Abstract
Major depressive disorder is an often chronic and recurring illness. Left untreated, major depressive disorder may result in progressive alterations in brain morphometry and circuit function. Recent findings, however, suggest that pharmacotherapy may halt and possibly reverse those effects. These findings, together with evidence that a delay in treatment is associated with poorer clinical outcomes, underscore the urgency of rapidly treating depression to full recovery. Early optimized treatment, using measurement-based care and customizing treatment to the individual patient, may afford the best possible outcomes for each patient. The aim of this article is to present recommendations for using a patient-centered approach to rapidly provide optimal pharmacological treatment to patients with major depressive disorder. Offering major depressive disorder treatment determined by individual patient characteristics (e.g., predominant symptoms, medical history, comorbidities), patient preferences and expectations, and, critically, their own definition of wellness provides the best opportunity for full functional recovery.
Keywords: depression, function, pharmacotherapy, treatment optimization
Introduction
Major depressive disorder (MDD) is the leading cause of disability worldwide, affecting an estimated 350 million individuals globally (World Health Organization, 2012). In addition to mood symptoms, individuals with MDD experience impairments in physical, occupational, and social functioning (Kessler et al., 2003; American Psychiatric Association, 2013). Their return to previous functioning may have a slower trajectory compared with symptomatic improvement during treatment (Miller et al., 1998; Bech, 2005; Papakostas, 2009; IsHak et al., 2011). Ongoing functional impairment may interfere with integration back into daily life and in turn delay full functional recovery. Conversely, a higher level of functioning at baseline may be associated with better outcomes after treatment (McIntyre et al., 2017).
The Canadian Network for Mood and Anxiety Treatments (CANMAT) recommends pharmacotherapy as a first-line treatment for moderate to severe MDD (Kennedy et al., 2016). Numerous antidepressants are available for the treatment of MDD, including selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors, and other antidepressants (agomelatine, bupropion, mianserin, mirtazapine, and vortioxetine), all of which have Level 1 evidence and are therefore recommended as first-line treatments (Kennedy et al., 2016). Despite the range of pharmacotherapy options, treatment of MDD to full symptomatic and functional recovery remains challenging. Rates of remission are low for any given drug (approximately ≤50%) across groups of patients evaluated in antidepressant clinical trials (Thase et al., 2005; Machado et al., 2006) and may be lower among patients treated in clinical practice (Trivedi et al., 2006; Moller, 2008). The achievement of both symptom remission and full functional recovery after a trial of an antidepressant treatment is even more difficult (Soares et al., 2014b), and functional recovery can lag behind symptom remission (Sheehan et al., 2011). Therefore, successful management of MDD necessitates development of a personalized treatment plan that allows the individual patients to achieve full functional recovery in the most effective and efficient manner (McIntyre et al., 2015; National Institute of Mental Health, 2015). The aim of this article is to present recommendations for using a patient-centered approach to rapidly provide optimal pharmacological treatment to patients with MDD. First, we establish the importance of providing early, optimized treatment of MDD based on recent research exploring the effects of depression on brain structure and function, using the hippocampus as a well-studied example. Then, we present a consensus of expert opinion on best practices for physicians to address both depressive symptoms and functional impairment in MDD, with a focus on treating with a sense of urgency in the clinical practice setting.
The Need for Early Optimized Treatment
Clinicians have historically used a “start low and go slow” approach to pharmacotherapy for MDD, prescribing an initial antidepressant trial for up to 6 to 8 weeks before concluding that the treatment has failed and an adjustment is warranted (Work Group on Major Depressive Disorder, 2010; Qaseem et al., 2008). However, evidence suggests that MDD should be treated with a greater sense of urgency (Habert et al., 2016). When depression is not treated with urgency, patient suffering is prolonged. Furthermore, delaying effective treatment may reduce the likelihood of both asymptomatic remission and functional recovery, and increase the time to achieve remission (Gormley et al., 1999; Okuda et al., 2010; Bukh et al., 2013; Ghio et al., 2014). Failure to rapidly and effectively treat major depression may have lasting effects on patients’ brain structure and function, which may worsen progressively with successive depressive episodes (Moylan et al., 2013).
Results of recent brain imagining research provide clear rationale for treating MDD as rapidly as possible. Untreated MDD is associated with damage to the brain, evident as loss of volume in various brain areas, including the hippocampus (Videbech and Ravnkilde, 2004; McKinnon et al., 2009; MacQueen and Frodl, 2011). Hippocampal volume loss is more severe in patients with a longer duration of untreated depression (Sheline et al., 2003; McKinnon et al., 2009; MacQueen and Frodl, 2011). More recently, research has focused on whether antidepressant treatment can alter the course of brain deficits associated with MDD (Duric and Duman, 2013; Rotheneichner et al., 2014). To underscore the need for optimizing treatment for patients with depression as rapidly as possible, we review here English language publications over the past 5 years examining effects of depression and antidepressant treatment on hippocampal volume. While other brain areas have also been examined, we focused this discussion on the hippocampus as an example, because it has been examined in numerous studies in treated and untreated depressed patients in both cross-sectional and longitudinal studies. Excluding bipolar and psychotic depression, our search yielded a total of 235 articles. A total of 33 primary articles reported research in human depression, 19 of which were found to be relevant and are summarized below (Table 1).
Table 1.
Recent Literature on Antidepressant Effects on Hippocampal Volume Deficits
| Reference | Method | Comparison | Findings |
|---|---|---|---|
| Cross-sectional analyses | |||
| Geerlings et al., 2012 | MRI | ≥65 y; depressed, HC | • Smaller volume in depressed (defined by CES-D score or AD use) vs HC • Smaller volume in AD users vs no AD use (defined as HC) |
| Huang et al., 2013 | MRI | Treated depressed (AD), untreated depressed (no AD; 10-pt lower HAM-D17), HC | • Lower hippocampus, CA1-3, and DG volume in depressed vs HC and no AD vs AD • AD intermediate between no AD and HC for CA1-3 |
| Nugent et al., 2013 | MRI | Depressed, remitted, HC | • Smaller volume hippocampus and thalamus in depressed vs remitted and vs HC • Remitted and HC did not differ |
| Arnone et al., 2013* | MRI | Depressed, remitted, HC | • Volume reduced in depressed vs remitted and HC; no difference between remitted and HC |
| Zhao et al., 2014 | MRI, meta-analysis | Untreated depressed, HC | • Smaller volume for depressed vs HC • Smaller volume in subgroup with first episode depression vs HC • No association with number of episodes |
| Travis et al., 2015 | MRI | Depressed (most taking AD), HC | • Similar volume between groups, but differences in DG • Duration of depression was negatively correlated with volume • No association between volume and memory scores |
| Schmaal et al., 2016 | MRI, meta-analysis | Depressed (some taking AD), HC | • Smaller volume for depressed vs HC • No volume difference in patients with first episode depression vs HC; smaller volume in patients with recurrent episodes • No significant effect of AD |
| Longitudinal analyses | |||
| Schermuly et al., 2011 | MRI, 4+ mo AD treatment |
Depressed, HC | • No difference between depressed and HC • Volume increased in depressed during treatment; change in volume was associated with baseline function but not depression scores |
| Sheline et al., 2012 | MRI 12 wk sertraline |
≥60 y; depressed, HC Remitters, nonremitters |
• Smaller volume for depressed vs HC at baseline • Smaller volume predicted poorer MADRS score • Smaller volume in nonremitters vs remitters |
| Husarova et al., 2012 | MRS 7–11 wk escitalopram or venlafaxine (MADRS responders only) |
Depressed; baseline vs postbaseline | • Myoinositol/creatine and phosphocreatine ratio negatively correlated with MADRS score at baseline • Lac/creatine and phosphocreatine ratio, an indicator of damage or dysfunction, decreased after AD treatment |
| Arnone et al., 2013* | MRI 8 wk citalopram |
Depressed, HC | • Volume increase after treatment • No difference between medicated and unmedicated remitters • Significant negative correlation between duration of untreated depression and hippocampal volume |
| Furtado et al., 2013 | MRI 3 mo TMS (some taking AD) |
Responders vs nonresponders | • Reduction in volume in nonresponders vs responders • No correlation with HAM-D17 score |
| Tendolkar et al., 2013 | MRI ECT treatment (TRD; previous AD treatment) |
Depressed; baseline vs posttreatment | • Increased volume after ECT |
| Godlewska et al., 2014 | MRI 8 wk escitalopram |
Responders vs nonresponders | • No significant effects • Trend toward normalization of volume in responders |
| Taylor et al., 2014 | MRI 2-y AD treatment (mostly sertraline) |
≥60 y; depressed, HC | • No difference from baseline for HC or remitted • Volume decrease over time for nonremitters • Significantly less volume for nonremitters vs HC at follow up • Worsening or nonimproving MADRS associated with decreasing volume |
| Elbejjani et al., 2015 | MRI 4 yr AD treatment (nonrandomized) |
≥65 y; history of depression vs no history | • Baseline: smaller volume with greater symptoms in women • Faster volume loss in women with history of depression vs no history • Slower loss with AD vs no AD in men with history of depression |
| Phillips et al., 2015 | MRI 6–12 mo AD treatment |
TRD; depressed, HC Remitters, nonremitters |
• No difference between depressed and HC at baseline • Remitters increased in volume, nonremitters decreased |
| Postmortem studies | |||
| Boldrini et al., 2012 | IHC; neural progenitor cells capillaries |
Untreated depressed, treated depressed (SSRI or TCA), NDC | • More neural progenitor cells and capillary area in individuals treated with SSRIs vs untreated depressed and vs NDC • Capillary area, number of progenitor cells, and volume were correlated in treated depressed subjects |
| Boldrini et al., 2013 | IHC; granule neurons | Untreated depressed, treated depressed (SSRI or TCA), NDC | • More granule cells in some areas of the DG in individuals treated with SSRIs or TCAs; numbers were between untreated depressed and NDC • No difference in glia cell numbers |
| Duric et al., 2013 | Gene expression; cytoskeletal proteins |
Depressed, NDC | • Dysregulation of pre- and postsynaptic genes in depressed subjects vs NDC |
Abbreviations: AD, antidepressant; CES-D, Center for Epidemiologic Studies-Depression; DG, denate gyrus; ECT, electroconvulsive therapy; HAM-D17, 17-item Hamilton Rating Scale for Depression; HC, healthy control; IHC, immunohistochemistry; MADRS, Montgomery-Åsberg Depression Rating Scale; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; NDC, nondepressed control; TCA, tricyclic antidepressant; TMS, transcranial magnetic stimulation; TRD, treatment-resistant depression; SSRI, selective serotonin reuptake inhibitor.
*Included both cross-sectional and longitudinal analyses.
Recently published research identified in this search confirmed earlier findings that depression is associated with alterations in brain morphometry and circuit dysfunction that can be observed as volume loss in the hippocampus and other areas implicated in depression (Geerlings et al., 2012; Sheline et al., 2012; Arnone et al., 2013; Huang et al., 2013; Nugent et al., 2013; Zhao et al., 2014; Schmaal et al., 2016). A significant reduction in hippocampal volume has been reported as early as the first depressive episode (Zhao et al., 2014), although other studies have found no volumetric differences between first episode patients and healthy controls (McKinnon et al., 2009; Schmaal et al., 2016). More severe symptoms of depression are associated with greater hippocampal volume loss (Huang et al., 2013; Taylor et al., 2014; Elbejjani et al., 2015).
Treatment of depression is generally associated with an increase in hippocampal volume (Schermuly et al., 2011; Huang et al., 2013; Tendolkar et al., 2013) or at least a slowing of volume loss (Elbejjani et al., 2015), although this has not been observed in all studies (Schmaal et al., 2016). Structural changes may differ in patients with MDD depending on clinical response to treatment. Increased hippocampus volume has also been correlated with improvement in depressive symptoms: In several cross-sectional studies, hippocampal volume was significantly greater in patients who achieved remission from depression compared with nonremitters (Arnone et al., 2013; Nugent et al., 2013). Volume did not differ significantly between remitted MDD patients and healthy individuals (Arnone et al., 2013; Nugent et al., 2013; Taylor et al., 2014). In unmedicated patients with depression, an inverse relation between duration of untreated depression and hippocampal volume has been reported (Arnone et al., 2013). Results of longitudinal studies are mixed; an association between improvement in depression and increased hippocampus volume has been observed in some but not all studies (Schermuly et al., 2011; Arnone et al., 2013; Phillips et al., 2015). One study found that volumetric changes were associated with cognitive performance at baseline but not with depression severity or improvement (Schermuly et al., 2011). Volume differences between remitters and nonremitters in some studies appeared to be due at least in part to hippocampal volume loss over time in nonremitters rather than volume recovery in remitted patients (Furtado et al., 2013; Taylor et al., 2014; Phillips et al., 2015). In fact, a smaller hippocampal volume was associated with lack of improvement in depression rating scale scores (Taylor et al., 2014) and was a significant predictor of poorer depression outcomes (Sheline et al., 2012) in patients with late-life depression. The continued presence of depressive symptoms may promote chronic neuronal loss and suppress neurogenesis in the hippocampus (Boldrini et al., 2012, 2013; Duric et al., 2013; Furtado et al., 2013; Phillips et al., 2015), and preclinical work (Duman and Aghajanian, 2012; Licznerski and Duman, 2013; Bortolotto et al., 2014) supports the hypothesis that depression may disrupt neural connections in mood-related circuits and provides preliminary evidence that treatment may ameliorate these processes.
Evidence from these recent publications suggests that treatment may halt and even reverse progressive damage to brain areas associated with depression, underscoring the urgency of rapidly and fully treating depression. Additionally, the duration of untreated illness is a significant predictor of response, remission, and time to remission (Gormley et al., 1999; Okuda et al., 2010; Bukh et al., 2013; Altamura et al., 2015). Related measures such as age at the onset of depression, time since the onset of depression, duration of the current episode, and the number of previous episodes may also predict treatment outcome (Warden et al., 2007; Howland et al., 2008; Fava et al., 2009; Seemuller et al., 2010; Joel et al., 2014). Reducing the time between the onset of depression and optimization of treatment results in better short-term clinical outcomes, increases the likelihood of achieving full functional recovery (Habert et al., 2016), and, notably, may reduce the burden of damage to brain areas (Arnone et al., 2013; Nugent et al., 2013).
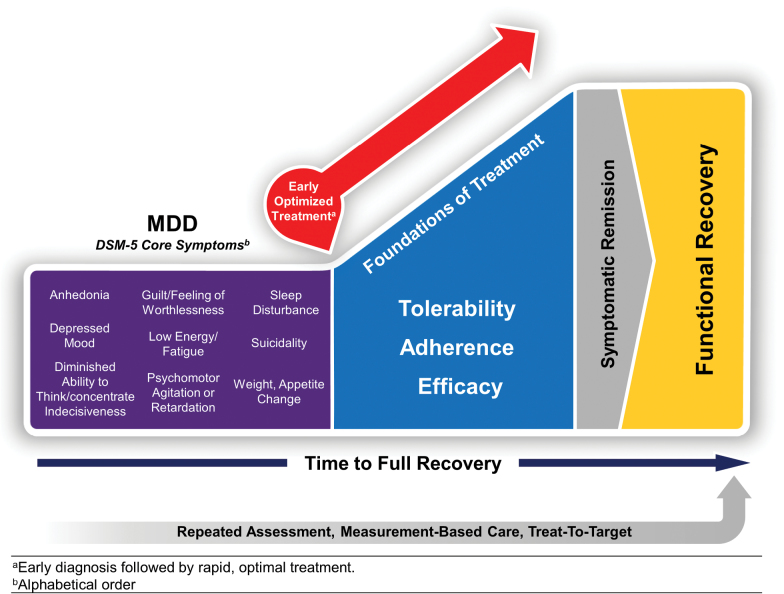
To effectively restore a patient to full function, clinicians must strive to deliver early optimized treatment (Figure 1) (Habert et al., 2016). Optimal treatment of depression should address all symptoms and functional impairment, minimize antidepressant side effects, address barriers to adherence, provide strategies for relapse prevention, and elevate patients’ overall sense of well-being and quality of life (Gelenberg et al.; Zimmerman et al., 2012; Kennedy et al., 2016; Lam et al., 2016). A patient’s recovery must always be addressed in terms of their own definition of “well.” Zimmerman and colleagues noted the primary goal of depression treatment for patients is “presence of positive mental health…feeling their usual self, returning to their usual level of functioning, and feeling in emotional control….” (Zimmerman et al., 2006). Ultimately, while the remission of symptoms is an important outcome, for patients, recovering from depression implies more than the absence of depressive symptoms (Zimmerman et al., 2006). What steps can clinicians take to ensure each patient receives the best possible individualized care, with no unnecessary delay? The following section presents guidance, based of published evidence and the combined experience of the authors, on providing optimal pharmacological treatment to patients with MDD, as rapidly as possible.
Figure 1.
Early optimized treatment is critical to bringing patients to full symptomatic and functional recovery. From (Habert et al., 2016).
How Does the Clinician Provide Early Optimized Treatment for the Individual Patient with MDD?
Screening and Diagnosis
Identifying MDD patients as early as possible after illness onset is an essential first step in optimizing treatment. However, numerous agencies do not recommend routine screening for depression in adults unless adequate resources and services are available for subsequent diagnostic assessment and management (National Institute for Health and Clinical Excellence, 2009; Joffres et al., 2013; Lam et al., 2016; Siu et al., 2016). Concerns with screening all adult patients include the lack of robust evidence supporting routine screening (due to the paucity of available data on its benefits and harms), the high likelihood that patients who screen positive will have been previously identified as suffering from depression, and the risk of identifying very mild depression that does not require treatment (Joffres et al., 2013; Keshavarz et al., 2013; O’Connor et al., 2016). However, the assertion that mild depression does not require treatment may not consider the possibility that an individual may have mild depressive symptoms related to a resolving (but not yet resolved) depressive episode or may be experiencing the onset of an impending, more severe depressive episode. Other subgroups of people in this mildly depressed population may include those individuals with chronically lower hedonic tone, who could be more susceptible to develop either a major depressive episode or what is described in the DSM-5 as dysthymia (or persistent depressive disorder) (Sternat et al., 2014; Sternat and Katzman, 2016).
Still, the literature at present suggests that routine screening is not recommended; rather, clinicians should consider screening patients with risk factors for depression, as outlined in Box 1 (National Institure for Health and Clinical Excellence, 2009; Lam et al., 2016), or be alert to the possibility of depression in those high-risk patients and address symptoms when they are observed (Joffres et al., 2013; Lam et al., 2016). For adolescents (12–18 years), the US Preventive Services Task Force recommends screening when resources are available to ensure an accurate MDD diagnosis with appropriate care and follow-up. There is little evidence to support screening patients younger than 12 years of age (US Preventive Services Task Force, 2009).
Box 1. Depression Screening in Primary and Secondary Care Settings
| CANMAT guidelines recommend screening patients with risk factors for depression (Lam et al., 2016 ): | ||
|---|---|---|
|
Clinical Risk Factors
History of depression Family history of depression Psychosocial adversity High users of the medical system Chronic medical conditions (especially cardiovascular disease, diabetes, and neurological disorders) Other psychiatric conditions Times of hormonal challenge (e.g., peripartum) |
Symptom Risk Factors
Unexplained physical symptoms Chronic pain Fatigue Insomnia Anxiety Substance abuse |
|
| Screening | ||
| A 2-question screen can be used for identifying patients that may require more detailed assessment (National Institute for Health and Clinical Excellence, 2009) | ||
| 1. In the last month, have you been bothered by little interest or pleasure in doing things? 2. In the last month, have you been feeling down, depressed or hopeless? |
||
| Patients who respond “yes” to either of these questions should be assessed with an instrument such as the PHQ (National Institute for Health and Clinical Excellence, 2009; Patient Health Questionnaire, 1999; Lam et al., 2016) | ||
| Over the past 2 weeks, how often have you been bothered by any of the following problems, scored 0 (not at all) to 3 (nearly every day)? | ||
| 1. Little interest or pleasure in doing things 2. Feeling down, depressed, or hopeless 3. Trouble falling asleep, staying asleep, or sleeping too much 4. Feeling tired or having little energy 5. Poor appetite or overeating 6. Feeling bad about yourself or that you’re a failure or have let yourself or your family down 7. Trouble concentrating on things, such as reading the newspaper or watching television 8. Moving or speaking so slowly that other people could have noticed. Or, the opposite - being so fidgety or restless that you have been moving around a lot more than usual 9. Thoughts that you would be better off dead or of hurting yourself in some way |
||
Abbreviation: CANMAT, Canadian Network for Mood and Anxiety Treatments.
An initial screen for depression can be as simple as asking the 2 questions recommended in the National Institute for Health and Clinical Excellence (NICE) guidelines: “During the last month, have you often been bothered by feeling down, depressed, or hopeless? During the last month, have you often been bothered by having little interest or pleasure in doing things?” A more in-depth assessment using a validated instrument should follow, if there is an affirmative response to either question. Given that no biological markers have been established for depression (Huang and Lin, 2015), objective screening questionnaires are critical tools to employ when assessing a depressed patient. Just as diabetes or hypertension would not be diagnosed without blood glucose levels or blood pressure assessments, a diagnosis of MDD requires the measurement of symptoms and associated functional impairment (American Psychiatric Association, 2013). A number of validated assessment tools that incorporate MDD diagnostic criteria are available, including both symptom scales and functional assessments (Table 2). The authors recommend the use of the 9-item Patient Heath Questionnaire (PHQ-9), a brief, self-administered assessment incorporating DSM diagnostic criteria that patients can complete quickly and that may be administered repeatedly to monitor symptom response to treatment (Kroenke and Spitzer, 2002). For a definitive diagnosis, the use of any assessment tools should always be coupled with a clinician’s good judgement, a psychiatric interview, and additional assessments to rule out other disorders (Lam et al., 2016).
Table 2.
Screening/Monitoring Tools for the Clinic (Endicott and Dorries, 2009; Lam et al., 2016)
| Instrument | Structure | Reference |
|---|---|---|
| Symptoms | ||
| Patient Health Questionnaire | 9 items, each scored 0–3 | Kroenke and Spitzer, 2002 |
| Quick Inventory for Depressive Symptomatology, Self-Rated | 16 items, each scored 0–3 | Rush et al., 2003 |
| Clinically Useful Depression Outcome Scale | 18 items, each scored 0–4 | Zimmerman et al., 2008 |
| Behavior and Symptom Identification Scale (BASIS-32) | 32 items, each scored 0–4 | Eisen et al., 1994) |
| Function/quality of life | ||
| Sheehan Disability Scale | 3 items, each scored 0–10 | Sheehan et al., 1996 |
| World Health Organization Disability Assessment Schedule II |
36 items in 6 domains, each scored 1–5 | Posl et al., 2007 |
| Lam Employment Absence and Productivity Scale | 10 items, 3 open response and 7 scored on a 5-point, Likert scale | Lam et al., 2009) |
| Social Adjustment Scale, Self-Report Version | 48 items in 6 domains, each rated on a 5-point, Likert scale | Weissman and Bothwell, 1976 |
| Social and Occupational Functioning Assessment Scale | Single scale, scored 0–100 | Rybarczyk, 2011 |
| EuroQoL-5D | Single scale, scored 0–100 | EuroQoL, 1990 |
Assessment tools also provide the clinician with a measure of the severity of specific depression symptoms or the degree of functional impairment. Determining both the episode specifiers and the severity of depression at diagnosis is critical to developing an appropriate treatment plan and establishing baseline measurements to monitor improvement during treatment (Kennedy et al., 2016; Lam et al., 2016). However, criteria for depression severity provided by various guidelines are not consistent (Davidson, 2010). The DSM-5 and the International Classification of Diseases, Tenth Revision (American Academy of Professional Coders, 2013) (ICD-10) definitions for depression severity differ based on the threshold numbers of symptoms present during a depressive episode, intensity of symptoms, and the resulting impairments/disability (Davidson, 2010). Although the criteria are similar, a patient meeting the ICD-10 criteria for mild depression, for example, might have subthreshold depression based on DSM-5 criteria (Davidson, 2010). The DSM-5 and ICD-10 criteria for severity include both number of symptoms and degree of impairment or disability (Davidson, 2010) The American Psychiatric Association, the British Association for Psychopharmacology, NICE, and CANMAT recommendations define MDD severity according to DSM criteria only (Work Group on Major Depressive Disorder, 2010; Anderson et al., 2008; National Instutute for Health and Clinical Excellence, 2009; Bauer et al., 2013; MacQueen et al., 2016). CANMAT and American Psychiatric Association guidelines address psychiatrists; NICE and British Association for Psychopharmacology guidelines address both specialists and primary care physicians.
Finally, because medical comorbidities can complicate depression screening and diagnosis, clinicians should be aware of their role in both under- or misdiagnosis of MDD (Huerta-Ramirez et al., 2013; McGuire et al., 2014). Depression symptoms may be missed in patients presenting with symptoms of comorbid conditions, resulting in delayed diagnosis and treatment (McGuire et al., 2014; Lam et al., 2016; Thase, 2016). Likewise, not ruling out the presence of another psychiatric disorder, such as obsessive-compulsive disorder, posttraumatic stress disorder, or bipolar disorder, when a patient presents with depression commonly results in treatment-resistance because the comorbid condition is inappropriately or inadequately treated. When clinicians understand which conditions may be associated with a higher risk of MDD (e.g., substance abuse, low hedonic tone, or attention deficit hyperactivity disorder [ADHD]; Kessler et al., 2003; Sternat et al., 2014), they are better able to remain alert for signs of depression in patients with those conditions (Epstein et al., 2014). An undiagnosed medical or psychiatric comorbidity can interfere with response to MDD treatment (McIntyre et al., 2015; Bron et al., 2016). The 2015 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults recommends differential screening for psychiatric and medical comorbidities (Florida Agency for Health Care Administration, 2015), which is in line with the approach set forth by the National Institute of Mental Health. The National Institute of Mental Health recommends the progression to more patient-centered diagnostic approaches that consider an individual’s genetic, physiologic, and behavioral profiles to achieve a specific and informative diagnosis (National Institute of Mental Health, 2015). Although subsets of patients with an increased risk for MDD have been noted (Box 1) (National Institute for Health and Clinical Excellence, 2009; Lam et al., 2016), risk factors serve only as a guide. Ultimately, the clinician must determine which individual patients require additional screening for an MDD diagnosis.
Individualized Treatment Plan
The development of an individualized treatment plan includes identifying specific treatment goals and determining the best strategies to employ to accomplish these goals (Lam et al., 2016). The ideal treatment plan takes into account the patient’s specific array of symptoms and associated functional impairment, together with the medical and lifestyle characteristics that might contribute to the success or failure of a given treatment, as outlined by the RDoC Initiative for developing new treatments. The RDoC Initiative recommended treatment be based on individual patient characteristics, personal and medical history, and quality of life (National Institute of Mental Health, 2015). An individual patient’s treatment goals should encompass the concept of “treating-to-target”—treating the patient to achieve the goal of full and sustained functional recovery across all aspects of MDD (McIntyre et al., 2015). A treating-to-target approach includes asymptomatic remission, but also functional recovery, including improvement in social, interpersonal, work, and family domains. It is essential to consider that a patient’s concept of wellness may differ substantially from that of the treating physician (Zimmerman et al., 2006; McIntyre et al., 2015), and ultimately, it is the patient’s concept that is the most important treatment goal.
A collaborative approach to developing and following the treatment plan is needed to achieve treating-to-target goals. The physician and patient should decide together how best to address bothersome symptoms and return the patient to pre-illness functioning. The treatment team may also include family members, other clinicians (nurses, social workers, specialists), and those involved in other aspects of recovery, for instance addiction counselors (Work Group on Major Depressive Disorder, 2010; Lam et al., 2016). This approach yields a more comprehensive MDD treatment plan and might also improve outcomes for comorbid conditions (Teh et al., 2010; Jiang et al., 2011).
Treatment plans often evolve over time, as patients progress or encounter obstacles to recovery (McIntyre et al., 2015). Given that about one-half of patients who initiate an antidepressant will fail to achieve remission (Thase et al., 2005; Machado et al., 2006; Thase et al., 2007), several treatment steps may be needed to achieve successful outcomes. Treatment failures may result from the lack of treatment efficacy, noncompliance, or poor tolerability. An effective treatment plan adjusts to address these obstacles to optimize treatment as rapidly as possible (Culpepper et al., 2015; Habert et al., 2016). For patients with MDD who do not respond to an initial treatment, physicians may consider increasing the dose, adding adjunctive treatment, or switching to an antidepressant with a different mechanism of action (Kennedy et al., 2016). However, evidence suggests that outcomes are less robust for patients who require subsequent treatment steps or when changes to an effective treatment are delayed. Remission rates for patients in 4 successive treatment steps in the Sequenced Treatment Alternatives to Relieve Depression trial dropped progressively, from 37% at step 1 to 13% at step 4 (Rush et al., 2006b). Patients who remitted during the first treatment step experienced greater improvements in function compared with patients who remitted at step 2 (Trivedi et al., 2013). Providing a longer treatment trial (8 weeks vs 4 weeks) before switching is associated with poorer functional outcomes, even among patients who remit on the second treatment (Romera et al., 2012). Research examining early response to antidepressant treatment suggests that treatment adjustment can be considered after just 2 to 4 weeks of treatment (Habert et al., 2016; Kennedy et al., 2016). Taken together, these findings suggest that developing a plan that is focused on early optimization of treatment, with continued measurement-based follow-up to ensure the patient is progressing, is a critical step in bringing the patient to full functional recovery.
A successful treatment plan also includes patient education regarding therapy options, possible associated adverse effects, and the expected course of recovery. A patient who knows what to expect from an antidepressant treatment comprehends when and why treatment adjustments might occur and understands the value of long-term antidepressant treatment is more likely to be adherent to the treatment plan and achieve positive outcomes (Ashton et al., 2005; Cameron et al., 2014; Culpepper et al., 2015).
Antidepressant Treatment Selection
A key factor in achieving treat-to-target goals is the selection of a treatment best matched to the individual patient. Rapid treatment optimization requires that antidepressant drug and dose selection is based on patients’ individual clinical characteristics rather than a habit-based, “one-size fits all” approach (National Institute of Mental Health, 2015; Habert et al., 2016). To date, pharmacogenetics/genomic biomarkers have yet to demonstrate a positive impact on health outcomes in clinical practice; however, future research may eventually prove biomarkers to be beneficial in selecting appropriate antidepressants in specific patient populations (Dunlop and Mayberg, 2014; Leuchter et al., 2014; Thase, 2014a). Some evidence indicates that antidepressant efficacy or tolerability may vary based on patient genotype. For example, in clinical trials of venlafaxine (extended release or immediate release), the cytochrome P450 (CYP) 2D6 poor metabolizer genotype is associated with significantly smaller mean improvements in depressive symptoms and significantly lower rates of remission vs those with the extensive metabolizer phenotype (Lobello et al., 2010). In depressed patients administered escitalopram, CYP2D6 genotypes yielding more rapid metabolism were significantly associated with a slower time to remission, and CYP2C19 poor metabolizers had significantly higher serum escitalopram levels compared with extensive metabolizers (Tsai et al., 2010). Plasma vortioxetine levels are reported to be twice as high in CYP2D6 poor metabolizers compared with CYP2D6 extensive metabolizers (Zhang et al., 2015). These findings suggest that certain patients may require therapeutic drug monitoring and/or dose adjustment for efficacy or safety if antidepressant drugs whose exposure or efficacy vary with metabolizer genotype are prescribed (Probst-Schendzielorz et al., 2015; Zhang et al., 2015).
In the absence of reliable biomarkers, before selecting an antidepressant, all relevant clinical factors that may be associated with differences in drug efficacy should be considered. Such factors include age, illness severity, predominant symptoms (e.g., agitation vs retardation), comorbidities, areas of functional impairment, and previous antidepressant treatment history (Uher et al., 2012; Kennedy et al., 2016). While antidepressants may be similar in efficacy overall (Work Group on Major Depressive Disorder, 2010; Gartlehner et al., 2011; Schueler et al., 2011), published literature suggests there may be potential benefits of some drugs in individuals with specific symptoms or comorbid conditions (Sternat and Katzman, 2016). The CANMAT guidelines include recommendations for specific antidepressant drugs or classes based on clinical specifiers and dimensions of MDD, although the evidence is not of the highest quality (Kennedy et al., 2016). Analysis of antidepressant efficacy in patient subpopulations has not been published for all antidepressant drugs, and understanding effectiveness of treatment among individuals with specific demographic, medical, or genetic characteristics remains an important research goal (National Institute of Mental Health, 2015).
Characteristics that may affect treatment tolerability or adherence for individual patients are also important considerations. These include past response, potential sensitivity to side effects, family history, potential for drug-drug interactions, simplicity of use, issues associated with abrupt discontinuation, cost, and branded vs generic formulations (Kennedy et al., 2016). Tolerability and compliance can be important differentiators when it is not clear that one antidepressant drug or class may hold an efficacy advantage for a particular patient (Kennedy et al., 2016). Although adverse events are associated with all drugs, specific antidepressants are associated with higher rates of particularly troublesome side effects, such as weight gain and sexual dysfunction (Ashton et al., 2005; Serretti and Chiesa, 2009; Serretti and Mandelli, 2010; Clayton et al., 2014). Tolerability concerns are heightened for patients who have comorbid conditions that could be worsened by antidepressant effects, or who take concomitant medications likely to contribute to drug-drug interactions (Manolopoulos et al., 2012; Kennedy et al., 2016; Thase et al., 2016). Efficacy and tolerability differences between drugs must be balanced based on the needs of the individual patient (Gartlehner et al., 2005; Machado et al., 2006; Gartlehner et al., 2008a, 2008b; Cipriani et al., 2009).
Physicians may also consider the importance of prescription insurance coverage when selecting an antidepressant treatment for a specific patient. A recent report suggests that most US healthcare plans implement restriction strategies other than complete exclusion (e.g., tier placement, administrative restrictions) to manage the cost and utilization of newer antidepressant treatments (Hodgkin et al., 2015). Most older, less expensive agents are unrestricted. The selection of branded vs generic medications should be considered. Generic medications provide a lower cost option. However, even though approval of a generic medication requires a demonstration of bioequivalence, which does sometimes fail (Chenu et al., 2009; Woodcock et al., 2012), there nonetheless may be efficacy or tolerability differences for some patients taking generic drugs, possibly related to difference in excipients (Gallelli et al., 2013; Andrade, 2015). Discussing insurance coverage may inform antidepressant selection, as restrictions and higher copay requirements may preclude patients from following through with their prescription.
A review of nonpharmacological interventions is beyond the scope of this article; however, it is important to note that psychological therapies are recommended as first-line treatment or as an adjunctive to pharmacological therapy for some patients (Parikh et al., 2016). Complementary and alternative medicines have proven beneficial for some patients with MDD, particularly those with mild to moderate depression, or as adjunctive treatment in patients with moderate to severe depression (Ravindran et al., 2016). As full functional recovery is all too commonly not achieved with pharmacological treatment alone, consideration of nonpharmacological interventions may prove essential to achieving optimized treatment (McIntyre et al., 2015).
Assessment of Early Improvement for Early Optimized Treatment
Once an initial antidepressant selection is made, physicians should be thorough and persistent in monitoring patient response through clinical interviews and by using validated measurement tools (Papakostas, 2016). Maintaining initial treatment for up to 8 weeks (Work Group on Major Depressive Disorder, 2010) before making adjustments is outdated. Instead, clinicians must shift to evidence-based and patient-specific prescribing with rapid optimization of treatment, closely monitoring efficacy and tolerability over the first 1 to 4 weeks of treatment to heighten adherence and reduce time spent on ineffective medication (Habert et al., 2016; Lam et al., 2016).
A growing body of research has demonstrated that early improvements in depressive symptoms and functioning predict eventual remission or recovery (reviewed in Habert et al., 2016). In the majority of studies, improvement was defined as a percentage change in depression scale scores from baseline (often ≥20% change) assessed at 1 to 4 weeks after treatment initiation. Importantly, lack of early improvement (negative predictive value) appears to be a more accurate predictor of eventual treatment outcome than is achieving early improvement (positive predictive value) (Habert et al., 2016). These data suggest that in clinical practice, physicians can use a lack of improvement early in treatment as an indicator that modification of therapy is needed.
Most studies examining the predictive value of early improvement used a 2-week time point to assess early treatment response (Habert et al., 2016). Although 2 weeks is not an adequate trial for efficacy, information from the first week or two can be useful for making dose adjustments (Kennedy et al., 2016). Given that increases of 2 to 3 times an initial dose are common, delaying dose adjustments results in patients being under-dosed and increases time to recovery (Mohr et al., 2015). Baseline and weekly assessments of both depressive symptoms and functioning are therefore recommended (Szegedi et al., 2009; Lam et al., 2011).
The CANMAT algorithm for managing inadequate antidepressant response states that a dose increase should be the first step in optimizing treatment and recommends considering a switch or addition of adjunctive treatment after 2 to 4 weeks if no improvement is noted with a dose adjustment, or a dose increase is not tolerated (Kennedy et al., 2016). As with initial decisions regarding treatment choice, a range of clinical factors should be considered in determining a second treatment step (Table 3) (Kennedy et al., 2016). Switching to a new antidepressant may be appropriate when the initial drug has poor tolerability or when a patient prefers a simpler regimen with a single medication. An adjunctive medication can complement the initial antidepressant drug, targeting specific side effects and residual symptoms when there is a partial response, while giving the first drug a longer trial (Cameron et al., 2014; Kennedy et al., 2016).
Table 3.
Factors to Consider in Choosing Between Switching to Another Antidepressant Monotherapy or Adding an Adjunctive Medication (Level 3 Evidence)
| Consider switching to another antidepressant when: | |
| It is the first antidepressant trial. | |
| There are poorly tolerated side effects to the initial antidepressant. | |
| There is no response (<25% improvement) to the initial antidepressant.a | |
| There is more time to wait for a response (less severe, less functional impairment). | |
| Patient prefers to switch to another antidepressant. | |
| Consider an adjunctive medication when: | |
| There have been 2 or more antidepressant trials. | |
| The initial antidepressant is well tolerated. | |
| There is partial response (>25% improvement) to the initial antidepressant. | |
| There are specific residual symptoms or side effects to the initial antidepressant that can be targeted. | |
| There is less time to wait for a response (more severe, more functional impairment). | |
| Patient prefers to add on another medication. | |
With permission from (Kennedy et al., 2016).
aFor the initial antidepressant trial. In subsequent trials, lack of response (<25% improvement) may not be a factor for choosing between switch and adjunctive strategies.
Measurement-based care requires the use of validated tools for monitoring early improvement in symptoms and function (Lam et al., 2016), and numerous tools are available for the clinical setting (Table 2). Symptom assessments used in clinical trials (17-item Hamilton Rating Scale for Depression, Montgomery–Åsberg Depression Rating Scale) are too time-consuming and burdensome for application in daily clinical practice. Guideline-based validated instruments such as the PHQ-9 or Quick Inventory of Depressive Symptomatology, in contrast, are fast, easy, and self-administered (Kroenke and Spitzer, 2002; Rush et al., 2006a; Thase, 2014b).
Because treatment goals include a full functional recovery and return to premorbid quality of life, improvements in functional impairment should also be monitored (Lam et al., 2016). The Sheehan Disability Scale is a useful tool for identifying functional impairment and severity of functional disability (Sheehan, 2000; Sheehan and Sheehan, 2008). Similarly, various patient-administered scales are available for evaluating quality of life (Endicott and Dorries, 2009; Lam et al., 2016). Improvement in function and quality of life often lags behind symptom remission in patients with MDD (Bech, 2005; Sheehan and Sheehan, 2008; IsHak et al., 2011). Similar to symptom improvement, functional improvement in the first 2 weeks of treatment is predictive of functional outcomes (Soares et al., 2014b), indicating that weekly assessment of function and quality of life can be valuable for considering adjustments to the treatment plan. Other specialized outcome scales may be needed to screen for comorbid conditions, both at initial assessment and when adjusting the treatment plan in the case of treatment failure. Because patients with MDD have a 2-fold higher prevalence of ADHD relative to the general population and the presence and severity of comorbid ADHD can inform treatment selection for both conditions (Canadian Attention Deficit Hyperactivity Disorder Resource Alliance, 2011; Bond et al., 2012), the authors recommend assessing for ADHD using the Adult ADHD Self-Report Scale (Kessler et al., 2005) in patients with treatment-resistant depression.
In addition to in-office assessments, internet-based tools allow patients to monitor depressive symptoms and functional impairment between office visits. One such tool is MoodFx, an interactive website focusing on depression and anxiety (Work With Us Program, 2014; Mohr et al., 2015; MoodFX website, 2016). Web-based tools allow patients to monitor their symptoms, cognitive deficits, and workplace functioning using validated questionnaires (PHQ-9, the Generalized Anxiety Disorder Questionnaire-7, the Lam Employment Absence and Productivity Scale, the Perceived Deficits Questionnaire-5, and items on general functioning and quality of life from the Clinically Useful Depression Outcome Scale). Patients can monitor improvement and share results with their clinician, which supports their ongoing engagement in the treatment process.
Treatment Adherence
Medication adherence is critical to achieving full, functional recovery and is one of the major challenges hindering optimal treatment of MDD (Kohler et al., 2012; Ho et al., 2015; Lam et al., 2016). Lack of adherence to antidepressant treatment is associated with poor treatment outcomes, including a greater risk of relapse (Gopinath et al., 2007), increased impairments in functioning (Burton et al., 2007), and greater risk of suicide (Ruengorn et al., 2012). Obstacles to adherence include poor tolerability, social stigma, inadequate patient education, low motivation, medication cost, delayed onset of efficacy, weight gain, sexual dysfunction, failure of patients to perceive benefits of treatment, and premature discontinuation of treatment after symptoms have improved (Masand, 2003; Ashton et al., 2005; Burra et al., 2007; Fortney et al., 2011). Clinical experience has shown that patients are reluctant to start another antidepressant following a long trial of an ineffective antidepressant. Compliance with the second antidepressant may also be compromised if a patient mistakes withdrawal symptoms from the previous medication for side effects of the new one. Using a crossover technique to taper one antidepressant while initiating a new one may reduce or eliminate discontinuation symptoms (Masand, 2005). SwitchRx.ca offers suggested crossover schedules for antidepressant switches (Canadian Network for Mood and Anxiety Treatments, 2017).
To prevent early discontinuation and optimize patient adherence to treatment, physicians should choose antidepressants with improved tolerability profiles, use the fewest medications possible, and adjust dosages/treatment to minimize adverse effects (Cameron et al., 2014; Culpepper et al., 2015). Data from the Medical Expenditure Panel Survey for 1996 to 2001 showed that >40% of patients treated for a first depressive episode discontinued antidepressant treatment within the first 30 days (Olfson et al., 2006). Poor tolerability was identified as a primary reason for noncompliance (Demyttenaere et al., 2001). In a survey of 350 adults with MDD, adverse events were the second-most common cause of discontinuing an antidepressant (after lack of efficacy) and second-most common reason for noncompliance (after forgetting to take medication) (Ashton et al., 2005). Weight gain, sexual dysfunction, and fatigue/lack of energy were the most troublesome side effects and among the most likely to result in noncompliance or treatment discontinuation (Ashton et al., 2005). Physicians should closely monitor antidepressant tolerability, including the most common and most troublesome side effects for the prescribed drug or class, utilizing dose adjustment, pharmacological or nonpharmacological treatment of the adverse effect, or switching to a different drug to mitigate the impact on compliance (Anderson et al., 2008; Work Group on Major Depressive Disorder, 2010).
Additional steps to optimize patient adherence may involve patient education, setting realistic patient expectations, employing collaborative care systems, and providing adequate follow-up care (Cameron et al., 2014; Culpepper et al., 2015). Patients who are closely monitored by their healthcare providers have higher rates of treatment adherence. Physicians can maintain patient communication via regular office visits or augment scheduled appointments using internet-based tools such as MoodFx (Work With Us Program, 2014) or MedLink (Mohr et al., 2015). Tools for assisting the physician in monitoring adherence include electronic monitoring, pill counts, blister-packing medications, medication diaries, patient self-reporting, chart reviews, easy availability of prescription renewal, and pharmacy records (Osterberg and Blaschke, 2005; Velligan et al., 2006; Byerly et al., 2007; Nakonezny et al., 2008; van Onzenoort et al., 2012; Faurholt-Jepsen et al., 2014; Sutton et al., 2014; Orrell et al., 2015).
Long-Term Treatment Plan
Patients benefit from understanding the chronic nature of MDD and the need for a long-term plan to ensure they return to pre-illness functioning and lessen the risk of relapse or recurrence. Clinical evidence supports maintenance treatment of MDD with pharmacotherapy, psychotherapy, and other non-pharmacotherapies (Moller, 2008; Glue et al., 2010; Lam et al., 2016). The expected duration of antidepressant treatment should be discussed, and patients should be made aware of medication side effects that may occur later in treatment as well as possible discontinuation symptoms. Long-term maintenance pharmacotherapy should be considered for patients who have risk factors for recurrence of depression (Work Group on Major Depressive Disorder, 2010; Lam et al., 2016). Nonpharmacological interventions for MDD, including psychotherapy, behavior modification, and implementation of positive lifestyle habits (i.e., physical fitness, nutrition, weight loss, stress reduction/tolerability exercises, establishing a strong social network), may have long-term benefits, including mitigation of recurrence (Houle et al., 2013; Clarke et al., 2015; Lam et al., 2016). CANMAT recommends cognitive behavioral therapy as a first-line maintenance treatment for depression (Parikh et al., 2016). The long-term treatment plan must be individualized to each patient and evolve as the patient progresses through treatment (McIntyre et al., 2015).
Conclusions
Recent studies suggest that delaying the treatment of MDD can result in progressive damage to brain areas associated with depression and that pharmacotherapy may halt and even reverse those effects, underscoring the importance of rapidly treating depression to full recovery. Our recommendations for delivering rapidly optimized care are summarized in Box 2. Early optimized treatment of MDD, using measurement-based care, and customizing treatment to the individual patient may afford the best possible outcomes for each patient and increase the likelihood they will achieve full functional recovery. Using a patient-centered approach throughout MDD treatment entails aligning the treatment plan with each patient’s individual characteristics and preferences and expectations for treatment, including, most critically, their own definition of wellness and goals for recovery.
Box 2. Q and A
| When do you screen for depression, and what tools do you use? • Consider screening patients with risk factors for depression, or be alert to the possibility of depression in high-risk patients, following up when clinical symptoms are noted (Joffres et al., 2013; Lam et al., 2016) • A positive response on an initial 2-question screen (“During the last month, have you often been bothered by feeling down, depressed or hopeless? During the last month, have you often been bothered by having little interest or pleasure in doing things?”) can be followed up using the PHQ-9 (Kroenke and Spitzer, 2002) |
| How do you determine treatment goals for an individual patient? • An individual patient’s treatment goals focus on full and sustained functional recovery across all aspects of MDD (McIntyre et al., 2015). The goal should include symptomatic remission, but also functional recovery, including societal, interpersonal, work, and family domains, and any other health outcomes defined by the individual patient and/or clinician (Zimmerman et al., 2006; McIntyre et al., 2015). • The patient’s concept of remission may differ from that of the treating physician (Zimmerman et al., 2006). |
| What factors do you consider in selecting an antidepressant? • The individual patient’s diagnostic specifiers, symptoms and severity, areas of impairment, other clinical characteristics, and medical history together with patient biases and preferences for treatment should be considered when choosing an antidepressant (National Institute of Mental Health, 2015; Habert et al., 2016; Kennedy et al., 2016). • A range of factors (age, gender, MDD severity, predominant symptoms, diagnostic subtype, and comorbidities) could be associated with differences in efficacy or tolerability for specific drugs or classes (Uher et al., 2012; Kennedy et al., 2016). |
| What baseline assessments (for symptoms and function) do you use? How do you assess progress during treatment? What scales do you use? • The PHQ-9 and SDS are brief, validated scales that can be used to measure baseline symptoms of depression and functional impairment, respectively (Kroenke and Spitzer, 2002). • The same instruments can be administered weekly during acute treatment to assess changes in symptoms and function during treatment. • Patients can also monitor changes in symptoms and share results and concerns with their clinician via mobile device applications (Mood Disorders Society of Canada 2014; Mohr et al., 2015). |
| How early do you optimize the treatment step when needed? • Early improvements in depressive symptoms and in functioning, measured 1 to 4 weeks after initiation of treatment, predict later remission or recovery (Koran et al., 1995; Szegedi et al., 2003; Henkel et al., 2009; Kok et al., 2009; Szegedi et al., 2009; Lin et al., 2011; Joel et al., 2014; Lam et al., 2014; Soares et al., 2014a) • Information from the first week or 2 of treatment can be useful for making dose adjustments (Kennedy et al., 2016); a switch to another antidepressant or addition of adjunctive treatment should be considered after 2 to 4 weeks if no improvement is noted with a dose adjustment or if the patient does not tolerate the dose increase (Kennedy et al., 2016). |
| What are the major obstacles to adherence? How do you monitor for adherence? • Obstacles to adherence include poor tolerability, social stigma, inadequate patient education, lack of patient motivation, concerns about medication cost, weight gain, sexual dysfunction, delayed onset of efficacy, failure of patients to perceive benefits of treatment, and premature discontinuation of treatment after symptoms have improved (Masand, 2003; Ashton et al., 2005; Burra et al., 2007; Fortney et al., 2011) • Tools for monitoring adherence to antidepressant medication include electronic monitoring, pill counts, medication diaries, patient self-reporting, chart reviews, prescription renewal, and pharmacy records (Osterberg and Blaschke, 2005; Velligan et al., 2006; Byerly et al., 2007; Nakonezny et al., 2008; Faurholt-Jepsen et al., 2014; Sutton et al., 2014; Orrell et al., 2015). |
| How do comorbid conditions (psychiatric or general medical) affect a long-term treatment plan? • Because the presence of comorbid psychopathologies is a risk factor for recurrence of depression, long-term treatment is recommended for patients with comorbid conditions (Lam et al., 2016). • Treatment efficacy for both antidepressant and psychological treatments may vary with medical of psychiatric comorbidities, so these should be taken into account when developing a long-term treatment plan (Kennedy et al., 2016; Parikh et al., 2016). • The long-term treatment plan should also include addressing comorbid conditions in the maintenance period (Lam et al., 2016). |
Abbreviations: MDD, major depressive disorder; PHQ-9, 9-item Patient Health Questionnaire; SDS, Sheehan Disability Scale.
Statement of Interest
Dr. Oluboka has served on advisory boards or similar committees for Janssen, Pfizer, Lundbeck, Bristol-Myers Squibb, Sunovion, and Otsuka; participated in clinical trials or studies for Otsuka and Lundbeck; has received honoraria or other fees for Janssen, Lundbeck, Pfizer, Bristol-Myers Squibb, Otsuka, and Sunovion; and has received research grants for AstraZeneca, Lundbeck, and Otsuka. Dr. Katzman has received grant support, participated in advisory boards, and/or received honoraria for lectures from the Canadian Foundation for Innovation, Lotte & John Hecht Memorial Foundation, Allergan, AstraZeneca, Bedrocan, Biotics, Bristol-Myers Squibb, Eli Lilly, Genuine Health, Janssen, Lundbeck, Merck, Otsuka, Pfizer, Purdue, Shire, Sunovion, and Tweed. Dr. Habert has served on advisory boards and as a speaker or consultant for Pfizer, Amgen, Bristol-Myers Squibb, Boehringer, Eli Lilly, Allergan, Lundbeck, Bayer, AstraZeneca, Purdue, Novo-Nordisk, and Servier. Dr. McIntosh has served as a speaker and/or on advisory boards for Abbvie, Pfizer, Purdue, Lundbeck, Eli Lilly, Janssen, Shire, Otsuka, Bristol-Myers Squibb, Sunovion, and Allergan. Dr. MacQueen has received honoraria for serving as a speaker or on advisory boards for Lilly, Lundbeck, Pfizer, Allergan, and Janssen; and has chaired the Canadian Psychiatry Research Program for Pfizer. Dr. Milev has served on advisory boards or similar committees for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Lundbeck, Otsuka, Pfizer, Servier, and Shire; has participated in clinical trials or studies for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Lundbeck, Brain Cells, and Pfizer; has received honoraria or other fees from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Lundbeck, Otsuka, Pfizer, and Sunovion; and has received research grants from CIHR, OMHF, OPGRS, OBI, and CANBIND. Dr. McIntyre has received research or grants from private industries or nonprofit funds from Stanley Medical Research Institute, National Alliance for Research on Schizophrenia and Depression (NARSAD), and the National Institutes of Mental Health; has served on advisory boards for Janssen-Ortho, Eli Lilly, Lundbeck, Pfizer, Shire, Otsuka, Purdue, Takeda, and Allergan; has served on speaker bureaus for AstraZeneca, Bristol-Myers Squibb, Eli Lilly; Lundbeck, Pfizer, Shire, Otsuka, Purdue, Takeda, Janssen-Ortho, and Allergan; and has received research grants from AstraZeneca, Bristol-Myers Squibb, Janssen-Ortho, Eli Lilly, Lundbeck, Pfizer, Shire, Otsuka, Purdue, Takeda, and Allergan. Dr Blier has received grant funding and/or honoraria for lectures and/or for participation in advisory boards for Allergan, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Euthymics, Janssen, Lundbeck, Merck, Otsuka, Pfizer, Pierre Fabre, Servier, Shire, Takeda, and Valeant.
Acknowledgments
This review was supported by Pfizer Canada Inc. Medical writing support was provided by Kathleen Dorries at Peloton Advantage and was funded by Pfizer.
References
- Altamura AC, Serati M, Buoli M(2015)Is duration of illness really influencing outcome in major psychoses?Nord J Psychiatry 69:1–15. [DOI] [PubMed] [Google Scholar]
- American Academy of Professional Coders (2013)ICD-10 resource: coding for major depressive disorder http://cloud.aapc.com/documents/Depressive-Disorder-ICD-10-BH.pdf.
- American Psychiatric Association (2013)Major depressive disorder. In: Diagnostic and statistical manual of mental disorders (DSM-5), 5th ed, pp160–168. Washington, DC: American Psychiatric Association. [Google Scholar]
- Anderson IM, Ferrier IN, Baldwin RC, Cowen PJ, Howard L, Lewis G, Matthews K, McAllister-Williams RH, Peveler RC, Scott J, Tylee A(2008)Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British Association for Psychopharmacology guidelines. J Psychopharmacol 22:343–396. [DOI] [PubMed] [Google Scholar]
- Andrade C.(2015)Bioequivalence of generic drugs. J Clin Psychiatry 76:e1130–1131. [DOI] [PubMed] [Google Scholar]
- Arnone D, McKie S, Elliott R, Juhasz G, Thomas EJ, Downey D, Williams S, Deakin JF, Anderson IM(2013)State-dependent changes in hippocampal grey matter in depression. Mol Psychiatry 18:1265–1272. [DOI] [PubMed] [Google Scholar]
- Ashton AK, Jamerson BD, W LW, Wagoner C(2005)Antidepressant-related adverse effects impacting treatment compliance: results of a patient survey. Curr Ther Res Clin Exp 66:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Pfennig A, Severus E, Whybrow PC, Angst J, Moller HJ(2013)World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry 14:334–385. [DOI] [PubMed] [Google Scholar]
- Bech P.(2005)Social functioning: should it become an endpoint in trials of antidepressants?CNS Drugs 19:313–324. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, Arango V(2012)Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry 72:562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, Arango V, John Mann J(2013)Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology 38:1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond DJ, Hadjipavlou G, Lam RW, McIntyre RS, Beaulieu S, Schaffer A, Weiss M(2012)The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid attention-deficit/hyperactivity disorder. Ann Clin Psychiatry 24:23–37. [PubMed] [Google Scholar]
- Bortolotto V, Cuccurazzu B, Canonico PL, Grilli M(2014)NF-kappaB mediated regulation of adult hippocampal neurogenesis: relevance to mood disorders and antidepressant activity. Biomed Res Int 2014:612798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron TI, Bijlenga D, Verduijn J, Penninx BW, Beekman AT, Kooij JJ(2016)Prevalence of ADHD symptoms across clinical stages of major depressive disorder. J Affect Disord 197:29–35. [DOI] [PubMed] [Google Scholar]
- Bukh JD, Bock C, Vinberg M, Kessing LV(2013)The effect of prolonged duration of untreated depression on antidepressant treatment outcome. J Affect Disord 145:42–48. [DOI] [PubMed] [Google Scholar]
- Burra TA, Chen E, McIntyre RS, Grace SL, Blackmore ER, Stewart DE(2007)Predictors of self-reported antidepressant adherence. Behav Med 32:127–134. [DOI] [PubMed] [Google Scholar]
- Burton WN, Chen CY, Conti DJ, Schultz AB, Edington DW(2007)The association of antidepressant medication adherence with employee disability absences. Am J Manag Care 13:105–112. [PubMed] [Google Scholar]
- Byerly MJ, Thompson A, Carmody T, Bugno R, Erwin T, Kashner M, Rush AJ(2007)Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatr Serv 58:844–847. [DOI] [PubMed] [Google Scholar]
- Cameron C, Habert J, Anand L, Furtado M(2014)Optimizing the management of depression: primary care experience. Psychiatry Res 220:45–S57. [DOI] [PubMed] [Google Scholar]
- Canadian Attention Deficit Hyperactivity Disorder Resource Alliance (CADDRA) (2011)Canadian ADHD Practice Guidelines, 3rd ed Available online at https://www.caddra.ca/pdfs/caddraGuidelines2011.pdf. Retrieved 21 Apr 2017. [Google Scholar]
- Canadian Network for Mood and AnxietyTreatments (2017). SwitchRx: switching antipsychotic medications. In: Canadian Network for Mood and Anxiety Treatments. Available online at http://switchrx.ca/. Retrieved 21 Apr 2017. [Google Scholar]
- Chenu F, Batten LA, Zernig G, Ladstaetter E, Hebert C, Blier P(2009)Comparison of pharmacokinetic profiles of brand-name and generic formulations of citalopram and venlafaxine: a crossover study. J Clin Psychiatry 70:958–966. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C(2009)Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 373:746–758. [DOI] [PubMed] [Google Scholar]
- Clarke K, Mayo-Wilson E, Kenny J, Pilling S(2015)Can non-pharmacological interventions prevent relapse in adults who have recovered from depression? A systematic review and meta-analysis of randomised controlled trials. Clin Psychol Rev 39:58–70. [DOI] [PubMed] [Google Scholar]
- Clayton AH, Croft HA, Handiwala L(2014)Antidepressants and sexual dysfunction: mechanisms and clinical implications. Postgrad Med 126:91–99. [DOI] [PubMed] [Google Scholar]
- Clerc GE, Ruimy P, Verdeau-Palles J(1994)A double-blind comparison of venlafaxine and fluoxetine in patients hospitalized for major depression and melancholia. The Venlafaxine French Inpatient Study Group. Int Clin Psychopharmacol 9:139–143. [DOI] [PubMed] [Google Scholar]
- Culpepper L, Muskin PR, Stahl SM(2015)Major depressive disorder: understanding the significance of residual symptoms and balancing efficacy with tolerability. Am J Med 128:S1–S15. [DOI] [PubMed] [Google Scholar]
- Davidson JR.(2010)Major depressive disorder treatment guidelines in America and Europe. J Clin Psychiatry 71:e04. [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, Enzlin P, Dewe W, Boulanger B, De Bie J, De Troyer W, Mesters P(2001)Compliance with antidepressants in a primary care setting, 1: beyond lack of efficacy and adverse events. J Clin Psychiatry 62:30–33. [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK(2012)Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Mayberg HS(2014)Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues Clin Neurosci 16:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, Duman RS(2013)Depression and treatment response: dynamic interplay of signaling pathways and altered neural processes. Cell Mol Life Sci 70:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, Jurjus GJ, Dieter L, Duman RS(2013)Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol 16:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen SV, Dill DL, Grob MC(1994)Reliability and validity of a brief patient-report instrument for psychiatric outcome evaluation. Hosp Community Psychiatry 45:242–247. [DOI] [PubMed] [Google Scholar]
- Elbejjani M, Fuhrer R, Abrahamowicz M, Mazoyer B, Crivello F, Tzourio C, Dufouil C(2015)Depression, depressive symptoms, and rate of hippocampal atrophy in a longitudinal cohort of older men and women. Psychol Med 45:1931–1944. [DOI] [PubMed] [Google Scholar]
- Endicott J, Dorries KM(2009)Functional outcomes in MDD: established and emerging assessment tools. Am J Manag Care 15:S328–S334. [PubMed] [Google Scholar]
- Epstein I, Szpindel I, Katzman MA(2014)Pharmacological approaches to manage persistent symptoms of major depressive disorder: rationale and therapeutic strategies. Psychiatry Res 220:S15–S33. [DOI] [PubMed] [Google Scholar]
- EuroQol Group (1990)EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 16:199–208. [DOI] [PubMed] [Google Scholar]
- Faurholt-Jepsen M, Vinberg M, Frost M, Christensen EM, Bardram J, Kessing LV(2014)Daily electronic monitoring of subjective and objective measures of illness activity in bipolar disorder using smartphones--the MONARCA II trial protocol: a randomized controlled single-blind parallel-group trial. BMC Psychiatry 14:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Wiltse C, Walker D, Brecht S, Chen A, Perahia D(2009)Predictors of relapse in a study of duloxetine treatment in patients with major depressive disorder. J Affect Disord 113:263–271. [DOI] [PubMed] [Google Scholar]
- Florida Agency for Health Care Administration (2015)Florida Best Practice Psychotherapeutic Medication Guidelines for Adults: The University of South Florida, Florida Medicaid Drug Therapy Management Program Available online at http://www.medicaidmentalhealth.org/_assets/file/Guidelines/Web_2015-Psychotherapeutic%20Medication%20Guidelines%20for%20Adults_Final_Approved1.pdf. Retrieved 17 Apr 2017.
- Fortney JC, Pyne JM, Edlund MJ, Stecker T, Mittal D, Robinson DE, Henderson KL(2011)Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry 72:827–834. [DOI] [PubMed] [Google Scholar]
- Furtado CP, Hoy KE, Maller JJ, Savage G, Daskalakis ZJ, Fitzgerald PB(2013)An investigation of medial temporal lobe changes and cognition following antidepressant response: a prospective rTMS study. Brain Stimul 6:346–354. [DOI] [PubMed] [Google Scholar]
- Gallelli L, Palleria C, De Vuono A, Mumoli L, Vasapollo P, Piro B, Russo E(2013)Safety and efficacy of generic drugs with respect to brand formulation. J Pharmacol Pharmacother 4:S110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartlehner G, Hansen RA, Carey TS, Lohr KN, Gaynes BN, Randolph LC(2005)Discontinuation rates for selective serotonin reuptake inhibitors and other second-generation antidepressants in outpatients with major depressive disorder: a systematic review and meta-analysis. Int Clin Psychopharmacol 20:59–69. [DOI] [PubMed] [Google Scholar]
- Gartlehner G, Thieda P, Hansen RA, Gaynes BN, Deveaugh-Geiss A, Krebs EE, Lohr KN (2008a) Comparative risk for harms of second-generation antidepressants: a systematic review and meta-analysis. Drug Saf 31:851–865. [DOI] [PubMed] [Google Scholar]
- Gartlehner G, Gaynes BN, Hansen RA, Thieda P, Deveaugh-Geiss A, Krebs EE, Moore CG, Morgan L, Lohr KN (2008b) Comparative benefits and harms of second-generation antidepressants: background paper for the American College of Physicians. Ann Intern Med 149:734–750. [DOI] [PubMed] [Google Scholar]
- Gartlehner G, Hansen RA, Morgan LC, Thaler K, Lux L, Van Noord M, Mager U, Thieda P, Gaynes BN, Wilkins T, Strobelberger M, Lloyd S, Reichenpfader U, Lohr KN(2011)Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis. Ann Intern Med 155:772–785. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Brickman AM, Schupf N, Devanand DP, Luchsinger JA, Mayeux R, Small SA(2012)Depressive symptoms, antidepressant use, and brain volumes on MRI in a population-based cohort of old persons without dementia. J Alzheimers Dis 30:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelenberg AJ, Freeman MP, Markowitz JC, Rosenbaum JF, Thase ME, Trivedi MH, Van Rhoads RS (2010)Practice guideline for the treatment of patients with major depressive disorder: American Psychiatric Association Available online at http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Retrieved March 24, 2017.
- Ghio L, Gotelli S, Marcenaro M, Amore M, Natta W(2014)Duration of untreated illness and outcomes in unipolar depression: a systematic review and meta-analysis. J Affect Disord 152–154:45–51. [DOI] [PubMed] [Google Scholar]
- Glue P, Donovan MR, Kolluri S, Emir B(2010)Meta-analysis of relapse prevention antidepressant trials in depressive disorders. Aust N Z J Psychiatry 44:697–705. [DOI] [PubMed] [Google Scholar]
- Godlewska BR, Hasselmann HW, Igoumenou A, Norbury R, Cowen PJ(2014)Short-term escitalopram treatment and hippocampal volume. Psychopharmacology (Berl) 231:4579–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath S, Katon WJ, Russo JE, Ludman EJ(2007)Clinical factors associated with relapse in primary care patients with chronic or recurrent depression. J Affect Disord 101:57–63. [DOI] [PubMed] [Google Scholar]
- Gormley N, O’Leary D, Costello F(1999)First admissions for depression: is the ‘no-treatment interval’ a critical predictor of time to remission?J Affect Disord 54:49–54. [DOI] [PubMed] [Google Scholar]
- Habert J, Katzman MA, Oluboka OJ, McIntyre RS, McIntosh D, MacQueen GM, Khullar A, Milev RV, Kjernisted KD, Chokka PR, Kennedy SH(2016)Functional recovery in major depressive disorder: focus on early optimized treatment. Prim Care Companion CNS Disord 18. [DOI] [PubMed] [Google Scholar]
- Henkel V, Seemuller F, Obermeier M, Adli M, Bauer M, Mundt C, Brieger P, Laux G, Bender W, Heuser I, Zeiler J, Gaebel W, Mayr A, Moller HJ, Riedel M(2009)Does early improvement triggered by antidepressants predict response/remission? Analysis of data from a naturalistic study on a large sample of inpatients with major depression. J Affect Disord 115:439–449. [DOI] [PubMed] [Google Scholar]
- Ho SC, Chong HY, Chaiyakunapruk N, Tangiisuran B, Jacob SA(2015)Clinical and economic impact of non-adherence to antidepressants in major depressive disorder: a systematic review. J Affect Disord 193:1–10. [DOI] [PubMed] [Google Scholar]
- Hodgkin D, Horgan CM, Creedon TB, Merrick EL, Stewart MT(2015)Management of newer antidepressant medications in U.S. commercial health plans. J Ment Health Policy Econ 18:165–173. [PMC free article] [PubMed] [Google Scholar]
- Houle J, Gascon-Depatie M, Belanger-Dumontier G, Cardinal C(2013)Depression self-management support: a systematic review. Patient Educ Couns 91:271–279. [DOI] [PubMed] [Google Scholar]
- Howland RH, Wilson MG, Kornstein SG, Clayton AH, Trivedi MH, Wohlreich MM, Fava M(2008)Factors predicting reduced antidepressant response: experience with the SNRI duloxetine in patients with major depression. Ann Clin Psychiatry 20:209–218. [DOI] [PubMed] [Google Scholar]
- Huang TL, Lin CC(2015)Advances in biomarkers of major depressive disorder. Adv Clin Chem 68:177–204. [DOI] [PubMed] [Google Scholar]
- Huang Y, Coupland NJ, Lebel RM, Carter R, Seres P, Wilman AH, Malykhin NV(2013)Structural changes in hippocampal subfields in major depressive disorder: a high-field magnetic resonance imaging study. Biol Psychiatry 74:62–68. [DOI] [PubMed] [Google Scholar]
- Huerta-Ramirez R, Bertsch J, Cabello M, Roca M, Haro JM, Ayuso-Mateos JL(2013)Diagnosis delay in first episodes of major depression: a study of primary care patients in Spain. J Affect Disord 150:1247–1250. [DOI] [PubMed] [Google Scholar]
- Husarova V, Bittsansky M, Ondrejka I, Kerna V, Dobrota D(2012)Hippocampal neurometabolite changes in depression treatment: a (1)H magnetic resonance spectroscopy study. Psychiatry Res 201:206–213. [DOI] [PubMed] [Google Scholar]
- IsHak WW, Greenberg JM, Balayan K, Kapitanski N, Jeffrey J, Fathy H, Fakhry H, Rapaport MH(2011)Quality of life: the ultimate outcome measure of interventions in major depressive disorder. Harv Rev Psychiatry 19:229–239. [DOI] [PubMed] [Google Scholar]
- Jiang W, Krishnan R, Kuchibhatla M, Cuffe MS, Martsberger C, Arias RM, O’Connor CM(2011)Characteristics of depression remission and its relation with cardiovascular outcome among patients with chronic heart failure (from the SADHART-CHF Study). Am J Cardiol 107:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel I, Begley AE, Mulsant BH, Lenze EJ, Mazumdar S, Dew MA, Blumberger D, Butters M, Reynolds CF 3rd(2014)Dynamic prediction of treatment response in late-life depression. Am J Geriatr Psychiatry 22:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffres M, Jaramillo A, Dickinson J, Lewin G, Pottie K, Shaw E, Connor Gorber S, Tonelli M(2013)Recommendations on screening for depression in adults. CMAJ 185:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, Hasnain M, Jollant F, Levitt AJ, MacQueen GM, McInerney SJ, McIntosh D, Milev RV, Muller DJ, Parikh SV, Pearson NL, Ravindran AV, Uher R(2016)Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 3. Pharmacological Treatments. Can J Psychiatry 61:540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarz H, Fitzpatrick-Lewis D, Streiner DL, Maureen R, Ali U, Shannon HS, Raina P(2013)Screening for depression: a systematic review and meta-analysis. CMAJ Open 1:E159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS(2003)The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289:3095–3105. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, Howes MJ, Jin R, Secnik K, Spencer T, Ustun TB, Walters EE(2005)The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med 35:245–256. [DOI] [PubMed] [Google Scholar]
- Kohler S, Hoffmann S, Unger T, Steinacher B, Fydrich T(2012)Adherence to guidelines and effectiveness of inpatient treatment for unipolar depression. Int J Psychiatry Clin Pract 16:103–112. [DOI] [PubMed] [Google Scholar]
- Kok RM, van Baarsen C, Nolen WA, Heeren TJ(2009)Early response as predictor of final remission in elderly depressed patients. Int J Geriatr Psychiatry 24:1299–1303. [DOI] [PubMed] [Google Scholar]
- Koran LM, Hamilton SH, Hertzman M, Meyers BS, Halaris AE, Tollefson GD, Downs JM, Folks DG, Jeste DV, Lazarus LW(1995)Predicting response to fluoxetine in geriatric patients with major depression. J Clin Psychopharmacol 15:421–427. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL(2002)The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann 32:1–7. [Google Scholar]
- Lam RW, Michalak EE, Yatham LN(2009)A new clinical rating scale for work absence and productivity: validation in patients with major depressive disorder. BMC Psychiatry 9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam RW, Filteau MJ, Milev R(2011)Clinical effectiveness: the importance of psychosocial functioning outcomes. J Affect Disord 132Suppl 1:S9–S13. [DOI] [PubMed] [Google Scholar]
- Lam RW, Endicott J, Hsu MA, Fayyad R, Guico-Pabia C, Boucher M(2014)Predictors of functional improvement in employed adults with major depressive disorder treated with desvenlafaxine. Int Clin Psychopharmacol 29:239–251. [DOI] [PubMed] [Google Scholar]
- Lam RW, McIntosh D, Wang J, Enns MW, Kolivakis T, Michalak EE, Sareen J, Song WY, Kennedy SH, MacQueen GM, Milev RV, Parikh SV, Ravindran AV(2016)Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 1. Disease Burden and Principles of Care. Can J Psychiatry 61:510–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchter AF, Hunter AM, Krantz DE, Cook IA(2014)Intermediate phenotypes and biomarkers of treatment outcome in major depressive disorder. Dialogues Clin Neurosci 16:525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licznerski P, Duman RS(2013)Remodeling of axo-spinous synapses in the pathophysiology and treatment of depression. Neuroscience 251:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Lane HY, Chen CC, Juo SH, Yen CF(2011)Early prediction of fluoxetine response for Han Chinese inpatients with major depressive disorder. J Clin Psychopharmacol 31:187–193. [DOI] [PubMed] [Google Scholar]
- Lobello KW, Preskorn SH, Guico-Pabia CJ, Jiang Q, Paul J, Nichols AI, Patroneva A, Ninan PT(2010)Cytochrome P450 2D6 phenotype predicts antidepressant efficacy of venlafaxine: a secondary analysis of 4 studies in major depressive disorder. J Clin Psychiatry 71:1482–1487. [DOI] [PubMed] [Google Scholar]
- Machado M, Iskedjian M, Ruiz I, Einarson TR(2006)Remission, dropouts, and adverse drug reaction rates in major depressive disorder: a meta-analysis of head-to-head trials. Curr Med Res Opin 22:1825–1837. [DOI] [PubMed] [Google Scholar]
- MacQueen G, Frodl T(2011)The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research?Mol Psychiatry 16:252–264. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Frey BN, Ismail Z, Jaworska N, Steiner M, Van Lieshout RJ, Kennedy SH, Lam RW, Milev RV, Parikh SV, Ravindran AV(2016)Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 6. Special Populations: Youth, Women, and the Elderly. Can J Psychiatry 61:588–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolopoulos VG, Ragia G, Alevizopoulos G(2012)Pharmacokinetic interactions of selective serotonin reuptake inhibitors with other commonly prescribed drugs in the era of pharmacogenomics. Drug Metabol Drug Interact 27:19–31. [DOI] [PubMed] [Google Scholar]
- Masand PS.(2003)Tolerability and adherence issues in antidepressant therapy. Clin Ther 25:2289–2304. [DOI] [PubMed] [Google Scholar]
- —— (2005)A review of pharmacologic strategies for switching to atypical antipsychotics. Prim Care Companion J Clin Psychiatry 7:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AW, Eastwood JA, Hays RD, Macabasco-O’Connell A, Doering LV(2014)Depressed or not depressed: untangling symptoms of depression in patients hospitalized with coronary heart disease. Am J Crit Care 23:106–116. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Lee Y, Mansur RB(2015)Treating to target in major depressive disorder: response to remission to functional recovery. CNS Spectr 20:20–30; quiz 31. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Florea I, Tonnoir B, Loft H, Lam RW, Christensen MC(2017)Efficacy of vortioxetine on cognitive functioning in working patients with major depressive disorder. J Clin Psychiatry 78:115–121. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM(2009)A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci 34:41–54. [PMC free article] [PubMed] [Google Scholar]
- Miller IW, Keitner GI, Schatzberg AF, Klein DN, Thase ME, Rush AJ, Markowitz JC, Schlager DS, Kornstein SG, Davis SM, Harrison WM, Keller MB(1998)The treatment of chronic depression, part 3: psychosocial functioning before and after treatment with sertraline or imipramine. J Clin Psychiatry 59:608–619. [DOI] [PubMed] [Google Scholar]
- Mohr DC, Stiles-Shields C, Brenner C, Palac H, Montague E, Kaiser SM, Carty-Fickes E, Duffecy J(2015)MedLink: a mobile intervention to address failure points in the treatment of depression in general medicine. International Conference on Pervasive Computing Technologies for Healthcare: [proceedings] International Conference on Pervasive Computing Technologies for Healthcare2015:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller HJ.(2008)Outcomes in major depressive disorder: the evolving concept of remission and its implications for treatment. World J Biol Psychiatry 9:102–114. [DOI] [PubMed] [Google Scholar]
- Mood Disorders Society of Canada. Work With Us Program (2014)Work With Us partners with MoodFx to feature new mood tracker app http://www.mooddisorderscanada.ca/documents/New-Views/Work%20With%20Us%20%20MoodFX_NR_2014-12-01_FINAL.pdf. Retrieved 27 Oct 2016.
- MoodFX web site (2016)MoodFX. In. Vancouver, Canada: Mood Disorders Centre University of British Columbia’s eHealth Strategy Office; https://www.moodfx.ca/. Retrieved 27 Oct 2016. [Google Scholar]
- Moylan S, Maes M, Wray NR, Berk M(2013)The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 18:595–606. [DOI] [PubMed] [Google Scholar]
- Nakonezny PA, Byerly MJ, Rush AJ(2008)Electronic monitoring of antipsychotic medication adherence in outpatients with schizophrenia or schizoaffective disorder: an empirical evaluation of its reliability and predictive validity. Psychiatry Res 157:259–263. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence (2009)Depression in Adults: Recognition and Management https://www.nice.org.uk/guidance/cg90/resources/depression-in-adults-recognition-and-management-975742636741. Retrieved 27 Oct 2016.
- National Institute of Mental Health (2015)Strategic Plan for Research http://www.nimh.nih.gov/about/strategic-planning-reports/nimh_strategicplanforresearch_508compliant_corrected_final_149979.pdf. Retrieved 27 Oct 2016.
- Nugent AC, Davis RM, Zarate CA Jr, Drevets WC(2013)Reduced thalamic volumes in major depressive disorder. Psychiatry Res 213:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor E, Rossom RC, Henninger M, Groom HC, Burda BU, Henderson JT, Bigler KD, Whitlock EP(2016)Screening for depression in adults: an updated systematic evidence review for the U.S. Preventive Services Task Force: Agency for Healthcare Research and Quality, Rockville, MD. [PubMed] [Google Scholar]
- Okuda A, Suzuki T, Kishi T, Yamanouchi Y, Umeda K, Haitoh H, Hashimoto S, Ozaki N, Iwata N(2010)Duration of untreated illness and antidepressant fluvoxamine response in major depressive disorder. Psychiatry Clin Neurosci 64:268–273. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Tedeschi M, Wan GJ(2006)Continuity of antidepressant treatment for adults with depression in the United States. Am J Psychiatry 163:101–108. [DOI] [PubMed] [Google Scholar]
- Orrell C, Cohen K, Mauff K, Bangsberg DR, Maartens G, Wood R(2015)A randomized controlled trial of real-time electronic adherence monitoring with text message dosing reminders in people starting first-line antiretroviral therapy. J Acquir Immune Defic Syndr 70:495–502. [DOI] [PubMed] [Google Scholar]
- Osterberg L, Blaschke T(2005)Adherence to medication. N Engl J Med 353:487–497. [DOI] [PubMed] [Google Scholar]
- Papakostas GI.(2009)Major depressive disorder: psychosocial impairment and key considerations in functional improvement. Am J Manag Care 15:S316–S321. [PubMed] [Google Scholar]
- Papakostas GI.(2016)Identifying patients with depression who require a change in treatment and implementing that change. J Clin Psychiatry 77:16–21. [DOI] [PubMed] [Google Scholar]
- Parikh SV, Quilty LC, Ravitz P, Rosenbluth M, Pavlova B, Grigoriadis S, Velyvis V, Kennedy SH, Lam RW, MacQueen GM, Milev RV, Ravindran AV, Uher R(2016)Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 2. Psychological treatments. Can J Psychiatry 61:524–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patient health questionnaire (PHQ-9) New Hampshire Medical Society website Available online at http://www.nhms.org/sites/default/files/Pdfs/PHQ9-Depression-Scale.pdf. Retrieved 17 Apr 2017.
- Phillips JL, Batten LA, Tremblay P, Aldosary F, Blier P(2015)A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment-resistant depression. Int J Neuropsychopharmacol 18:pyv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posl M, Cieza A, Stucki G(2007)Psychometric properties of the WHODASII in rehabilitation patients. Qual Life Res 16:1521–1531. [DOI] [PubMed] [Google Scholar]
- Probst-Schendzielorz K, Viviani R, Stingl JC(2015)Effect of Cytochrome P450 polymorphism on the action and metabolism of selective serotonin reuptake inhibitors. Expert Opin Drug Metab Toxicol 11:1219–1232. [DOI] [PubMed] [Google Scholar]
- Qaseem A, Snow V, Denberg TD, Forciea MA, Owens DK(2008)Using second-generation antidepressants to treat depressive disorders: a clinical practice guideline from the American College of Physicians. Ann Intern Med 149:725–733. [DOI] [PubMed] [Google Scholar]
- Ravindran AV, Balneaves LG, Faulkner G, Ortiz A, McIntosh D, Morehouse RL, Ravindran L, Yatham LN, Kennedy SH, Lam RW, MacQueen GM, Milev RV, Parikh SV(2016)Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 5. Complementary and alternative medicine treatments. Can J Psychiatry 61:576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera I, Perez V, Menchon JM, Schacht A, Papen R, Neuhauser D, Abbar M, Picard H, Gilaberte I(2012)Early vs. conventional switching of antidepressants in patients with MDD and moderate to severe pain: a double-blind randomized study. J Affect Disord 143:47–55. [DOI] [PubMed] [Google Scholar]
- Rotheneichner P, Lange S, O’Sullivan A, Marschallinger J, Zaunmair P, Geretsegger C, Aigner L, Couillard-Despres S(2014)Hippocampal neurogenesis and antidepressive therapy: shocking relations. Neural Plast 2014:723915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruengorn C, Sanichwankul K, Niwatananun W, Mahatnirunkul S, Pumpaisalchai W, Patumanond J(2012)Factors related to suicide attempts among individuals with major depressive disorder. Int J Gen Med 5:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC, Shores-Wilson K, Biggs MM, Woo A, Nierenberg AA, Fava M (2006a) An evaluation of the quick inventory of depressive symptomatology and the hamilton rating scale for depression: a sequenced treatment alternatives to relieve depression trial report. Biol Psychiatry 59:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB(2003)The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 54:573–583. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M (2006b) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917. [DOI] [PubMed] [Google Scholar]
- Rybarczyk B.(2011)Social and occupational functioning assessment scale (SOFAS). In: Encyclopedia of Clinical Neuropsychology (Kreutzer J, DeLuca J, Caplan B, eds), p2313 New York: Springer. [Google Scholar]
- Schermuly I, Wolf D, Lieb K, Stoeter P, Fellgiebel A(2011)State dependent posterior hippocampal volume increases in patients with major depressive disorder. J Affect Disord 135:405–409. [DOI] [PubMed] [Google Scholar]
- Schmaal L, et al. (2016)Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 21:806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schueler YB, Koesters M, Wieseler B, Grouven U, Kromp M, Kerekes MF, Kreis J, Kaiser T, Becker T, Weinmann S(2011)A systematic review of duloxetine and venlafaxine in major depression, including unpublished data. Acta Psychiatr Scand 123:247–265. [DOI] [PubMed] [Google Scholar]
- Seemuller F, Riedel M, Obermeier M, Bauer M, Adli M, Kronmuller K, Holsboer F, Brieger P, Laux G, Bender W, Heuser I, Zeiler J, Gaebel W, Dichgans E, Bottlander R, Musil R, Moller HJ(2010)Outcomes of 1014 naturalistically treated inpatients with major depressive episode. Eur Neuropsychopharmacol 20:346–355. [DOI] [PubMed] [Google Scholar]
- Serretti A, Chiesa A(2009)Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol 29:259–266. [DOI] [PubMed] [Google Scholar]
- Serretti A, Mandelli L(2010)Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry 71:1259–1272. [DOI] [PubMed] [Google Scholar]
- Sheehan DV.(2000)Sheehan Disability Scale. In: Handbook of Psychiatric Measures (Rush AJ, Pincus HA, First MB, eds), pp113–115. Washington, DC: American Psychiatric Association. [Google Scholar]
- Sheehan DV, Harnett-Sheehan K, Raj BA(1996)The measurement of disability. Int Clin Psychopharmacol 11:89–95. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Harnett-Sheehan K, Spann ME, Thompson HF, Prakash A(2011)Assessing remission in major depressive disorder and generalized anxiety disorder clinical trials with the discan metric of the Sheehan disability scale. Int Clin Psychopharmacol 26:75–83. [DOI] [PubMed] [Google Scholar]
- Sheehan KH, Sheehan DV(2008)Assessing treatment effects in clinical trials with the discan metric of the Sheehan Disability Scale. Int Clin Psychopharmacol 23:70–83. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC(2003)Untreated depression and hippocampal volume loss. Am J Psychiatry 160:1516–1518. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Disabato BM, Hranilovich J, Morris C, D’Angelo G, Pieper C, Toffanin T, Taylor WD, MacFall JR, Wilkins C, Barch DM, Welsh-Bohmer KA, Steffens DC, Krishnan RR, Doraiswamy PM(2012)Treatment course with antidepressant therapy in late-life depression. Am J Psychiatry 169:1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu AL, Bibbins-Domingo K, Grossman DC, Baumann LC, Davidson KW, Ebell M, Garcia FA, Gillman M, Herzstein J, Kemper AR, Krist AH, Kurth AE, Owens DK, Phillips WR, Phipps MG, Pignone MP(2016)Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. JAMA 315:380–387. [DOI] [PubMed] [Google Scholar]
- Soares CN, Fayyad RS, Guico-Pabia CJ (2014a) Early improvement in depressive symptoms with desvenlafaxine 50 mg/d as a predictor of treatment success in patients with major depressive disorder. J Clin Psychopharmacol 34:57–65. [DOI] [PubMed] [Google Scholar]
- Soares CN, Endicott J, Boucher M, Fayyad RS, Guico-Pabia CJ (2014b) Predictors of functional response and remission with desvenlafaxine 50 mg/d in patients with major depressive disorder. CNS Spectr 19:519–527. [DOI] [PubMed] [Google Scholar]
- Sternat T, Katzman MA(2016)Neurobiology of hedonic tone: the relationship between treatment-resistant depression, attention-deficit hyperactivity disorder, and substance abuse. Neuropsychiatr Dis Treat 12:2149–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternat T, Lodzinski A, Katzman MA(2014)Hedonic tone: a bridge between the psychobiology of depression and its comorbidities. J Depress Anxiety 3147. [Google Scholar]
- Sutton S, Kinmonth AL, Hardeman W, Hughes D, Boase S, Prevost AT, Kellar I, Graffy J, Griffin S, Farmer A(2014)Does electronic monitoring influence adherence to medication? Randomized controlled trial of measurement reactivity. Ann Behav Med 48:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegedi A, Muller MJ, Anghelescu I, Klawe C, Kohnen R, Benkert O(2003)Early improvement under mirtazapine and paroxetine predicts later stable response and remission with high sensitivity in patients with major depression. J Clin Psychiatry 64:413–420. [DOI] [PubMed] [Google Scholar]
- Szegedi A, Jansen WT, van Willigenburg AP, van der Meulen E, Stassen HH, Thase ME(2009)Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients. J Clin Psychiatry 70:344–353. [DOI] [PubMed] [Google Scholar]
- Taylor WD, McQuoid DR, Payne ME, Zannas AS, MacFall JR, Steffens DC(2014)Hippocampus atrophy and the longitudinal course of late-life depression. Am J Geriatr Psychiatry 22:1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh CF, Zaslavsky AM, Reynolds CF 3rd, Cleary PD(2010)Effect of depression treatment on chronic pain outcomes. Psychosom Med 72:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tendolkar I, van Beek M, van Oostrom I, Mulder M, Janzing J, Voshaar RO, van Eijndhoven P(2013)Electroconvulsive therapy increases hippocampal and amygdala volume in therapy refractory depression: a longitudinal pilot study. Psychiatry Res 214:197–203. [DOI] [PubMed] [Google Scholar]
- Thase ME. (2014a) Translating clinical science into effective therapies. J Clin Psychiatry 75:e11. [DOI] [PubMed] [Google Scholar]
- Thase ME. (2014b) Using biomarkers to predict treatment response in major depressive disorder: evidence from past and present studies. Dialogues Clin Neurosci 16:539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME.(2016)Managing medical comorbidities in patients with depression to improve prognosis. J Clin Psychiatry 77:22–27. [DOI] [PubMed] [Google Scholar]
- Thase ME, Gommoll C, Chen C, Kramer K, Sambunaris A(2016)Effects of levomilnacipran extended-release on motivation/energy and functioning in adults with major depressive disorder. Int Clin Psychopharmacol 31:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, Pritchett YL, Ossanna MJ, Swindle RW, Xu J, Detke MJ(2007)Efficacy of duloxetine and selective serotonin reuptake inhibitors: comparisons as assessed by remission rates in patients with major depressive disorder. J Clin Psychopharmacol 27:672–676. [DOI] [PubMed] [Google Scholar]
- Thase ME, Haight BR, Richard N, Rockett CB, Mitton M, Modell JG, VanMeter S, Harriett AE, Wang Y(2005)Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: a meta-analysis of original data from 7 randomized controlled trials. J Clin Psychiatry 66:974–981. [DOI] [PubMed] [Google Scholar]
- Travis S, Coupland NJ, Silversone PH, Huang Y, Fujiwara E, Carter R, Seres P, Malykhin NV(2015)Dentate gyrus volume and memory performance in major depressive disorder. J Affect Disord 172:159–164. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Morris DW, Wisniewski SR, Lesser I, Nierenberg AA, Daly E, Kurian BT, Gaynes BN, Balasubramani GK, Rush AJ(2013)Increase in work productivity of depressed individuals with improvement in depressive symptom severity. Am J Psychiatry 170:633–641. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M(2006)Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163:28–40. [DOI] [PubMed] [Google Scholar]
- Tsai MH, Lin KM, Hsiao MC, Shen WW, Lu ML, Tang HS, Fang CK, Wu CS, Lu SC, Liu SC, Chen CY, Liu YL(2010)Genetic polymorphisms of cytochrome P450 enzymes influence metabolism of the antidepressant escitalopram and treatment response. Pharmacogenomics 11:537–546. [DOI] [PubMed] [Google Scholar]
- Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, Hauser J, Dernovsek MZ, Souery D, Bajs M, Maier W, Aitchison KJ, Farmer A, McGuffin P(2012)Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med 42:967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Preventive Services Task Force (2009)Screening and treatment for major depressive disorder in children and adolescents: US Preventive Services Task Force Recommendation Statement. Pediatrics 123:1223–1228. [DOI] [PubMed] [Google Scholar]
- van Onzenoort HA, Neef C, Verberk WW, van Iperen HP, de Leeuw PW, van der Kuy PH(2012)Determining the feasibility of objective adherence measurement with blister packaging smart technology. Am J Health Syst Pharm 69:872–879. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Lam YW, Glahn DC, Barrett JA, Maples NJ, Ereshefsky L, Miller AL(2006)Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophr Bull 32:724–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B(2004)Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry 161:1957–1966. [DOI] [PubMed] [Google Scholar]
- Warden D, Rush AJ, Trivedi MH, Fava M, Wisniewski SR(2007)The STAR*D Project results: a comprehensive review of findings. Curr Psychiatr Rep 9:449–459. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bothwell S(1976)Assessment of social adjustment by patient self-report. Arch Gen Psychiatry 33:1111–1115. [DOI] [PubMed] [Google Scholar]
- Woodcock J, Khan M, Yu LX(2012)Withdrawal of generic budeprion for nonbioequivalence. N Engl J Med 367:2463–2465. [DOI] [PubMed] [Google Scholar]
- Work Group on Major Depressive Disorder. Practice Guideline For the Treatment of Patients With Major Depressive Disorder, edition 3. American Psychiatric Association (2010)World Health Organization. Depression: a Global Public Health Concern http://www.who.int/mental_health/management/depression/who_paper_depression_wfmh_2012.pdf?ua=1. Retrieved 27 Oct 2016.
- Zhang J, Mathis MV, Sellers JW, Kordzakhia G, Jackson AJ, Dow A, Yang P, Fossom L, Zhu H, Patel H, Unger EF, Temple RJ(2015)The US Food and Drug Administration’s perspective on the new antidepressant vortioxetine. J Clin Psychiatry 76:8–14. [DOI] [PubMed] [Google Scholar]
- Zhao YJ, Du MY, Huang XQ, Lui S, Chen ZQ, Liu J, Luo Y, Wang XL, Kemp GJ, Gong QY(2014)Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychol Med 44:2927–2937. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Chelminski I, McGlinchey JB, Posternak MA(2008)A clinically useful depression outcome scale. Compr Psychiatry 49:131–140. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, McGlinchey JB, Posternak MA, Friedman M, Attiullah N, Boerescu D(2006)How should remission from depression be defined? The depressed patient’s perspective. Am J Psychiatry 163:148–150. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Martinez JA, Attiullah N, Friedman M, Toba C, Boerescu DA, Rahgeb M(2012)Why do some depressed outpatients who are in remission according to the Hamilton Depression Rating Scale not consider themselves to be in remission?J Clin Psychiatry 73:790–795. [DOI] [PubMed] [Google Scholar]