Abstract
HIV is most prevalent among men who have sex with men (MSM), and although most MSM use condoms consistently during casual sex, some take risks. To better understand the psychology of those risky decisions, we examined neural correlates of playing a virtual sexual ‘hook up’ game in an functional magnetic resonance imaging scanner in MSM who had, in the past 90 days, been sexually risky (N = 76) or safe (N = 31). We found that during potentially risky sexual choices, previously risky MSM had more right insula activity than previously safe MSM. Real-life sexual risk was related to trait positive and negative urgency. Insula activity that differentiated risky and safe MSM was related to trait positive and negative urgency. Future work should further examine if, and to what extent, insula activation during safe sex negotiation drives MSM’s rash risky sexual decision-making.
Keywords: risky decision-making, sexual health, men who have sex with men, insula, negative urgency, fMRI
Introduction
Risky sexual behavior increases the risk of contracting sexual diseases, including HIV (Bearinger et al., 2007), which continue to spread among men who have sex with men (MSM) in the United States (CDC, 2015). Sexually risky decisions (SRDs) ‘in the heat of the moment’ often precipitate risky sexual behavior (Noar et al., 2006), so examining sexual behavior within that context could provide a better understanding of factors contributing to sexually risky behavior. Retrospective reporting of desires, urges and feelings may be inaccurate and thus inadequate to capture qualities of those ‘in the moment’ decision-making experiences (Mustanski, 2007) particularly given that a high state of sexual arousal is correlated with sexual risk behaviors in MSM (Ariely and Loewenstein, 2006). To address this issue, we used a virtual ‘hook up’ computer game to provoke responses similar to those participants would have while making real life sex-related decisions.
The current article presents the first study to capture brain responses in an ecologically valid ‘heat of the moment’ sexual risk simulation task. Participants played the computer game during functional magnetic resonance imaging (fMRI) scanning and their decision-time neural responses were captured and analyzed. This enabled us to combine neurological correlates with behavioral and personality correlates of risky sex to explore why people make risky choices in spite of the possible health consequences.
To select candidate neural factors of risk-taking during decision-making, we identified key decision-making sub-regions using Bechara’s (2005) neurobiological theory of decision-making (Noël et al., 2013). The theory describes three key subsystems determining decision-making in an immediate-reward context: (1) a reflective subsystem; (2) an impulsive subsystem and (3) an interoceptive-urge subsystem.
Reflective subsystem components include parts of the lateral prefrontal cortex (lPFC) and anterior cingulate cortex, thought to be important in cognitive control and response inhibition (Ridderinkhof et al., 2004; Hazy et al., 2007), and the ventromedial prefrontal cortex (PFC), implicated in integrating and assessing value of alternatives (Basten et al., 2010). In this article, we divide the reflective system into two sub-components: the dorsal PFC system and the ventral PFC system, considering the dorsal PFC’s role in top-down controlled processing and the ventral PFC’s role in valuation of alternatives and affective consideration of future consequences (Bechara, 2005).
The impulsive subsystem is centered around the striatum (Balleine et al., 2007) and the amygdala. The interoceptive urge system, comprised of the insula, is the source of interoceptive signals. It is linked to deviations from homeostasis that give rise to the feeling of urge and desire (Naqvi and Bechara, 2009; Xue et al., 2010; Berntson et al., 2011; Droutman et al., 2015a,b), and it drives a salience and attention network (Sridharan et al., 2008; Uddin, 2014). Droutman et al. (2015a,b) highlighted the insula’s role in stimulus-driven interoceptive urge (Garavan, 2010) or craving in risky conditions (Naqvi et al., 2014), salience (Menon and Uddin, 2010), attention switching (Sridharan et al., 2008), and task set maintenance (Dosenbach et al., 2007) and tracking reward value (Paulus and Frank, 2006) during decisions involving uncertainty (Weller et al., 2007). Through the insula’s role in salience and attention, it could perform a role in a cognitive control network (Cole and Schneider, 2007), driving attention to stimuli and a facilitating interoceptive-driven decision-making response to an interoceptive trigger, particularly under risky conditions. In particular, its role in task set maintenance (Dosenbach et al., 2007) during decision processing could explain how the insula produces salience and attention functions within the broader cognitive control network, driving bottom-up attentional redirection to give it a role in cognition and decision-making. Interoceptive urge, salience, valuation and cognitive control appear to be most evident in the right anterior dorsal insula (Droutman et al., 2015a,b). Risky participants may experience greater desire or interoceptive urge upon exposure to highly salient or arousing stimuli, driving insula activity (Bechara, 2005; Craig, 2010; Droutman et al., 2015a,b). Thus, during decision-making, greater insula activity could indicate a more viscerally-felt somatic response that drives decision-making.
It is useful to further characterize any activity found by examining known psychological factors that correlate with sexual risk. We specifically focused on trait positive and negative urgency because they have been related both to risky sexual behavior (Zapolski et al., 2009; Simons et al., 2010) and to insula activation in a risky decision-making task (Xue et al., 2010). Trait urgency, usually defined and measured separately as positive and negative urgency, is defined as ‘the tendency to engage in rash action in response to extreme positive (negative) affect,’ respectively (Cyders and Smith, 2008, p. 807). In a longitudinal study, Zapolski et al. (2009) found that positive urgency predicts an increase in risky sexual behavior in college students, while other longitudinal research (Deckman and DeWall, 2011) found that negative urgency was particularly important for risky sexual behavior. In Xue et al. (2010), riskier decision-making was likely to recruit insula during risky decision-making and during feedback following risk-taking; the right anterior insula activity found was correlated with trait urgency; Droutman et al. (2015a,b) found that this is particularly true for the anterior dorsal and posterior insula. The finding is consistent with Chester et al.’s (2016) work linking insula activity during decision-making to interoceptive urge and risk-taking. Thus, we collected these measures with real-life sexual risk, in order to facilitate interpretation of risk group differences in neural activity during SR (sexually risky) decision-making in the task.
Research questions
Using the neurobiological model described above, we considered four hypotheses regarding potential neural differences between men who do and do not engage in high-risk sexual behavior in real life. These predictions, including the interoceptive urge prediction and its relationship with the insula, were described a priori before data was collected in NIDA R01DA031626, available online.1
Interoceptive urge subsystem
We hypothesized that additional interoception-driven desire, identifiable by more insula activity, distinguishes participants in the risky group from their safe group counterparts in our task.
Impulsive subsystem
Risky and safe participants should display differences in the impulsive decision-making subsystem. Risky behavior during the task could be explained by heightened sensitivity to reward and punishment (and hence impulsivity), visible as striatal region BOLD activity. Thus, in simulated sexual decisions, we predict additional striatal activity in the sexually risky group relative to the safe group. While insula subsystem activity suggests a somatosensory-driven response, impulsive subsystem activity simply suggests heightened sensitivity to reward and punishment.
Controlled processing subsystem
We considered potential controlled processing subsystem differences between sexual risk groups. A difference in controlled processing subsystem between risky and safe participants would suggest that control and response inhibition subsystem differences distinguish risky from safe participants during SRD-making.
Trait urgency
We also examined trait urgency and its link both to sexual behavior and neural activity in the game. We hypothesized that there would be a link between a trait measure of urgency and MSM’s past risky sexual behavior.
Materials and methods
Self report measures described herein as well as the game itself, playable as an executable on Microsoft Windows computers, are accessible online in the Open Science Framework database using the URL http://osf.io/t7nzy.
Participants
One hundred and twenty participants recruited for a larger study participated in this experiment. Qualified participants were sexually active non-monogamous MSM, had anal intercourse in the past 90 days, were HIV negative (tested within last 6 months), free of neurological history, non-binge drinkers2 and met all safety requirements for MRI scanning. Informed consent was obtained from each participant before the experiment. The protocol of the fMRI study was approved by the relevant Institutional Review Board. For this larger study, fMRI data were collected from 177 participants. Game data were collected from 120 participants and of those, due to equipment and data storage problems, data from 113 participants were used for the analysis. Six further participants were excluded because they did not meet the inclusion criteria for any of the three risk groups, described below, leaving 107 MSM (MSM; including multi-racial participants, 38 Black, 49 Hispanic, and 32 White, aged 18–31, M = 24.1, s.d. = 3.1).
This was part of a wider study in which we examined the differences between sexually risky and sexually safe men and also, for sexually risky men, differences between men who have used methamphetamine and men who have not. Within the population (MSM in the Greater Los Angeles Area), methamphetamine use during sex is common (Landovitz et al., 2013) and methamphetamine use is associated with increased risk of HIV infection (Buchacz et al., 2005; Allerton and Blake, 2008), so we were interested in including a methamphetamine-using group for other analyses not reported here. Consequently, the men were selected to fit into one of three groups: those who reported neither having condomless anal intercourse in the previous 90 days (CAI90), nor ever using methamphetamine (Safe group; N = 31), participants who had reported CAI in the previous 90 days but had reported never using methamphetamine (risky, no-methamphetamine group; N = 42) and participants who reported having used methamphetamine at least once in a lifetime and reported having CAI in the previous 90 days (risky methamphetamine-using group; N = 34). For the current article, we were exclusively interested in sexual risk, so participants were subsequently grouped by sexual risk-taking: one group [safe participants] only had protected AI, and one group [risky participants] had at least one instance of CAI90.
Self-report measures
In an online screening survey, participants reported their frequency of different sexual behaviors and their drug and alcohol use. Sexual practice data included the amount of CAI participants had in the previous 90 days and the proportion of the sex that they had in the previous 90 days that was unsafe or condomless (operationalized as anal sex without a condom). Participants were asked to report experienced cravings for drugs and alcohol as well as filling out a Sexual Functioning and Methamphetamine Use scale, developed from Appleby et al. (2003).
Participants completed a survey taken immediately before the scan answering questions on the UPPS-P scale (Lynam et al., 2006), designed to measure aspects of impulsive behavior (Lynam et al., 2006). The subscales of this measure include Positive and Negative Urgency, Lack of Premeditation, Lack of Perseverance and Sensation-Seeking. For exploratory purposes participants also filled out an Attachment scale (Collins, 2008), the Big-5 Aspects scale (DeYoung et al., 2007), a BIS/BAS scale (Carver and White, 1994) and other scales not related to these analyses.
Game task and measures
The fMRI Game task was a video game in which participants had the opportunity to meet attractive young male computer characters and ‘hook up’ (have sex) with them on a virtual date. Through their onscreen avatar, players had opportunities to make choices about safe sex practice before having explicitly depicted sex with a virtual partner. The exact number of opportunities participants had to make a SRD varied based on the choices the participant made in the game, but the median number of decisions made was seven. The game is described in more detail in the Supplementary Material (1.3. Subsystems).
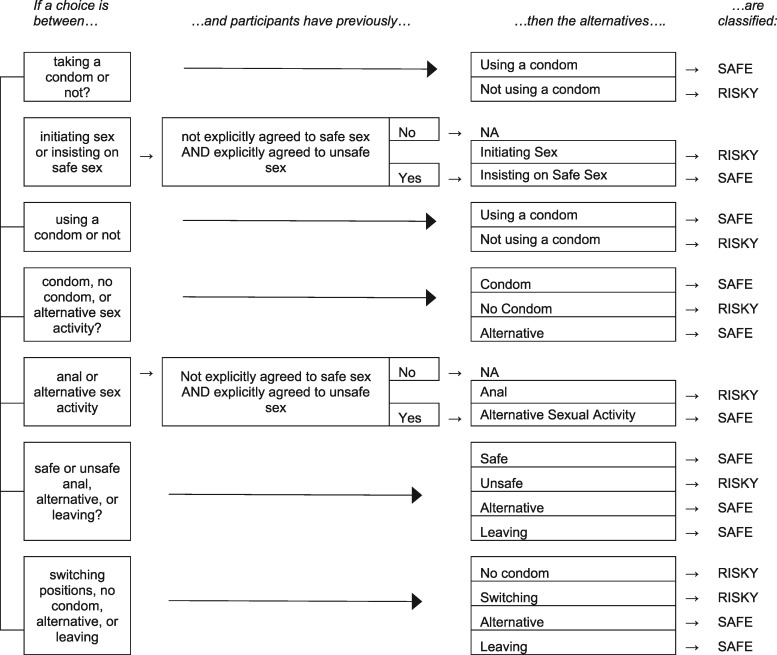
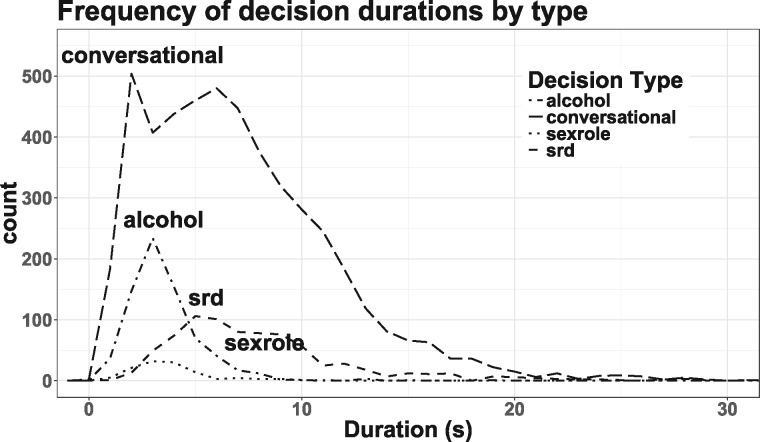
Decision-making events were separated into four decision categories: SRDs, sex-role, alcohol and conversational decisions; the text for all choices is listed in the Supplementary Material (Supplementary Table S1). Time for each event was determined by the amount of time it took subjects to actually make the decision each time (Figure 1). SRDs were categorized as ‘Safe’ or ‘Risky’ choices according to the flowchart described in Figure 2.
Fig. 1.
Procedure used to determine whether a SRD choice is safe or risky, taking into account the context of the choice. Each box in the first level of multiple alternatives in this chart describes all the alternatives participants had for a particular SRD. ‘Previously agreed to safe sex’ describes a ‘NA’ indicates a particular choice is not relevant to sexual risk-taking at all. Some choices are considered ‘risky’ merely relative to others. For instance, switching positions is not inherently risky, but if participants have not previously agreed to safe sex, it is deemed risky relative to choosing an alternative sexual activity with virtually no HIV risk.
Fig. 2.
Histogram of duration of in-game decisions by type: alcohol, conversational, sex role or SRDs.
In a prior national sample of 377 participants exposed to the game task over the Internet reported in Miller et al. (in preparation), participants were asked to indicate their level of agreement before and subsequent to game play via a five-item scale asking how aroused, turned on, sexually hot, sexually excited and sensuous they felt on 6 point scales from ‘not at all’ to ‘very strongly.’ Across participants, self-reported sexual arousal rose significantly from a mean of 3.91 (s.d. = 1.53) before gameplay to 4.17 (s.d. = 1.44) subsequent to game-play, an 0.26-point increase [CI:[0.14, 0.38], t(376) = 4.189, P < 0.001].
fMRI analysis
Preprocessing is described in the Supplementary Material. fMRI statistical analyses were carried out using FEAT 6 (FMRIB software library, version 4.1.8, http://www.fmrib.ox.ac.uk/fsl) and the XFSL package (a BASH script library complementing FSL using grid computing; http://xfsl.fmri.cn).
Data were modeled at the first level using a general linear model. Regressors were created for each of the four decision categories (alcohol, sex role, SRDs and conversational). Alcohol and sex-role decision categories were included to eliminate variance not related to the study hypotheses. Note that for each category, decisions were considered together regardless of whether the outcome was risky or safe, because there were not enough risky sexual choices made to get a measure of activity unique to the choice of a risky outcome. Each regressor was compared against an implicit baseline in a general linear model.
Alcohol, sex role and SRDs were contrasted against conversational decision-making in linear models, so in total, eight contrasts were examined–two for each of alcohol, sex role and SRD choices (contrasting each first against the implicit baseline and second against the conversational task), one for the conversational task contrasted with the baseline and one examining all of the decisions together, contrasted with the baseline. The implicit baseline included all activity that was not part of the four participant decision types. This included times when the participant was navigating around the 3D environment; times when the computer character was saying something to the participant; any sexual activity; any future self presentations (the ‘future self’ functioned as a narrator’) and any other activity that was not a decision the participant was making.
Comparing each decision type with both the implicit baseline and the conversational decisions is useful for two reasons. First, we can be more confident that any positive contrast found across both contrasts is not due to negative activation within the conversational task, because activity was present in contrast with implicit baseline. Second, identifying activity across both contrasts helps us to be confident that it relates to the particular decision-making category (e.g. SRDs in particular), and is not an artifact of decision-making via the game interface, (i.e. moving a mouse to select a response from a response menu), because the activity appeared significant even where contrasted against conversational decision-making. This is important because of the movement coordination and other brain processing required for mundane, ecologically irrelevant processing like selecting items from a menu. By comparing SRDs with conversational decision-making, these activities can be controlled for.
A temporal derivative of each regressor was added to each model and the result was convolved with a double-gamma hemodynamic response function. At the second level, a fixed-effects analysis aggregated these eight activity contrasts across both runs for each participant.
Finally, at the third level, we used FSL’s randomise permutation testing tool (Winkler et al., 2014), calculating 500 permutations, to generate separate threshold-free cluster-enhanced (TFCE; Smith and Nichols, 2009) contrasts in each of the four anatomical regions of interest (interoceptive, valuative, controlled-processing and impulsive regions).
Since the game is participant-driven, not all participants made sex role decisions during their sessions. The narrative required that all engage in sexual activity, but some participants consistently chose oral or manual over anal sex and only those who chose anal sex had sex role decisions to make. For the two sex role activity contrasts, 81 (20 in Safe Group and 61 in Risky Sex Group) participants who had at least one sex role decision were included.
Additionally, in order to check for within-group neural differences related to risky sex, risky sex was tested as a covariate at the whole-brain level. Two separate covariate analyses were run, treating risky sex as a continuous variable covariate so that within-group neural differences could be detected. One included methamphetamine use as an additional regressor in order to control for it, while the other did not. In all of these analyses, no interaction within prior-defined decision-making regions could be found, so this is not further discussed.
Results
Behavioral analysis
Risk-taking and other behavior within the game is related to similar activities in real life. Participants who made at least one unsafe SRD during gameplay reported more unprotected sex in the previous 90 days than participants who made no SRDs during gameplay (Miller et al., in preparation). Chosen in-game sex positions (i.e. top or bottom) were correlated with sex positions taken in real life, and in-game alcohol consumption was also related to real-life alcohol consumption. Details of these results are provided in Supplementary Material (2.1 Behavioral results).
The frequency and durations of participants’ decisions are recorded in Table 1. Of the 34 participants in the risky methamphetamine-using group, only 15 (44%) reported using methamphetamine in the previous 90 days and 11 reported using methamphetamine in the previous 90 days during sex.
Table 2.
Risky sex behavior by group: mean and s.d. of instances of CAI90
| Condomless anal intercourse in previous 90 days by group | ||
|---|---|---|
| Group | Mean | s.d. |
| 1. Safe group | 0 | 0 |
| 2. Risky group | 9.16 | 13.12 |
Table 1.
Behavioral statistics for each decision type
| Alcohol | Conversational | Sexrole | SRD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of decisions per participant | (Mean) | 6.7 | 1.1 | 45.1 | 10.8 | 1.1 | 0.8 | 7.3 | 2.4 |
| (Median) | 7.0 | 45.0 | 1.0 | 7.0 | |||||
| Decision duration (s) | (Mean) | 3.5 | 1.7 | 7.1 | 4.5 | 4.0 | 2.2 | 8.1 | 4.6 |
| (Median) | 3.2 | 6.4 | 3.5 | 7.0 | |||||
Notes: SRDs are the primary decision of interest, and conversational decisions were used as a contrast for them. Other decisions are reported as context about participant behavior, but are not further analyzed here.
Small type denotes s.d. of adjacent figure. SRD, Sexually Risky Decision-making.
Total scan time per subject, on average, was 320 s for conversational decisions, 59 s for SRDs, 24 s for alcohol decisions and 6 s for sexrole decisions; the total scan time per subject did not significantly differ across the sexual risk groups for any of the four decision categories. Subjects rarely made a risky choice when faced with an SRD: over all 107 subjects, only 21 made any risky choices at all. Due to the way subjects in each group played the game, there was a marginal difference in the number of SRDs (Safe = 6.7 SRDs; Risky = 7.7 SRDs, t[56]= –1.94, P = 0.058) faced by each group, though there was no significant difference in the total duration of time spent making SRDs per subject (Safe = 55.51 s; Risky = 60.5 s, t[0.97], P = 0.33) or in the mean time spent per SRD per subject (Safe = 8.6 s; Risky = 7.9 s; t[49]= –1.56, P = 0.13). Thus, it seems unlikely group differences in decisions faced could be responsible for any group contrast differences.
There was typically a short to moderate delay between SRDs and the decisions that immediately preceded them (M = 10.1 s, s.d. = 15.8 s, Med = 2.7 s, IQR = 16.2 s). There were longer delays from SRDs to their subsequent decisions (M = 25.5 s, s.d. = 21.7 s, Med = 30.8 s, IQR = 46.1 s), reflecting that often, narrative activity not involving decision-making occurred subsequent to decisions. Conversational decisions had relatively shorter delays prior (Mean = 10.1 s, s.d. = 15.9 s, Med = 3.0 s, IQR = 12.5 s) and subsequent (Mean = 7.9 s, s.d. = 14.2 s, Med = 1.9 s, IQR = 8.7 s), reflecting that most conversational decisions occurred during periods where there were a lot of choices.
Neuroimaging
In this section, we report neuroimaging findings related to the interoceptive network. This includes examining main effects across all subjects as well as group contrast effects that signal possible links to risky decision-making. We then examine possible links between insula activity and positive and negative urgency during SRD-making. Main effects in the other four decision-making regions examined are described in the Supplementary Material (2.2.1. Main Effects: SRDs in TFCE ROI analysis), though there were no significant contrasts between Risky and Safe groups in those regions. Data in this section are also accessible in an electronic form in the Open Science Framework database using the URL http://osf.io/t7nzy.
Insula activity
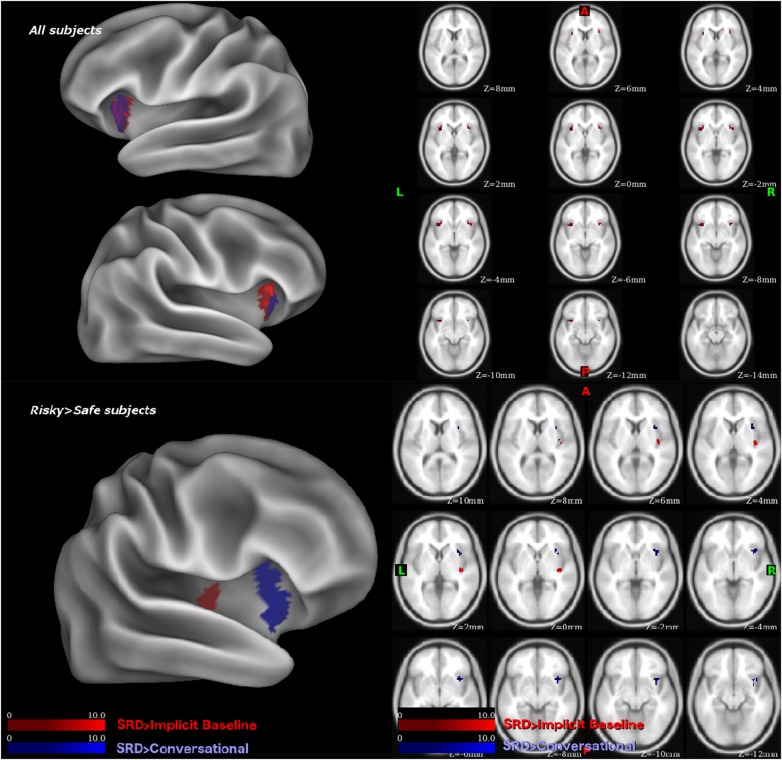
Effect across risk groups. Across all subjects, we examined the insula cluster map during SRD > Implicit baseline and SRD > Conversational. Bilateral anterior insula activity was evident in both the SRD > Implicit Baseline (Left: 162 voxels at [–30, 14, –12]; Right: 106 voxels at [34, 18, –8]) and SRD > Conversational (Left: 108 voxels at [–30, –18, –8]; Right: 18 voxels at [32, 20 –8]) contrasts. In other words, the anterior insula was differentially active for SRD compared to Conversational activity and compared to implicit baseline across the groups.
Difference in risk groups. We contrasted risky and safe subjects, and examined the insula cluster map during SRD > Implicit Baseline and SRD > Conversational decision contrasts. Comparing the Risky and Safe groups, the SRD > Implicit Baseline contrast showed a significant cluster of activity in the right posterior insula (53 voxels peaking at [42, –16, 4]), while the SRD > Conversational contrast showed a significant cluster of activity in the right anterior dorsal insula (155 voxels peaking at [36, 14, –8]) (Figure 3, Table 3).
Fig. 3.
Above: Across all subjects. Below: Risky-Sex > Safe-Sex contrast. Highlighted regions are activity contrast meeting the randomise TFCE <0.05 cluster significance threshold, in the SRD > Implicit Baseline (Red) or SRD > Conversational (Blue) activity contrasts.
Table 3.
TFCE Clusters from Risky-Sex and Safe-Sex between-group examining the left and right insulas as distinct contrasts
| Group | Contrast | Cluster voxel count | Peak z-stat | Cluster peak |
Decision-making system | ||
|---|---|---|---|---|---|---|---|
| (MNI-152 space) |
Regions covereda | ||||||
| X | Y | Z | |||||
| Risky-sex > Safe-sex | Conversational | 155 | 3.90 | 36 | 14 | –8 | R anterior dorsal and ventral insula |
| Implicit baseline | 53 | 3.87 | 42 | –16 | 4 | R Posterior insula | |
Note: L, Left; R, Right.
Insula regions as defined in Deen et al. (2011).
Trait-level personality explanations
In order to search for possible explanations for the observed differences in Safe-Sex and Risky-Sex groups during SRDs, we examined relationships between participants’ scores on positive and negative urgency, as well as 23 exploratory personality subscale values with the real life incidences of risky sex (CAI90) (Table 4). Due to the inclusion of the safe group the data was highly skewed and all correlations were Spearman’s correlations. Using false discovery rate correction (Benjamini and Yekutieli, 2001) for 25 multiple comparisons, there were 5 significant correlations (P < 0.05 corrected) with incidences of risky sex, the strongest of which was negative urgency (rs= 0.44, P < 0.001 uncorrected). To match the risk measure we used in the fMRI contrast, we ran a follow-up analysis treating sexual risk as a dichotomized value and measuring the difference in personality factors between risky and safe participants, using a Welch’s t-test, (t = 5.427, P < 0.001). In a follow-up logistic regression analysis predicting risk group (Safe or Risky) from both positive and negative urgency, the negative urgency term was significant (β = 2.27, P < 0.001) while the positive urgency term was not. When a positive and negative urgency interaction term was added, to include three predictors in total, only the negative urgency term was significant (β = 3.91, P < 0.001).
Table 4.
Risky sex (CAI90) Spearman’s rho correlations with personality variables with FDR corrected P < 0.05
| Personality factor |
rs |
t |
|||
|---|---|---|---|---|---|
| P | P | ||||
| UPPS negative urgency | 0.435 | [0.258, 0.674] | <0.001 | 5.427 | <0.001 |
| Big5: neuroticism/withdrawal | 0.355 | [0.163, 0.579] | 0.006 | 3.863 | 0.003 |
| UPPS positive urgency | 0.347 | [0.154, 0.570] | 0.006 | 3.898 | 0.003 |
| Big5: Neuroticism | 0.315 | [0.118, 0.534] | 0.014 | 3.680 | 0.003 |
| Premeditation | 0.279 | [0.079, 0.494] | 0.036 | 3.353 | 0.008 |
Notes: Using Benjamini-Hochberg False discovery rate with all P-values corrected for 25 multiple comparisons. t-score P-values are uncorrected and derived from a follow-up analysis in which the two sexual risk groups were compared along each factor, to demonstrate the associations held for the same groups used to define the fMRI contrasts. Confidence intervals calculated using Fisher’s r-to-z transformation.
Negative and positive urgency and insula-related activity
Consistent with previous findings outlined in the Introduction, negative urgency was the personality factor most strongly correlating with risky real life sexual behavior in our dataset. As outlined in the introduction, negative urgency has been previously linked to the anterior dorsal insula activity, found to significantly differentiate risky and safe subjects here. Thus, we examined the relationship between urgency and insula activity using 3 mm cluster peaks for the two right insula clusters found in the cluster analysis above. Significant correlations of the insula cluster peaks were found for positive and negative urgency (Supplementary Table S2). Although we did identify negative urgency as a potential variable of interest ahead of time before running the study (https://tinyurl.com/riskysexualdecisionmaking), we also found it was strongly related to group (as described in the previous section). For this reason, to the extent that the relationship between Group and negative urgency prompted further analysis of urgency and other variables, the correlations in Table 5 must be treated with caution and are somewhat inflated by the known relationship between positive and negative urgency and group, as described in Table 4.
Table 5.
Spearman’s correlations of activity at contrast 3 mm cluster peaks, when examining activity in each contrast using masks derived from each contrast
| Activity | Mask | Negative urgency | Positive urgency |
|---|---|---|---|
| SRD-implicit baseline | SRD-conversational | 0.13 | 0.13 |
| SRD-implicit baseline | SRD-implicit baseline | 0.23* | 0.18 |
| SRD-Conversational | SRD-conversational | 0.22* | 0.25* |
| SRD-Conversational | SRD-implicit baseline | 0.11 | 0.1 |
Notes: The first column refers to the contrast from which the activity was sampled; the second column refers to the contrast from which the 3 mm cluster peaks were created. For instance, the first row contains activity as identified in the SRD-Implicit Baseline contrast, within the bounds of the cluster peak identified in the SRD-Implicit Baseline contrast.
P < 0.05 uncorrected.
Insula activity across baselines and meth use
Two potential issues remain when interpreting these results. First, the clusters detected in the posterior insula during SRD, and the anterior insula during SRD-Conversational did not overlap. Thus, we need to ensure that each of the contrast effects (in the anterior and posterior insula, respectively) were not primarily driven by either of the baseline contrasts (Implicit, or Conversational). Second, because we combined all of the risky subjects in a single group, the observed differential may reflect a difference between meth users and non-meth users rather than sexually risky and safe subjects.
Anatomical ROIs confirm risk group differences
In order to ensure the effects we found could be observed across baseline and conversational contrasts, we conducted further ROI tests defined solely anatomically. The contrast means for the right insula and three subregions of the right insula cortex from the Safe-Sex and Risky-Sex groups were compared using both the baseline and conversational contrasts. Anatomical regions of interest were defined using three standardized anatomical masks (Deen et al., 2011)–anterior dorsal insula, anterior ventral insula and posterior insula, which various investigations have described as distinct structures (Chang et al., 2013; Droutman et al., 2015a,b). There was only one expected direction for an interpretable effect, so we used a one-tailed t-test, only testing for an effect in that direction, i.e. more SRD activity in the risky conditions than safe condition.
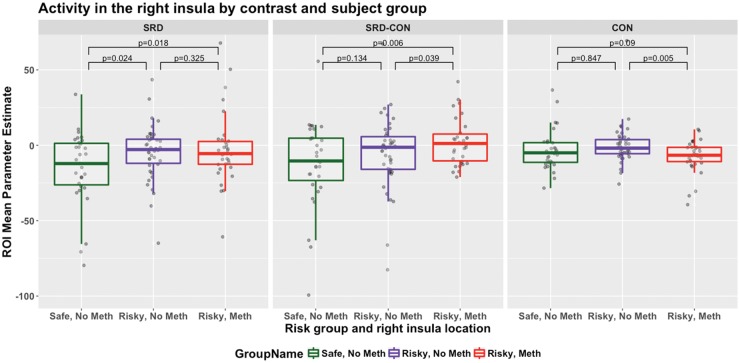
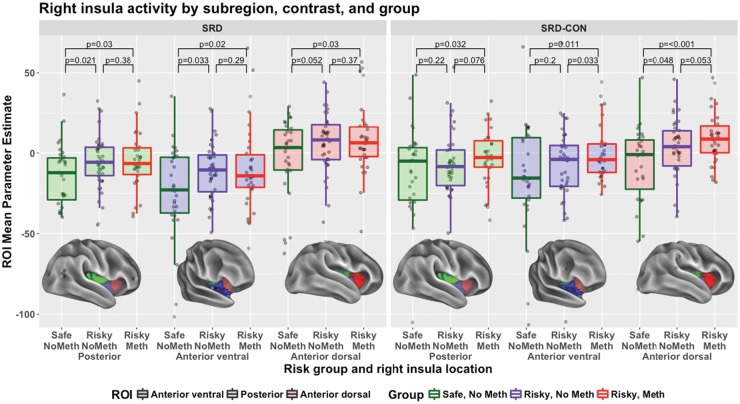
Both risky groups, considered separately, show significantly (P < 0.05) more right insula activity during SRD than the safe group (Figure 4, left panel). We also considered the three main regions of the right insula. In the right anterior dorsal insula, there was significantly (P < 0.05) more activity in each Risky Group, considered separately, than the safe group, during SRD, regardless of baseline used (Implicit or Conversational; both panels). In the right posterior and right anterior ventral insula, there was significantly (P < 0.05) more SRD activity in each Risky group, considered separately, than the safe group (Figure 4, left panel).
Fig. 4.
Activity in the right insula by contrast and subject group. Each data point represents one participant. P-values describe one-tailed t-tests, for SRD and SRD-CON, testing for greater activity in the riskier groups and for CON, testing for greater activity in the safer groups.
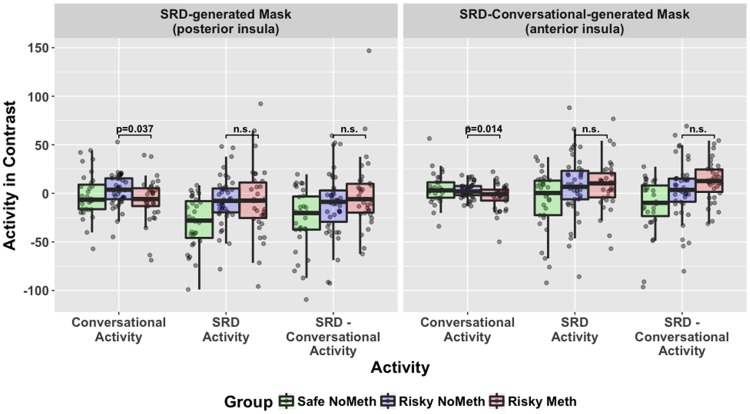
Fig. 6.
The graph shows activity at each 3 mm spherical cluster peak in each frame. The first frame shows activity in the SRD-generated mask in the posterior insula while the second frame shows activity in the SRD-Conversational-generated mask in the anterior insula. For SRD activity, there is consistently no difference between the two risk groups. For conversational activity, there does appear to be a difference between Meth and Non-Meth groups. Anatomical ROIs are shown in the previous section.
There was some evidence of a difference in Risky Meth and Risky No Meth groups, but not across both baselines. Across the right insula, the Risky Meth group had more activity than the Risky No Meth group, but only when contrasted with Conversational decisions (Figure 4, center panel), and so this may be attributable to the Risky No Meth group’s greater activity during Conversational Decision-making (Figure 4, right panel). Examining SRD-Conversational contrasts within the three insula subregions (Figure 5), there was significantly (P < 0.05) greater activity in the Meth group in the anterior ventral right insula and marginally significantly more activity in the posterior and anterior dorsal insula. Again, however, the complete absence of any significant difference when not contrasting with the Conversational baseline suggests that this effect could be due to the significantly greater activity observed for the Risky No Meth group during Conversational decision-making.
Fig. 5.
SRD Insula activity (SRD-Implicit Baseline and SRD-Conversational) by location and Subject Group. Each data point represents one participant; regions highlighted are the regions from which the z-stat activity means were taken. P-values described are of one-tailed t-tests testing for greater PE in the risky compared to safe group.
No significant difference at cluster peaks attributable to meth use
In order to investigate differences between Meth Users and Non-Meth users further, we measured activity in 3 mm spherical cluster peaks found for each of three risk groups (Safe No Meth, Risky No Meth and Risky Meth) and measuring activity during SRD contrasting with either the implicit or conversational baselines. We compared group differences in activity at the 3 mm peaks of the clusters (Supplementary Figure S6) across both baselines (implicit or conversational) and for each group contrast (Risky Meth > Safe; Risky No Meth > Safe) when each group is considered separately. For comparison, Supplementary Figure S6 also includes conversational decision-making activity in each group in the same cluster peaks. As expected, for conversational decision-making, there were no Safe vs Risky differences that were consistent over Meth and No Meth Risk subgroups, or between the implicit and conversational baselines. We used t-tests to compare Risky No Meth and Risky Meth groups (because these groups were of different sizes, respectively, N = 43 and N = 34, there is potential for concern about bias in such a test; see Kriegeskorte et al., 2009, but the group size difference is relatively small).
There was a significant difference between Risky Meth and Risky No Meth groups during conversational activity (P < 0.05), but there were no significant differences between Risky Meth and Risky No Meth groups for either of the SRD-Implicit Baseline or SRD-Conversational Baseline contrasts. Thus, the additional insula activity found for risky but not safe subjects during SRD-making at both of the cluster peaks (found using SRD-Implicit Baseline or SRD-Conversational) seems unlikely to be due to Meth Use or to either the Meth Group or Non Meth group in isolation.
Discussion
The current results support several conclusions. First, neural processes during SRD-making distinguish previously risky MSM from safe MSM, suggesting that individual differences in neural processing in the moment of decision-making impact risk outcomes. Second, of the decision-making regions in Bechara’s (2005) decision-making network, it is insula activity that distinguishes risky from safe MSM during SRD-making; and we did not find any evidence for impulsive-region or controlled-processing distinctions. Third, there is a relationship between real-life sexual risk and traits including negative and positive urgency. Fourth, we found that SRD activity in right insula regions that distinguish risky from safe MSM may be correlated with positive and negative urgency. This may suggest that people higher on trait negative urgency are more prone to make risky decisions in the heat of the moment, and that the neural mechanism that produces this difference includes the right posterior and anterior dorsal insula. Overall, we have identified neural correlates of risky sexual decision-making and identified some possible psychological interpretations for those correlates.
A competing explanation for individual variation in sexual risk is that safe men are safer because they do not put themselves in risky situations in the first place. Our finding that neural processes differ between risk groups during SRD-making, in a decision-making relevant region of the brain, suggests that at least some individual variation in sexual risk is related to variation in decision-making processes in sexual situations themselves, rather than through avoiding risky situations altogether. There are clear signs of neural processing differences in the insula and other regions, and this could be leading riskier participants to take risks.
The game is designed to simulate real-life decision-making, and because it is also designed as an intervention tool, it is possible that the SRD-Conversational and SRD comparison activity are due to differences in comparison conditions not related to SRD-making in particular. In particular, both the Implicit Baseline and Conversational contrast could introduce signal not specifically relevant to risky sexual decision-making. We note two features of the design that make it more likely that it is SRD-making in particular which is responsible for the difference. First, we have only interpreted activity that is apparent with both the Baseline and Conversational contrasts. By using Conversational contrasts we can be confident that the effect is not due to decision-making in general; nor does it reflect the irrelevant stimulus actions like selecting items from the on-screen menu. By using baseline contrasts, we can also be confident that the effect is not due to negative activity contrast within the Conversational task. Second, the SRDs include activity during sexually charged scenes, but also include activity during more mundane scenes, like the first scene of the game where the participant is about to leave the house and decides whether to take a condom (Supplementary Table S1). Conversely, the Conversational decisions and the implicit Baseline with which the SRDs are contrasted also occur in the context of a mix of mundane and sexually charged settings. We also note that the power of this paradigm is somewhat limited, due to the small number of decision-points throughout the task. We only measured a mean of 7.3 SRDs per subject, which in turn, considering the mean SRD duration of 8.1 s, affects the total number of images available to measure decision-making. This was because our game task was designed for intervention purposes and thus, design decisions were made in order to maximize real-life correspondence. We suggest that future fMRI examinations of this kind of task seek to maximize the number of decision points while maintaining a level of ecological validity.
We examined four distinct decision-making systems from Bechara’s (2005) decision-making network: the dlPFC, the striatum, the valuative orbitofrontal-ventromedial PFC and the insula system. Within the four decision-making regions selected a priori, only right insula activity distinguished risky participants from safe participants during SRD-making in a way that was clearly distinct from other, non-sexually risky forms of decision-making, i.e. consistent regardless of baseline.
The finding was most robust in the right anterior dorsal insula, where the insula-masked TFCE analysis suggested more activity for risky than safe subjects during SRD-making; a follow-up analysis suggested an effect regardless of baseline (implicit or conversational). Anatomical ROIs showed the same thing. There also appeared to be activity in the right posterior insula, except that in the anatomical-only contrast, the posterior insula activity is only observed while not contrasting with conversational decision-making. Averages of 3 mm point spheres around TFCE cluster peaks suggested that for both of the Risk Groups–the No Meth group and the Meth Group–there was significantly more insula activity during SRDs for risky subjects than safe subjects.
Explanations for the finding that risky men had more SRD activity in the right insula than safe men can be found in literature about right insula activity during decision-making. According to the Somatic Marker Hypothesis (Bechara and Damasio, 2005), the insula plays an important role in decision-making in transmitting interoceptive signals to the PFC. Other work has identified the posterior insula as responsible for sensorimotor processing (Droutman et al., 2015a,b; Wager and Barrett, 2017), while the dorsal anterior insula has been implicated in risky decision-making (Droutman et al., 2015a,b) as well as salience (Menon and Uddin, 2010), task set maintenance (Dosenbach et al., 2007) and cognitive control (Cole and Schneider, 2007). The finding may be related to previous research by Häcker et al., (2014), which found additional insula activity when participants observed an individual they perceived as high-risk compared to during observation of an individual perceived as low-risk. The dorsal anterior insula has also been previously related to trait urgency in risky decision-making, (Xue et al., 2010), as was explicitly found here. One possibility is that risky subjects had a stronger insula reaction to stimuli; subjects high on trait urgency had a particularly strong reaction, as in Xue et al. (2010). This would drive decision-making activity based around the right anterior dorsal insula–previously having been implicated in valuation during risky decision-making (Paulus and Frank, 2006; Weller et al., 2007). Activity in the right posterior insula supports this interpretation, because it could indicate a somatosensory-driven response (Craig, 2002). Thus, the difference found between risky and safe participants, across both the posterior and anterior dorsal insula, could indicate a difference in interoceptive signal leading to a response under risky conditions.
There appears to be a relationship between real-life sexual risk and traits including negative and positive urgency, consistent with the role of negative and positive urgency (Cyders and Smith, 2008; Zapolski et al., 2009; Cyders et al., 2010; Simons et al., 2010) in risky decision-making. A follow-up logistic regression of our behavioral data also suggested that negative urgency in particular may be the key factor predicting risky decision-making among our study population. Unfortunately, we did not collect self report data from our subjects about affect during the game, and our team plans to incorporate this in the next revision of the game environment. We did find that the same insula regions distinguishing risky from safe participants during SRD-making may be correlated with trait negative urgency. This is consistent with previous data described above, linking the insula to trait negative urgency during a risky gambling task (Xue et al., 2010), the posterior and anterior dorsal insula with urge processing and generation (Droutman et al., 2015a,b), and with Chester et al. (2016), who found that in a negatively-valenced decision-making task, greater insula activity predicted substance abuse 1 month and 1 year later among subjects high in negative urgency.
Prior literature has identified a role of both positive (Zapolski et al., 2009) and negative urgency (Deckman and DeWall, 2011) in risky sexual behavior. Zapolski et al. (2009) found that positive urgency was related to risky sexual behavior in a predominantly Caucasian (86%) female (73%) undergraduate sample. Deckman and DeWall (2011)’s sample of predominantly White (76%) female (80%) undergraduates also found that, of the other forms of impulsivity in the UPPS + P scale, sensation seeking, lack of premeditation and positive urgency were associated with risky sexual behavior. In contrast–possibly due to differences in predominant sexual orientation, race or gender, our data did not suggest that sensation seeking was at all related to variance in risky sexual behavior. Additionally, while our own study only classified unprotected casual sex in the prior 90 days as risky, their study included lifetime occurrences of a one night stand regardless of any protection, sex with a promiscuous partner and other conditions. The present research adds to the broader picture about personality traits related to different types of risky sexual behavior in specific population groups.
Behavioral (e.g. problem gambling) and drug addictions are both related to striatal, reflective and impulsive-system dysfunction (Leeman and Potenza, 2012). The current study does not find differences between risky and safe subjects in any of those systems, though it did reveal an insula-based distinction. This could help point to a distinction between problem gambling and drug use on the one hand, and problematic sexual risk-taking on the other. Whereas problem gambling and drug use are both related to a wide variety of neurological pathologies, it seems that problematic sexual risk-taking may be specifically related to differences in urge response.
The results presented here suggest a role of the insula in risky sexual decision-making. The insula is associated with interoceptive input and processing of social risk in the decision-making process (Xue et al., 2010; Droutman et al., 2015a,b). Previous research relates the insula to negative urgency. The current findings are consistent with that relationship: negative urgency appears to be related to insula activity during risky sexual decision-making.
Conclusions
Positive and negative urgency are associated with risky sexual behavior. The right insula appears to be more active during SRD-making for risky subjects compared to safe subjects. Cluster peaks differentiating risky from safe subjects during SRD-making are correlated with negative urgency. Taken together, it appears that negative urgency could play a somatically-driven role through the insula to disrupt potential safe choices during SRD-making.
Funding
Research reported in this article was supported by the National Institute on Drug Abuse under R01DA031626 awarded to Stephen Read (PI), by the National Institute of Mental Health under RMH082671A, awarded to Lynn Miller (PI) and the California HIV/AIDS Research Program (CHRP) of the University of California, Grant Number ID01-USC-029 awarded to Paul Robert Appleby, PhD (PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDA NIMH, or CHRP.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Supplementary Material
Footnotes
The abstract with these predictions is publicly accessible on the NIH website https://projectreporter.nih.gov/ using the application ID 1R01DA031626, when searching for “All projects”, or directly from the NIH website via the shortcut URL https://tinyurl.com/riskysexualdecisionmaking.
Binge drinkers were defined as having 3 or more drinks each day or 6 or more drinks per session on multiple occasions
References
- Allerton M., Blake W. (2008). The ‘Party Drug’ crystal methamphetamine: risk factor for the acquisition of HIV. The Permanente Journal, 12(1), 56–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby P., Miller L., Ayala A. (2003). Why Methamphetamine Contributes to Risky Sex for MSM. Los Angeles, California: University of Southern California, Annenberg School for Communication. [Google Scholar]
- Ariely D., Loewenstein G. (2006). The heat of the moment: the effect of sexual arousal on sexual decision making. Journal of Behavioral Decision Making, 19(2), 87–98.http://dx.doi.org/10.1002/bdm.501 [Google Scholar]
- Balleine B.W., Delgado M.R., Hikosaka O. (2007). The role of the dorsal striatum in reward and decision-making. The Journal of Neuroscience, 27(31), 8161–5.http://dx.doi.org/10.1523/JNEUROSCI.1554-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten U., Biele G., Heekeren H.R., Fiebach C.J. (2010). How the brain integrates costs and benefits during decision making. Proceedings of the National Academy of Sciences, 107(50), 21767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearinger L.H., Sieving R.E., Ferguson J., Sharma V. (2007). Global perspectives on the sexual and reproductive health of adolescents: patterns, prevention, and potential. The Lancet, 369(9568), 1220–31. [DOI] [PubMed] [Google Scholar]
- Bechara A. (2005). Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience, 8(11), 1458–63.http://dx.doi.org/10.1038/nn1584 [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio A.R. (2005). The somatic marker hypothesis: a neural theory of economic decision. Games and Economic Behavior, 52(2), 336–72.http://dx.doi.org/10.1016/j.geb.2004.06.010 [Google Scholar]
- Benjamini Y., Yekutieli D. (2001). The control of the false discovery rate in multiple testing under dependency. Annals of Statistics, 29(4), 1165–88. [Google Scholar]
- Berntson G.G., Norman G.J., Bechara A., Tranel D., Bruss J., Cacioppo J.T. (2011). The insula, the amygdala and evaluative processes. Psychological Science, 22(1), 80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchacz K., McFarland W., Kellogg T.A., et al. (2005). Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. Aids, 19(13), 1423–4.http://dx.doi.org/10.1097/01.aids.0000180794.27896.fb [DOI] [PubMed] [Google Scholar]
- Carver C.S., White T.L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology, 67(2), 319..http://dx.doi.org/10.1037/0022-3514.67.2.319 [Google Scholar]
- CDC. (2015). HIV Surveillance Report, 2013. Retrieved from: Http://Www. Cdc. Gov/Hiv/Library/Reports/Surveillance, 25.
- Chang L.J., Yarkoni T., Khaw M.W., Sanfey A.G. (2013). Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cerebral Cortex (New York, N.Y.: 1991), 23(3), 739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester D.S., Lynam D.R., Milich R., Powell D.K., Andersen A.H., DeWall C.N. (2016). How do negative emotions impair self-control? A neural model of negative urgency. NeuroImage, 132, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M.W., Schneider W. (2007). The cognitive control network: integrated cortical regions with dissociable functions. NeuroImage, 37(1), 343–60.http://dx.doi.org/10.1016/j.neuroimage.2007.03.071 [DOI] [PubMed] [Google Scholar]
- Collins N.L. (2008). 28 Item Short Form of the Experiences in Close Relationships Scale. Santa Barbara, Santa Barbara, CA: University of California. [Google Scholar]
- Craig A.D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–66.http://dx.doi.org/10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Craig A.D.B. (2010). The sentient self. Brain Structure and Function, 214(5–6), 563–77.http://dx.doi.org/10.1007/s00429-010-0248-y [DOI] [PubMed] [Google Scholar]
- Cyders M.A., Smith G.T. (2008). Emotion-based dispositions to rash action: positive and negative urgency. Psychological Bulletin, 134(6), 807..http://dx.doi.org/10.1037/a0013341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders M.A., Zapolski T.C., Combs J.L., Settles R.F., Fillmore M.T., Smith G.T. (2010). Experimental effect of positive urgency on negative outcomes from risk taking and on increased alcohol consumption. Psychology of Addictive Behaviors, 24(3), 367.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckman T., DeWall C.N. (2011). Negative urgency and risky sexual behaviors: a clarification of the relationship between impulsivity and risky sexual behavior. Personality and Individual Differences, 51(5), 674–8.http://dx.doi.org/10.1016/j.paid.2011.06.004 [Google Scholar]
- Deen B., Pitskel N.B., Pelphrey K.A. (2011). Three systems of insular functional connectivity identified with cluster analysis. Cerebral Cortex, 21(7), 1498–506.http://dx.doi.org/10.1093/cercor/bhq186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–80. [DOI] [PubMed] [Google Scholar]
- DeYoung C.G., Quilty L.C., Peterson J.B. (2007). Between facets and domains: 10 aspects of the Big Five. Journal of Personality and Social Psychology, 93(5), 880..http://dx.doi.org/10.1037/0022-3514.93.5.880 [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Miezin F.M., et al. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences, 104(26), 11073–8.http://dx.doi.org/10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droutman V., Bechara A., Read S.J. (2015a). Roles of the different sub-regions of the insular cortex in various phases of the decision-making process. Frontiers in Behavioral Neuroscience, 9, 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droutman V., Read S.J., Bechara A. (2015b). Revisiting the role of the insula in addiction. Trends in Cognitive Sciences, 19(7), 414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, 113(28), 7900–5.http://dx.doi.org/10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H. (2010). Insula and drug cravings. Brain Structure and Function, 214(5–6), 593–601.http://dx.doi.org/10.1007/s00429-010-0259-8 [DOI] [PubMed] [Google Scholar]
- Grov C., Parsons J.T., Bimbi D.S. (2007). Sexual risk behavior and venues for meeting sex partners: an intercept survey of gay and bisexual men in LA and NYC. AIDS and Behavior, 11(6), 915–26.http://dx.doi.org/10.1007/s10461-006-9199-y [DOI] [PubMed] [Google Scholar]
- Häcker E.K.F., Schmälzle R., Renner B., Schupp H.T. (2014). Neural correlates of HIV risk feelings. Social cognitive and affective neuroscience, 10(4), 612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazy T.E., Frank M.J., O’Reilly R.C. (2007). Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philosophical Transactions of the Royal Society B: Biological Sciences, 362(1485), 1601–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Simmons W.K., Bellgowan P.S.F., Baker C.I. (2009). Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience, 12(5), 535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landovitz R.J., Tseng C.-H., Weissman M., et al. (2013). Epidemiology, sexual risk behavior, and HIV prevention practices of men who have sex with men using GRINDR in Los Angeles, California. Journal of Urban Health, 90(4), 729–39.http://dx.doi.org/10.1007/s11524-012-9766-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman R.F., Potenza M.N. (2012). Similarities and differences between pathological gambling and substance use disorders: a focus on impulsivity and compulsivity. Psychopharmacology, 219(2), 469–90.http://dx.doi.org/10.1007/s00213-011-2550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynam D.R., Smith G.T., Whiteside S.P., Cyders M.A. (2006). The UPPS-P: Assessing Five Personality Pathways to Impulsive Behavior. West Lafayette, IN: Purdue University. [Google Scholar]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 214(5–6), 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K., Lepsien J., Möller H.E., Lohmann G. (2017). Commentary: Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Frontiers in human neuroscience, 11, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustanski B. (2007). The influence of state and trait affect on HIV risk behaviors: a daily diary study of MSM. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 26(5), 618–26.http://dx.doi.org/10.1037/0278-6133.26.5.618 [DOI] [PubMed] [Google Scholar]
- Naqvi N.H., Bechara A. (2009). The hidden island of addiction: the insula. Trends in Neurosciences, 32(1), 56–67.http://dx.doi.org/10.1016/j.tins.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi N.H., Gaznick N., Tranel D., Bechara A. (2014). The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Annals of the New York Academy of Sciences, 1316, 53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noar S.M., Carlyle K., Cole C. (2006). Why communication is crucial: meta-analysis of the relationship between safer sexual communication and condom use. Journal of Health Communication, 11(4), 365–90.http://dx.doi.org/10.1080/10810730600671862 [DOI] [PubMed] [Google Scholar]
- Noël X., Brevers D., Bechara A. (2013). A neurocognitive approach to understanding the neurobiology of addiction. Current Opinion in Neurobiology, 23(4), 632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus M.P., Frank L.R. (2006). Anterior cingulate activity modulates nonlinear decision weight function of uncertain prospects. NeuroImage, 30(2), 668–77.http://dx.doi.org/10.1016/j.neuroimage.2005.09.061 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K.R., van den Wildenberg W.P.M., Segalowitz S.J., Carter C.S. (2004). Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition, 56(2), 129–40. [DOI] [PubMed] [Google Scholar]
- Simons J.S., Maisto S.A., Wray T.B. (2010). Sexual risk taking among young adult dual alcohol and marijuana users. Addictive Behaviors, 35(5), 533–6.http://dx.doi.org/10.1016/j.addbeh.2009.12.026 [DOI] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage, 44(1), 83–98.http://dx.doi.org/10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences, 105(34), 12569–74.http://dx.doi.org/10.1073/pnas.0800005105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley D., Yamamoto T., Hirose K., Keele L., Imai K. (2014). Mediation: R package for causal mediation analysis. Journal of Statistical Software, 59(5), Retrieved from: http://dspace.mit.edu/handle/1721.1/91154. [Google Scholar]
- Uddin L.Q. (2014). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16(1), 55–61.http://dx.doi.org/10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- Wager T.D., Barrett L.F. (2017). From affect to control: functional specialization of the insula in motivation and regulation. bioRxiv, 102368. https://doi.org/10.1101/102368
- Weller J.A., Levin I.P., Shiv B., Bechara A. (2007). Neural correlates of adaptive decision making for risky gains and losses. Psychological Science, 18(11), 958–64.http://dx.doi.org/10.1111/j.1467-9280.2007.02009.x [DOI] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. (2014). Permutation inference for the general linear model. Neuroimage, 92, 381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G., Lu Z., Levin I.P., Bechara A. (2010). The impact of prior risk experiences on subsequent risky decision-making: the role of the insula. NeuroImage, 50(2), 709–16.http://dx.doi.org/10.1016/j.neuroimage.2009.12.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapolski T.C., Cyders M.A., Smith G.T. (2009). Positive urgency predicts illegal drug use and risky sexual behavior. Psychology of Addictive Behaviors, 23(2), 348..http://dx.doi.org/10.1037/a0014684 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.