Abstract
Background & objectives:
Milk proteins play a beneficial role in the regulation of food intake, postprandial glycaemia and enteroendocrine hormone secretions and thus are receiving considerable attention for the management of metabolic inflammatory disorders such as type 2 diabetes mellitus (T2DM). The objective of this study was to evaluate the efficacy of peptide/s obtained from milk proteins (casein and whey) as well as from the milk fermented with Lactobacillus helveticus as secretagogues for gut hormones and to purify and characterize the active peptides.
Methods:
Effect of hydrolysates of casein protein (CP) and whey protein (WP) and L. helveticus fermented milk on the expression of proglucagon, pro-gastric inhibitory peptide (GIP) and cholecystokinin (CCK) genes was monitored by real-time quantitative polymerase chain reaction. The active glucagon-like peptide-1 (GLP-1) secretion was also quantitatively measured using ELISA.
Results:
Hydrolysates of CP and WP as well as fermentates of L. helveticus induced the proglucagon, pro-GIP and CCK expression and secretion of GLP-1 in STC-1 (pGIP/Neo) cells. However, intact casein exhibited maximum GLP-1 secretion and proglucagon expression. Two active peptides (F5 and F7) derived from CP1 and WP3 hydrolysates having the ability to upregulate the GLP-1 secretion by 1.6 and 1.8 folds were obtained, and the mass was found to be 786 and 824 Da, respectively, as determined by electrospray ionization-mass spectrometry. However, no single active peptide from L. helveticus fermented milk could be obtained.
Interpretation & conclusions:
Casein as well as fermentates obtained from L. helveticus fermented milk showed higher potential for GLP-1 induction. These can be explored as novel therapeutics to T2DM effectively after demonstrating their in vivo efficacy in appropriate animal models.
Keywords: Glucagon-like peptide-1, incretins, Lactobacillus, milk proteins, milk protein hydrolysates, type 2 diabetes mellitus
Consumption of milk and dairy products has been shown to be associated with decreased prevalence of metabolic disorders including type 2 diabetes mellitus (T2DM). The evidence from experimental studies suggests the role of dietary proteins in the prevention of T2DM1. Milk proteins, both casein protein (CP) and whey protein (WP), have been recognized as complete proteins by virtue of having almost all the essential amino acids besides showing more satiating effects in comparison to fat and carbohydrates. These proteins also serve as precursors of biologically active peptides, exhibiting a multitude of physiological activities that are considered important from human health perspectives2. The protein hydrolysates have also been reported to be effective secretagogues for gut hormones such as glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic polypeptide or gastric inhibitory peptide (GIP) and cholecystokinin (CCK), which are secreted in the gut in response to the food intake and also act as satiety signals3. These gut hormones particularly CCK and the incretins (GIP and GLP-1) have been found to play a key role in the maintenance of metabolic health. Both of these have the capacity to promote differentiation and proliferation of β-cells of the pancreas, potentiate insulin secretion from β-cells and are also associated with the regulation of food intake. The key enteroendocrine hormones, such as GLP-1 and GIP, stimulate postprandial insulin secretion by pancreatic β-cells4.
Milk protein hydrolysates, particularly WP and CP hydrolysates, prepared with enzymatic digestion, have demonstrated insulinotropic properties5, besides serving as stimulators of incretins and other gut hormones which lead to insulin release and reduction of postprandial blood glucose level in type 2 diabetic subjects6. The fermented milk has also been reported for its insulinotropic property7. However, the mechanisms governing the insulinotropic properties of milk protein hydrolysates are not completely understood.
Though considerable efforts have been made to explore the clinical efficacy of milk protein hydrolysates as potential modulators for secretion of most of the gut hormones (GLP-1, GIP and CCK) in human, no systematic attempt has been made to study the comparative evaluation of milk protein hydrolysates obtained from enzymatic digestion and the milk fermented with Lactobacillus helveticus and their purified fractions in vitro in cell culture models on the expression of gut hormones. Hence, this study was undertaken to (i) investigate the comparative analysis of bioactive fractions derived from in vitro enzymatic digestion of CP and WP and bioactive peptides derived from the milk fermented with L. helveticus on the expression of gut hormones in vitro in an enteroendocrine STC-1 (pGIP/Neo) cell line model by real-time quantitative polymerase chain reaction (RT-qPCR) and GLP-1 secretion, and (ii) purify and characterize the active peptide/s.
Material & Methods
The study was conducted at Molecular Biology Unit, division of Dairy Microbiology, National Dairy Research Institute (NDRI), Karnal, Haryana, India.
Preparation of milk protein hydrolysates by in vitro enzymatic digestion: Aqueous dispersions of WPC-70 (1.4% w/v, Modern Dairies Ltd., Karnal, India.) and sodium caseinate (1% w/v) were prepared, and their protein content was determined8. Hydrolysis of CP and WP was carried out by incubating their aqueous dispersions at constant pH and temperature in shaker water bath with pepsin (pH 2.0, 37°C) and pancreatin (pH 7.5, 40°C) separately at enzyme-substrate ratio (E:S) of 1:25 on protein basis. Samples of WPs digested with pepsin were withdrawn after 60, 120 and 240 min and coded, respectively, as WP1, WP2 and WP3 and those of casein as CP1, CP2 and CP3, respectively. Similarly, samples were coded as WT1, WT2 and WT3, respectively, for WP hydrolysates prepared with pancreatin and those of casein as CT1, CT2 and CT3. In addition, to simulate gastrointestinal digestion, two-stage hydrolysis was carried out. For this, protein samples (WP and CP) were first hydrolyzed with pepsin (E:S 1:50; pH 2.0; temperature 37°C) for 60 min, followed by 60 min hydrolysis with pancreatin (E:S 1:50; pH 7.5; temperature 40°C) and coded as W2 and C2. Enzymes in all reactions were inactivated at the end of desired incubation period by heating samples at 90°C for 20 min. The hydrolysates were used immediately or stored at −80°C until further use. The extent of protein hydrolysis was determined in terms of per cent degree of hydrolysis (DH) by O-phthaldialdehyde (OPA) method9. The hydrolysates were also analyzed with sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE)10.
Preparation of bioactive peptides with proteolytic Lactobacillus helveticus: Proteolytic lactobacilli L. helveticus NCDC 288 and L. helveticus NCDC 292 were procured from National Collection of Dairy Cultures (NCDC), NDRI, Karnal, and maintained in de Man, Rogosa and Sharpe (MRS) broth at 37°C for 16-18 h. The purity of cultures was ascertained before use by catalase test and microscopic examination after Gram staining.
For generation of bioactive peptides, reconstituted skim milk (11%) was autoclaved and inoculated with overnight grown culture of L. helveticus (106 cells/ml) followed by incubation at 37°C for 24 h. After 24 h, the curd was broken by vigorous shaking and centrifuged at 12,000 ×g for 10 min. The supernatant was neutralized to pH 7.5 and filtered through 0.2 μm filter. The protein content of this supernatant was estimated8 and analyzed further for the expression of gut hormones. Proteolysis in fermented milk was measured using OPA method11.
In vitro evaluation of protein hydrolysates on gut hormone expression and secretion using mouse enteroendocrine STC-1 (pGIP/Neo) cell line
Cell culture maintenance and propagation: STC-1 (pGIP/Neo) cell line (a gift from Dr. Brain D Green, Queen's University Belfast, UK) maintained in Dulbecco's Modified Eagle Medium (DMEM) + GlutaMaX (GIBCO, UK) supplemented with 100 μg/ml streptomycin, 100 U/ml penicillin, 1000 ng/ml G418 and 10 per cent (v/v) of foetal bovine serum (FBS). Cells were grown under an atmosphere of 5 per cent CO2-95 per cent air at 37°C and were sub-cultured every 3-4 days using 0.02 per cent EDTA and 0.05 per cent trypsin.
Toxicity of test protein hydrolysates on the STC-1 (pGIP/Neo) cells was determined using trypan blue exclusion assay12 using different concentrations of protein (0-100 μg/ml) for two hours. Hydrolysates showing more than 95 per cent viability were considered as non-toxic.
Challenging STC-1 (pGIP/Neo) cells with protein hydrolysates: For assessing the effect of milk protein hydrolysates (fermented milk and in vitro digested protein hydrolysates) on gut hormones, STC-1 (pGIP/Neo) cells (1×106) were grown in 12-well plates till confluency. After attaining confluency, cells were washed with HEPES buffer (Sigma, USA) and incubated for 30 min in the same buffer under an atmosphere of 5 per cent CO2-95 per cent air at 37°C. After 30 min, cells were washed and incubated with stimulation buffer (HEPES + dextrose) along with protein hydrolysates (10, 25 and 50 μg/ml protein concentration) for two hours. Forskolin (30 μM) was used as the positive control. Supernatant was used for secretion analysis of GLP-1 and cells for RNA isolation.
Expression analysis of gut hormones: The effect of milk protein hydrolysates on gut hormones (proglucagon, pro-GIP and CCK) was assessed at transcriptional level by quantifying the relative expression using RT-qPCR. Before analyzing the effect at transcriptional level, stability of housekeeping genes was evaluated. A total of eight housekeeping genes viz. glyceraldehyde 3-phosphate dehydrogenase (GAPDH), beta-glucuronidase (GUS B), hypoxanthine-guanine phosphoribosyltransferase (HPRT1), phosphoglycerate kinase 1 (PGK-1), peptidylprolyl isomerase A (PPIA), beta-actin (ACTB), TATA-binding protein (TBP1), and tyrosine 3/tryptophan 5-monooxygenase activation protein, zeta polypeptide (YWHAZ) were selected. The product size and specificity of primers were again re-confirmed by in silico analysis (data not shown) and got synthesized commercially from the Integrated DNA Technologies, USA. The RT-qPCR reactions were performed using Light Cycler 480 Real-Time PCR (Roche Molecular Biochemicals, Germany). Upon completion of real-time PCR, data were analyzed using geNorm (version 2.3 software, Belgium). In the analysis using geNorm, the reference genes were ranked according to the expression stability value M (average pair-wise variation of a gene with all other tested candidate reference genes).
Expression analysis of the target genes viz., proglucagon, pro-GIP and CCK was performed with the help of real-time PCR. The primer sequences for proglucagon (Forward 5’-ggc aca ttc acc agc gac tac-3’; Reverse 5’-caa tgg cga ctt ctt ctg gg-3’), pro-GIP (Forward 5’-gaa gac ctg ctc tct gtt gct ggt-3’ Reverse 5’-cag agc tct gct tgg tcc acc atc-3’) and CCK (Forward 5’-tga tttc ccc atc caa agc-3’ Reverse 5’-gct tct gca ggg act acc g-3’) were designed based on earlier published cDNA sequences13,14,15,16 Total RNA was isolated using Tri Reagent (Sigma Aldrich, USA), RNA was quantified by A260 measurement (Synergy HT Multi-Mode Microplate Reader, Biotek, USA) and quality of RNA was assessed by agarose gel electrophoresis. The cDNA synthesis was performed using QuantiTect® Reverse Transcription kit (Qiagen, USA). The real-time PCR was performed in Roche Light cycler 480 using SYBR GREEN as intercalating dye (Roche Diagnostics, USA) following the instruction manual. The data analysis was performed using REST 2009 software (Qiagen, USA)
Secretion analysis of glucagon-like peptide-1: Supernatant obtained after incubation of two hours was immediately analyzed for GLP-1 secretion using ELISA assay kit [GLP-1 (7-36) Active ELISA kit Millipore, USA] as per the manufacturer's instructions. The GLP-1 secretion was normalized with the total amount of secreted protein and the data represented as pmol GLP-1 secreted/mg of secreted protein.
Purification and partial characterization of protein hydrolysates
Ultrafiltration: The milk protein hydrolysates (fermented milk and in vitro digested protein hydrolysates) were centrifuged at 12,000 ×g for 20 min at 4°C and supernatant thus obtained was filtered through 0.2 μm filter. The filtered samples were ultrafiltered through 10, 3 and 1 kDa MWCO membranes, sequentially using stirred cell assembly (Amicon, USA) and OMEGA MWCO membrane discs (dia. 62 mm, Pall Life Sciences, USA) under nitrogen pressure (50 psi) at 4°C. Permeate of each (10, 3 and 1 kDa) was collected and analyzed for GLP-1 secretion. The selected 1 kDa permeate was further analyzed for expression of gut hormones (proglucagon, pro-GIP and CCK).
Characterization of 1 kDa filtrate: The selected 1 kDa permeates were assessed for amino acid content and by Fourier transform infrared (FTIR) spectroscopy analysis. Amino acid analysis of 1 kDa fraction was performed according to the method of Heinrikson & Meredith17. The IR spectra were recorded on FT-IR RX-1 spectrometer (Perkin Elmer, USA) in the 200-4000/cm range using standard potassium bromide pellet technique.
Reversed phase chromatography (RPC) for purification of active molecule: The 1 kDa permeates of selected hydrolysates were further purified by RPC (AKTA purifier, GE Healthcare, India). The hydrolysates were resolved using SOURCE 15RPC column (6.4 mm × 100 mm, particle size 15 μm, GE Healthcare) with mobile phase consisting of water added with 0.1 per cent TFA (A) and 70 per cent acetonitrile with 0.01 per cent TFA (B). For separation of analytes, a gradient programme (0-100% B for 20 column volumes) was used with flow rate of 1 ml/min. The absorbance of the eluent was monitored at 214 nm. The fractions were collected manually and concentrated using lyophilizer. Protein content in individual fractions was estimated8 and each fraction (25 μg/ml) was analyzed for the GLP-1 secretion. Active fractions for GLP-1 secretion were further analyzed for their effect on expression of gut hormones. Mass of the active fractions obtained from RPC was determined using electrospray ionization-mass spectrometry (ESI) on micro mass Quattro II triple quadrupole mass spectrometer (Agilent 4000 QTRAP, USA). The samples were dissolved in water and introduced into the ESI source through a syringe pump at the rate of 10 ml/min. During this whole process, ESI capillary was set at 3.5 KV and the cone voltage was 40 V.
Statistical analysis: Statistical analysis was carried out with ANOVA followed by Newman–Keuls multiple range post hoc test. All data for mRNA expression are presented as relative fold change obtained using REST software from three independent experiments.
Results
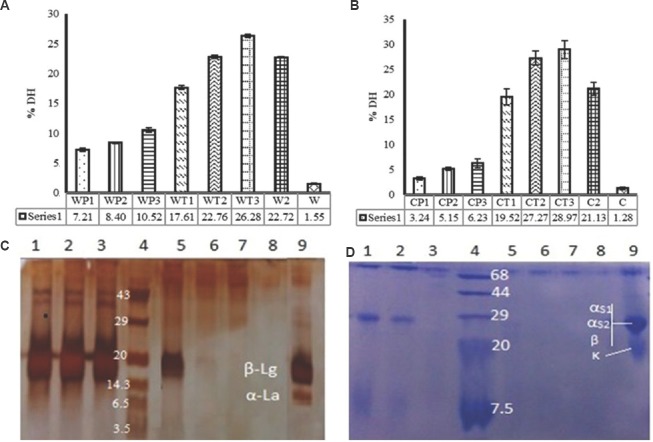
Degree of hydrolysis (DH): The DH for WP and casein in unhydrolyzed conditions were found to be 1.55 and 1.28 per cent, respectively. The DH for WP hydrolysates (WP1, WP2, WP3, WT1, WT2, WT3 and W2) were found to be 7.21, 8.40, 10.52, 17.61, 22.76, 26.28 and 22.7 per cent, respectively. Similarly, DH for casein hydrolysates were 3.24, 5.15, 6.23, 19.52, 27.27, 28.97 and 21.13 per cent, respectively (Fig. 1A and B). All the hydrolyzed and crude (whey and casein) samples were subjected to SDS-PAGE (Fig. 1C and D) and the SDS-PAGE profiles of casein and whey hydrolysates were found to be in accordance with DH. The SDS-PAGE profile of WP hydrolysates with pepsin differed considerably in comparison to hydrolysates with pancreatin (Fig. 1C). α-Lactalbumin (WP1, WP2 and WP3) was more susceptible to pepsin in comparison to β-lactoglobulin, with no residual α-lactalbumin in SDS image, whereas the β-lactoglobulin was unaffected or underwent very little proteolysis with pepsin treatment (Lanes 1, 2 and 3; Fig. 1C). Treatment with pancreatin resulted in no residual α-lactalbumin and β-lactoglobulin. In the initial phase, the hydrolysis was slow with no α-lactalbumin and less β-lactoglobulin (WT1; Lane 5; Fig. 1C). However, with increased incubation time, both subunits got hydrolyzed and no clear bands were visible in WP hydrolyzed with pancreatin for 120 and 240 min (WT2 and WT3; lanes 6 and 7; Fig. 1C), suggesting their extensive hydrolysis. Pancreatin used in the study was a mixture of enzymes (trypsin and chymotrypsin), which hydrolyzes both α-lactalbumin and β-lactoglobulin. In two-stage hydrolysis, both subunits underwent extensive proteolysis. The digestion of casein with pepsin was progressive with time [Fig. 1D (lane 1, 2 and 3)]. The small molecular weight subunit of casein (k-casein; 19 kDa) completely disappeared whereas large molecular weight subunits (αs1, αs2 and β casein; 23.6, 25.2 and 23.9 kDa) disappeared slowly with time. The band of intact casein (lane 9) became lighter (less intense) during 60 min of hydrolysis with pepsin (CP1, lane 1). During 120 min of hydrolysis (CP2, lane 2), it became further lighter, and during 240 min of hydrolysis (CP3, lane 3), it disappeared almost completely (Fig. 1D).
Fig. 1.
Degree of hydrolysis (DH) (A) in whey protein with pepsin (WP), pancreatin (WT) and both (W2). (B) Casein digested with pepsin (CP), pancreatin (CT) and both (C2). (C) sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) of whey protein hydrolysates (lane 1, 2 & 3, whey protein treated with pepsin for 60, 120 and 240 min, respectively; lane 4, protein marker; lanes 5, 6 & 7, whey protein treated with pancreatin for 60, 120 and 240 min, respectively; lane 8, two stage hydrolysis of whey protein; lane 9, whey protein. (D) SDS-PAGE of casein hydrolysates (lanes 1, 2 & 3, casein treated with pepsin for 60, 120 & 240 min, respectively; lane 4, protein marker; lanes 5, 6 & 7, casein treated with pancreatin for 60, 120 & 240 min, respectively; lane 8, two stage hydrolysis of casein; lane 9, casein protein). α-La, α-lactalbumin; β-Lg, β-lactoglobulin; αs1, αs1-casein; αs2, αs2 casein; β, β-casein; κ, κ-casein.
The degree of proteolysis/DH in milk fermented by L. helveticus strains was determined by measuring the release of free NH2 groups using the OPA method. The proteolysis in the milk fermented with L. helveticus NCDC 292 and L. helveticus NCDC 288 was found to be 429.74 and 300.51 μg/ml of leucine, respectively.
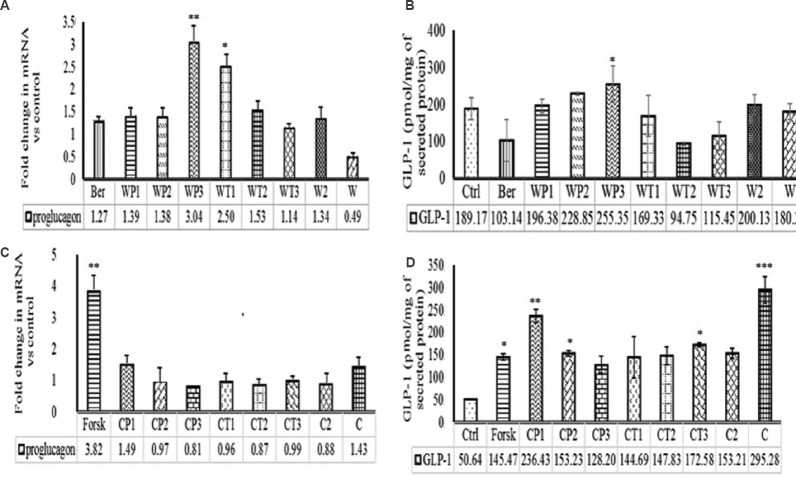
Effect of milk protein hydrolysates on proglucagon expression and GLP-1 secretion: The GAPDH was found to be the most stable housekeeping gene for all the test samples and was used for further studies. Since proglucagon showed pleiotropic effects in our initial experiments, we measured the expression level of proglucagon only. The data presented in Fig. 2A indicated that the relative expressions of proglucagon in STC-1 (pGIP/Neo) cells stimulated with WP and its hydrolysates i.e. WP1, WP2, WP3, WT1, WT2, WT3, W2 were 1.39-, 1.38-, 3.04-, 2.5-, 1.53-, 1.14- and 1.34-fold, respectively. Treatment with WP3, WT1 and WT2 upregulated the expression of proglucagon. However, the maximum upregulation (3.04-fold) was recorded with WP3. Active GLP-1 was also measured with ELISA kit and GLP-1 secretion was normalized with secreted protein. The WP1, WP2, WP3, WT1, WT2, WT3, W2 and WP modulated the GLP-1 secretion by 1.04, 1.21, 1.35, 0.9, 0.50, 0.61, 1.06 and 0.95 folds, respectively (Fig. 2B). Only WP2 and WP3 upregulated the GLP-1 secretion with maximum fold value in WP3 (1.35-fold) treatment. Similarly, intact casein (C), CP1, CP2, CP3, CT1, CT2, CT3 and C2 increased the proglucagon expression by 1.43-, 1.49-, 0.97-, 0.81-, 0.96-, 0.87-, 0.99- and 0.88-fold, respectively, in comparison to control (Fig. 2C). The GLP-1 secretion with casein (C), CP1, CP2, CP3, CT1, CT2, CT3 and C2 increased by 5.83-, 4.68-, 3.03-, 2.53-, 2.86-, 2.92-, 3.41- and 3.02- fold, respectively, relative to control (Fig. 2D).
Fig. 2.
Effect of protein hydrolysates on proglucagon mRNA expression and glucagon-like peptide-1 (GLP-1) secretion. (A) Whey protein hydrolysates on proglucagon expression and (B) GLP-1 secretion. (C) Casein hydrolysates on proglucagon expression and (D) GLP-1 secretion. WP1, 2 & 3, whey protein treated with pepsin for 60, 120 and 240 min, respectively; WT1, 2 & 3, whey proteins treated with pancreatin for 60, 120 and 240 min, respectively; W2, whey proteins treated with pepsin and pancreatin by two stage hydrolysis; W, untreated whey proteins; CP1, 2 & 3, casein proteins treated with pepsin for 60, 120 and 240 min, respectively; CT1, 2 & 3, casein proteins treated with pancreatin for 60, 120 and 240 min, respectively; C2, casein proteins treated with pepsin and pancreatin by two stage hydrolysis; C, untreated casein proteins; Ber, Berberine; Forsk, Forskolin. All data for GLP-1 secretion are presented as mean±SD (n=3). P*<0.05,**<0.01 and ***<0.001 compared to control. Values presented below the individual bars are relative fold change due to treatment.
Effect of L. helveticus fermented milk on proglucagon, GIP and CCK expression and GLP-1 secretion: The bioactive peptides present in the whey samples derived from the milk fermented with L. helveticus NCDC 292 and L. helveticus NCDC 288 separately were able to upregulate the expression of proglucagon by 1.82- and 2.35-fold, respectively, relative to control. Similarly, the secretion of GLP-1 expressed in STC-1 (pGIP/Neo) cells on stimulation with L. helveticus NCDC 292 and L. helveticus NCDC 288 was found to be 3.4- and 3.8-fold higher, respectively, relative to GLP-1 secretion in control (Fig. 3).
Fig. 3.
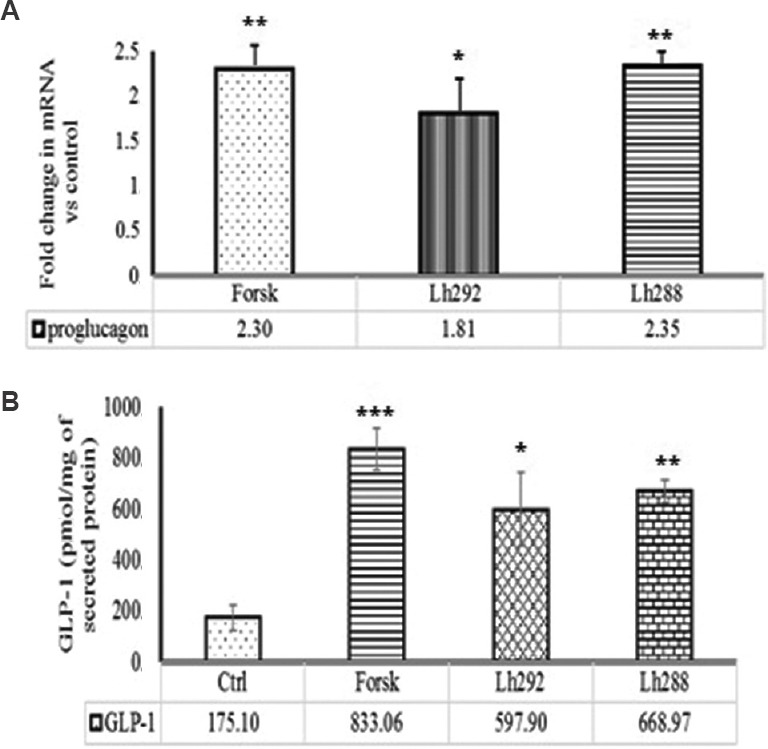
Effect of fermented milk on (A) proglucagon mRNA expression and (B) GLP-1 secretion. Ctrl, control; Forsk, Forskolin; Lh292, Lactobacillus helveticus 92; Lh288, L. helveticus 288. All data for GLP-1 secretion presented as mean±SD (n=3). P*<0.05,**<0.01 and ***<0.001 compared to control. Values presented below the individual bars are relative fold change due to treatment.
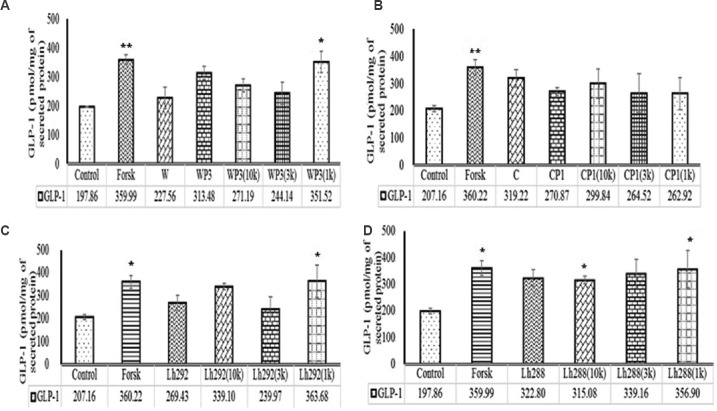
Purification and characterization: The 1 kDa fractions which elicited higher GLP-1 secretion (3.07-, 2.71-, 1.78- and 1.27-fold by L. helveticus NCDC 288, L. helveticus NCDC 292, WP3 and CP1, respectively, relative to control; Fig. 4) for fermented milk as well as in vitro digested milk protein hydrolysates were characterized for FTIR and amino acid content.
Fig. 4.
Effect of ultrafiltrated fractions (10 kDa, 3 kDa & 1 kDa) of protein hydrolysates on GLP-1 secretion. (A) WP3 (10K, 3K & 1K), fractions of whey proteins treated with pepsin for 240 min. (B) CP1(10K, 3K & 1K), fractions of casein proteins treated with pepsin for 60 min. (C) Lh292 (10K, 3K & 1K), fractions of milk fermented with Lactobacillus helveticus Lh292 and (D) Lh288 (10K, 3K & 1K), milk fermented with L. helveticus Lh288. P*<0.05 and **<0.01, compared to control. Values presented below the individual bars are relative fold change due to treatment.
In 1 kDa fraction of WP3, proline, valine, isoleucine and phenylalanine were found to be the major amino acids whereas, in 1 kDa fractions derived from CP1, proline and phenylalanine were the major amino acids. Similarly, in 1 kDa fraction of L. helveticus NCDC 288 and L. helveticus NCDC 292 fermented milk, proline, valine, isoleucine and phenylalanine were found to be the major amino acids. The IR spectrum of the compound (data not shown) indicated the presence of carbonyl function group (C=O) (1654/cm) and ether linkage (1215/cm) in the compound. The appearance of the band near 3400/cm may be attributed to the presence of unassociated O-H or N-H stretching, whereas bands at 3019/cm are due to asymmetrical and symmetrical C-H stretching of –CH2 group, respectively. Besides, C-H bending vibrations were also recorded at 1384/cm. Skeletal vibrational band of thioether was also recorded at 669/cm, suggesting the presence of peptide moiety having some thioether group.
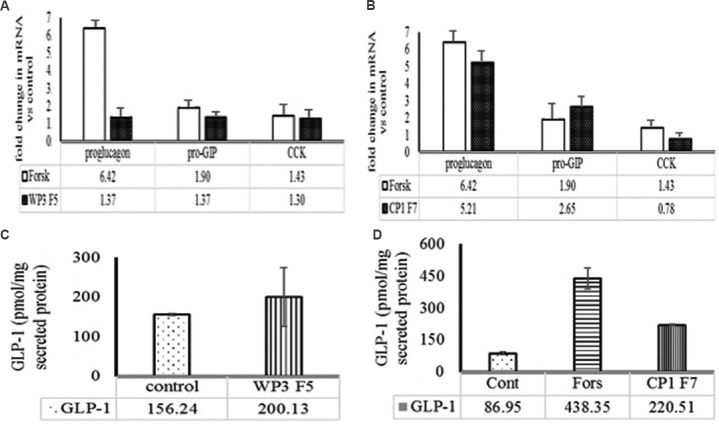
Effect of ultrafiltrate and active fractions on gut hormone expression and GLP-1 secretion: The fraction 5 (F5) of WP3 reverse phase chromatogram significantly upregulated the secretion of active GLP-1 by 1.8-fold relative to control (data not shown). Similarly, F7 of CP1 induced maximum active GLP-1 secretion in STC-1 cells and fold increase was found to be 1.6 relative to control (data not shown). It was further demonstrated that F5 (from WP3 <1 kDa) fraction upregulated the expression of proglucagon, pro-GIP and CCK by 1.37-, 1.37- and 1.3-fold, respectively, relative to control. Similarly, the F7 (from CP1< 1 kDa) was found to upregulate the expression of proglucagon and pro-GIP by 5.21-, 2.65-fold, respectively. However, no difference was recorded with regard to expression of CCK in STC-1 (pGIP/Neo) cells (Fig. 5A and B). In addition, WP3-F5 (F5 fraction from WP3) and CP1-F7 (F7 fraction from CP1) were able to induce secretion of GLP-1 to the level of 200.13 and 220.51 pmol/mg, respectively from STC-1 cells (Fig. 5C and 5D).
Fig. 5.
Effect of selected reversed phase chromatography fraction on gut hormone expression (proglucagon, pro-gastric inhibitory peptide (Pro-GIP) and cholecystokinin (CCK) and glucagon-like peptide-1 (GLP-1) secretion. Effect of (A) WP3F5 (5th fraction of whey protein hydrolysate WP3) and (B) CP1F7 (7th fraction of casein protein hydrolysate CP1) on proglucagon mRNA expression. Effect of WP3F5 (C) and CP1F7 (D) on GLP-1 secretion. Values presented below the individual bars are relative fold change due to treatment.
The active 1 kDa fractions were further purified by using RPC. The individual peaks obtained from RPC of 1 kDa fractions of L. helveticus NCDC 288 and L. helveticus NCDC 292 failed to modulate the secretion of GLP-1 (data not shown). However, 1 kDa fractions of L. helveticus NCDC 288 and L. helveticus NCDC 292 modulated the gut hormone expression. The proglucagon expression was increased 1.44- and 3.98-fold with L. helveticus NCDC 292 and L. helveticus NCDC 288 <1 kDa fractions, respectively, relative to control. Pro-GIP expression was also increased by 3.78- and 1.58-fold in STC-1 (pGIP/Neo) cells when subjected to 1 kDa fractions obtained from the milk fermented with L. helveticus NCDC 292 and NCDC 288, respectively, relative to control. However, L. helveticus 292 and L. helveticus NCDC 288 (<1 kDa) fractions also modulated the expression of CCK by upregulating the expression of mRNA by 3.23- and 4.57-fold, respectively, relative to control (Fig. 6). L. helveticus NCDC 288 showed more potential to modulate the expression of proglucagon and CCK, whereas L. helveticus NCDC 292 modulated the expression of pro-GIP and CCK more efficiently in comparison to proglucagon expression.
Fig. 6.
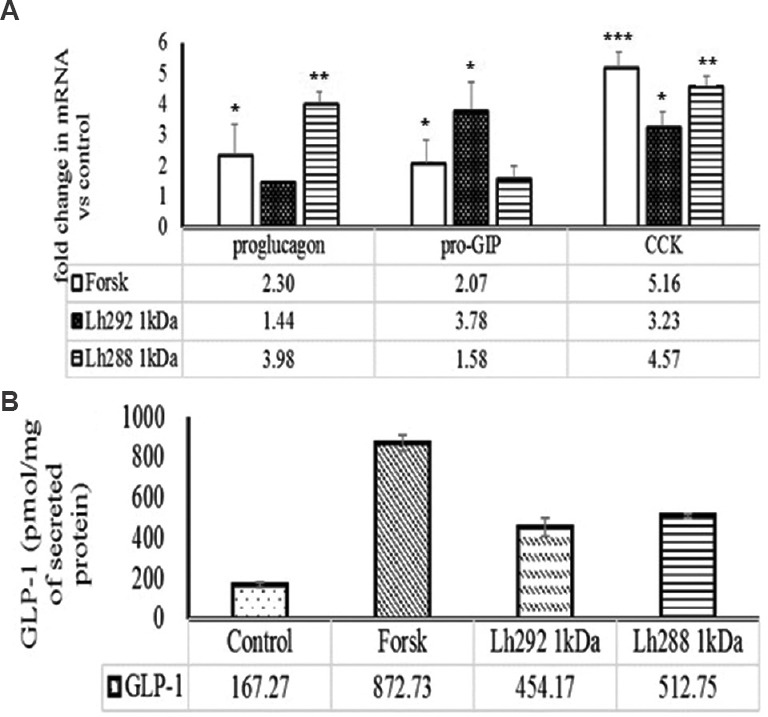
Effect of 1 kDa reversed phase chromatography fraction of milk, fermented with Lactobacillus helveticus Lh288 (Lh288 1kDa) and Lh292 (Lh288 1kDa) on (A) mRNA expression of gut hormones [proglucagon, pro-gastric inhibitory peptide (pro-GIP) and cholecystokinin (CCK)] and (B) secretion of GLP-1. Forsk, forskolin used as positive control. P*<0.05,**<0.01 and ***<0.001, compared to control. Values presented below the individual bars are relative fold change due to treatment.
The active mass of peak was determined and found to be 824 and 786 Da for fifth peak of WP3 and seventh peak from CP1 RPC, indicating that these active peaks might be composed of 7-8 amino acid residues.
Discussion
It has been documented that the major milk protein fractions, casein and whey, have the potential to exert differential effects on gastrointestinal hormone secretion and appetite18. Hence, these can play an important role in weight management, obesity and diabetes. It has also been reported that the protein component of dairy milk might be important for weight loss and diabetes than milk calcium in overweight adults, thereby suggesting that casein and whey are the likely candidates for such beneficial effects of dairy milk proteins19. In this study, two methods of milk protein hydrolysis (in vitro digestion and milk fermentation) were used and evaluated for their effects on GLP-1 secretion and proglucagon, pro-GIP and CCK expression. The extent of hydrolysis was relatively low with pepsin digestion but increased with pancreatin digestion. This could be attributed to solubility of both proteins at low pH at which WPs were more soluble whereas the casein tended to precipitate at low pH, which delayed the hydrolysis of casein. The phenomenon observed in peptic phase of in vitro digestion of casein can be supported by similar findings recorded in a previous study20. During pancreatic hydrolysis at pH values closer to neutrality, the solubility of casein was significantly increased and peptide bonds became more susceptible to proteolytic cleavage. As a result, relatively high levels of hydrolysis were recorded in casein compared to WP. In our study, we observed 7-28 per cent DH for whey, which was consistent with an earlier study21 that recorded a wide range of DH between 4 and 37 per cent for whey with various enzymes (pepsin, trypsin, chymotrypsin, crude enzymes). In the present study we recorded 3-6 per cent DH of casein with pepsin, which was in agreement with the findings of Tam and Whitaker22. The SDS-PAGE profiles of WP and casein hydrolysates were found to be in accordance with DH. The DH for the milk fermented with two proteolytic bacteria was found to be higher in L. helveticus NCDC 292 strain relative to NCDC 288 strain. These differences in hydrolytic activity might be attributed to strain-specific enzymatic action.
To evaluate the expression and secretion of gut hormones with protein hydrolysates, mouse enteroendocrine STC-1 (pGIP/Neo) cells were used as the model. The STC-1 (pGIP/Neo) cells are enteroendocrine in nature and are frequently used in the secretion and expression study of various gut hormones viz. proglucagon, pro-GIP and CCK14,15,16. In addition, GLP-1 has pleiotropic effect in comparison to GIP and CCK; therefore, more emphasis was given on proglucagon expression and GLP-1 secretion in studies with crude hydrolysates (WP and WT; CP and CT and whey fractions of milk fermentate). However, after purification, pro-GIP and CCK expression was also evaluated along with proglucagon expression and GLP-1 secretion.
In our study, active peptide of casein hydrolysate (CP1; F7) upregulated the expression of proglucagon, pro-GIP and GLP-1 secretion in comparison to control. However, it failed to modulate the expression of CCK. Similarly, the active peptide of whey hydrolysates (WP3; F5) upregulated the expression of proglucagon, pro-GIP and CCK. Whey fractions isolated from the milk fermented with L. helveticus were also found to upregulate the expression of proglucagon and secretion of GLP-1 and that was higher in comparison to hydrolysates obtained with enzymatic digestion (in vitro) of milk proteins. The reason of higher GLP-1 secretion and proglucagon expression could be attributed to combined effect of milk-derived bioactive peptides and bioactive peptides of microbial origin. The differential modulation of gut hormone expression and GLP-1 secretion with L. helveticus strain might be attributed to their genetic makeup. The different DH/degree of proteolysis also confirmed our hypothesis that both cultures have different proteolytic activity and thus differentially modulate the gut hormones. Fraction with high DH exhibited lower GLP-1 secretion as well as proglucagon expression. The reason behind this could be the hydrolysis of active peptide by endopeptidase enzymes of the proteolytic cultures.
The molecular weight of the active peptide obtained from whey (F5, whey hydrolysate) and casein hydrolytic (F7, casein hydrolysate) fractions, suggested that peptide moieties were composed of 7-8 amino acids. However, the individual peaks obtained from whey fraction (>1 kDa) of fermented milk failed to modulate the gut hormones expression and GLP-1 secretion. The reason could be combined effect of milk and bacterial generated peptides on GLP-1 secretion that might have been separated during purification.
On comparative evaluation of the data generated with protein hydrolysates in STC-1 (pGIP/Neo) cells, it was observed that milk protein hydrolysates (both casein and whey) could stimulate the expression (proglucagon, pro-GIP and CCK) and secretion (GLP-1) of gut hormones. Reimer23 also reported that milk protein hydrolysates could induce the secretion of GLP-1 during the in vitro study. Whey and casein hydrolysate in our study, it was observed that whey hydrolysates were more effective in inducing the expression of proglucagon and secretion of GLP-1 in comparison to casein hydrolysates. Contrary to this, the expression of proglucagon and GLP-1 secretion was more pronounced with intact casein relative to both casein and whey hydrolysates in our study. In some studies, whey has been found to stimulate one or both the incretin hormones to a greater extent than other protein sources, such as casein24,25,26, whereas another study27 observed casein to be more satiating than whey in the long term28. Other studies reported no difference between whey and casein28,29. In addition, WP hydrolysates have been found to stimulate plasma CCK, GLP-1 and GIP level in obese individuals in a dose-dependent manner30. The different behavior of CP and WP in terms of differential expression of proglucagon, pro-GIP and secretion of GLP-1 could be attributed to multiple factors such as different absorption rates of the two proteins and their hydrolysates, composition and nature of amino acids (essential versus non-essential amino acids and branched chain amino acids), intact protein and their peptides and also the nature of enteroendocrine cells used in this study. Hall et al18 demonstrated that post-absorptive increases in plasma amino acids served as potential mediators of the increased satiety response to the fast protein i.e. whey. Calbet and Holst31 showed that similar gastric emptying rates and GLP-1 secretion were elicited by both the proteins and their protein hydrolysates, thereby leaving ample scope to do more extensive work on these lines at molecular level. Although the in vitro enzymatic digestion of whey and casein resulted in upregulation of GLP-1 secretion and proglucagon expression, the whey fraction of fermented milk had the higher potential for the same activity. This could be due to combined effect of milk protein hydrolysate and microbial peptide, of which the latter fraction was absent in in vitro hydrolysis with enzymes. Hence, it would be interesting to further investigate the mechanism of action of the individual fractions after their complete purification and characterization in animal models to establish their functional efficacy.
In conclusion, although the WP and casein hydrolysates harboured novel biologically active peptide fractions that were able to stimulate not only the expression of the key gut hormones such as proglucagon, pro-GIP and CCK but also the secretion of GLP-1 in STC-1 (pGIP/Neo) cells by virtue of expressing their ability to modulate the expression of key gut hormones in STC-1 (pGIP/Neo) cells, but the potential of fermented milk was comparatively higher for these activities. Therefore, fermented milk with L. helveticus could be further explored as novel dietary strategy to manage obesity and associated weight gain effectively after demonstrating their in vivo chemical efficacy in appropriate animal models.
Acknowledgment
This work was funded by the Indian Council of Medical Research (ICMR) (grant no. 5/3/8/75/2009-RHN), New Delhi, India. Authors thank the Director, Indian Council of Agricultural Research - National Dairy Research Institute (ICAR-NDRI), Karnal, for providing facilities and infrastructure for the study. The Junior Research Fellowship awarded to the first author (DDC) by Indian Council of Agricultural Research (New Delhi, India) is duly acknowledged.
Footnotes
Conflicts of Interest: None.
References
- 1.Bendtsen LQ, Lorenzen JK, Bendsen NT, Rasmussen C, Astrup A. Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: A review of the evidence from controlled clinical trials. Adv Nutr. 2013;4:418–38. doi: 10.3945/an.113.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma S, Singh R, Rana S. Bioactive peptides: A review. Int J Bioautomation. 2012;15:223–50. [Google Scholar]
- 3.Jakubowicz D, Froy O. Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and Type 2 diabetes. J Nutr Biochem. 2013;24:1–5. doi: 10.1016/j.jnutbio.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Diakogiannaki E, Gribble FM, Reimann F. Nutrient detection by incretin hormone secreting cells. Physiol Behav. 2012;106:387–93. doi: 10.1016/j.physbeh.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nongonierma AB, FitzGerald RJ. Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides. 2013;39:157–63. doi: 10.1016/j.peptides.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Ma J, Stevens JE, Cukier K, Maddox AF, Wishart JM, Jones KL, et al. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care. 2009;32:1600–2. doi: 10.2337/dc09-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostman EM, Liljeberg Elmståhl HG, Björck IM. Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. Am J Clin Nutr. 2001;74:96–100. doi: 10.1093/ajcn/74.1.96. [DOI] [PubMed] [Google Scholar]
- 8.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 9.Nielsen PM, Petersen D, Dambmann C. Improved method for determining food protein degree of hydrolysis. J Food Sci. 2001;66:642–6. [Google Scholar]
- 10.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Church FC, Swaisgood HE, Porter DH, Catignani L. Spectrophotometric assay using O-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J Dairy Sci. 1983;66:1219–27. [Google Scholar]
- 12.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001;21:3B:A.3B.1–A.3B.2. doi: 10.1002/0471142735.ima03bs21. [DOI] [PubMed] [Google Scholar]
- 13.Foltz M, Ansems P, Schwarz J, Tasker MC, Lourbakos A, Gerhardt CC. Protein hydrolysates induce CCK release from enteroendocrine cells and act as partial agonists of the CCK1 receptor. J Agric Food Chem. 2008;56:837–43. doi: 10.1021/jf072611h. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima S, Hira T, Eto Y, Asano K, Hara H. Soybean beta 51-63 peptide stimulates cholecystokinin secretion via a calcium-sensing receptor in enteroendocrine STC-1 cells. Regul Pept. 2010;159:148–55. doi: 10.1016/j.regpep.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Daly K, Al-Rammahi M, Moran A, Marcello M, Ninomiya Y, Shirazi-Beechey SP. Sensing of amino acids by the gut-expressed taste receptor T1R1-T1R3 stimulates CCK secretion. Am J Physiol Gastrointest Liver Physiol. 2013;304:G271–82. doi: 10.1152/ajpgi.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kouadjo KE, Nishida Y, Cadrin-Girard JF, Yoshioka M, St-Amand J. Housekeeping and tissue-specific genes in mouse tissues. BMC Genomics. 2007;8:127. doi: 10.1186/1471-2164-8-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinrikson RL, Meredith SC. Amino acid analysis by reverse-phase high-performance liquid chromatography: Precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984;136:65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- 18.Hall WL, Millward DJ, Long SJ, Morgan LM. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr. 2003;89:239–48. doi: 10.1079/BJN2002760. [DOI] [PubMed] [Google Scholar]
- 19.Bowen J, Noakes M, Clifton PM. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J Clin Endocrinol Metab. 2006;91:2913–9. doi: 10.1210/jc.2006-0609. [DOI] [PubMed] [Google Scholar]
- 20.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94:14930–5. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peña-Ramos EA, Xiong YL. Antioxidative activity of whey protein hydrolysates in a liposomal system. J Dairy Sci. 2001;84:2577–83. doi: 10.3168/jds.S0022-0302(01)74711-X. [DOI] [PubMed] [Google Scholar]
- 22.Tam JJ, Whitaker JR. Rates and extents of hydrolysis of several caseins by pepsin, rennin, Endothia parasitica protease and Mucor pusillus protease. J Dairy Sci. 1972;55:1523–31. [Google Scholar]
- 23.Reimer RA. Meat hydrolysate and essential amino acid-induced glucagon-like peptide-1 secretion, in the human NCI-H716 enteroendocrine cell line, is regulated by extracellular signal-regulated kinase1/2 and p38 mitogen-activated protein kinases. J Endocrinol. 2006;191:159–70. doi: 10.1677/joe.1.06557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: A meta-regression 1. Am J Clin Nutr. 2006;83:260–74. doi: 10.1093/ajcn/83.2.260. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie AL, Calderwood D, Hobson L, Green BD. Whey proteins have beneficial effects on intestinal enteroendocrine cells stimulating cell growth and increasing the production and secretion of incretin hormones. Food Chem. 2015;189:120–8. doi: 10.1016/j.foodchem.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Veldhorst MAB, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJAH, Westerterp KR, Engelen MPKJ, et al. Dose-dependent satiating effect of whey relative to casein or soy. Physiol Behav. 2009;96:675–82. doi: 10.1016/j.physbeh.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Alfenas Rde CG, Bressan J, Paiva AC. Effects of protein quality on appetite and energy metabolism in normal weight subjects. Arq Bras Endocrinol Metabol. 2010;54:45–51. doi: 10.1590/s0004-27302010000100008. [DOI] [PubMed] [Google Scholar]
- 28.Calbet JAL, MacLean DA. Plasma glucagon and insulin responses depend on the rate of appearance of amino acids after ingestion of different protein solutions in humans. J Nutr. 2002;132:2174–82. doi: 10.1093/jn/132.8.2174. [DOI] [PubMed] [Google Scholar]
- 29.Veldhorst MAB, Nieuwenhuizen AG, Hochstenbach-Waelen A, Westerterp KR, Engelen MPKJ, Brummer RJM, et al. Effects of complete whey-protein breakfasts versus whey without GMP-breakfasts on energy intake and satiety. Appetite. 2009;52:388–95. doi: 10.1016/j.appet.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Hutchison AT, Feinle-Bisset C, Fitzgerald PCE, Standfield S, Horowitz M, Clifton PM, et al. Comparative effects of intraduodenal whey protein hydrolysate on antropyloroduodenal motility, gut hormones, glycemia, appetite, and energy intake in lean and obese men. Am J Clin Nutr. 2015;102:1323–31. doi: 10.3945/ajcn.115.114538. [DOI] [PubMed] [Google Scholar]
- 31.Calbet JA, Holst JJ. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur J Nutr. 2004;43:127–39. doi: 10.1007/s00394-004-0448-4. [DOI] [PubMed] [Google Scholar]