Key Points
Question
Is cooling for 120 hours, cooling to 32.0°C, or both more neuroprotective than cooling for 72 hours at 33.5°C, the current standard of care, among neonates with moderate or severe hypoxic-ischemic encephalopathy at birth?
Findings
In this randomized clinical trial that included 364 neonates, there was no significant difference in the probability of death or disability at 18 months of age comparing cooling at 120 hours (31.6%) vs 72 hours (31.8%) or to a depth of 32.0°C (31.5%) vs 33.5°C (31.9%).
Meaning
These findings do not support change from the current regimen of cooling for 72 hours at 33.5°C for neonates with moderate or severe encephalopathy; however, statistical interactions between duration and depth of cooling support the possibility of higher mortality with the combination of longer and deeper cooling, suggesting that further investigation may be warranted.
Abstract
Importance
Hypothermia for 72 hours at 33.5°C for neonatal hypoxic-ischemic encephalopathy reduces death or disability, but rates continue to be high.
Objective
To determine if cooling for 120 hours or to a temperature of 32.0°C reduces death or disability at age 18 months in infants with hypoxic-ischemic encephalopathy.
Design, Setting, and Participants
Randomized 2 × 2 factorial clinical trial in neonates (≥36 weeks’ gestation) with hypoxic-ischemic encephalopathy at 18 US centers in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network between October 2010 and January 2016.
Interventions
A total of 364 neonates were randomly assigned to 4 hypothermia groups: 33.5°C for 72 hours (n = 95), 32.0°C for 72 hours (n = 90), 33.5°C for 120 hours (n = 96), or 32.0°C for 120 hours (n = 83).
Main Outcomes and Measures
The primary outcome was death or moderate or severe disability at 18 to 22 months of age adjusted for center and level of encephalopathy. Severe disability included any of Bayley Scales of Infant Development III cognitive score less than 70, Gross Motor Function Classification System (GMFCS) level of 3 to 5, or blindness or hearing loss despite amplification. Moderate disability was defined as a cognitive score of 70 to 84 and either GMFCS level 2, active seizures, or hearing with amplification.
Results
The trial was stopped for safety and futility in November 2013 after 364 of the planned 726 infants were enrolled. Among 347 infants (95%) with primary outcome data (mean age at follow-up, 20.7 [SD, 3.5] months; 42% female), death or disability occurred in 56 of 176 (31.8%) cooled for 72 hours and 54 of 171 (31.6%) cooled for 120 hours (adjusted risk ratio, 0.92 [95% CI, 0.68-1.25]; adjusted absolute risk difference, −1.0% [95% CI, −10.2% to 8.1%]) and in 59 of 185 (31.9%) cooled to 33.5°C and 51 of 162 (31.5%) cooled to 32.0°C (adjusted risk ratio, 0.92 [95% CI, 0.68-1.26]; adjusted absolute risk difference, −3.1% [95% CI, −12.3% to 6.1%]). A significant interaction between longer and deeper cooling was observed (P = .048), with primary outcome rates of 29.3% at 33.5°C for 72 hours, 34.5% at 32.0°C for 72 hours, 34.4% at 33.5°C for 120 hours, and 28.2% at 32.0°C for 120 hours.
Conclusions and Relevance
Among term neonates with moderate or severe hypoxic-ischemic encephalopathy, cooling for longer than 72 hours, cooling to lower than 33.5°C, or both did not reduce death or moderate or severe disability at 18 months of age. However, the trial may be underpowered, and an interaction was found between longer and deeper cooling. These results support the current regimen of cooling for 72 hours at 33.5°C.
Trial Registration
clinicaltrials.gov Identifier: NCT01192776
This randomized 2 × 2 factorial trial compares the effects of hypothermia depth (32.0°C vs 33.5°C) and duration (72 vs 120 hours) on death and disability among infants with moderate or severe hypoxic-ischemic encephalopathy.
Introduction
Hypothermia at 33.5°C for 72 hours initiated within 6 hours of birth among neonates born at full term with evidence of moderate or severe hypoxic-ischemic encephalopathy has reduced the rates of death or survival with disability in infancy and childhood. Data from animal studies published 10 years after the initial clinical neuroprotection trials demonstrated that cooling to a depth of 32.0°C or for a duration longer than 72 hours is neuroprotective. In an attempt to further decrease the rate of death or disability associated with hypoxic-ischemic encephalopathy, the current randomized clinical trial was designed to test whether longer cooling (120 hours), deeper cooling (32.0°C), or both decreases death or disability at 18 months of age compared with cooling at 33.5°C for 72 hours. A previous report from this study included 4 secondary outcomes (neonatal intensive care unit [NICU] deaths, acute adverse events, multiorgan dysfunction, and NICU length of stay). This article reports the primary outcomes of death or disability (moderate or severe) at age 18 to 22 months.
Methods
The study was conducted at all 18 US sites in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Multicenter Neonatal Research Network between October 2010 and January 2016, with RTI International as the data coordinating center. Criteria for eligibility and details of cooling and rewarming were similar to the first NICHD randomized clinical trial of therapeutic hypothermia and published previously. Random assignment was stratified by center and level of encephalopathy (moderate or severe) in a 2 × 2 factorial design to 33.5°C or 32.0°C and to 72 hours or 120 hours. Neonates who were born at 36 weeks’ or greater gestation were enrolled. The trial protocol is available in Supplement 1 . An independent data and safety monitoring committee monitored interim data and evaluated safety.
The protocol was approved by the institutional review board at each site. Written informed consent was obtained from parents of study participants.
Outcomes
The primary outcome was death or moderate or severe disability at 18 to 22 months of age. This composite outcome was selected because death is a competing outcome for disability. Infants were evaluated at 18 to 22 months of age; the families of those who did not return for follow-up were contacted by telephone to obtain information about the primary outcome. Data on growth, vision, and hearing were obtained, and neurological and developmental testing was performed by trained examiners who were masked to intervention status.
Neuromotor disability was based on the presence of cerebral palsy, and functional disability was graded according to the Gross Motor Function Classification System (GMFCS, levels 1-5; level 1 includes children who walk but gait is not fluent; level 2 includes those who are unable to walk but who can pull to stand and take steps holding on to furniture; level 3 includes those who use hands for sitting support and are unable to crawl, level 4 includes those for whom support is needed for sitting; and level 5 includes those who require adult assistance to move). Cognitive outcome was assessed by the Bayley Scales of Infant Development III (reported mean, 100 [SD, 15]). Severe disability was defined as any of the following: a Bayley III cognitive score of less than 70, a GMFCS level of 3 to 5, blindness, or profound hearing loss (inability to understand commands despite amplification). Moderate disability was defined as a Bayley III cognitive score of 70 to 84 and either a GMFCS level of 2, seizure disorder, or a hearing deficit requiring amplification to understand commands. Mild disability was defined as a cognitive score of 70 to 84, or a cognitive score of 85 or higher and any of the following: presence of a GMFCS level 1 or 2, seizure disorder, or hearing loss not requiring amplification. Normal neurocognitive status was defined as a cognitive score of 85 or higher in the absence of any neurosensory deficits or seizures after NICU discharge.
Prespecified secondary outcomes in this report include post-NICU discharge mortality; level of disability by stage of encephalopathy; rates of vision, hearing, and multiple disabilities; cognitive and motor scores; cerebral palsy rates; rehospitalization rates; and growth measurements. Neonatal brain magnetic resonance imaging findings will be reported separately.
Statistical Analysis
A sample size of 726 neonates (363 in each group to compare the 2 durations of cooling and the 2 depths of cooling) was based on a 2-tailed α=.05, a statistical power of 80%, a 5% loss to follow-up, and a comparison of death or disability of 37.5% and 27.5% in the 2 durations and depth-of-cooling groups. Generalized estimating equation (GEE) models with log link were used to obtain relative risk (RR) estimates for binary outcomes, adjusting for level of encephalopathy and intracenter correlations. Continuous outcomes were similarly assessed using GEE linear regression after log transformation. Treatment interactions between the 2 factors (deeper and longer cooling) were assessed for the primary outcome and key secondary outcomes. All reported P values are 2-sided and not adjusted for multiple comparisons. P<.05 was considered statistically significant.
To obtain probability estimates of treatment effect for the 3 experimental groups, a Bayesian analysis of death or disability or death alone was conducted. A log binomial model with level of encephalopathy and main effects of cooling duration and depth and their interaction was used to estimate posterior median of the RRs and 95% credible intervals (CrIs). The model also included a random center effect and used neutral priors for treatment effects centered at an RR of 1 (95% prior interval, 0.5-2.0). Weakly informative priors were used for all other parameters to exclude large treatment effects and produce conservative estimates of treatment effects (see eAppendix in Supplement 2 for details). The statistical software used was SAS, version 9.3 (SAS Institute Inc).
Results
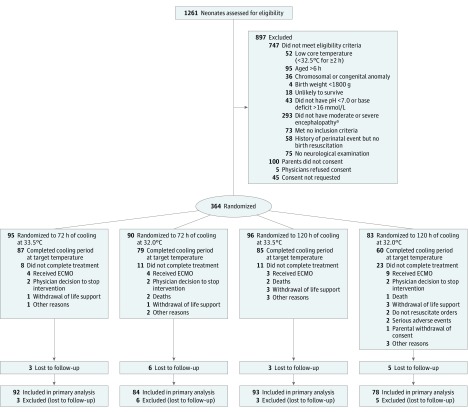
The trial was closed for in-hospital mortality and futility concerns by the data safety and monitoring committee after 1261 neonates had been screened, 514 were eligible, and 364 were enrolled (Figure). At 18 to 22 months of age, the primary outcome was available for 176 infants in the 72-hour group, 171 in the 120-hour group, 185 in the 33.5°C group, and 162 in the 32.0°C group. The baseline maternal and neonatal characteristics of the 72-hour and 120-hour groups and the 33.5°C and 32.0°C groups are shown in Table 1 and those across all 4 randomization groups are presented in the eTable in Supplement 2. The primary outcome of death or moderate or severe disability occurred in 56 of 176 infants (31.8%) in the 72-hour group, 54 of 171 infants (31.6%) in the 120-hour group, 59 of 185 infants (31.9%) in the 33.5°C group, and 51 of 162 infants (31.5%) in the 32.0°C group (Table 2). The adjusted RRs for the primary outcome for duration or depth of cooling were not significantly different. The primary outcome was similar among infants with moderate or severe encephalopathy between the 120-hour and 72-hour groups and between the 32.0°C and 33.5°C groups.
Figure. Flow of Neonates Through a Trial of Cooling for 120 Hours vs 72 Hours and Cooling to 32.0°C vs 33.5°C for Hypoxic-Ischemic Encephalopathy.
ECMO indicates extracorporeal membrane oxygenation.
aModerate or severe encephalopathy was defined as the presence of 1 or more signs in at least 3 of the following 6 categories: (1) level of consciousness (moderate is lethargic, severe is stupor or coma); (2) spontaneous activity (moderate is decreased activity, severe is no activity); (3) posture (moderate is distal flexion or complete extension, severe is decerebrate); (4) tone (moderate is hypotonia, severe is flaccid); (5) primitive reflexes (moderate is a weak suck, severe is an absent suck, or moderate is incomplete Moro reflex and severe is absent); and (6) autonomic nervous system—either pupil (moderate is constricted; severe is deviated, dilated, or nonreactive to light), heart rate (moderate is bradycardia, severe is variable heart rate), or respiration (moderate is periodic breathing, severe is apnea).
Table 1. Maternal and Neonatal Characteristics for the Duration of Cooling and Depth of Cooling Groupsa.
| Characteristics | Duration of Cooling | Depth of Cooling | ||
|---|---|---|---|---|
| 72 h (n = 176) |
120 h (n = 171) |
33.5°C (n = 185) |
32.0°C (n = 162) |
|
| Maternal characteristics | ||||
| Race, No. (%)b | ||||
| Black | 51 (29) | 56 (33) | 58 (32) | 49 (31) |
| White | 113 (65) | 98 (58) | 112 (61) | 99 (62) |
| Otherb | 11 (6) | 14 (8) | 13 (7) | 12 (8) |
| Age, mean (SD), y | 28.1 (6.6) | 28.1 (7.0) | 28.3 (6.6) | 27.9 (7.0) |
| Married, No. (%) | 93 (53) | 89 (52) | 100 (55) | 82 (51) |
| Gravida, median (IQR) | 2 (1-3) | 2 (1-3) | 2 (1-3) | 2 (1-3) |
| Parity, median (IQR) | 1 (1-3) | 1 (1-3) | 1 (1-3) | 1 (1-2) |
| Pregnancy complications, No. (%) | ||||
| Chronic hypertension | 37 (21) | 33 (20) | 33 (18) | 37 (23) |
| Antepartum hemorrhage | 22 (13) | 16 (9) | 20 (11) | 18 (11) |
| Thyroid dysfunction | 11 (6) | 3 (2) | 6 (3) | 8 (5) |
| Diabetes | 21 (12) | 24 (14) | 22 (12) | 23 (14) |
| Intrapartum complications, No. (%) | ||||
| Decelerations in fetal heart rate | 134 (77) | 134 (79) | 144 (79) | 124 (77) |
| Cord prolapse, rupture, or compression | 26 (15) | 20 (12) | 28 (15) | 18 (11) |
| Uterine rupture | 11 (6) | 11 (6) | 9 (5) | 13 (8) |
| Maternal pyrexia (≥37.6°C) | 23 (13) | 18 (11) | 17 (9) | 24 (15) |
| Shoulder dystocia | 14 (8) | 14 (8) | 14 (8) | 14 (9) |
| Maternal hemorrhage | 25 (14) | 28 (16) | 29 (16) | 24 (15) |
| Rupture of membranes (spontaneous or induced), No. (%) | ||||
| None | 44 (26) | 49 (30) | 55 (31) | 38 (25) |
| ≤18 h prior to delivery | 111 (65) | 94 (58) | 108 (60) | 97 (63) |
| >18 h prior to delivery | 16 (9) | 20 (12) | 17 (9) | 19 (12) |
| Rupture of membranes, h prior to delivery | ||||
| Mean (SD) | 11.1 (19.6) | 10.8 (16.8) | 10.9 (16.5) | 11.0 (20.0) |
| Median (IQR) | 7.6 (2.7-14.7) | 5.9 (2.1-14.3) | 6.4 (2.3-14.1) | 7.3 (1.8-15.1) |
| Emergency cesarean delivery, No. (%) | 111 (63) | 107 (63) | 113 (61) | 105 (65) |
| Neonatal characteristics | ||||
| Age at randomization, mean (SD), h | 5.0 (1.1) | 4.9 (1.4) | 4.9 (1.1) | 4.9 (1.4) |
| Transferred from birth hospital, No. (%) | 114 (65) | 111 (65) | 121 (65) | 104 (64) |
| Male, No. (%) | 102 (58) | 100 (58) | 99 (54) | 103 (64) |
| Apgar score ≤5, No. (%) | ||||
| 5 min after birth | 147 (84) | 143 (85) | 157 (85) | 133 (83) |
| 10 min after birth | 102 (66) | 109 (71) | 111 (70) | 100 (67) |
| Birth weight, mean (SD), g | 3300 (530) | 3429 (649) | 3297 (610) | 3440 (567) |
| Gestational age, mean (SD), wk | 38.6 (1.5) | 38.7 (1.4) | 38.5 (1.4) | 38.7 (1.5) |
| Length, mean (SD), cm | 50.6 (2.9) | 50.8 (3.1) | 50.5 (2.9) | 50.9 (3.1) |
| Head circumference, mean (SD), cm | 34.0 (1.9) | 34.3 (1.7) | 34.0 (1.6) | 34.2 (2.0) |
| Intubation in delivery room, No. (%) | 135 (77) | 136 (80) | 146 (79) | 125 (78) |
| Continued resuscitation at 10 min, No. (%) | 149 (85) | 152 (90) | 163 (89) | 138 (86) |
| Time to spontaneous respiration >10 min, No. (%) |
70 (42) | 76 (48) | 81 (47) | 65 (42) |
| Cord blood | ||||
| pH, mean (SD) | 6.9 (0.2) | 6.9 (0.2) | 6.9 (0.2) | 7.0 (0.2) |
| Base deficit, mean (SD), mmol/L | 16.0 (7.8) | 15.9 (6.6) | 16.0 (7.5) | 15.9 (6.9) |
| Seizuresc | 46 (26) | 56 (33) | 57 (31) | 45 (28) |
| Moderate encephalopathyd | 135 (77) | 128 (75) | 146 (79) | 117 (72) |
| Severe encephalopathyd | 41 (23) | 43 (25) | 39 (21) | 45 (28) |
| Inotropic supportc | 42 (24) | 33 (19) | 35 (19) | 40 (25) |
| Anticonvulsantsc | 29 (18) | 25 (17) | 30 (19) | 24 (17) |
Abbreviation: IQR interquartile range.
Percentages are based on the number of mothers or neonates for whom data were available. Because of rounding, not all percentages sum to 100.
Other race includes American Indian or Alaskan Native, Asian, Native Hawaiian or other Pacific Islander, and more than 1 race.
Data are from time of randomization.
Encephalopathy was defined as the presence of either moderate or severe signs in at least 3 of the following 6 categories: (1) level of consciousness (moderate is lethargic, severe is stupor or coma); (2) spontaneous activity (moderate is decreased activity, severe is no activity); (3) posture (moderate is distal flexion or complete extension, severe is decerebrate); (4) tone (moderate is hypotonia, severe is flaccid); (5) primitive reflexes (moderate is a weak suck, severe is an absent suck, or moderate is incomplete Moro reflex and severe is absent); and (6) autonomic nervous system—either pupil (moderate is constricted; severe is deviated, dilated, or nonreactive to light), heart rate (moderate is bradycardia, severe is variable heart rate), or respiration (moderate is periodic breathing, severe is apnea). The number of moderate or severe signs determined the extent of encephalopathy; if signs were equally distributed, the designation was based on level of consciousness.
Table 2. Outcome at 18 to 22 Months of Age by Duration of Cooling and Depth of Cooling Groupsa.
| Outcomes | Duration of Cooling | Depth of Cooling | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neonates, No./Total (%) | Adjusted Risk Difference, % (95% CI) | Adjusted RR (95% CI) |
P Value | Neonates, No./Total (%) | Adjusted Risk Difference, % (95% CI) | Adjusted RR (95% CI) |
P Value | |||
| 72 h (n = 176) |
120 h (n = 171) |
33.5°C (n = 185) |
32.0°C (n = 162) |
|||||||
| Primary outcome | ||||||||||
| Death or moderate or severe disability | 56/176 (31.8) | 54/171 (31.6) | −1.0 (−10.2 to 8.1) | 0.92 (0.68-1.25) | .60 | 59/185 (31.9) | 51/162 (31.5) | −3.1 (−12.3 to 6.1) | 0.92 (0.68-1.26) | .62 |
| Secondary outcomes | ||||||||||
| Death | 23/176 (13) | 33/171 (19) | 6.2 (−0.9 to 13.4) | 1.39 (1.02-1.90) | .04 | 26/185 (14) | 30/162 (19) | 2.4 (−4.8 to 9.6) | 1.17 (0.67-2.04) | .58 |
| Among infants with moderate encephalopathy | 8/135 (6) | 11/128 (9) | 3.0 (−3.5 to 9.5) | 1.39 (0.60-3.24) | .44 | 11/146 (8) | 8/117 (7) | −0.6 (−7.2 to 6.0) | 0.90 (0.34-2.36) | .83 |
| Among infants with severe encephalopathy | 15/41 (37) | 22/43 (51) | 21.2 (−1.4 to 43.8) | 1.44 (0.94-2.21) | .09 | 15/39 (38) | 22/45 (49) | 0.6 (−24.0 to 25.2) | 1.24 (0.70-2.18) | .46 |
| Death or moderate or severe disability | ||||||||||
| Among infants with moderate encephalopathy | 27/135 (20) | 28/128 (22) | 1.9 (−8.3 to 12.0) | 1.11 (0.72-1.69) | .64 | 33/146 (23) | 22/117 (19) | −4.6 (−14.8 to 5.7) | 0.82 (0.46-1.46) | .50 |
| Among infants with severe encephalopathy | 29/41 (71) | 26/43 (60) | −4.3 (−26.0 to 17.4) | 0.86 (0.55-1.35) | .52 | 26/39 (67) | 29/45 (64) | −14.9 (−37.6 to 7.9) | 0.95 (0.73-1.22) | .67 |
| Death or severe disability | 52/176 (30) | 54/171 (32) | 1.3 (−7.8 to 10.3) | 0.98 (0.71-1.35) | .90 | 58/185 (31) | 48/162 (30) | −4.3 (−13.4 to 4.8) | 0.89 (0.64-1.23) | .47 |
| Among survivors | ||||||||||
| Moderate or severe disability | 33/153 (22) | 21/138 (15) | −6.4 (−15.4 to 2.7) | 0.68 (0.41-1.11) | .13 | 33/159 (21) | 21/132 (16) | −5.1 (−14.2 to 3.9) | 0.71 (0.36-1.39) | .32 |
| Moderate disability | 4/153 (3) | 0/138 (0) | −2.6 (−5.4 to 0.2) | NA | NA | 1/159 (1) | 3/132 (2) | 1.6 (−1.1 to 4.4) | NA | NA |
| Severe disability | 29/153 (19) | 21/138 (15) | −3.8 (−12.6 to 5.0) | 0.77 (0.45-1.29) | .32 | 32/159 (20) | 18/132 (14) | −6.8 (−15.5 to 2.0) | 0.63 (0.31-1.28) | .20 |
| Mild disabilityb | 31/150 (21) | 40/135 (30) | 7.7 (−2.7 to 18.1) | NA | NA | 38/153 (25) | 33/132 (25) | −0.6 (−11.0 to 9.7) | NA | NA |
| Visual impairmentc | 13/153 (9) | 6/138 (4) | −3.6 (−9.3 to 2.1) | 0.50 (0.20-1.26) | .14 | 9/159 (6) | 10/132 (8) | 1.5 (−4.2 to 7.2) | 1.23 (0.50-3.05) | .65 |
| Hearing impairmentd | 6/153 (4) | 5/138 (4) | −0.8 (−5.3 to 3.7) | 0.91 (0.42-1.98) | .81 | 8/159 (5) | 3/132 (2) | −3.3 (−7.7 to 1.2) | 0.41 (0.09-1.93) | .26 |
| No disability (normal)b | 86/150 (57) | 74/135 (55) | −1.3 (−12.9 to 10.3) | 0.92 (0.82-1.03) | .16 | 82/153 (54) | 78/132 (59) | 6.5 (−5.0 to 17.9) | 1.11 (0.81-1.52) | .52 |
| Multiple disabilitiese | 24/153 (16) | 12/138 (9) | −6.7 (−14.3 to 1.0) | 0.55 (0.28-1.10) | .09 | 22/159 (14) | 14/132 (11) | −2.9 (−10.5 to 4.7) | 0.73 (0.31-1.72) | .47 |
| Rehospitalizations after dischargef | 43/149 (29) | 22/135 (16) | −12.1 (−21.7 to −2.5) | 0.57 (0.36-0.93) | .02 | 39/152 (26) | 26/132 (20) | −6.4 (−16.0 to 3.2) | 0.74 (0.49-1.12) | .15 |
| Gastrostomy or tube feedingsf | 27/149 (18) | 18/135 (13) | −5.6 (−14.0 to 2.9) | 0.70 (0.41-1.20) | .20 | 28/152 (18) | 17/132 (13) | −4.9 (−13.3 to 3.6) | 0.66 (0.36-1.21) | .18 |
| Height <10th percentileg | 32/144 (22) | 24/132 (18) | −3.1 (−12.6 to 6.5) | 0.78 (0.53-1.15) | .21 | 32/145 (22) | 24/131 (18) | −4.7 (−14.2 to 4.7) | 0.80 (0.47-1.37) | .41 |
| Weight <10th percentileg | 16/147 (11) | 11/133 (8) | −1.4 (−8.5 to 5.7) | 0.75 (0.37-1.53) | .43 | 13/148 (9) | 14/132 (11) | 2.2 (−4.9 to 9.2) | 1.18 (0.53-2.61) | .68 |
| Head circumference <10th percentileg | 33/145 (23) | 21/131 (16) | −6.3 (−15.8 to 3.2) | 0.70 (0.42-1.17) | .18 | 24/145 (17) | 30/131 (23) | 5.3 (−4.1 to 14.8) | 1.35 (0.89-2.03) | .16 |
| Cerebral palsy | 29/153 (19) | 18/138 (13) | −5.2 (−13.6 to 3.3) | 0.65 (0.36-1.16) | .15 | 26/159 (16) | 21/132 (16) | −0.1 (−8.5 to 8.4) | 0.94 (0.51-1.73) | .85 |
| Quadriplegic | 14/29 (48) | 6/18 (33) | 9/26 (35) | 11/21 (52) | ||||||
| Diplegic | 3/29 (10) | 2/18 (11) | 4/26 (15) | 1/21 (5) | ||||||
| Hemiplegic | 2/29 (7) | 2/18 (11) | 2/26 (8) | 2/21 (10) | ||||||
| Dystonic | 3/29 (10) | 3/18 (17) | 3/26 (12) | 3/21 (14) | ||||||
| Athetotic | 1/29 (3) | 1/18 (6) | 2/26 (8) | 0 | ||||||
| Ataxic | 1/29 (3) | 2/18 (11) | 2/26 (8) | 1/21 (5) | ||||||
| Unclassified | 2/29 (7) | 2/18 (11) | 1/26 (4) | 3/21 (14) | ||||||
| Adjudicatedh | 3/29 (10) | 0 | 3/26 (12) | 0 | ||||||
| Disabling (moderate or severe)i | 24/153 (16) | 14/138 (10) | −4.8 (−12.6 to 3.0) | 0.63 (0.31-1.27) | .20 | 23/159 (14) | 15/132 (11) | −2.7 (−10.5 to 5.0) | 0.76 (0.36-1.60) | .47 |
| Seizuresj | 27/153 (18) | 16/137 (12) | −5.2 (−13.2 to 2.8) | 0.67 (0.38-1.21) | .19 | 25/158 (16) | 18/132 (14) | −3.2 (−11.1 to 4.8) | 0.83 (0.48-1.46) | .53 |
| Bayley III cognitive scorek | ||||||||||
| ≥85 | 99/147 (67) | 97/136 (71) | 1 [Reference] | 104/151 (69) | 92/132 (70) | 1 [Reference] | ||||
| 70-84 | 24/147 (16) | 23/136 (17) | −1.1 (−11.4 to 9.3) | 0.97 (0.70-1.34) | .83 | 23/151 (15) | 24/132 (18) | 1.1 (−9.2 to 11.4) | 1.13 (0.65-1.96) | .67 |
| <70 | 24/147 (16) | 16/136 (12) | −4.9 (−14.9 to 5.0) | 0.72 (0.48-1.07) | .10 | 24/151 (16) | 16/132 (12) | −3.9 (−13.8 to 5.9) | 0.73 (0.35-1.55) | .41 |
| Bayley III language scorel | ||||||||||
| ≥85 | 78/141 (55) | 79/134 (59) | 1 [Reference] | 78/145 (54) | 79/130 (61) | 1 [Reference] | ||||
| 70-84 | 34/141 (24) | 30/134 (22) | −1.5 (−13.7 to 10.7) | 0.91 (0.57-1.45) | .70 | 37/145 (26) | 27/130 (21) | −10.9 (−23.0 to 1.1) | 0.74 (0.50-1.10) | .14 |
| <70 | 29/141 (21) | 25/134 (19) | −2.6 (−14.7 to 9.6) | 0.87 (0.57-1.33) | .51 | 30/145 (21) | 24/130 (18) | −5.6 (−17.7 to 6.4) | 0.80 (0.45-1.43) | .45 |
| Bayley III motor scorem | ||||||||||
| ≥85 | 100/144 (69) | 108/133 (81) | 1 [Reference] | 108/149 (72) | 100/128 (78) | 1 [Reference] | ||||
| 70-84 | 17/144 (12) | 8/133 (6) | −9.0 (−17.2 to −0.7) | 0.46 (0.21-1.03) | .06 | 13/149 (9) | 12/128 (9) | −0.5 (−8.9 to 8.0) | 0.995 (0.51-1.95) | .99 |
| <70 | 27/144 (19) | 17/133 (13) | −7.4 (−16.9 to 2.1) | 0.60 (0.36-0.98) | .04 | 28/149 (19) | 16/128 (13) | −6.2 (−15.7 to 3.3) | 0.65 (0.30-1.41) | .28 |
Abbreviations: NA, not applicable due to small sample size; RR, relative risk.
All models adjust for center and severity of hypoxic-ischemic encephalopathy. Relative risks are shown for 120 hours vs 72 hours (duration of cooling) and for 32.0°C vs 33.5°C (depth of cooling). Encephalopathy was defined as the presence of either moderate or severe signs in at least 3 of the following 6 categories: (1) level of consciousness (moderate is lethargic, severe is stupor or coma); (2) spontaneous activity (moderate is decreased activity, severe is no activity); (3) posture (moderate is distal flexion or complete extension, severe is decerebrate); (4) tone (moderate is hypotonia, severe is flaccid); (5) primitive reflexes (moderate is a weak suck, severe is an absent suck, or moderate is incomplete Moro reflex and severe is absent); and (6) autonomic nervous system; either pupil (moderate is constricted, severe is deviated, dilated, or nonreactive to light), heart rate (moderate is bradycardia, severe is variable heart rate), or respiration (moderate is periodic breathing, severe is apnea). The number of moderate or severe signs determined the extent of the encephalopathy; if signs were equally distributed, the designation was based on the level of consciousness. Severe disability was defined as any of the following: a Bayley III cognitive score <70, a Gross Motor Function Classification System (GMFCS) level of 3-5, blindness, or profound hearing loss (inability to understand commands despite amplification). Moderate disability was defined as a Bayley III cognitive score of 70-84 and either a GMFCS level of 2, seizure disorder, or a hearing deficit requiring amplification to understand commands. Mild disability was defined by a cognitive score of 70-84 or a cognitive score ≥85 and any of the following: presence of a GMFCS level 1 or 2, seizure disorder, or hearing loss not requiring amplification. No disability (normal) was defined as a cognitive score ≥85 in the absence of any neurosensory deficits or seizures after neonatal intensive care unit discharge.
Six infants were missing data for no disability and mild disability. These infants were adjudicated to no/mild disability, but sufficient information was unavailable to distinguish between no disability and mild disability.
Visual impairment was defined as bilateral blindness with some/no useful vision.
Hearing impairment was defined as hearing impairment despite amplification.
Multiple disabilities was defined based on available data as ≥2 of the following: disabling cerebral palsy, GMFCS level 3-5, Bayley III cognitive score <70, blindness, or deafness.
Seven infants were missing data for rehospitalizations after discharge and gastrostomy or tube feedings. Numbers shown are based on available data. This is an exploratory variable.
Fifteen infants were missing data for height, 11 infants were missing data for weight, and 15 infants were missing data for head circumference. Numbers shown are based on available data.
Adjudicated cerebral palsy is based on caretaker interview/medical chart review by a small group of investigators unaware of treatment status.
Disabling cerebraly palsy was defined as that which was moderate or severe, with infants requiring support for sitting, unable to crawl, or requiring adult assistance to move.
One infant was missing data on seizures. Numbers shown are based on available data.
Eight infants were missing data on Bayley III cognitive score.
Sixteen infants were missing data on Bayley III language score.
Fourteen infants were missing data on Bayley III motor score.
Most of the secondary outcomes did not differ by duration or depth of cooling, with the exception of more deaths but fewer rehospitalizations after discharge and infants with motor scores less than 70 with duration of cooling of 120 hours vs 72 hours (Table 2). Very few surviving infants had moderate disability. An interaction test between depth and duration of cooling in this 2 × 2 factorial design trial was statistically significant for the primary outcome (P = .048). Rates of the primary outcomes in the 4 groups were 29.3% (27 of 92 infants) in the group with 33.5°C for 72 hours, 34.5% (29 of 84 infants) in the group with 32.0°C for 72 hours, 34.4% (32 of 93 infants) in the group with 33.5°C for 120 hours, and 28.2% (22 of 78 infants) in the group with 32.0°C for 120 hours (Table 3). The Bayesian analyses of the posterior probabilities of reducing death or moderate or severe disability with deeper cooling, longer cooling, or both compared with standard cooling (probability of RR <1.0) were 58%, 50%, and 78%, respectively. There was no statistically significant interaction for death (P = .11) or any other secondary outcome other than a Bayley III cognitive score of 70 to 84 vs 85 or higher (P = .049) and a Bayley III motor score of less than 70 vs 85 or higher (P = .04).
Table 3. Outcomes at 18 to 22 Months of Age by the 4 Hypothermia Groupsa.
| Outcomes | Neonates, No./Total (%) | Adjusted Risk Difference, % (95% CI) |
Adjusted RR (95% CI) |
Neonates, No./Total (%) | Adjusted Risk Difference, % (95% CI) |
Adjusted RR (95% CI) | Neonates, No./Total (%) | Adjusted Risk Difference, % (95% CI) |
Adjusted RR (95% CI)a |
|
|---|---|---|---|---|---|---|---|---|---|---|
| For 72 h at 33.5°C | For 72 h at 32.0°C | For 120 h at 33.5°C | For 120 h at 32.0°C | |||||||
| Primary outcome | ||||||||||
| Death or moderate or severe disability | 27/92 (29.3) | 29/84 (34.5) | 5.0 (−7.8 to 17.8) | 1.23 (0.76-1.98) | 32/93 (34.4) | 6.7 (−5.8 to 19.3) | 1.21 (0.78-1.87) | 22/78 (28.2) | −4.9 (−18.1 to 8.3) | 0.85 (0.53-1.35) |
| Secondary outcomes | ||||||||||
| Death | 8/92 (9) | 15/84 (18) | 9.5 (−0.6 to 19.5) | 2.16 (0.55-8.49) | 18/93 (19) | 12.9 (3.1 to 22.7) | 2.52 (1.06-5.95) | 15/78 (19) | 8.3 (−1.9 to 18.6) | 1.85 (0.79-4.31) |
| Among infants with moderate encephalopathy | 4/71 (6) | 4/64 (6) | 1.2 (−8.0 to 10.4) | 1.06 (0.26-4.29) | 7/75 (9) | 4.4 (−4.4 to 13.2) | 1.55 (0.49-4.92) | 4/53 (8) | 2.3 (−7.4 to 12.0) | 1.27 (0.26-6.20) |
| Among infants with severe encephalopathy | 4/21 (19) | 11/20 (55) | 24.1 (−7.9 to 56.2) | 2.68 (0.61-11.8) | 11/18 (61) | 53.6 (20.1 to 87.1) | 3.14 (1.31-7.51) | 11/25 (44) | 20.8 (−8.7 to 50.3) | 2.25 (1.06-4.77) |
| Death or moderate or severe disability | ||||||||||
| Among infants with moderate encephalopathy | 14/71 (20) | 13/64 (20) | −1.0 (−15.3 to 13.4) | 1.02 (0.48-2.15) | 19/75 (25) | 4.8 (−9.0 to 18.5) | 1.29 (0.74-2.25) | 9/53 (17) | −3.4 (−18.4 to 11.7) | 0.86 (0.41-1.80) |
| Among infants with severe encephalopathy | 13/21 (62) | 16/20 (80) | 2.5 (−28.8 to 33.7) | 1.29 (0.80-2.07) | 13/18 (72) | 17.7 (−15.0 to 50.4) | 1.17 (0.67-2.05) | 13/25 (52) | −14.9 (−43.7 to 13.9) | 0.84 (0.48-1.46) |
| Among survivorsb | ||||||||||
| Moderate or severe disability | 19/84 (23) | 14/69 (20) | −1.0 (−13.5 to 11.5) | 0.95 (0.49-1.85) | 14/75 (19) | −2.4 (−14.7 to 9.9) | 0.89 (0.47-1.69) | 7/63 (11) | −12.2 (−25.1 to 0.7) | 0.44 (0.16-1.16) |
| Moderate disability | 1/84 (1) | 3/69 (4) | 3.2 (−0.6 to 7.0) | 0/75 (0) | −1.0 (−4.8 to 2.7) | 0/63 (0) | −1.2 (−5.1 to 2.7) | |||
| Visual impairmentc | 7/84 (8) | 6/69 (9) | 0.8 (−7.1 to 8.7) | 1.18 (0.53-2.61) | 2/75 (3) | −4.3 (−12.1 to 3.5) | 0.38 (0.08-1.83) | 4/63 (6) | −2.0 (−10.2 to 6.1) | 0.68 (0.17-2.67) |
| Hearing impairmentd | 4/84 (5) | 2/69 (3) | −1.4 (−7.6 to 4.8) | 0.67 (0.15-3.05) | 4/75 (5) | 1.0 (−5.1 to 7.1) | 1.34 (0.45-3.99) | 1/63 (2) | −4.4 (−10.8 to 2.0) | 0.29 (0.03-2.93) |
| No disability (normal)e | 46/81 (57) | 40/69 (58) | 0.4 (−15.5 to 16.4) | 0.99 (0.67-1.47) | 36/72 (50) | −7.2 (−23.1 to 8.7) | 0.82 (0.63-1.08) | 38/63 (60) | 5.9 (−10.5 to 22.3) | 1.03 (0.77-1.39) |
| Mild disabilitye | 16/81 (20) | 15/69 (22) | 1.2 (−13.1 to 15.5) | NA | 22/72 (31) | 9.5 (−4.8 to 23.8) | NA | 18/63 (29) | 6.9 (−7.9 to 21.6) | NA |
| Multiple disabilitiesf | 15/84 (18) | 9/69 (13) | −3.4 (−14.0 to 7.1) | 0.81 (0.34-1.89) | 7/75 (9) | −7.1 (−17.5 to 3.3) | 0.60 (0.28-1.27) | 5/63 (8) | −9.5 (−20.4 to 1.3) | 0.42 (0.12-1.47) |
| Rehospitalizations after discharge | 29/80 (36) | 14/69 (20) | −14.7 (−27.8 to −1.6) | 0.58 (0.30-1.13) | 10/72 (14) | −20.2 (−33.3 to −7.1) | 0.42 (0.21-0.84) | 12/63 (19) | −17.7 (−31.2 to −4.1) | 0.52 (0.30-0.89) |
| Gastrostomy or tube feedingsg | 16/80 (20) | 11/69 (16) | −2.9 (−14.5 to 8.8) | 0.79 (0.49-1.28) | 12/72 (17) | −3.6 (−15.2 to 8.0) | 0.84 (0.48-1.47) | 6/63 (10) | −10.8 (−22.8 to 1.3) | 0.44 (0.16-1.18) |
| Height <10th percentile | 19/75 (25) | 13/69 (19) | −7.0 (−20.2 to 6.2) | 0.77 (0.41-1.46) | 13/70 (19) | −5.4 (−18.6 to 7.9) | 0.76 (0.42-1.39) | 11/62 (18) | −7.8 (−21.4 to 5.9) | 0.65 (0.33-1.30) |
| Weight <10th percentile | 7/78 (9) | 9/69 (13) | 5.0 (−4.8 to 14.8) | 1.46 (0.57-3.77) | 6/70 (9) | 1.4 (−8.4 to 11.2) | 0.98 (0.38-2.49) | 5/63 (8) | 0.5 (−9.6 to 10.6) | 0.85 (0.28-2.57) |
| Head circumference <10th percentile | 15/76 (20) | 18/69 (26) | 6.1 (−7.0 to 19.2) | 1.37 (0.91-2.07) | 9/69 (13) | −5.3 (−18.5 to 7.9) | 0.69 (0.33-1.45) | 12/62 (19) | −1.1 (−14.6 to 12.5) | 0.94 (0.47-1.88) |
| Cerebral palsy | 16/84 (19) | 13/69 (19) | 1.9 (−9.8 to 13.6) | 1.18 (0.63-2.20) | 10/75 (13) | −3.2 (−14.7 to 8.3) | 0.79 (0.35-1.80) | 8/63 (13) | −5.6 (−17.6 to 6.5) | 0.61 (0.26-1.45) |
| Quadriplegic | 6/19 (38) | 8/13 (62) | 3/10 (30) | 3/8 (38) | ||||||
| Diplegic | 2/19 (13) | 1/13 (8) | 2/10 (20) | 0 | ||||||
| Hemiplegic | 1/19 (6) | 1/13 (8) | 1/10 (10) | 1/8 (13) | ||||||
| Dystonic | 1/19 (6) | 2/13 (15) | 2/10 (20) | 1/8 (13) | ||||||
| Athetotic | 1/19 (6) | 0 | 1/10 (10) | 0 | ||||||
| Ataxic | 1/19 (6) | 0 | 1/10 (10) | 1/8 (13) | ||||||
| Unclassified | 1/19 (6) | 1/13 (8) | 0 | 2/8 (25) | ||||||
| Adjudicatedh | 3/19 (19) | 0 | 0 | 0 | ||||||
| Disabling (moderate or severe)i | 14/84 (17) | 10/69 (14) | −0.4 (−11.2 to 10.4) | 0.98 (0.48-2.01) | 3/10 (30) | −2.6 (−13.2 to 8.0) | 0.81 (0.36-1.84) | 5/63 (8) | −7.9 (−19.0 to 3.2) | 0.45 (0.13-1.53) |
| Seizures | 15/84 (18) | 12/69 (17) | −1.6 (−12.6 to 9.4) | 1.03 (0.53-1.99) | 10/74 (14) | −3.7 (−14.5 to 7.2) | 0.83 (0.38-1.82) | 6/63 (10) | −8.6 (−19.9 to 2.7) | 0.54 (0.20-1.44) |
| Bayley III cognitive score | ||||||||||
| Median (IQR) | 90 (80-100) | 90 (75-100) | 90 (80-100) | 90 (80-95) | ||||||
| ≥85 | 51 (65) | 48 (70) | 1 [Reference] | 53 (73) | 1 [Reference] | 44 (70) | 1 [Reference] | |||
| 70-84 | 14 (18) | 10 (14) | −5.7 (−20.3 to 8.9) | 0.80 (0.38-1.68) | 9 (12) | −7.7 (−22.1 to 6.7) | 0.67 (0.41-1.10) | 14 (22) | 0.4 (−14.3 to 15.1) | 1.09 (0.57-2.10) |
| <70 | 13 (17) | 11 (16) | −1.1 (−14.8 to 12.6) | 0.95 (0.41-2.20) | 11 (15) | −2.2 (−15.7 to 11.3) | 0.92 (0.50-1.71) | 5 (8) | −9.9 (−24.4 to 4.6) | 0.45 (0.16-1.28) |
| Bayley III language score | ||||||||||
| >85 | 39 (53) | 39 (57) | 39 (54) | 40 (65) | ||||||
| 70-84 | 18 (25) | 16 (24) | −7.9 (−25.0 to 9.2) | 0.87 (0.45-1.70) | 19 (26) | 1.1 (−15.8 to 18.0) | 1.05 (0.59-1.88) | 11 (18) | −13.2 (−30.9 to 4.4) | 0.64 (0.32-1.28) |
| <70 | 16 (22) | 13 (19) | −4.2 (−21.3 to 12.9) | 0.87 (0.44-1.70) | 14 (19) | −1.2 (−18.3 to 15.9) | 0.94 (0.53-1.69) | 11 (18) | −8.2 (−25.5 to 9.0) | 0.70 (0.30-1.61) |
| Bayley III motor score | ||||||||||
| >85 | 53 (68) | 47 (71) | 55 (77) | 53 (85) | ||||||
| 70-84 | 10 (13) | 7 (11) | −2.6 (−14.6 to 9.3) | 0.88 (0.44-1.78) | 3 (4) | −11.1 (−22.9 to 0.6) | 0.35 (0.15-0.82) | 5 (8) | −9.3 (−21.0 to 2.5) | 0.53 (0.17-1.63) |
| <70 | 15 (19) | 12 (18) | 0.1 (−13.2 to 13.4) | 1.02 (0.48-2.16) | 13 (18) | −1.5 (−14.4 to 11.3) | 0.89 (0.46-1.69) | 4 (6) | −14.5 (−28.0 to −0.9) | 0.29 (0.09-0.97) |
Abbreviations: IQR, interquartile range; NA, not applicable due to small sample size; RR, relative risk.
Adjusted risk differences and RRs compare each experimental group with the usual care group (cooling for 72 hours at 33.5°C). For definitions of encephalopathy and severe, moderate, mild, or no disability, see footnote a in Table 2.
Missing data: 17 neonates were lost to follow-up 3 in the group with 72 hours of cooling at 33.5°C; 6 in the group with 72 hours of cooling at 32.0°C; 3 in the group with 120 hours of cooling at 33.5°C; and 5 in the group with 72 hours of cooling at 32.0°C.
Visual impairment is defined as bilateral blindness with some/no useful vision.
Hearing impairment is defined as hearing impairment despite amplification.
Six infants were missing data for no disability (normal) and mild disability. These infants were adjudicated to no/mild disability, but sufficient information was unavailable to distinguish between normal and mild disability. These include 3 infants in in the group with 72 hours of cooling at 33.5°C and 3 in the in the group with 120 hours of cooling at 33.5°C.
Multiple disabilities was defined as ≥2 of the following: disabling cerebral palsy, Gross Motor Function Classification System level 3-5, Bayley III cognitive score <70, blindness, or deafness. Multiple disabilities occurred only in the severe disability group.
Exploratory outcome.
Adjudicated cerebral palsy is based on review caretaker interview/medical chart review by a small group of investigators unaware of treatment status.
Disabling cerebral palsy was defined as that which was moderate or severe, with infants requiring support for sitting, unable to crawl, or requiring adult assistance to move.
The mortality rates in the 4 hypothermia groups are noted in the eFigure in Supplement 2. The adjusted RR for death was higher in the group with 33.5°C for 120 hours vs the group with 33.5°C for 72 hours (RR, 2.52; 95% CI, 1.06-5.95) (Table 3). The Bayesian estimates of the adjusted RRs for mortality for the 3 intervention groups compared with standard cooling were 1.08 (95% CrI, 0.74-1.59) for the group with 32.0°C for 72 hours, 1.33 (95% CrI, 0.91-1.93) for the group with 33.5°C for 120 hours, and 1.36 (95% CrI, 0.81-2.21) for the group with 32.0°C for 120 hours. The posterior probabilities of increasing death with deeper cooling, longer cooling, or both compared with standard cooling (probability of RR >1.0) were 66%, 93%, and 89%, respectively.
Discussion
This trial demonstrated that neither longer nor deeper cooling reduced mortality or appeared neuroprotective in term neonates with moderate or severe hypoxic-ischemic encephalopathy. The rates of death or moderate or severe disability among infants randomized to the usual care group (cooling to 33.5°C for 72 hours) was 29%; this is lower than the rate of 44% from the cooled group in the first NICHD Neonatal Research Network cooling trial and in other trials. This lower rate may reflect the lower rate of severe encephalopathy, lower acuity, and earlier initiation of cooling in this trial.
In this study, there was a higher mortality rate noted among infants who underwent cooling for 120 hours compared with 72 hours. The infants undergoing cooling for 120 hours at 32.0°C had the highest mortality rates but lowest disability rates. This finding needs to be interpreted with caution in view of the composite outcome, the early closure of the study, and the significant interaction between depth and duration of cooling. The Bayesian analyses indicated a 78% probability that longer and deeper cooling reduces death or disability; however, this low disability rate (11%) was offset by the increased mortality. Some earlier preclinical studies demonstrated that extending the duration or depth of cooling was neuroprotective; other recent studies have not.
The strengths of this study are that 71% of eligible infants were enrolled and primary outcome data were available for 95% of infants. Functional and cognitive outcome was evaluated by certified examiners unaware of treatment status. The limitations of this study are that the study was not powered to examine interactions and no adjustments were made for multiple comparisons.
Conclusions
Among neonates of at least 36 weeks’ gestational age with moderate or severe hypoxic-ischemic encephalopathy, cooling for longer than 72 hours, cooling to a depth lower than 33.5°C, or both did not reduce death or disability at 18 months of age. However, the trial may be underpowered, and an interaction was found between longer and deeper cooling. These results support the current regimen of cooling for 72 hours at 33.5°C.
Trial Protocol
eAppendix. Material for Bayesian Analysis of Optimizing Cooling Trial
eTable. Maternal and Neonatal Characteristics by the Four Hypothermia Groups
eFigure. Kaplan-Meier Survival Analysis
References
- 1.Shankaran S, Laptook AR, Ehrenkranz RA, et al. ; National Institute of Child Health and Human Development Neonatal Research Network . Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574-1584. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1(1):CD003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankaran S, Pappas A, McDonald SA, et al. ; Eunice Kennedy Shriver NICHD Neonatal Research Network . Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366(22):2085-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzopardi D, Strohm B, Marlow N, et al. ; TOBY Study Group . Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371(2):140-149. [DOI] [PubMed] [Google Scholar]
- 5.Thoresen M, Bågenholm R, Løberg EM, Apricena F, Kjellmer I. Posthypoxic cooling of neonatal rats provides protection against brain injury. Arch Dis Child Fetal Neonatal Ed. 1996;74(1):F3-F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennet L, Roelfsema V, George S, Dean JM, Emerald BS, Gunn AJ. The effect of cerebral hypothermia on white and grey matter injury induced by severe hypoxia in preterm fetal sheep. J Physiol. 2007;578(Pt 2):491-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankaran S, Laptook AR, Pappas A, et al. ; Eunice Kennedy Shriver NICHD Neonatal Research Network . Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA. 2014;312(24):2629-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214-223. [DOI] [PubMed] [Google Scholar]
- 9.Bayley N. Manual for the Bayley Scales of Infant and Toddler Development. 3rd ed San Antonio, TX: Hardcourt Assessment; 2006. [Google Scholar]
- 10.Pedroza C, Tyson JE, Das A, Laptook A, Bell EF, Shankaran S; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Advantages of Bayesian monitoring methods in deciding whether and when to stop a clinical trial: an example of a neonatal cooling trial. Trials. 2016;17(1):335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson JO, Yuill CA, Zhang FG, Wassink G, Bennet L, Gunn AJ. Extending the duration of hypothermia does not further improve white matter protection after ischemia in term-equivalent fetal sheep. Sci Rep. 2016;6:25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alonso-Alconada D, Broad KD, Bainbridge A, et al. Brain cell death is reduced with cooling by 3.5°C to 5°C but increased with cooling by 8.5°C in a piglet asphyxia model. Stroke. 2015;46(1):275-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood T, Osredkar D, Puchades M, et al. Treatment temperature and insult severity influence the neuroprotective effects of therapeutic hypothermia. Sci Rep. 2016;6(6):23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Material for Bayesian Analysis of Optimizing Cooling Trial
eTable. Maternal and Neonatal Characteristics by the Four Hypothermia Groups
eFigure. Kaplan-Meier Survival Analysis