Abstract
Background
The aim of this study was to investigate the protective effects of neutrophil gelatinase-associated lipocalin (NGAL) on hypoxia/reoxygenation (H/R) induced acute kidney injury (AKI) in vitro.
Material/Methods
We used NRK-52E cells and H/R treatments to mimic ischemia/reperfusion injury (IRI) in vitro. Experimental groups were: the control group, the H/R group, the 3-methyladenine (3-MA)+H/R group, the NGAL (0.25, 0.5, and 1 ug/mL)+H/R group, and the NGAL (0.25, 0.5, 1 ug/mL)+3-MA+H/R group. After 24 hours of culture, cell proliferation was analyzed by CCK-8 assay. Expression of LC3-II was detected by immunoblot assay. Autophagy was detected by electron microscopy.
Results
The expression of LC3-II was increased in the H/R group compared with normoxic condition (p<0.05) and proliferation also improved. Autophagy was significantly inhibited by 3-MA, with downregulated of LC3-II, followed by decreased cell viability (p<0.05). We further detected the effect of different doses of NGAL in H/R induced injury, and found that low doses of NGAL alone slightly increased LC3-II protein accumulation, and autophagy was further induced with higher dose of NGAL treatment. Meanwhile, cell viability assays showed induced cell survival. We found that in the NGAL+3-MA group, cell viability assays revealed reduced cell damage, followed concomitantly with depressed autophagy. The formulation of autophagosomes were correlated with LC3-II protein expression in each group.
Conclusions
Autophagy plays a renoprotective role in H/R injury, as well in AKI. NGAL might be related to attenuated tubular epithelial cell damage via adjusting autophagy.
MeSH Keywords: Acute Kidney Injury, Autophagy, Cell Hypoxia, Lipocalins
Background
Acute kidney injury (AKI) is a severe disease and appears as a rapid decline of renal function that can result in extreme poor outcomes for patients [1]. Renal ischemia/reperfusion injury (IRI) is the most common risk factor of AKI. AKI still represents a diagnostic and therapeutic challenge to physicians, despite advances in research.
Renal proximal tubular epithelium (PTE) injury represents the most prominent cause of AKI following exposure to stressful conditions such as IRI, nephrotoxins, and inflammation [2]. Among apoptosis, necrosis, and other cell death pathways, autophagy has been increasingly recognized as an important process that can determine cell survival or death. Previous studies have shown that autophagy can be activated in AKI and protect renal PTE against IRI [3,4]. Meanwhile, other studies have reported that autophagy may induced cell death in AKI [5]. The function of autophagy in ischemia/reperfusion (IR)-AKI remains controversial.
Neutrophil gelatinase-associated lipocalin (NGAL) is a small siderophoric protein that is upregulated early in the post-ischemic kidney, and has been shown to be a novel early urinary biomarker for IR-AKI [6]. In addition, studies have shown that NGAL is involved in the processes of cell proliferation, differentiation, and apoptosis. It plays an adaptive role in hypoxia/reoxygenation (H/R) injury by regulation of cell apoptosis [7]. Thus, we investigated whether NGAL protects or damages cells by regulating autophagy in AKI.
Material and Methods
Materials
NRK-52E, a rat kidney proximal tubular cell line, was originally obtained from Dr. Mei (The Second Military Medical School, Shanghai, China). The cells were maintained for experiments as described previously [8]. Proteins and antibodies were from the following sources: human NGAL recombinant protein was purchased from R&D Systems (Minneapolis, MN, USA), anti-LC3 was purchased from Cell Signaling Technology (MA, USA), and 3-methyladenine was purchased from Sigma (Saint Louis, MI, USA).
Cell culture and I/R injury
The NRK-52E cells were cultured as a monolayer in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% (v/v) heat-inactivated fetal bovine serum (FBS) in a humidified atmosphere containing 5% CO2 at 37°C.
To mimic IRI in vitro, the model of H/R injury in the NRK-52E cells was established in an incubator as described previously [8]. The cells were seeded into 10 cm plastic dishes and incubated in low-glucose DMEM medium without FBS for 24 hours. Then cells were exposed to hypoxia (5% CO2, 1% O2, and 94% N2) for six hours. Reoxygenation was achieved by switching to full culture medium, and the cells were subsequently maintained at 37°C in a humidified atmosphere containing 5% CO2 for various periods of time.
Drug treatment
To examine the effects of NGAL on autophagy in I/R-induced cell injury, different concentrations of human NGAL recombinant protein (0.25, 0.5, and 1 ug/mL) were added to the medium during H/R in each experiment. To determine the effects of autophagy on renal I/R injury, 5 mM 3-methyladenine (a class III phosphatidylinositol 3-kinase inhibitor which can block the initial autophagic sequestration and autophagosome formation) was added to the medium one hour before each experiment.
Immunoblot analysis
Western blotting was used to quantify the levels of LC3-II protein in the cells. The NRK-52E cells were lysed in radio-immunoprecipitation assay (RIPA) buffer containing protease inhibitors at 4°C for 30 minutes. Protein concentration was determined by bicinchoninic acid (BCA) assay. Equal amounts of protein were loaded for each lane and separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred onto polyvinyl difluoride membranes at 200 mA for two hours.
The western blots were subsequently blocked with 5% non-fat milk in Tris-buffered saline containing 0.05% Tween-20 for one hour at room temperature, and then incubated with anti-LC3 (1: 1,000) and anti-glyceraldenhyde-3-phosphate dehydrogenase (GAPDH; 1: 1,000) primary antibodies at 4°C overnight. After washing with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1: 10,000) for two hours at room temperature. After several washes with TBST, immunoreactive proteins were visualized via enhanced chemiluminescent (ECL) detection. The densitometry values of scanned immunoreactive protein bands were corrected by the intensity of GAPDH bands.
Electron microscopy
Samples for electron microscopy analysis were fixed with a fixative containing 2% glutaraldehyde, 1% formaldehyde, and 10 mM sodium phosphate, pH 7.2; then dehydrate in a graded series of acetone, and embedded in epoxy resins. Ultrathin sections were cut with an ultramicrotome, followed by the addition of 2% uranyl acetate and lead citrate, and then processed by standard procedures and photographed under an electron microscope.
Statistical analysis
Results are expressed as the means ± standard deviation (SD). Statistics analysis used an independent Student t-test. For comparison of group data, one-way analyze of variance (ANOVA) was performed. A p-value <0.05 was considered statistically significant. All data was performed using SPSS statistics software version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Autophagy was induced in NRK-52E cells during H/R injury
The NRK-52E cells were subjected to hypoxia for six hours followed by reoxygenation at 0, 6, 12, 24, and 48 hours. We first examined the effects of H/R injury on cell viability in cells over time. Cell viability, measured by CCK-8 assay, was significantly decreased after 24 hours with H/R treatment (Figure 1A).
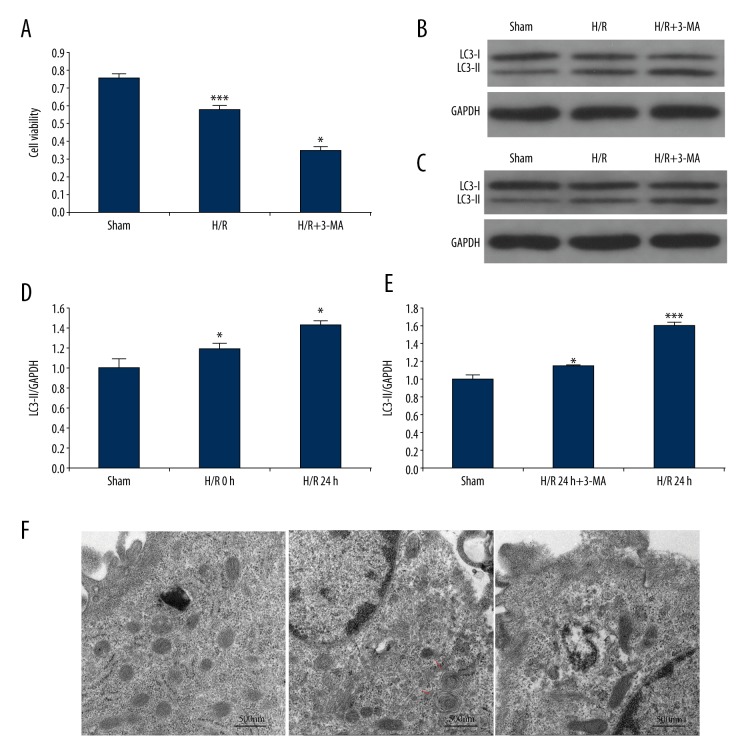
Figure 1.
Autophagy in NRK-52E cells induced by H/R injury. (A) The cell viability during H/R condition with or without 3-MA pretreatment. (B) Time course effect of LC3-II expression in NRK-52E cells were determined by western blot in response to H/R injury. (C) LC3-II expression was determined by western blot in cells at 24 hours after reoxygenation with or without 3-MA treatment. (D) Quantification of LC3-II levels in B. (E) Quantification of LC3-II expression in C. Densitometric analysis of the bands of LC3-II were normalized to GAPDH (D) and (E). (F) Electron micrographs showing autophagic vacuoles in different group, there were more autophagic vacuoles in the cells from H/R group than control and H/R+3-MA group. Arrows indicate autophagic vacuoles, scale bar: 500 nm, (7,800x). In A, D, and E, the data are presented as the mean ±SDs in three independent experiments; * p<0.05 significantly different from the sham group; ** p<0.05, significantly different from the H/R group. H/R – hypoxia/reoxygenation; 3-MA – 3-methyladenine.
To determine the occurrence of autophagy during H/R injury, we focused on autophagy-related protein LC3-II, the autophagic form of LC3, which was considered as an indication of autophagy. As shown in Figure 1B and 1C, we used western immunoblot detection. The protein expression of LC3-II was significantly elevated at the initial point of reoxygenation compared to controls, and showed the highest accumulation at 24 hours of H/R incubation (Figure 1B, 1D). These findings suggest that autophagy may be induced by hypoxia and heightened by reoxygenation.
We further investigated the effects of H/R injury on autophagosome generation in NRK-52E cells using electron microscopy. Compared with the cells before induction of H/R injury, autophagosomes containing cytoplasm or other undigested organelles with characteristic double or multiple membranes (Figure 1F) were identified in the cells after 24 hours of reoxygenation, which correlated with the time course of protein LC3-II accumulation (Figure 1B).
Suppression of autophagy by 3-methyladenine worsened I/R injury in vitro
In varying experimental conditions, autophagy can kill cells or be cytoprotective. To verify the pathophysiological role of autophagy during renal I/R injury, we used 3-MA, which is an inhibitor of autophagy, in an in vitro model. We determined the optimum reagent concentrations and catalytic time for effective autophagy inhibition and minimum cytotoxicity, and then collected cell lysates for immunoblot analysis of LC3-II. As shown in Figure 1C and 1E, the accumulation of LC3-II in the H/R injury group was notably increased (Figure 1C, Lane 3). We found that by blocking the initial autophagic sequestration, 3-MA treatment resulted in a reduction of LC3-II accumulation (Figure 1C, Lane 2). We also examined autophagosomes formation in the presence of 3-MA. We found, consistent with earlier results (Figure 1C), that H/R injury enhanced formation of autophagosome; in the H/R+3-MA group the appearance was remarkably different (Figure 1F). This finding was further supported by the results of the cell viability test, which found cell death aggravated by 3-MA treatment during hypoxia and reoxygenation (Figure 1A). Overall, these results indicated the protective role of autophagy in NRK-52E cells during I/R injury.
NGAL attenuated I/R injury in NRK-52E cells through autophagy
Former studies have implicated the role of NGAL in cell apoptosis during IR and nephrotoxicity injury [9–11]. Of note, a recent study by Sweeney et al. suggested that NGAL may also be involved in the regulation of autophagy [12]. Thus, we investigated the effects of human NGAL recombinant protein on H/R-induced autophagy in NRK-52E cells. First, we examined the accumulation of the autophagy marker LC3-II in renal cells by western blots. As shown in Figure 2A and 2B, a minimum level of LC3-II expression was shown in the sham control group (Figure 2A, Lane 1), which was induced by 24 hours of reoxygenation (Figure 2A, Lane 5). Cells incubated with a lower concentration of recombinant protein NGAL (Figure 2A, Lane 4) did not effect LC3-II during H/R, while higher concentrations (Figure 2A, Lane 2 and 3) showed increased levels of LC3-II. Densitometry analysis of western immunoblots from separate conditions corroborated LC3-II accumulation during H/R injury with or without NGAL (Figure 2B). Those results suggested that NGAL upregulated autophagy activity during reoxygenation in a concentration dependent manner.
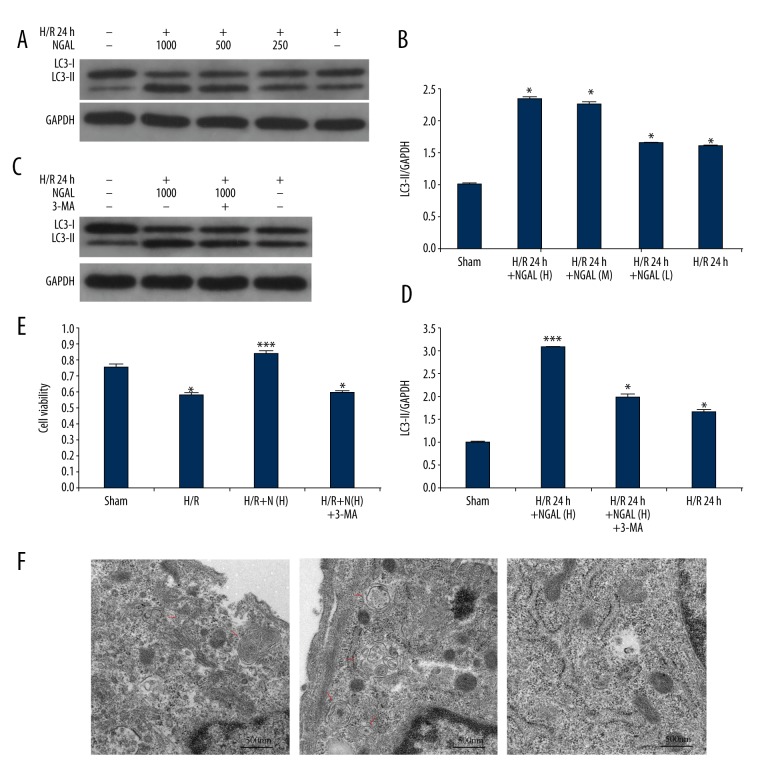
Figure 2.
Changes of autophagic vacuoles and cell viability in NRK-52E cells after exposure to NGAL protein. (A) NRK-52E cells were incubated with H/R for 24 hours and treated with different concentration of NGAL. Cell lysates were prepared and collected for immunoblot analysis of LC3-II and GAPDH. (B) Quantification of LC3-II levels in A. (C) Cells were treated with NGAL in the presence or absence of 3-MA; LC3-II expression levels in cell lysates was determined by western blot. (D) Quantification of LC3-II levels in C; densities of the bands in each lane were analyzed and normalized to GAPDH (B, D). (E) Following incubated with NGAL, cells were treated in the presence or absence of 3-MA; the cell viability was assessed by CCK-8 assay. (F) Electron micrographs showing autophagic vacuoles in different group. There were more autophagic vacuoles in the cells from H/R 24-hour group with high doses of NGAL treatment than others. Autophagic vacuoles indicated by arrows; scale bar: 500 nm, (7,800×). Data in B and D are expressed as mean ±SDs in each experiment; * p<0.05, significantly different from the sham group; ** p<0.05, significantly different from the H/R group. H/R – hypoxia/reoxygenation; 3-MA – 3-methyladenine; NGAL – (H) 1 ug/mL human NGAL recombinant protein were used in experiment; (L) 0.25 ug/mL human NGAL recombinant protein were used in experiment; (M) 0.5 ug/mL human NGAL recombinant protein were used in experiment.
To extend these in vitro findings of NGAL as a trigger for autophagy during H/R treatment, we also looked for autophagosomes in recombinant protein NGAL treated NRK-52E cells by electron microscopy. As shown in Figure 2F, the high concentration of NGAL incubation induced autophagosomes formation compared to controls. This phenomenon was further affirmed by the cell viability tests (Figure 2E). NRK-52E cells exposed to the highest dose of NGAL combined with 3-MA had worse cell viability compared with cells with NGAL pretreatment alone (Figure 2E), which is in accordance with the protein expression of LC3-II (Figure 2C, 2D). Together, these results suggested that NGAL attenuated H/R injury in NRK-52E cells via autophagy activation.
Discussion
Data in this experiment demonstrated that autophagy occurred in NRK-52E cells in response to hypoxia, and heightened after reoxygenation, as indicated by LC3-II formation and the accumulation of autophagic vacuoles. Autophagy is an evolutionarily conserved catabolic process of degradation of damaged organelles, protein aggregates, and other macromolecules through the hydrolases of lysosomes [13]. Traditionally, autophagy is recognized as occurring at a baseline level to maintain cellular homeostasis by digesting damaged proteins and recycling nutrients for cell survival [14]. In pathological conditions, a wide range of cellular stresses, such as hypoxia, oxidant injury, and other organ damaging factors, contribute to the induction of autophagy [15]. Over the past few years, autophagy has been intensely studied in both physiological and pathogenesis processes in diseases, but much is still unknown with regards to the process in kidneys. In our in vitro model, we cultured renal PTE under H/R conditions to mimic IRI, which is a major cause of AKI in the clinical setting.
AKI includes etiological conditions in a variety of settings where there is a sudden occurrence of elevated serum creatinine, decreased glomerular filtration rate, and variably urinary output. Renal PTE cells are a key target in AKI. Histological studies have indicated that the severity of renal histopathological changes is more correlated with tubular and interstitial damage than glomerular damage [1,16,17]. Furthermore, necrosis and apoptosis are the basic characteristics of tubular cell death following IRI. Nowadays, the pathogenesis of AKI involves multiple stress forms, including ischemic and other stresses such as necrosis and apoptosis, as were found concomitantly with autophagy in IRI. The activation of autophagy in renal tubular epithelia cells has been reported in both rat models and cell experiments [17–20]. In this study, we established a cell culture model of H/R injury to model IRI in the kidney. We used immunoblot analysis to observe the change in LC3-II expression after H/R injury; LC3-II is proteolytically processed from LC3 and localized on the autophagic vesicle for a hallmark of autophagy [21,22]. Our study results showed that LC3-II was expressed at a much lower level in the control group with non-injury. These results were in accordance with former reports [13]. After hypoxia and reoxygenation, LC3-II accumulation was greatly increased.
Although autophagy has been shown to be involved in the development of kidney disease, it remains controversial as to whether autophagy activation protects renal cells from injury [19]. To explore the role of autophagy in IRI, we used 3-MA, a class III phosphatidylinositol 3-kinase inhibitor that can block autophagy activation. We detected LC3-II by western blot; the data showed that pretreatment with 3-MA resulted in the lowest expression of LC3-II and reduced the number of autophagic vacuoles. The cell viability test also confirmed that inhibition of autophagy could induce more severe loss of cell survival. This compelling evidence suggests that inhibition of autophagy by 3-MA will worsen IRI. In contrast, autophagy played a protective role in this experimental model, suggesting that activation of autophagy could be effective in renal IRI. Nevertheless, the precise mechanism by which autophagy regulates renal IRI needs to be further investigated. More importantly, the present study provided evidence for the intensity of autophagy in response to NGAL. This was identified by the accumulation of LC3-II, induction of acidic autophagic vacuoles, and autophagolysosomes. At the same time, the cell viability test showed upregulated autophagy led to better cell survival. These results demonstrate that NGAL might induce autophagy and protect renal cells from injury under H/R condition, even AKI.
AKI has been well-studied during the past decades. Despite the rapid progress in treatment strategies for AKI, the disease remains a major global public health concern associated with high mortality. Approximately 13% of AKI patients suffer from chronic kidney disease and end-stage renal disease [1,23,24]. Efforts to attenuate the poor clinical outcome of AKI have been hampered by lack of knowledge about AKI pathophysiology and early diagnosis markers. Such knowledge would allow for initiation of appropriate treatment regimens during AKI treatable phases, before the disease become irreversible. Accordingly, there has been a concerted effort to explore novel early diagnosis biomarkers of AKI [25,26]. NGAL has emerged as one of the highly predictive urinary biomarkers and is an important step forward in the study of early diagnosis biomarkers.
NGAL, also known as lipocalin-2, is a member of the lipocalin superfamily. Normally, NGAL is synthesized in the loop of Henle and collecting ducts; however, in response to ischemic or nephrotoxin damaged, the concentration of NGAL precedes serum creatinine and increases dramatically in both cortical tubulars and proximal tubules. Thus, it meets most of the requirements of a proper “real-time” renal biomarker [6,27,28]. Mori et al. [10] reported that in a mouse IRI model, a single dose of NGAL might protect renal and mitigated azotemia significantly in the early stage of renal failure. Mishra also showed the protective effects of exogenous NGAL on the kidney [9]. The results of these studies indicated that NGAL could play an important protective role in IR renal injury. The structure of NGAL supports a renoprotective effect as it forms a barrel-shaped tertiary structure with a hydrophobic calyx that can bind siderophores and other small molecules. NGAL can then deliver iron into intracellular sites, which then stop producing oxygen radicals that can cause cell apoptosis. The iron might also protect and enhance cell repair for multiple reasons [11,29,30]. Specifically, recent research has shown that autophagy has an important role in iron homoeostasis and iron can regulate autophagy, in which nuclear receptor co-activator 4 (NCOA4) is involved and uses quantitative proteomics as the cargo receptor that mediates autophagy of ferritin [31].
Based on these studies, we focused on NGAL and autophagy in tubular epithelial cells’ death. At our initial research, we found that NGAL induced autophagy within hours of I/R injury in a rat renal IRI model. Moreover, significant autophagosome formation increased. The early occurrence of this phenomenon suggested that NGAL might be involved in AKI by regulating autophagy. To further investigate this relationship between NGAL and autophagy, we applied our hypothesis to the IR-AKI cell model, and our study revealed that autophagy occurred after hypoxia and reoxygenation, and inhibition of autophagy by 3-MA worsened H/R injury. Therefore, based on these findings, we pretreated NRK-52E cells with different doses of NGAL. We found that elevated levels of NGAL enhanced LC3-II levels and accumulation of autophagosomes. This effect was notably reversed by treatments that combined NGAL and 3-MA. The induction of autophagy by NGAL combined with 3-MA could lead to a sharp decline in cell viability. Taken together, our data suggested that NGAL might have a renoprotective role via inducing autophagy in renal tubular epithelial cells during IRI.
In conclusion, our study demonstrated that autophagy was moderately induced in the pathological physiology process of renal IRI. Moreover, exogenous NGAL can heighten autophagy and maintain cell survival to protect against renal injury. There were some limitations in our study. We pretreated cells with NGAL at different doses during the effective therapeutic window; however, further experiments are needed to investigate the specific mechanisms involved. However, this could be a new target for diagnostic and therapeutic treatment of renal injury.
Conclusions
Autophagy plays a renoprotective role in H/R injury; NGAL might be related to attenuate tubular epithelial cell damage via adjusting autophagy. This could be a new target for diagnostic and therapeutic treatment of AKI.
Acknowledgements
We thank all the individuals who helped us in this study.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the research grants from Yangzhou Science and Technology Bureau (Grant Number YZ2015118)
References
- 1.Zuk A, Bonventre JV. Acute kidney injury. Ann Rev Med. 2016;67:293–307. doi: 10.1146/annurev-med-050214-013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lombardi D, Becherucci F, Romagnani P. How much can the tubule regenerate and who does it? An open question. Nephrol Dial Transplant. 2016;31:1243–50. doi: 10.1093/ndt/gfv262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang M, Wei Q, Dong G, et al. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–83. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He L, Livingston MJ, Dong Z. Autophagy in acute kidney injury and repair. Nephron Clin Pract. 2014;127:56–60. doi: 10.1159/000363677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki C, Isaka Y, Takabatake Y, et al. Participation of autophagy in renal ischemia/reperfusion injury. Biochem Biophys Res Commun. 2008;368:100–6. doi: 10.1016/j.bbrc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 6.Martensson J, Bellomo R. The rise and fall of NGAL in acute kidney injury. Blood Purif. 2014;37:304–10. doi: 10.1159/000364937. [DOI] [PubMed] [Google Scholar]
- 7.Sanjeevani S, Pruthi S, Kalra S, et al. Role of neutrophil gelatinase-associated lipocalin for early detection of acute kidney injury. Int J Crit Illn Inj Sci. 2014;4(3):223–28. doi: 10.4103/2229-5151.141420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basnakian AG, Ueda N, Hong X, et al. Ceramide synthase is essential for endonuclease-mediated death of renal tubular epithelial cells induced by hypoxia-reoxygenation. Am J Physiol Renal Physiol. 2005;288:F308–14. doi: 10.1152/ajprenal.00204.2004. [DOI] [PubMed] [Google Scholar]
- 9.Mishra J. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004;15:3073–82. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 10.Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–21. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt-Ott KM, Mori K, Kalandadze A, et al. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens. 2006;15:442–49. doi: 10.1097/01.mnh.0000232886.81142.58. [DOI] [PubMed] [Google Scholar]
- 12.Sung HK, Chan YK, Han M, et al. Lipocalin-2 (NGAL) attenuates autophagy to exacerbate cardiac apoptosis induced by myocardial ischemia. J Cell Physiol. 2017;232:2125–34. doi: 10.1002/jcp.25672. [DOI] [PubMed] [Google Scholar]
- 13.Kaushal GP, Shah SV. Autophagy in acute kidney injury. Kidney Int. 2016;89:779–91. doi: 10.1016/j.kint.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizushima N, Komatsu M. Autophagy: Renovation of cells and tissues. Cell. 2011;147:728–41. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11:709–30. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linkermann A, Chen G, Dong G, et al. Regulated cell death in AKI. J Am Soc Nephrol. 2014;25:2689–701. doi: 10.1681/ASN.2014030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang YL, Zhang J, Cui LY, et al. Autophagy activation attenuates renal ischemia-reperfusion injury in rats. Exp Biol Med (Maywood) 2015;240:1590–98. doi: 10.1177/1535370215581306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura T, Takabatake Y, Takahashi A, et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol. 2011;22:902–13. doi: 10.1681/ASN.2010070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livingston MJ, Dong Z. Autophagy in acute kidney injury. Semin Nephrol. 2014;34:17–26. doi: 10.1016/j.semnephrol.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mei S, Livingston M, Hao J, et al. Autophagy is activated to protect against endotoxic acute kidney injury. Sci Rep. 2016;6:22171. doi: 10.1038/srep22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: A comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:1–43. doi: 10.1007/s00210-007-0183-5. [DOI] [PubMed] [Google Scholar]
- 22.McLeland CB, Rodriguez J, Stern ST. Autophagy monitoring assay: Qualitative analysis of MAP LC3-I to II conversion by immunoblot. Methods Mol Biol. 2011;697:199–206. doi: 10.1007/978-1-60327-198-1_21. [DOI] [PubMed] [Google Scholar]
- 23.Chawla LS, Eggers PW, Star RA, et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta RL, Cerdá J, Burdmann EA, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet. 2015;385:2616–43. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 25.Obermuller N, Geiger H, Weipert C, Urbschat A. Current developments in early diagnosis of acute kidney injury. Int Urol Nephrol. 2014;46:1–7. doi: 10.1007/s11255-013-0448-5. [DOI] [PubMed] [Google Scholar]
- 26.Urbschat A, Obermuller N, Haferkamp A. Biomarkers of kidney injury. Biomarkers. 2011;16(Suppl 1):S22–30. doi: 10.3109/1354750X.2011.587129. [DOI] [PubMed] [Google Scholar]
- 27.Clerico A, Galli C, Fortunato A, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker of acute kidney injury: A review of the laboratory characteristics and clinical evidences. Clin Chem Lab Med. 2012;50:1505–17. doi: 10.1515/cclm-2011-0814. [DOI] [PubMed] [Google Scholar]
- 28.Devarajan P. Review: Neutrophil gelatinase-associated lipocalin: A troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–28. doi: 10.1111/j.1440-1797.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- 29.Flower DR. Experimentally determined lipocalin structures. Biochim Biophys Acta. 2000;1482:46–56. doi: 10.1016/s0167-4838(00)00147-3. [DOI] [PubMed] [Google Scholar]
- 30.Goetz DH, Holmes MA, Borregaard N, et al. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–43. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 31.Chan YK, Sung HK, Sweeney G. Iron metabolism and regulation by neutrophil gelatinase-associated lipocalin in cardiomyopathy. Clin Sci (Lond) 2015;129:851–62. doi: 10.1042/CS20150075. [DOI] [PubMed] [Google Scholar]