelic diversity and antibody recognition of Plasmodium falciparum merozoite surface protein 1 during hypoendemic malaria transmission in the Brazilian amazon region. Infect Immun. 1999;67:5906–16. doi: 10.1128/iai.67.11.5906-5916.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Färnert A, Rooth I, Svensson, Snounou G, Björkman A. Complexity of Plasmodium falciparum infections is consistent over time and protects against clinical disease in Tanzanian children. J Infect Dis. 1999;179:989–95. doi: 10.1086/314652. [DOI] [PubMed] [Google Scholar]
- 12.Konaté L, Zwetyenga J, Rogier C, Bischoff E, Fontenille D, Tall A, et al. Variation of Plasmodium falciparum msp1 block 2 and msp2 allele prevalence and of infection complexity in two neighbouring Senegalese villages with different transmission conditions. Trans R Soc Trop Med Hyg. 1999;93(Suppl 1):21–8. doi: 10.1016/s0035-9203(99)90323-1. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Tables of Malaria Vaccine Projects Globally. 2011. [accessed on July 17, 2016]. Available from: http://www.who.int/vaccine_research/links/Rainbow/en/
- 14.Borre MB, Dziegiel M, Høgh B, Petersen E, Rieneck K, Riley E, et al. Primary structure and localization of a conserved immunogenic Plasmodium falciparum glutamate rich protein (GLURP) expressed in both the preerythrocytic and erythrocytic stages of the vertebrate life cycle. Mol Biochem Parasitol. 1991;49:119–31. doi: 10.1016/0166-6851(91)90135-s. [DOI] [PubMed] [Google Scholar]
- 15.Pratt-Riccio LR, Bianco C, Jr, Totino PR, Perce-Da-Silva Dde S, Silva LA, Riccio EK, et al. Antibodies against the Plasmodium falciparum glutamate-rich protein from naturally exposed individuals living in a Brazilian malaria-endemic area can inhibit in vitro parasite growth. Mem Inst Oswaldo Cruz. 2011;106(Suppl 1):34–43. doi: 10.1590/s0074-02762011000900005. [DOI] [PubMed] [Google Scholar]
- 16.Mohapatra PK, Prakash A, Bhattacharyya DR, Mahanta J. Epidemiological importance of younger age group during malaria epidemic in PHC Tamulpur, Assam. J Commun Dis. 1998;30:229–32. [PubMed] [Google Scholar]
- 17.Yadav K, Dhiman S, Rabha B, Saikia P, Veer V. Socio-economic determinants for malaria transmission risk in an endemic primary health centre in Assam, India. Infect Dis Poverty. 2014;3:19. doi: 10.1186/2049-9957-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadav P, Sharma R, Kumar S, Kumar U. Magnetic resonance features of cerebral malaria. Acta Radiol. 2008;49:566–9. doi: 10.1080/02841850802020476. [DOI] [PubMed] [Google Scholar]
- 19.Sehgal PNM, Sharma ID, Gogai S. Resistance to chloroquine in falciparum malaria in Assam state, India. J Commun Dis. 1973;5:175–80. [Google Scholar]
- 20.Kumar D, Dhiman S, Rabha B, Goswami D, Deka M, Singh L, et al. Genetic polymorphism and amino acid sequence variation in Plasmodium falciparum GLURP R2 repeat region in Assam, India, at an interval of five years. Malar J. 2014;13:450. doi: 10.1186/1475-2875-13-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bharti PK, Shukla MM, Sharma YD, Singh N. Genetic diversity in the block 2 region of the merozoite surface protein-1 of Plasmodium falciparum in central India. Malar J. 2012;11:78. doi: 10.1186/1475-2875-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall TA. BioEdit: A user-friendly biological sequence alignment editor and analysis programme for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–8. [Google Scholar]
- 23.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 24.Lopez AC, Ortiz A, Coello J, Socha-Ochoa W, Torres RE, Banegas IE, et al. Genetic diversity of Plasmodium vivax and Plasmodium falciparum in Honduras. Malar J. 2012;11:391. doi: 10.1186/1475-2875-11-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranjit MR, Das A, Das BP, Das BN, Dash BP, Chhotray GP. Distribution of Plasmodium falciparum genotypes in clinically mild and severe malaria cases in Orissa, India. Trans R Soc Trop Med Hyg. 2005;99:389–95. doi: 10.1016/j.trstmh.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Mahajan RC, Farooq U, Dubey ML, Malla N. Genetic polymorphism in Plasmodium falciparum vaccine candidate antigens. Indian J Pathol Microbiol. 2005;48:429–38. [PubMed] [Google Scholar]
- 27.Hamid MM, Mohammed SB, El Hassan IM. Genetic diversity of Plasmodium falciparum field isolates in central Sudan inferred by PCR genotyping of merozoite surface protein 1 and 2. N Am J Med Sci. 2013;5:95–101. doi: 10.4103/1947-2714.107524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barry EA, Schultz L, Senn N, Nale J, Kiniboro B, Siba MP, et al. High levels of genetic diversity of Plasmodium falciparum populations in Papua New Guinea despite variable infection prevalence. Am J Trop Med Hyg. 2013;88:718–25. doi: 10.4269/ajtmh.12-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kun FJJ, Schmidt-Ott RJ, Lehman LG, Lell B, Luckner D, Greve B, et al. Merozoite surface antigen 1 and 2 genotypes and rosetting of Plasmodium falciparum in severe and mild malaria in Lambarene, Gabon. Trans R Soc Trop Med Hyg. 1998;92:110–14. doi: 10.1016/s0035-9203(98)90979-8. [DOI] [PubMed] [Google Scholar]
- 30.Joshi H, Valecha N, Verma A, Kaul A, Mallick PK, Shalini S, et al. Genetic structure of Plasmodium falciparum field isolates in eastern and north-eastern India. Malar J. 2007;6:60. doi: 10.1186/1475-2875-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulenge MF, Hunja CW, Magiri E, Culleton R, Kaneko A, Aman RA. Genetic diversity and population structure of Plasmodium falciparum in Lake Victoria Islands, a region of intense transmission. Am J Trop Med Hyg. 2016;95:1077–85. doi: 10.4269/ajtmh.16-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain MM, Sohail M, Kumar R, Branch OH, Adak T, Raziuddin M. Genetic diversity in merozoite surface protein-1 and 2 among Plasmodium falciparum isolates from malarious districts of tribal dominant state of Jharkhand, India. Ann Trop Med Parasitol. 2011;105:579–92. doi: 10.1179/2047773211Y.0000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranjit MR, Sharma YD. Genetic polymorphism of falciparum malaria vaccine candidate antigen genes among field isolates in India. Am J Trop Med Hyg. 1999;61:103–8. doi: 10.4269/ajtmh.1999.61.103. [DOI] [PubMed] [Google Scholar]
- 34.Rich SM, Ayala FJ. Population structure and recent evolution of Plasmodium falciparum. Proc Natl Acad Sci U S A. 2000;97:6994–7001. doi: 10.1073/pnas.97.13.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oeuvray C, Theisen M, Rogier C, Trape JF, Jepsen S, Druilhe P. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect Immun. 2000;68:2617–20. doi: 10.1128/iai.68.5.2617-2620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ping-Xin Z, Mei-Xin Z, Lei Z, Ping-Ya Y, Xin G. Sequence analysis and genotypes of glutamate rich protein of Plasmodium falciparum isolates from different malaria endemic areas in China. Biomed Environ Sci. 2002;15:1–7. [PubMed] [Google Scholar]
- 37.Mwingira F, Nkwengulila G, Schoepflin S, Sumari D, Beck HP, Snounou G, et al. Plasmodium falciparum msp-1, msp-2 and glurp allele frequency and diversity in sub-Saharan Africa. Malar J. 2011;10:79. doi: 10.1186/1475-2875-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ariey F, Chalvet W, Hommel D, Peneau C, Hulin A, Mercereau-Puijalon O, et al. Plasmodium falciparum parasites in French Guiana: Limited genetic diversity and high selfing rate. Am J Trop Med Hyg. 1999;61:978–85. doi: 10.4269/ajtmh.1999.61.978. [DOI] [PubMed] [Google Scholar]


