Abstract
The contractile function of skeletal muscle declines during intense or prolonged physical exercise, that is, fatigue develops. Skeletal muscle fibers fatigue acutely during highly intense exercise when they have to rely on anaerobic metabolism. Early stages of fatigue involve impaired myofibrillar function, whereas decreased Ca2+ release from the sarcoplasmic reticulum (SR) becomes more important in later stages. SR Ca2+ release can also become reduced with more prolonged, lower intensity exercise, and it is then related to glycogen depletion. Increased reactive oxygen/nitrogen species can cause long-lasting impairments in SR Ca2+ release resulting in a prolonged force depression after exercise. In this article, we discuss molecular and cellular mechanisms of the above fatigue-induced changes, with special focus on multiple mechanisms to decrease SR Ca2+ release to avoid energy depletion and preserve muscle fiber integrity. We also discuss fatigue-related effects of exercise-induced Ca2+ fluxes over the sarcolemma and between the cytoplasm and mitochondria.
The contractile function of skeletal muscle fibers declines during intense or prolonged physical exercise, that is, fatigue develops. Within the muscle fibers, fatigue is generally related to increased energy demands, in which effective ATP resynthesis is needed to match the dramatically increased ATP consumption during contractions. In contracting muscle fibers, ATP is mainly consumed by the molecular motors—the actomyosin cross-bridges; ion pumps—the sarcoplasmic reticulum (SR) Ca2+-pumps (SERCA); and, to a minor degree, the sarcolemmal Na+-K+-pumps. Adequate ATP delivery to these ATP-consuming proteins is essential for normal cell function and integrity, because depletion of ATP would have devastating consequences: constantly attached, noncycling cross-bridges and rigor development; insufficient SR Ca2+ pumping leading to an uncontrolled increase in the free cystolic [Ca2+] ([Ca2+]i); and inadequate maintenance of Na+ and K+ gradients over the sarcolemma resulting in impaired action potential propagation and muscle fibers eventually becoming inexcitable. Obviously, mechanisms to prevent these catastrophic consequences of ATP depletion exist within the muscle fibers. These mechanisms involve, on the one hand, effective metabolic systems to resynthesize ATP and, on the other hand, a fatigue-induced decline in ATP consumption. The latter fatigue mechanisms, which inhibit contraction-dependent ATP consumption, are a major focus of this review.
The activation of skeletal muscle fibers starts at the neuromuscular junction, where an action potential arriving at the presynaptic terminal of an α-motor neuron initiates the release of acetylcholine, which binds to receptors on the muscle fiber and subsequently triggers a sarcolemmal action potential. This action potential propagates along the surface of the muscle fiber and also into the t-tubular system, in which it activates the t-tubular voltage sensors, the dihydropyridine receptors (DHPRs). DHPR then activates the SR Ca2+ release channels, the ryanodine receptors type 1 (RyR1), whereby Ca2+ is released into the cytosol and [Ca2+]i increases. Ca2+ subsequently binds to the actin filament-regulating proteins, the troponin–tropomyosin complex. This complex undergoes conformational changes allowing the myosin heads to attach to actin, which initiates the ATP-dependent cross-bridge cycling and the muscle fiber contracts. Ca2+ is constantly pumped back into the SR by the ATP-consuming SERCA. [Ca2+]i, therefore, declines rapidly when α-motor neuron activation ceases and SR Ca2+ release stops, and the muscle fiber relaxes. Thus, strict control of intracellular Ca2+ fluxes is of fundamental importance for muscle fiber function, especially during stressful situations such as intense physical exercise.
Muscle fibers are acutely fatigued (i.e., within less than a minute to a few minutes) during intense contractile activity when the energy consumption exceeds the aerobic capacity and a large fraction of the required energy has to come from anaerobic metabolism. Accordingly, slow-twitch type 1 muscle fibers consume ATP relatively slowly, have a high aerobic capacity, and are therefore generally more fatigue resistant than fast-twitch type 2 muscle fibers (for reviews, see Allen et al. 2008; Wilson et al. 2012). Moreover, fatigue occurs more rapidly under hypoxic conditions, for example, at high altitude (Fan and Kayser 2016), when blood flow is restricted (Loenneke et al. 2014), or because of the limited O2 diffusion in in vitro experiments performed on isolated whole muscle (Zhang et al. 2006). A fundamental effect of endurance training is increased aerobic capacity and, hence, increased fatigue resistance. It is frequently proposed that a major component behind the increased fatigue resistance with endurance training is a switch toward more slow-twitch type 1 fibers, that is, a shift from type 2 to type 1 myosin heavy chain isoforms. Although some studies have shown an increase in the proportion of type 1 fibers with endurance training (Jansson et al. 1978; Howald et al. 1985), most studies did not detect any endurance training–induced shift between the major fiber types (for reviews, see Harridge 2007; Wilson et al. 2012). Thus, a shift from type 2 to type 1 fibers rarely occurs with endurance training and the improved performance is mainly because of increased aerobic capacity within the preexisting major fiber types (Harridge 2007; Westerblad et al. 2010).
A dominating ATP-consuming process in resting muscle fibers is protein synthesis and the global protein synthesis is blunted in working muscle (Rose and Richter 2009). The decreased protein synthesis will tend to reduce ATP consumption during exercise, but the overall impact is small because the ATP consumed by cross-bridges and SERCA during contractions is several-fold higher than that of protein synthesis (Rose and Richter 2009).
Glycogen, a branched polymer containing thousands of glucose residues, serves as a readily mobilized energy store in skeletal muscle. These glycogen stores can become depleted during prolonged physical exercise (i.e., many minutes to a few hours) and there is strong correlation between glycogen depletion and fatigue-induced decline in exercise performance (Bergström et al. 1967; Hermansen et al. 1967; Ørtenblad et al. 2013).
The production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) is considered to increase during physical exercise and these highly reactive molecules have been implicated in fatigue development. In addition, force recovery after fatiguing exercise can be very slow and even take days to be completed and ROS/RNS appear to have key roles in the induction of this severely delayed recovery (Bruton et al. 2008; Cheng et al. 2016). Intriguingly, an extra intake of antioxidants has been shown to hamper beneficial effects of endurance training (Gomez-Cabrera et al. 2008; Ristow et al. 2009; Paulsen et al. 2014) and there might be common Ca2+-dependent mechanisms underlying the ROS/RNS-related delayed recovery and the triggering of adaptive responses to endurance training (Place et al. 2015; Cheng et al. 2016).
In this article, we provide a concise overview of molecular and cellular aspects of exercise-induced skeletal muscle fatigue and recovery. We first discuss mechanisms behind acute fatigue with particular focus on the declining SR Ca2+ release in severely fatigued muscle fibers, which prevents fibers from the devastating consequences of ATP depletion. Next, we discuss the role of Ca2+ and ROS/RNS on the long-lasting force depression often seen after physical exercise and its relation to adaptations induced by endurance training. Finally, we discuss functional effects of exercise-induced Ca2+ fluxes over the sarcolemma and between the cytoplasm and mitochondria. The interest in these Ca2+ fluxes is increasing in view of the recent discoveries of key components: (1) the SR Ca2+ sensor, the stromal-interacting molecule 1 (STIM1), which activates the sarcolemmal Orai1 Ca2+ channel and which thereby controls store-operated Ca2+ entry (SOCE) (for recent reviews, see Cully and Launikonis 2013; Pan et al. 2014), and (2) the mitochondrial Ca2+ uniporter (MCU) and its associated regulating proteins that control mitochondrial Ca2+ uptake (for recent reviews, see Marchi and Pinton 2014; Williams et al. 2015; De Stefani et al. 2016).
ACUTE FATIGUE AND ITS DEPENDENCY ON ANAEROBIC METABOLISM
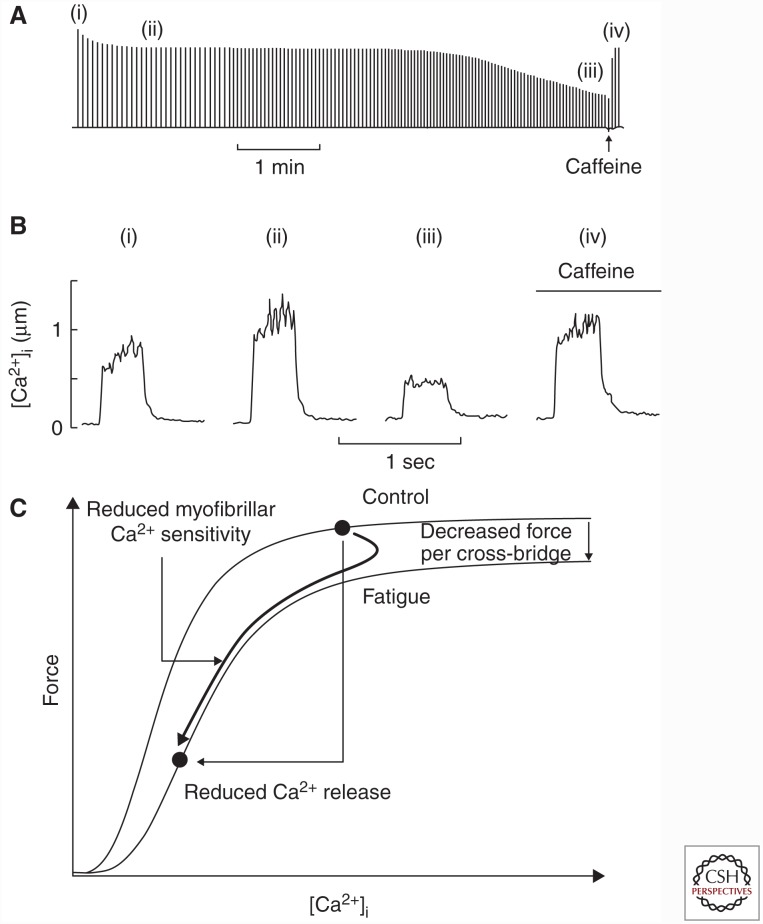
Fatigue develops rapidly during physical activities requiring a rate of ATP production that exceeds the aerobic capacity of the muscle fibers. Thus, this type of fatigue is closely related to the need for ATP produced by anaerobic metabolism. Experiments on single muscle fibers have revealed three components underlying the force decrease during this type of acute fatigue: decreased ability of the actomyosin cross-bridges to generate force, reduced myofibrillar Ca2+ sensitivity, and decreased SR Ca2+ release (Fig. 1) (Westerblad and Allen 1991; Allen et al. 2008). The first two of these components relate to impaired myofibrillar function and occur early during fatiguing stimulation. The third component, decreased SR Ca2+ release, generally becomes important in later stages of fatigue and will be discussed in more detail in the following sections.
Figure 1.
Three mechanisms underlying the force decrease during fatigue induced by repeated tetanic stimulation. Typical pattern of force decline (A), and selected [Ca2+]i records (B) obtained during fatigue induced by repeated tetanic stimulation in mouse flexor digitorum brevis (FDB) intact single fibers. Tetanic force initially decreases while tetanic [Ca2+]i increases (i to ii); hence, the initial force decline is caused by impaired myofibrillar function. The final force decrease is caused by reduced sarcoplasmic reticulum (SR) Ca2+ release (ii to iii). Application of caffeine facilitates SR Ca2+ release and the resulting increase in tetanic [Ca2+]i leads to a marked force increase (iv). (A and B adapted, with permission, from Lännergren and Westerblad 1991 and Westerblad and Allen 1991, respectively.) (C) The relation between force and [Ca2+]i during fatigue induced by repeated tetanic stimulation (thick line) plotted together with the force–[Ca2+]i relationship in the unfatigued state and during fatigue (thin lines). This assessment shows that acute fatigue involves initial decreases in force per cross-bridge and myofibrillar Ca2+ sensitivity, which are followed by decreased SR Ca2+ release.
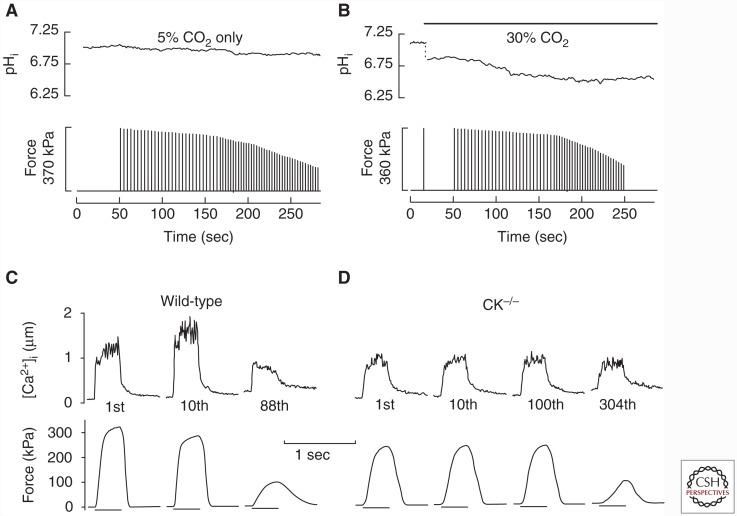
Anaerobic metabolism leads to accumulation of lactate and hydrogen ions, mainly because of glycogen breakdown, and increased creatine and inorganic phosphate (Pi) ions, because of creatine kinase (CK)-dependent phosphocreatine breakdown (Westerblad et al. 2010). Lactate and creatine ions have no major impact on myofibrillar contractile function (Posterino et al. 2001; Murphy et al. 2004), whereas increased cytoplasmic concentrations of both H+ (i.e., reduced pH or acidosis) and Pi have been shown to impair myofibrillar contractile function. Traditionally, “lactic acid” was considered the major cause of acute fatigue, but this viewpoint has been challenged. Many studies show a good temporal correlation between the extent of acidosis and the fatigue-induced decrease in contractile function (Cady et al. 1989; Kent-Braun 1999), but several pieces of evidence indicate that this correlation might not be causative; for instance, (1) studies have shown an early force decline during intense sustained voluntary contraction that was accompanied by alkalosis and the force recovery after fatiguing contractions can occur despite continued acidosis (Sahlin and Ren 1989; Degroot et al. 1993), (2) the force decline during fatiguing contractions was not faster when human muscle or isolated mouse flexor digitorum brevis (FDB) fibers were acidified before induction of fatigue (Fig. 2A,B) (Wilson et al. 1988; Bruton et al. 1998), and (3) at physiological temperatures, acidosis had little effect on maximum force production and shortening velocity in skinned muscle fibers (Pate et al. 1995), intact mouse muscle fibers (Westerblad et al. 1997), and whole mouse muscles (Wiseman et al. 1996). Thus, increased [Pi] rather than acidosis has been proposed to be the dominant cause of the declining force production during acute fatigue (Fig. 2C,D) (Dahlstedt et al. 2001; Westerblad et al. 2002; Allen et al. 2011). However, the role of acidosis in acute fatigue remains controversial and a major unresolved issue is whether the force-reducing effects of elevated [Pi] in fatigue are amplified by the concomitant acidosis (Fitts 2016; Westerblad 2016).
Figure 2.
Acidosis has no obvious effect on fatigue development, whereas fatigue is delayed when Pi accumulation is limited by inhibition of phosphocreatine breakdown. Representative records from isolated mouse flexor digitorum brevis (FDB) intact single fibers fatigued by repeated tetanic contractions. Fibers fatigued at the same rate under normal conditions (A) and after being acidified by ∼0.4 pH unit by bubbling the bath solution with 30% instead of 5% CO2 (B). (A and B adapted, with permission, from Bruton et al. 1998.) (C,D) Fatigue developed much faster in wild-type fibers than in creatine kinase–deficient (CK−/−) fibers, which cannot break down phosphocreatine and hence the accompanying increase in Pi is prevented. Representative records obtained in the first, tenth, and last (88th) fatiguing tetanic contraction in a wild-type fiber; the CK−/− fiber showed no fatigue during 100 tetanic contraction. (C and D adapted, with permission, from Dahlstedt et al. 2000.)
During most types of fatigue-inducing exercise, cytoplasmic [ATP] is held relatively constant as ATP consumption is matched by ATP resynthesis by the combined action of the above discussed anaerobic sources and mitochondrial oxidative phosphorylation (Bangsbo et al. 1996; Sahlin et al. 1998; Walter et al. 1999; Westerblad et al. 2010). However, under circumstances in which very intense exercise is performed (e.g., 25 sec of maximal effort cycling), [ATP] can decrease by up to 80% in individual fast-twitch type 2 fibers, reaching values as low as 0.7–1.7 mm (Karatzaferi et al. 2001). A reduction in [ATP] is accompanied by increases in ATP breakdown products, such as ADP, AMP, and IMP. Furthermore, Mg2+ in the cytosol is mostly bound to ATP, and a net ATP breakdown during fatigue causes an increase in the free cytoplasmic [Mg2+] ([Mg2+]i) (Westerblad and Allen 1992; Dahlstedt and Westerblad 2001). Thus, any or all of these metabolic changes could affect myofibrillar force generation in skeletal muscle.
During cross-bridge cycling, ATP is required to detach myosin from actin following a cross-bridge power stroke. However, a decrease in [ATP] does not have any marked effects on muscle contractility until falling below ∼0.5 mm, at which point it mostly affects contractile speed and submaximal forces (Dutka and Lamb 2004; Cooke 2007). ADP acts as a weak competitor to ATP binding on the catalytic site on myosin and increased ADP will increase rather than decrease isometric force, while it slows contractions (Cooke 2007). This is supported by observations in muscle fibers from adenylate kinase–deficient mice in which [ADP] was shown to increase to ∼1.5 mm during repeated contractions, which is 20–30 times greater than typically measured during fatigue, and this was accompanied by a tendency of increased isometric force and a marked slowing of relaxation (Hancock et al. 2005). Furthermore, modeling based on the decrease in maximum shortening velocity in severely fatigued single mouse muscle fibers indicates that [ADP] may transiently increase to ∼1.5 mm during tetanic contraction when the ATP-buffering phosphocreatine store has been depleted (Westerblad et al. 1998). Cytosolic concentrations of [Mg2+]i that can be reached during fatigue (<3 mm) do not affect actomyosin cross-bridge properties in skeletal muscle (Swenson et al. 2014). Thus, the reduction in [ATP] and accompanying changes observed during muscle fatigue may have some relatively minor effects on the actomyosin interaction.
REDUCED SR Ca2+ RELEASE
As discussed above, intrinsic mechanisms must exist to prevent muscle fibers from depleting their ATP stores during high-intensity exercise. The most effective way of reducing the energy consumed by contracting muscle fibers is to inhibit SR Ca2+ release, which reduces the number of ATP-consuming cycling cross-bridges and lessens the demand on ATP-dependent SR Ca2+ reuptake by SERCA. However, reducing SR Ca2+ release comes with the price of declining muscle performance, that is, fatigue gets more severe. We will next discuss mechanisms by which SR Ca2+ might be reduced during acute fatigue.
Acidosis
Early studies showed a marked inhibition of the open probability of isolated SR Ca2+ release channels, RyR1, when pH was lowered to values observed in fatigued muscle fibers (Ma et al. 1988). However, later studies observed much smaller inhibitory effects of acidosis on the activation of isolated RyR1 (Laver et al. 2000). Furthermore, the normal action potential–induced SR Ca2+ release was little, if at all, affected by lowering pH to values observed in acute fatigue (Westerblad and Allen 1993; Lamb and Stephenson 1994; Bruton et al. 1998). Thus, acidosis is not important for the decrease in SR Ca2+ release in acute fatigue. In fact, recent results even show that intracellular acidosis can have a protective effect during fatigue by decreasing the permeability of chloride channels, which would help preserve muscle excitability (Pedersen et al. 2004); this will be discussed in more detail below.
Increased Pi
In addition to the effect at the myofibrillar level, an increase in [Pi] has been proposed to affect SR Ca2+ release during acute fatigue. Experiments on intact isolated frog, mouse, and rat muscle fibers show a similar pattern of changes in tetanic [Ca2+]i during fatigue induced by repeated brief tetani. An initial increase followed by a decrease and the lag between the two depends on the fatigue resistance (Allen et al. 1989; Westerblad and Allen 1991; Lunde et al. 2001). CK and elevated [Pi] have a central role in this pattern of [Ca2+]i changes because the initial increase is blunted and the secondary decrease is severely delayed with CK inhibition induced either pharmacologically or genetically (CK−/− muscles) (Dahlstedt et al. 2000; Dahlstedt and Westerblad 2001). Moreover, when the CK deficiency of CK−/− fibers was acutely reversed by CK injection, the normal changes in tetanic [Ca2+]i during fatigue reappeared, that is, an early increase followed by a decrease (Dahlstedt et al. 2003). In the following two paragraphs, we discuss two mechanisms by which increased [Pi] may decrease SR Ca2+ release: Pi-induced inhibition of RyR1 and Ca2+-Pi precipitation within the SR.
Skinned fiber experiments have shown an inhibitory effect of increased [Pi] on depolarization-induced SR Ca2+ release, which was attributed to impaired activation of RyR1 (Duke and Steele 2001; Steele and Duke 2003). This Pi-induced inhibitory effect was markedly larger at the [Mg2+]i of fatigued (∼1.6 mm) than of rested (∼0.8 mm) mammalian muscle fibers (Westerblad and Allen 1992); this fits with a relationship between decreasing tetanic [Ca2+]i and increasing [Mg2+]i during fatigue under normal conditions, and which is completely lost with pharmacological CK inhibition (Dahlstedt and Westerblad 2001).
The free [Ca2+] in the SR is assumed to be ∼1 mm, and the in vitro measured Ca2+-Pi solubility product is ∼6 mm2 (Fryer et al. 1995). When [Pi] increases during fatigue, some Pi may enter the SR and if the Ca2+-Pi solubility product is exceeded, precipitation occurs and the Ca2+ available for release decreases (Fryer et al. 1995). This proposed SR Ca2+-Pi precipitation has not been directly shown, but indirect evidence exists both from experiments on skinned fibers with intact SR exposed to high [Pi] (Fryer et al. 1995; Duke and Steele 2001; Dutka et al. 2005) and intact fibers injected with Pi (Westerblad and Allen 1996). Several fatigue experiments also support the Ca2+-Pi precipitation mechanism. For instance, the [Ca2+]i response to a high dose of caffeine or 4-chloro-m-cresol (compounds that directly stimulate RyR1) was reduced in fatigued mouse and toad muscle fibers, which indicates a reduction of Ca2+ available for rapid release (Westerblad and Allen 1991; Kabbara and Allen 1999). Accordingly, estimates of the free [Ca2+] in the SR using low-affinity Ca2+ indicators loaded into the SR show a decline during fatiguing stimulation and a subsequent recovery in both isolated toad muscle fibers and mouse muscle studied in situ (Kabbara and Allen 2001; Allen et al. 2011). The recovery of the rapidly releasable SR Ca2+ pool occurred in the absence of extracellular Ca2+, that is, Ca2+ did not disappear from the cells during fatigue. On the other hand, the recovery was blocked by inhibition of mitochondrial respiration, showing that recovery of releasable SR Ca2+ depended on aerobic metabolism (Kabbara and Allen 1999).
Reduced ATP
RyR1 opening is strongly stimulated by ATP binding, its breakdown products ADP and AMP act as weak competitive agonists, and Mg2+ is a potent inhibitor (Meissner et al. 1986; Lamb and Stephenson 1994; Laver et al. 2001b; Dutka and Lamb 2004). However, rather large changes are required to induce a substantial inhibition of voltage-activated RyR1 Ca2+ release; for instance, cytoplasmic [ATP] has to be reduced to 0.5 mm to get an ∼20% decrease in SR Ca2+ release, and increasing [Mg2+]i to 3 mm reduces release by ∼40% (Dutka and Lamb 2004). Nevertheless, the combination of changes related to declining [ATP] may decrease SR Ca2+ release during very intense physical activities. For instance, the transient decrease in tetanic [Ca2+]i observed at the onset of high-intensity stimulation of CK−/− muscle fibers, where phosphocreatine cannot be used as a rapid energy buffer, was likely the result of the combined inhibitory actions of reduced [ATP] and increased [Mg2+]i on RyR1 opening (Dahlstedt et al. 2000).
Impaired Action Potential Propagation
Repeated firing of action potentials during physical exercise leads to changes in intra- versus extracellular Na+ and K+ concentrations, which may impair sarcolemmal excitability. For each action potential, there is an influx of Na+ during the depolarization phase and an efflux of K+ in the subsequent repolarization phase. The resulting disturbance in Na+ and K+ membrane gradients is counteracted by the sarcolemmal Na+-K+-pumps, which actively transport Na+ out of and K+ in to the cell. Nevertheless, marked changes in the concentration of these ions occur during intense exercise (McKenna et al. 2008). For instance, the interstitial (extracellular) K+ concentration can increase from ∼4 mm at rest to above 10 mm during high-intensity exercise (Juel et al. 2000; Mohr et al. 2004). In rested muscle fibers, such a change in the K+ concentration gradient over the sarcolemma results in impaired action potential propagation and, consequently, decreased force production. However, these problems are counteracted during exercise, for example, by the electrogenic effects of Na+-K+ pumps (McKenna et al. 2008). Moreover, Nielsen et al. (2001) showed that the impaired excitability and severely (∼75%) decreased force production induced by exposing isolated rat muscles to increased K+ (11 mm) were almost completely reversed by acidification. This protective effect of acidosis was subsequently shown to be mediated via inhibition of sarcolemmal Cl− (ClC-1) channels (Pedersen et al. 2004, 2005). These Cl− channels account for 80%–90% of the passive sarcolemmal conductance, which tends to clamp the membrane at the resting potential and limit the Na+ current-induced depolarization at the onset of action potentials. Thus, inhibition of ClC-1 channels facilitates the Na+ current-induced depolarization and protects against action potential failure (Pedersen et al. 2016). During early stages of fatigue, ClC-1 channels are inhibited by protein kinase C–mediated phosphorylation, and by acidosis, which counteracts the reduced excitability caused by a reduced K+ gradient over the sarcolemma (Pedersen et al. 2009a,b; de Paoli et al. 2013).
Opposite to the situation in early fatigue, the passive sarcolemmal conductance has been found to increase in late stages of fatigue in fast-twitch fibers, which promotes action potential failure (Pedersen et al. 2009b). This increased conductance was not observed in slow-twitch fibers, which indicates that it is controlled by energy metabolic factors. Moreover, experiments with pharmacological inhibitors indicate that the increased conductance is the result of parallel activation of ClC-1 channels and ATP-sensitive K+ (KATP) channels (Pedersen et al. 2009a; de Paoli et al. 2013). The latter channels are, as the name suggests, controlled by ATP and stay in a closed state until [ATP] is decreased below ∼2 mm (Spruce et al. 1985). There has been considerable interest in the possibility that KATP channels play an important role in the exercise-induced reduction in muscle force, but this remains uncertain because diverging results have been obtained both with pharmacological and genetic approaches (for review, see Flagg et al. 2010). Nevertheless, parallel opening of ClC-1 and KATP channels in late stages of fatigue constitutes a metabolically controlled mechanism by which SR Ca2+ release can be inhibited to prevent ATP depletion.
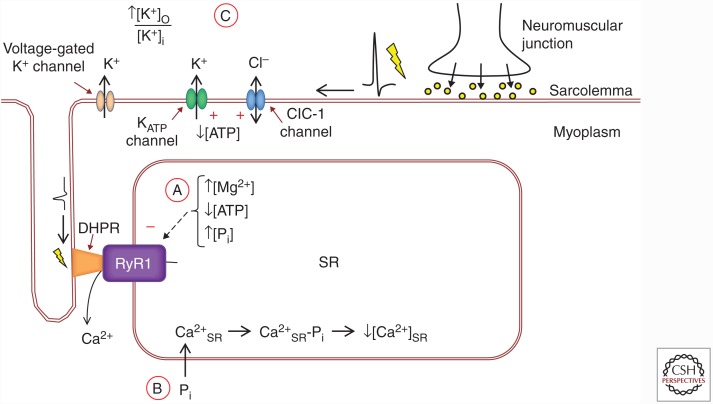
To sum up, several mechanisms may inhibit SR Ca2+ release in acute fatigue to prevent deleterious effects of ATP depletion (Fig. 3): inhibition of RyR1 caused by elevated [Pi], increased [Mg2+]i, and/or decreased [ATP]; Ca2+-Pi precipitation in the SR; decreased excitability because of a reduced K+ gradient over the sarcolemma and opening of ClC-1 and KATP channels. How these mechanisms interact and their relative importance in different types of fatiguing exercise remain to be established. Nevertheless, the fact that several mechanisms exist shows that the ability to limit SR Ca2+ release in situations of severe metabolic stress is an essential property of skeletal muscle fibers.
Figure 3.
Cellular mechanisms of decreased sarcoplasmic reticulum (SR) Ca2+ release in acute fatigue. (A) Increases in the cytoplasmic concentration of Mg2+ and Pi, as well as decreased ATP, can directly inhibit action potential-DHPR-mediated RyR1 opening. (B) When myoplasmic [Pi] increases, some Pi can enter the SR and form Ca2+-Pi precipitates that reduce the free Ca2+ available for release ([Ca2+]SR). (C) Action potential propagation can be impaired by extracellular K+ accumulation, as well as increased opening of Cl− (ClC-1) and ATP-sensitive K+ (KATP) channels. Red plus and minus symbols indicate excitatory or inhibitory influence on channels, respectively. DHPR, Dihydropyridine receptor; RyR1, ryanodine receptor 1; [K+]o, extracellular [K+]; [K+]i, intracellular [K+].
PROLONGED EXERCISE AND GLYCOGEN DEPLETION
One of the first studies using the needle biopsy technique to obtain samples of human skeletal muscle showed a marked muscle glycogen depletion after prolonged (∼2 h) cycling exercise and the time until exhaustion was correlated to the preexercise glycogen concentration (Bergström et al. 1967). Since then, many studies have confirmed that exercise capacity is severely compromised when muscle glycogen content is depleted to very low levels (Ørtenblad et al. 2013). However, the exact mechanism(s) by which glycogen depletion causes muscle fatigue remains uncertain.
Recent studies using electron microscopy imaging have shown that glycogen is preferentially located in three distinct subcellular compartments: subsarcolemmal, intermyofibrillar (i.e., between myofibrils), and intramyofibrillar (i.e., within the contracting myofibrils) (Marchand et al. 2002; Nielsen et al. 2009; Ørtenblad et al. 2013). Although the exact role of the different pools of glycogen is not fully understood, electron microscopy images show a preferential depletion of intramyofibrillar glycogen in fatigued muscle fibers from rodents (Nielsen et al. 2014, 2009) and humans (Marchand et al. 2007; Nielsen et al. 2011; Ørtenblad et al. 2011). Moreover, a correlation between reduced SR Ca2+ release and depletion in intramyofibrillar and, to some extent, intermyofibrillar glycogen was observed after repeated tetanic stimulation of intact single mouse muscle fibers (Nielsen et al. 2014). Thus, intramyofibrillar glycogen is preferentially reduced during fatigue and this is associated with decreased SR Ca2+ release and, hence, reduced force production. The exact mechanism(s) by which depletion of intramyofibrillar glycogen leads to decreased SR Ca2+ remains to be established. Future studies are also needed to elucidate links between the above-discussed mechanisms of reduced SR Ca2+ release in acute fatigue and localized glycogen depletion. For instance, the Pi-related impairments in myofibrillar function induced by repeated tetanic stimulation occur early during fatigue, whereas the reduced SR Ca2+ release attributed to Ca2+-Pi precipitation comes with a delay (Dahlstedt et al. 2003; Allen et al. 2008). One possible explanation for this delay is that Pi enters the SR via small conductance chloride channels that show an increased open probability at low [ATP] (Laver et al. 2001a), which might occur in their vicinity when intramyofibrillar glycogen becomes depleted (Nielsen et al. 2014). Similar reasoning can also be applied to the delayed opening of ClC-1 and KATP, which results in increased passive sarcolemmal conductance and impaired excitability.
REACTIVE OXYGEN/NITROGEN SPECIES
ROS/RNS are molecules with unpaired valence electron(s), which makes them highly reactive (Halliwell and Gutteridge 1984). Their effects are complex and depend on several factors: the type of ROS/RNS; the magnitude, duration, and location of production; and the defense systems consisting of both endogenous and exogenous antioxidants. It is generally accepted that the production of ROS/RNS increases during most types of physical activities (Powers and Jackson 2008), although exercise-induced increases in ROS/RNS are generally difficult to measure largely because of methodological limitations (Cheng et al. 2016). For instance, fluorescent indicators used to measure cellular ROS/RNS show relatively small increases in fluorescence in response to repeated contractions (e.g., Reid et al. 1992; Pye et al. 2007; Sakellariou et al. 2013; Cheng et al. 2015), which makes it difficult to detect ROS/RNS changes in a quantitative and spatially/temporally efficient way. Because of these problems, methods based on high-pressure liquid chromatography (HPLC) have been recommended (Kalyanaraman et al. 2012), but such methods are impractical when following ROS/RNS over time, for example, during exercise and the subsequent recovery. Recently developed indicators based on genetically engineered fusion proteins may improve the situation, and a major attraction with these is that they can be targeted to specific organelles or proteins (Hanson et al. 2004; Gutscher et al. 2009; Meyer and Dick 2010; Pal et al. 2013).
The primary ROS in cells are the superoxide anion (O2•−) and its downstream derivatives, such as hydrogen peroxide (H2O2). Mitochondria are traditionally considered to be the major site for O2•− production in contracting skeletal muscle and this viewpoint is, for instance, supported by recent studies employing either mitochondrial-targeted O2•− indicator or antioxidant (Wei et al. 2011; Cheng et al. 2015). On the other hand, other recent studies propose NADPH-oxidase 2 (NOX2) as the main ROS source in contracting skeletal muscles, because the contraction-mediated ROS increase was prevented by pharmacological inhibition or genetic knockdown of NOX2 (Michaelson et al. 2010; Pal et al. 2013; Sakellariou et al. 2013). Thus, the relative importance of different sources of O2•− in contracting muscle remains uncertain.
The primary RNS in cells are nitric oxide (NO•) along with its downstream derivatives, such as peroxynitrite (ONOO•−). The production of NO• increases in muscle fibers during repeated contractions (Pye et al. 2007; Cheng et al. 2015), mainly by increased neuronal nitric oxide synthase activity (Balon and Nadler 1994; Kobzik et al. 1994; Hirschfield et al. 2000).
Clear positive effects of decreased ROS/RNS on endurance have been shown in human studies in which fatigue was produced with submaximal contractions (Reid et al. 1992, 1994; Khawli and Reid 1994; Powers et al. 2011). Conversely, the effect is small or absent with high-intensity exercise and near-maximum contractions (Fig. 4A–D) (Reid et al. 1992; Matuszczak et al. 2005; Powers et al. 2011; Cheng et al. 2015), because in this case the above-discussed “classical” fatigue mechanisms probably dominate (Allen et al. 2008). The positive effects of reducing ROS/RNS during repeated submaximal contractions fit with the fact that acute effects of exogenously applied ROS/RNS are most marked on the steep part of the force–Ca2+ relationship, where small changes in myofibrillar Ca2+ sensitivity or SR Ca2+ release have large effects on force production (Lamb and Westerblad 2011; Cheng et al. 2016). However, the effects of ROS/RNS are highly complex and the results of some studies even imply that reducing ROS/RNS during fatigue would impair rather than improve performance. For instance, acute exogenous application of H2O2 results in a transient increase in myofibrillar Ca2+ sensitivity (Andrade et al. 1998, 2001; Cheng et al. 2015) and some skinned fiber experiments show increased rather than decreased myofibrillar Ca2+ sensitivity after fatiguing contractions (Gejl et al. 2015; Watanabe et al. 2015).
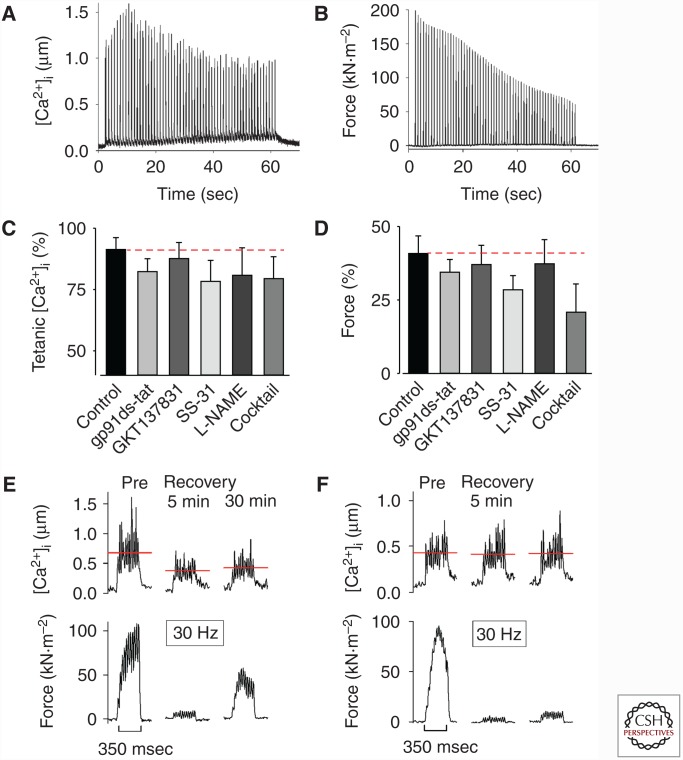
Figure 4.
The decrease in tetanic [Ca2+]i and force during fatigue induced by repeated tetanic contractions was not affected by the presence of reactive oxygen species (ROS)/reactive nitrogen species (RNS)-modulating compounds, whereas the delayed recovery process is ROS/RNS-sensitive. Typical fatigue profiles of [Ca2+]i (A), and force (B) from an intact single flexor digitorum brevis (FDB) fiber stimulated with repeated tetanic contractions. Relative tetanic [Ca2+]i (C), and force (D) at the end of fatiguing stimulation in standard Tyrode solution (control) compared with exposure to the NADPH-oxidase 2 (NOX-2) inhibitor gp91ds-tat; the NOX-4 inhibitor GKT137831; the mitochondrial-targeted antioxidant SS-31; the nitric oxide synthase (NOS) inhibitor L-NAME; and antioxidant-NOS-inhibitor cocktail. Data are mean ± SEM; dashed red lines indicate no difference from control. One-way ANOVA showed no difference between groups for either [Ca2+]i or force. Representative records of [Ca2+]i (upper row) and force (lower row) obtained in one control fiber (E) and one fiber superfused with mitochondrial-targeted antioxidant SS-31 (F) and stimulated at submaximal frequency (30 Hz) before (Pre) and 5 and 30 min after fatiguing stimulation. Note that the prolonged low-frequency force depression (PLFFD) was related to decreased tetanic [Ca2+]i in control but not in the presence of SS-31 (indicated by red dashed lines). (Adapted, with permission, from Cheng et al. 2015.)
After fatiguing exercise, muscle fibers frequently enter a prolonged state of severely depressed submaximal force, that is, prolonged low-frequency force depression (PLFFD) (Allen et al. 2008). At the muscle fiber level, depressed submaximal force can be caused by decreased SR Ca2+ release and/or reduced myofibrillar Ca2+ sensitivity. Acutely fatigued fast-twitch fibers of wild-type mice enter a marked PLFFD that is mainly caused by decreased SR Ca2+ release (Westerblad et al. 1993). On the other hand, the cause of PLFFD changes toward reduced myofibrillar Ca2+ sensitivity in genetically modified mouse FDB fibers overexpressing the mitochondrial matrix redox enzyme superoxide dismutase 2 (SOD2, converts O2•− to H2O2) (Bruton et al. 2008), in rat FDB fibers that endogenously express more SOD2 than mouse FDB fibers (Bruton et al. 2008), and in mouse FDB fibers treated with the mitochondria-targeted antioxidant SS-31 or the nitric oxide synthase inhibitor L-NAME (Fig. 4E,F) (Cheng et al. 2015). We recently exposed human subjects to one session of high-intensity interval training (six Wingate tests, i.e., 6 × 30 sec all-out cycling with 4 min rest in between) (Place et al. 2015). This exhaustive exercise caused a marked PLFFD accompanied by an unexpected RyR1 fragmentation in vastus lateralis muscles of recreationally active subjects. A similar PLFFD was induced in muscles of elite endurance athletes, but the RyR1 remained intact. The elite endurance athletes had higher levels of the antioxidant enzymes SOD2 and catalase in their muscles than recreationally active subjects. Furthermore, RyR1 fragmentation was induced by high-intensity stimulation of isolated mouse muscles and this fragmentation was blocked by the antioxidant N-acetyl cysteine (Place et al. 2015). To sum up, accumulation of O2•−, or ONOO•−, preferentially impairs SR Ca2+ release, likely via redox modifications of RyR1 (Bellinger et al. 2008a,b; Andersson et al. 2011; Lanner et al. 2012). On the other hand, decreased myofibrillar Ca2+ sensitivity is the major problem when O2•− is more effectively metabolized to H2O2, or its downstream products. Thus, antioxidants do not prevent PLFFD, but they can change the underlying mechanism from impaired SR Ca2+ release to reduced myofibrillar Ca2+ sensitivity (Cheng et al. 2016).
An increase in baseline [Ca2+]i can stimulate mitochondrial biogenesis and hence lead to improved muscle endurance (Wright et al. 2007; Bruton et al. 2010). As described above, PLFFD in muscle of wild-type mice and recreationally active humans was accompanied by RyR1 modification, which can elevate baseline [Ca2+]i, and thereby trigger mitochondrial biogenesis and other beneficial adaptations (Bruton et al. 2010; Place et al. 2015). Thus, ROS/RNS-induced modifications in RyR1 structure and function offer a mechanism as to why treatment with antioxidants hamper the beneficial effects of endurance training (Gomez-Cabrera et al. 2008; Ristow et al. 2009; Paulsen et al. 2014; Merry and Ristow 2016). However, RyR1 modification can be a double-edged sword and severe modifications, for example, a major RyR1 depletion of the associated stabilizing protein FKBP12, have been linked to muscle weakness after strenuous contractile activity (Aydin et al. 2008; Bellinger et al. 2008b), during aging (Andersson et al. 2011), or in various pathological conditions (Bellinger et al. 2008a; Waning et al. 2015).
Ca2+ FLUXES OVER THE SARCOLEMMA
The contractile machinery in skeletal muscle fibers is activated by Ca2+ ions being released from the SR. The absolute majority of the Ca2+ ions being released are subsequently pumped back into the SR by the SERCA (Balnave and Allen 1998; Cully and Launikonis 2013). However, a small amount of the released Ca2+ will leave the cell, mainly via the Na+-Ca2+ exchanger and plasma membrane Ca2+-ATPase (Hidalgo et al. 1986; Balnave and Allen 1998). This efflux of Ca2+ has to be countered by a Ca2+ influx to avoid Ca2+ depletion during physical activities. Accordingly, the rate of Ca2+ influx is increased during prolonged contractile activities and, if anything, the total muscle Ca2+ content increases (Everts et al. 1993; Gissel and Clausen 1999).
During contractions, SR Ca2+ release is initiated by action potential activation of DHPR, which in turn activates RyR1. DHPR, also called Cav1.1, is a voltage-dependent L-type Ca2+ channel that, because of its slow Ca2+ conductance, mainly acts as voltage sensors in skeletal muscle (Lamb 2000). Nevertheless, Ca2+ may enter muscle fibers via action potential activation of the DHPRs (Lee et al. 2015; Robin and Allard 2015).
Ca2+ can also enter muscle fibers via SOCE (Cully and Launikonis 2013; Pan et al. 2014). In this case, decreased SR [Ca2+] stimulates the intra-SR Ca2+ sensor, STIM1, which activates the sarcolemmal Orai1 Ca2+ channel mainly located in the t-tubular membrane. A recent study supports an important role of SOCE during fatiguing contractions: knockdown of STIM1 or expression of a dominant-negative Orai1 mutation in mouse FDB fibers accelerated the decline in [Ca2+]i during repeated tetanic stimulation; dominant-negative Orai1 mice displayed impaired performance in in vivo fatigue tests and muscles isolated from these mice fatigued more rapidly during repeated contractions (Wei-Lapierre et al. 2013). However, enhanced Ca2+ influx via SOCE induced by overexpressing STIM1 in mouse skeletal muscle resulted in severe pathological changes in muscle fibers resembling those in muscle dystrophy (Goonasekera et al. 2014). Thus, a fine-tuned balance between Ca2+ influx and efflux is essential for overall muscle function and integrity. For instance, increased efflux of Ca2+ during fatiguing contractions must be balanced by a similarly sized Ca2+ influx to keep the SR Ca2+ store intact. Accordingly, the decrease in tetanic [Ca2+]i observed during late stages of fatigue is caused by impaired SR Ca2+ release rather than cellular Ca2+ depletion (see Fig. 1A,B).
MITOCHONDRIAL Ca2+ UPTAKE
Mitochondria are located adjacent to the SR, and are tethered together with small (10 nm) linkages between the outer mitochondrial membrane and the SR (Boncompagni et al. 2009). The close proximity between mitochondria and SR implies important interactions between the two organelles. Ca2+ readily passes into the mitochondrial intermembrane space via the outer membrane voltage-dependent anion channel (VDAC) (Ben-Hail et al. 2014). Subsequently, Ca2+ can enter the mitochondrial matrix mainly via the mitochondrial calcium uniporter (MCU) (Kirichok et al. 2004; Baughman et al. 2011; De Stefani et al. 2011). Finally, Ca2+ is extruded from the mitochondrial matrix mainly by the mitochondrial Na+-Ca2+ exchanger (NCLX) (Palty et al. 2010). Thus, the free [Ca2+] in the mitochondrial matrix ([Ca2+]mito) is set by the dynamic balance between MCU Ca2+ entry and NCLX Ca2+ extrusion (Williams et al. 2015). To avoid deleterious effects of mitochondrial Ca2+ overload (Duchen 2000; Brookes et al. 2004), [Ca2+]mito must be tightly controlled and not simply reflect [Ca2+]i, which can increase ∼100-fold when skeletal muscle fibers contract. Accordingly, MCU is regulated by several inner mitochondrial membrane proteins, which either stimulate or inhibit Ca2+ entry (Williams et al. 2015). The existence of an intricate regulation of [Ca2+]mito is illustrated by studies with repeated tetanic contractions of mouse wild-type skeletal muscle fibers, where the repeated large increases in [Ca2+]i during contractions were accompanied by [Ca2+]mito remaining virtually constant (Lännergren et al. 2001; Bruton et al. 2003b; Aydin et al. 2009), increasing substantially in mitochondria close to the surface (Bruton et al. 2003a), or increasing substantially and globally (Rossi et al. 2011; Ainbinder et al. 2015).
Increased [Ca2+]mito is a known stimulator of mitochondrial respiration and ATP production (Brookes et al. 2004; Glancy et al. 2013). Accordingly, impaired performance in a treadmill running endurance test was observed in MCU-deficient mice (Pan et al. 2014). On the other hand, excessive mitochondrial matrix Ca2+ uptake induces apoptotic signaling and can even result in cell death (Duchen 2000; Brookes et al. 2004). This can be illustrated by the mouse myopathy model with skeletal muscle-specific disruption of mitochondrial transcription factor A (Tfam). In contrast to their controls, muscle fibers of the Tfam knockout mice showed a large increase in [Ca2+]mito during repeated contractions and this increase was partially inhibited by preexposure to cyclosporine A (Aydin et al. 2009). The muscle-specific Tfam knockout mice develop a progressive muscle weakness because of decreased SR Ca2+ stores and they die when they are ∼4 months old; treatment with cyclosporine A counteracted the development of muscle weakness by improving SR Ca2+ handling, and all treated mice were still alive at 4 months of age (Gineste et al. 2015). To sum up: (1) there are intricate, functionally important, and not yet fully understood interactions between SR and mitochondria, which involve bidirectional Ca2+-dependent signaling; and (2) [Ca2+]mito must be tightly controlled to maintain the fine balance between beneficial and deleterious Ca2+ effects.
There are still uncertainties whether mitochondrial Ca2+ uptake represents a substantial Ca2+ buffer in cells and in this way significantly influences transient changes in [Ca2+]i (Williams et al. 2013). Quantitative analyses and simulations indicate that mitochondrial Ca2+ uptake has no or very limited impact on [Ca2+]i transients under normal physiological conditions (Baylor and Hollingworth 2007; Williams et al. 2013), and this agrees with results obtained with repeated contractions of mouse soleus muscle fibers (Bruton et al. 2003a). Conversely, mitochondrial Ca2+ uptake appeared to affect tetanic [Ca2+]i in muscle fibers of Xenopus frogs (Lännergren et al. 2001), and the decreased mitochondrial Ca2+ uptake during contractions was accompanied by increased [Ca2+]i transients in mouse muscle fibers exposed to short-term knockdown of mitofusin-2, which participates in mitochondrial fusion (Ainbinder et al. 2015).
CONCLUDING REMARKS
Within skeletal muscle fibers, acute fatigue develops when fibers depend on anaerobic metabolism. Early stages of fatigue involve impairments in myofibrillar functions: decreased cross-bridge force-generating capacity and reduced Ca2+ sensitivity. Impaired SR Ca2+ release becomes more important in later stages of fatigue and protects against highly deleterious consequences of energy depletion. Several mechanisms can contribute to the decreased SR Ca2+ release induced by high-intensity fatiguing exercise (see Fig. 3). The reduction in SR Ca2+ release is related to glycogen depletion with more prolonged lower intensity exercise. Finally, increased ROS/RNS can cause prolonged impairments in SR Ca2+ release and/or myofibrillar Ca2+ sensitivity and, hence, prolonged force depression, which might protect muscle fiber integrity after extensive exercise. Increased ROS/RNS can also trigger beneficial adaptations to endurance exercise. Table 1 presents examples of exercises in which the above-described fatigue mechanisms might limit the performance.
Table 1.
Examples of exercises in which different fatigue mechanisms might limit performance
| Mechanism underlying impaired performance | Type of exercise |
|---|---|
| Decreased myofibrillar force-generating capacity | Three to five repetition maximum weight lifts |
| Decreased SR Ca2+ release caused by increased [Pi] | 400–800 m running; 100–200 m swimming |
| Decreased SR Ca2+ release caused by reduced [ATP] and increased [Mg2+]i | All-out Wingate cycling |
| Impaired action potential propagation | Sustained maximum voluntary contraction; neuromuscular electrical stimulation (NMES) |
| Muscle glycogen depletion | Half-marathon and marathon running; 50 km cross-country skiing |
| Increases in ROS/RNS | Recovery after high-intensity or prolonged exhaustive exercises |
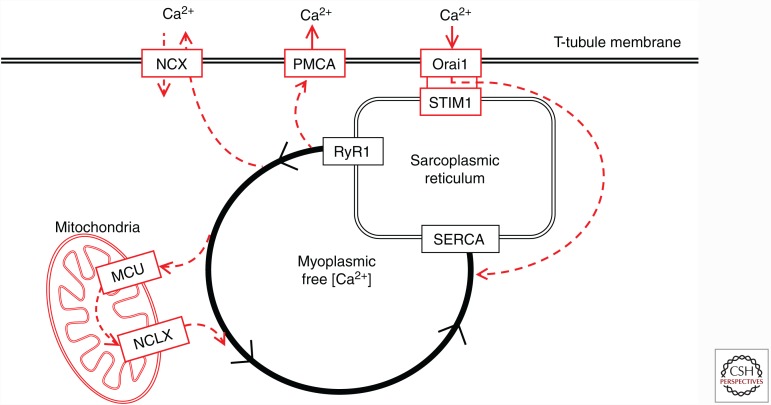
Figure 5 summarizes Ca2+ fluxes activated during repeated contractions. The absolute majority of Ca2+-ions released from the SR via RyR1 are actively pumped back into the SR by the SERCA. In addition, small amounts of Ca2+ ions leave the cell or enter the mitochondrial matrix. Although the latter fluxes are quantitatively small, they may affect fatigue development: impaired reuptake of extruded Ca2+ because of defective SOCE has been shown to accelerate fatigue development; modestly increased [Ca2+]mito speeds up mitochondrial respiration, whereas an excessive increase may trigger apoptotic signaling and even induce cell death.
Figure 5.
Ca2+ fluxes during contractions of skeletal muscle fibers. The contractile machinery of muscle fibers is triggered by Ca2+ released from the sarcoplasmic reticulum (SR) via the ryanodine receptor 1 (RyR1) Ca2+ channels. The absolute majority of Ca2+ ions are actively pumped back into SR by the SR Ca2+ pumps (SERCA). Few Ca2+ ions are extruded from the cell via the plasma membrane Ca2+ (PMCA) pumps or Na+-Ca2+ exchangers (NCX); the Ca2+ extrusion is balanced mainly by store-operated Ca2+ entry, which involves the SR Ca2+ sensor, the stromal-interacting molecule 1 (STIM1) that activates the sarcolemmal Orai1 Ca2+ channels. Some Ca2+ ions may enter the mitochondrial matrix mainly via the tightly controlled mitochondrial Ca2+ uniporter (MCU) and these are subsequently returned to the myoplasm via mitochondrial Na+-Ca2+ exchangers (NCLX).
ACKNOWLEDGMENTS
The authors acknowledge support from the Swedish Research Council and the Swedish National Centre for Sports Research.
Footnotes
Editors: Juleen R. Zierath, Michael J. Joyner, and John A. Hawley
Additional Perspectives on The Biology of Exercise available at www.perspectivesinmedicine.org
REFERENCES
- Ainbinder A, Boncompagni S, Protasi F, Dirksen RT. 2015. Role of mitofusin-2 in mitochondrial localization calcium uptake in skeletal muscle. Cell Calcium 57: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Lee JA, Westerblad H. 1989. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. J Physiol 415: 433–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. 2008. Skeletal muscle fatigue: Cellular mechanisms. Physiol Rev 88: 287–332. [DOI] [PubMed] [Google Scholar]
- Allen DG, Clugston E, Petersen Y, Röder IV, Chapman B, Rudolf R. 2011. Interactions between intracellular calcium and phosphate in intact mouse muscle during fatigue. J Appl Physiol (1985) 111: 358–366. [DOI] [PubMed] [Google Scholar]
- Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, Shiomi T, Zalk R, Lacampagne A, Marks AR. 2011. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 14: 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Allen DG, Westerblad H. 1998. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509: 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Westerblad H. 2001. Contractile response of skeletal muscle to low peroxide concentrations: Myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J 15: 309–311. [DOI] [PubMed] [Google Scholar]
- Aydin J, Shabalina IG, Place N, Reiken S, Zhang SJ, Bellinger AM, Nedergaard J, Cannon B, Marks AR, Bruton JD, et al. 2008. Nonshivering thermogenesis protects against defective calcium handling in muscle. FASEB J 22: 3919–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin J, Andersson DC, Hänninen SL, Wredenberg A, Tavi P, Park CB, Larsson NG, Bruton JD, Westerblad H. 2009. Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Human Mol Genet 18: 278–288. [DOI] [PubMed] [Google Scholar]
- Balnave CD, Allen DG. 1998. Evidence for Na+/Ca2+ exchange in intact single skeletal muscle fibers from the mouse. Am J Physiol 274: C940–C946. [DOI] [PubMed] [Google Scholar]
- Balon TW, Nadler JL. 1994. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol 77: 2519–2521. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Madsen K, Kiens B, Richter EA. 1996. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol 495: 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, et al. 2011. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476: 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor SM, Hollingworth S. 2007. Simulation of Ca2+ movements within the sarcomere of fast-twitch mouse fibers stimulated by action potentials. J Gen Physiol 130: 283–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger AM, Mongillo M, Marks AR. 2008a. Stressed out: The skeletal muscle ryanodine receptor as a target of stress. J Clin Invest 118: 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, Lacampagne A, et al. 2008b. Remodeling of ryanodine receptor complex causes “leaky” channels: A molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci 105: 2198–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hail D, Palty R, Shoshan-Barmatz V. 2014. Measurement of mitochondrial Ca2+ transport mediated by three transport proteins: VDAC1, the Na+/Ca2+ exchanger, and the Ca2+ uniporter. Cold Spring Harb Protoc 2014: 161–166. [DOI] [PubMed] [Google Scholar]
- Bergström J, Hermansen L, Hultman E, Saltin B. 1967. Diet, muscle glycogen and physical performance. Acta Physiol Scand 71: 140–150. [DOI] [PubMed] [Google Scholar]
- Boncompagni S, Rossi AE, Micaroni M, Beznoussenko GV, Polishchuk RS, Dirksen RT, Protasi F. 2009. Mitochondria are linked to calcium stores in striated muscle by developmentally regulated tethering structures. Mol Biol Cell 20: 1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. 2004. Calcium, ATP, and ROS: A mitochondrial love–hate triangle. Am J Physiol Cell Physiol 287: C817–C833. [DOI] [PubMed] [Google Scholar]
- Bruton JD, Lännergren J, Westerblad H. 1998. Effects of CO2-induced acidification on the fatigue resistance of single mouse muscle fibers at 28°C. J Appl Physiol 85: 478–483. [DOI] [PubMed] [Google Scholar]
- Bruton J, Tavi P, Aydin J, Westerblad H, Lännergren J. 2003a. Mitochondrial and myoplasmic [Ca2+] in single fibres from mouse limb muscles during repeated tetanic contractions. J Physiol 551: 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton JD, Dahlstedt AJ, Abbate F, Westerblad H. 2003b. Mitochondrial function in intact skeletal muscle fibres of creatine kinase deficient mice. J Physiol 552: 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton JD, Place N, Yamada T, Silva JP, Andrade FH, Dahlstedt AJ, Zhang SJ, Katz A, Larsson NG, Westerblad H. 2008. Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J Physiol 586: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton JD, Aydin J, Yamada T, Shabalina IG, Ivarsson N, Zhang SJ, Wada M, Tavi P, Nedergaard J, Katz A, et al. 2010. Increased fatigue resistance linked to Ca2+-stimulated mitochondrial biogenesis in muscle fibres of cold-acclimated mice. J Physiol 588: 4275–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady EB, Jones DA, Lynn J, Newham DJ. 1989. Changes in force and intracellular metabolites during fatigue of human skeletal muscle. J Physiol 418: 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AJ, Bruton JD, Lanner JT, Westerblad H. 2015. Antioxidant treatments do not improve force recovery after fatiguing stimulation of mouse skeletal muscle fibres. J Physiol 593: 457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AJ, Yamada T, Rassier D, Andersson DC, Westerblad H, Lanner JT. 2016. Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery. J Physiol 594: 5149–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. 2007. Modulation of the actomyosin interaction during fatigue of skeletal muscle. Muscle Nerve 36: 756–777. [DOI] [PubMed] [Google Scholar]
- Cully TR, Launikonis BS. 2013. Store-operated Ca2+ entry is not required for store refilling in skeletal muscle. Clin Exp Pharmacol Physiol 40: 338–344. [DOI] [PubMed] [Google Scholar]
- Dahlstedt AJ, Westerblad H. 2001. Inhibition of creatine kinase reduces the rate of fatigue-induced decrease in tetanic [Ca2+]i in mouse skeletal muscle. J Physiol 533: 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstedt AJ, Katz A, Wieringa B, Westerblad H. 2000. Is creatine kinase responsible for fatigue? Studies of skeletal muscle deficient of creatine kinase. FASEB J 14: 982–990. [DOI] [PubMed] [Google Scholar]
- Dahlstedt A, Katz A, Westerblad H. 2001. Role of myoplasmic phosphate in contractile function of skeletal muscle: Studies on creatine kinase-deficient mice. J Physiol 533: 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstedt AJ, Katz A, Tavi P, Westerblad H. 2003. Creatine kinase injection restores contractile function in creatine-kinase-deficient mouse skeletal muscle fibres. J Physiol 547: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroot M, Massie BM, Boska M, Gober J, Miller RG, Weiner MW. 1993. Dissociation of [H+] from fatigue in human muscle detected by high time resolution 31P-NMR. Muscle Nerve 16: 91–98. [DOI] [PubMed] [Google Scholar]
- de Paoli FV, Broch-Lips M, Pedersen TH, Nielsen OB. 2013. Relationship between membrane Cl− conductance and contractile endurance in isolated rat muscles. J Physiol 591: 531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. 2011. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Rizzuto R, Pozzan T. 2016. Enjoy the trip: Calcium in mitochondria back and forth. Annu Rev Biochem 85: 161–192. [DOI] [PubMed] [Google Scholar]
- Duchen MR. 2000. Mitochondria and Ca2+ in cell physiology and pathophysiology. Cell Calcium 28: 339–348. [DOI] [PubMed] [Google Scholar]
- Duke AM, Steele DS. 2001. Mechanisms of reduced SR Ca2+ release induced by inorganic phosphate in rat skeletal muscle fibers. Am J Physiol Cell Physiol 281: C418–C429. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Lamb GD. 2004. Effect of low cytoplasmic [ATP] on excitation–contraction coupling in fast-twitch muscle fibres of the rat. J Physiol 560: 451–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka TL, Cole L, Lamb GD. 2005. Calcium-phosphate precipitation in the sarcoplasmic reticulum reduces action potential-mediated Ca2+ release in mammalian skeletal muscle. Am J Physiol Cell Physiol 289: C1502–C1512. [DOI] [PubMed] [Google Scholar]
- Everts ME, Lømo T, Clausen T. 1993. Changes in K+, Na+ and calcium contents during in vivo stimulation of rat skeletal muscle. Acta Physiol Scand 147: 357–368. [DOI] [PubMed] [Google Scholar]
- Fan JL, Kayser B. 2016. Fatigue and exhaustion in hypoxia: The role of cerebral oxygenation. High Alt Med Biol 17: 72–84. [DOI] [PubMed] [Google Scholar]
- Fitts RH. 2016. The role of acidosis in fatigue: Pro perspective. Med Sci Sports Exerc 48: 2335–2338. [DOI] [PubMed] [Google Scholar]
- Flagg TP, Enkvetchakul D, Koster JC, Nichols CG. 2010. Muscle KATP channels: Recent insights to energy sensing and myoprotection. Physiol Rev 90: 799–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, Owen VJ, Lamb GD, Stephenson DG. 1995. Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. J Physiol 482: 123–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gejl KD, Hvid LG, Willis SJ, Andersson E, Holmberg HC, Jensen R, Frandsen U, Hansen J, Plomgaard P, Ørtenblad N. 2015. Repeated high-intensity exercise modulates Ca2+ sensitivity of human skeletal muscle fibers. Scand J Med Sci Sports 26: 488–497. [DOI] [PubMed] [Google Scholar]
- Gineste C, Hernandez A, Ivarsson N, Cheng AJ, Naess K, Wibom R, Lesko N, Bruhn H, Wedell A, Freyer C, et al. 2015. Cyclophilin D, a target for counteracting skeletal muscle dysfunction in mitochondrial myopathy. Hum Mol Genet 24: 6580–6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissel H, Clausen T. 1999. Excitation-induced Ca2+ uptake in rat skeletal muscle. Am J Physiol 276: R331–R339. [DOI] [PubMed] [Google Scholar]
- Glancy B, Willis WT, Chess DJ, Balaban RS. 2013. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry 52: 2793–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Vina J. 2008. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87: 142–149. [DOI] [PubMed] [Google Scholar]
- Goonasekera SA, Davis J, Kwong JQ, Accornero F, Wei-LaPierre L, Sargent MA, Dirksen RT, Molkentin JD. 2014. Enhanced Ca2+ influx from STIM1-Orai1 induces muscle pathology in mouse models of muscular dystrophy. Hum Mol Genet 23: 3706–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutscher M, Sobotta MC, Wabnitz GH, Ballikaya S, Meyer AJ, Samstag Y, Dick TP. 2009. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J Biol Chem 284: 31532–31540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. 1984. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock CR, Janssen E, Terjung RL. 2005. Skeletal muscle contractile performance and ADP accumulation in adenylate kinase-deficient mice. Am J Physiol Cell Physiol 288: C1287–C1297. [DOI] [PubMed] [Google Scholar]
- Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. 2004. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem 279: 13044–13053. [DOI] [PubMed] [Google Scholar]
- Harridge SD. 2007. Plasticity of human skeletal muscle: Gene expression to in vivo function. Exp Physiol 92: 783–797. [DOI] [PubMed] [Google Scholar]
- Hermansen L, Hultman E, Saltin B. 1967. Muscle glycogen during prolonged severe exercise. Acta Physiol Scand 71: 129–139. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, Gonzalez ME, Garcia AM. 1986. Calcium transport in transverse tubules isolated from rabbit skeletal muscle. Biochim Biophys Acta 854: 279–286. [DOI] [PubMed] [Google Scholar]
- Hirschfield W, Moody MR, O'Brien WE, Gregg AR, Bryan RM Jr, Reid MB. 2000. Nitric oxide release and contractile properties of skeletal muscles from mice deficient in type III NOS. Am J Physiol Regul Integr Comp Physiol 278: R95–R100. [DOI] [PubMed] [Google Scholar]
- Howald H, Hoppeler H, Claassen H, Mathieu O, Straub R. 1985. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflügers Arch 403: 369–376. [DOI] [PubMed] [Google Scholar]
- Jansson E, Sjödin B, Tesch P. 1978. Changes in muscle fibre type distribution in man after physical training. A sign of fibre type transformation? Acta Physiol Scand 104: 235–237. [DOI] [PubMed] [Google Scholar]
- Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. 2000. Interstitial K+ in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol 278: R400–R406. [DOI] [PubMed] [Google Scholar]
- Kabbara AA, Allen DG. 1999. The role of calcium stores in fatigue of isolated single muscle fibres from the cane toad. J Physiol 519: 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbara AA, Allen DG. 2001. The use of fluo-5N to measure sarcoplasmic reticulum calcium in single muscle fibres of the cane toad. J Physiol 534: 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, Ischiropoulos H. 2012. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic Biol Med 52: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatzaferi C, de Haan A, Ferguson RA, van Mechelen W, Sargeant AJ. 2001. Phosphocreatine and ATP content in human single muscle fibres before and after maximum dynamic exercise. Pflügers Arch 442: 467–474. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA. 1999. Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur J Appl Physiol Occup Physiol 80: 57–63. [DOI] [PubMed] [Google Scholar]
- Khawli FA, Reid MB. 1994. N-acetylcysteine depresses contractile function and inhibits fatigue of diaphragm in vitro. J Appl Physiol 77: 317–324. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. 2004. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427: 360–364. [DOI] [PubMed] [Google Scholar]
- Kobzik L, Reid MB, Bredt DS, Stamler JS. 1994. Nitric oxide in skeletal muscle. Nature 372: 546–548. [DOI] [PubMed] [Google Scholar]
- Lamb GD. 2000. Excitation-contraction coupling in skeletal muscle: Comparisons with cardiac muscle. Clin Exp Pharmacol Physiol 27: 216–224. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. 1994. Effects of intracellular pH and [Mg2+] on excitation–contraction coupling in skeletal muscle fibres of the rat. J Physiol 478: 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Westerblad H. 2011. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol 589: 2119–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanner JT, Georgiou DK, Dagnino-Acosta A, Ainbinder A, Cheng Q, Joshi AD, Chen Z, Yarotskyy V, Oakes JM, Lee CS, et al. 2012. AICAR prevents heat-induced sudden death in RyR1 mutant mice independent of AMPK activation. Nat Med 18: 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H. 1991. Force decline due to fatigue and intracellular acidification in isolated fibres from mouse skeletal muscle. J Physiol 434: 307–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H, Bruton JD. 2001. Changes in mitochondrial Ca2+ detected with Rhod-2 in single frog and mouse skeletal muscle fibres during and after repeated tetanic contractions. J Muscle Res Cell Motil 22: 265–275. [DOI] [PubMed] [Google Scholar]
- Laver DR, Eager KR, Taoube L, Lamb GD. 2000. Effects of cytoplasmic and luminal pH on Ca2+ release channels from rabbit skeletal muscle. Biophys J 78: 1835–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR, Lenz GK, Dulhunty AF. 2001a. Phosphate ion channels in sarcoplasmic reticulum of rabbit skeletal muscle. J Physiol 535: 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR, Lenz GKE, Lamb GD. 2001b. Regulation of the calcium release channel from rabbit skeletal muscle by the nucleotides ATP, AMP, IMP and adenosine. J Physiol 537: 763–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Dagnino-Acosta A, Yarotskyy V, Hanna A, Lyfenko A, Knoblauch M, Georgiou DK, Poche RA, Swank MW, Long C, et al. 2015. Ca2+ permeation and/or binding to CaV1.1 fine-tunes skeletal muscle Ca2+ signaling to sustain muscle function. Skelet Muscle 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenneke JP, Thiebaud RS, Abe T, Bemben MG. 2014. Blood flow restriction pressure recommendations: The hormesis hypothesis. Med Hypotheses 82: 623–626. [DOI] [PubMed] [Google Scholar]
- Lunde PK, Dahlstedt AJ, Bruton JD, Lännergren J, Thorén P, Sejersted OM, Westerblad H. 2001. Contraction and intracellular Ca2+ handling in isolated skeletal muscle of rats with congestive heart failure. Circ Res 88: 1299–1305. [DOI] [PubMed] [Google Scholar]
- Ma J, Fill M, Knudson CM, Campbell KP, Coronado R. 1988. Ryanodine receptor of skeletal muscle is a gap junction-type channel. Science 242: 99–102. [DOI] [PubMed] [Google Scholar]
- Marchand I, Chorneyko K, Tarnopolsky M, Hamilton S, Shearer J, Potvin J, Graham TE. 2002. Quantification of subcellular glycogen in resting human muscle: Granule size, number, and location. J Appl Physiol 93: 1598–1607. [DOI] [PubMed] [Google Scholar]
- Marchand I, Tarnopolsky M, Adamo KB, Bourgeois JM, Chorneyko K, Graham TE. 2007. Quantitative assessment of human muscle glycogen granules size and number in subcellular locations during recovery from prolonged exercise. J Physiol 580: 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S, Pinton P. 2014. The mitochondrial calcium uniporter complex: Molecular components, structure and physiopathological implications. J Physiol 592: 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszczak Y, Farid M, Jones J, Lansdowne S, Smith MA, Taylor AA, Reid MB. 2005. Effects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exercise. Muscle Nerve 32: 633–638. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Bangsbo J, Renaud JM. 2008. Muscle K+, Na+, and Cl disturbances and Na+-K+ pump inactivation: Implications for fatigue. J Appl Physiol 104: 288–295. [DOI] [PubMed] [Google Scholar]
- Meissner G, Darling E, Eveleth J. 1986. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry 25: 236–244. [DOI] [PubMed] [Google Scholar]
- Merry TL, Ristow M. 2016. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J Physiol 594: 5135–5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AJ, Dick TP. 2010. Fluorescent protein-based redox probes. Antioxid Redox Signal 13: 621–650. [DOI] [PubMed] [Google Scholar]
- Michaelson LP, Shi G, Ward CW, Rodney GG. 2010. Mitochondrial redox potential during contraction in single intact muscle fibers. Muscle Nerve 42: 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr M, Nordsborg N, Nielsen JJ, Pedersen LD, Fischer C, Krustrup P, Bangsbo J. 2004. Potassium kinetics in human muscle interstitium during repeated intense exercise in relation to fatigue. Pflügers Arch 448: 452–456. [DOI] [PubMed] [Google Scholar]
- Murphy RM, Stephenson DG, Lamb GD. 2004. Effect of creatine on contractile force and sensitivity in mechanically skinned single fibers from rat skeletal muscle. Am J Physiol Cell Physiol 287: C1589–C1595. [DOI] [PubMed] [Google Scholar]
- Nielsen OB, de Paoli F, Overgaard K. 2001. Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol 536: 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Schrøder HD, Rix CG, Ørtenblad N. 2009. Distinct effects of subcellular glycogen localization on tetanic relaxation time and endurance in mechanically skinned rat skeletal muscle fibres. J Physiol 587: 3679–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Holmberg HC, Schrøder HD, Saltin B, Ørtenblad N. 2011. Human skeletal muscle glycogen utilization in exhaustive exercise: Role of subcellular localization and fibre type. J Physiol 589: 2871–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Cheng AJ, Ørtenblad N, Westerblad H. 2014. Subcellular distribution of glycogen and decreased tetanic Ca2+ in fatigued single intact mouse muscle fibres. J Physiol 592: 2003–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørtenblad N, Nielsen J, Saltin B, Holmberg HC. 2011. Role of glycogen availability in sarcoplasmic reticulum Ca2+ kinetics in human skeletal muscle. J Physiol 589: 711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørtenblad N, Westerblad H, Nielsen J. 2013. Muscle glycogen stores and fatigue. J Physiol 591: 4405–4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R, Basu Thakur P, Li S, Minard C, Rodney GG. 2013. Real-time imaging of NADPH oxidase activity in living cells using a novel fluorescent protein reporter. PLoS ONE 8: e63989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, et al. 2010. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci 107: 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Brotto M, Ma J. 2014. Store-operated Ca2+ entry in muscle physiology and diseases. BMB Rep 47: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E, Bhimani M, Franks-Skiba K, Cooke R. 1995. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: Implications for fatigue. J Physiol 486: 689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen G, Cumming KT, Holden G, Hallen J, Ronnestad BR, Sveen O, Skaug A, Paur I, Bastani NE, Ostgaard HN, et al. 2014. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J Physiol 592: 1887–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, Nielsen OB, Lamb GD, Stephenson DG. 2004. Intracellular acidosis enhances the excitability of working muscle. Science 305: 1144–1147. [DOI] [PubMed] [Google Scholar]
- Pedersen TH, de Paoli F, Nielsen OB. 2005. Increased excitability of acidified skeletal muscle: Role of chloride conductance. J Gen Physiol 125: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, de Paoli FV, Flatman JA, Nielsen OB. 2009a. Regulation of ClC-1 and KATP channels in action potential-firing fast-twitch muscle fibers. J Gen Physiol 134: 309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, Macdonald WA, de Paoli FV, Gurung IS, Nielsen OB. 2009b. Comparison of regulated passive membrane conductance in action potential-firing fast- and slow-twitch muscle. J Gen Physiol 134: 323–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, Riisager A, de Paoli FV, Chen TY, Nielsen OB. 2016. Role of physiological ClC-1 Cl− ion channel regulation for the excitability and function of working skeletal muscle. J Gen Physiol 147: 291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place N, Ivarsson N, Venckunas T, Neyroud D, Brazaitis M, Cheng AJ, Ochala J, Kamandulis S, Girard S, Volungevicius G, et al. 2015. Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high-intensity interval exercise. Proc Natl Acad Sci 112: 15492–15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posterino GS, Dutka TL, Lamb GD. 2001. L+-lactate does not affect twitch and tetanic responses in mechanically skinned mammalian muscle fibres. Pflügers Arch 442: 197–203. [DOI] [PubMed] [Google Scholar]
- Powers SK, Jackson MJ. 2008. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Ji LL, Kavazis AN, Jackson MJ. 2011. Reactive oxygen species: Impact on skeletal muscle. Compr Physiol 1: 941–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pye D, Palomero J, Kabayo T, Jackson MJ. 2007. Real-time measurement of nitric oxide in single mature mouse skeletal muscle fibres during contractions. J Physiol 581: 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. 1992. Reactive oxygen in skeletal muscle. I: Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol 73: 1797–1804. [DOI] [PubMed] [Google Scholar]
- Reid MB, Stokic DS, Koch SM, Khawli FA, Leis AA. 1994. N-acetylcysteine inhibits muscle fatigue in humans. J Clin Invest 94: 2468–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. 2009. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci 106: 8665–8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin G, Allard B. 2015. Voltage-gated Ca2+ influx through L-type channels contributes to sarcoplasmic reticulum Ca2+ loading in skeletal muscle. J Physiol 593: 4781–4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Richter EA. 2009. Regulatory mechanisms of skeletal muscle protein turnover during exercise. J Appl Physiol 106: 1702–1711. [DOI] [PubMed] [Google Scholar]
- Rossi AE, Boncompagni S, Wei L, Protasi F, Dirksen RT. 2011. Differential impact of mitochondrial positioning on mitochondrial Ca2+ uptake and Ca2+ spark suppression in skeletal muscle. Am J Physiol Cell Physiol 301: C1128–C1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K, Ren JM. 1989. Relationship of contraction capacity to metabolic changes during recovery from a fatiguing contraction. J Appl Physiol 67: 648–654. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Tonkonogi M, Söderlund K. 1998. Energy supply and muscle fatigue in humans. Acta Physiol Scand 162: 261–266. [DOI] [PubMed] [Google Scholar]
- Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A, Jackson MJ. 2013. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal 18: 603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce AE, Standen NB, Stanfield PR. 1985. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature 316: 736–738. [DOI] [PubMed] [Google Scholar]
- Steele DS, Duke AM. 2003. Metabolic factors contributing to altered Ca2+ regulation in skeletal muscle fatigue. Acta Physiol Scand 179: 39–48. [DOI] [PubMed] [Google Scholar]
- Swenson AM, Trivedi DV, Rauscher AA, Wang Y, Takagi Y, Palmer BM, Malnasi-Csizmadia A, Debold EP, Yengo CM. 2014. Magnesium modulates actin binding and ADP release in myosin motors. J Biol Chem 289: 23977–23091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G, Vandenborne K, Elliott M, Leigh JS. 1999. In vivo ATP synthesis rates in single human muscles during high intensity. J Physiol 519: 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waning DL, Mohammad KS, Reiken S, Xie W, Andersson DC, John S, Chiechi A, Wright LE, Umanskaya A, Niewolna M, et al. 2015. Excess TGF-β mediates muscle weakness associated with bone metastases in mice. Nat Med 21: 1262–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Kanzaki K, Kuratani M, Matsunaga S, Yanaka N, Wada M. 2015. Contribution of impaired myofibril and ryanodine receptor function to prolonged low-frequency force depression after in situ stimulation in rat skeletal muscle. J Muscle Res Cell Motil 36: 275–286. [DOI] [PubMed] [Google Scholar]
- Wei L, Salahura G, Boncompagni S, Kasischke KA, Protasi F, Sheu SS, Dirksen RT. 2011. Mitochondrial superoxide flashes: Metabolic biomarkers of skeletal muscle activity and disease. FASEB J 25: 3068–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei-Lapierre L, Carrell EM, Boncompagni S, Protasi F, Dirksen RT. 2013. Orai1-dependent calcium entry promotes skeletal muscle growth and limits fatigue. Nat Commun 4: 2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H. 2016. Acidosis is not a significant cause of skeletal muscle fatigue. Med Sci Sports Exerc 48: 2339–2342. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. 1991. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol 98: 615–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. 1992. Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol 453: 413–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. 1993. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. J Physiol 466: 611–628. [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. 1996. The effects of intracellular injections of phosphate on intracellular calcium and force in single fibres of mouse skeletal muscle. Pflügers Arch 431: 964–970. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Duty S, Allen DG. 1993. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol 75: 382–388. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lännergren J. 1997. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J Physiol 500: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Dahlstedt AJ, Lännergren J. 1998. Mechanisms underlying reduced maximum shortening velocity during fatigue of intact, single fibres of mouse muscle. J Physiol 510: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG, Lännergren J. 2002. Muscle fatigue: Lactic acid or inorganic phosphate the major cause? News Physiol Sci 17: 17–21. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Katz A. 2010. Skeletal muscle: Energy metabolism, fiber types, fatigue and adaptability. Exp Cell Res 316: 3093–3099. [DOI] [PubMed] [Google Scholar]
- Williams GS, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ. 2013. Mitochondrial calcium uptake. Proc Natl Acad Sci 110: 10479–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GS, Boyman L, Lederer WJ. 2015. Mitochondrial calcium and the regulation of metabolism in the heart. J Mol Cell Cardiol 78: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JR, McCully KK, Mancini DM, Boden B, Chance B. 1988. Relationship of muscular fatigue to pH and diprotonated Pi in humans: A 31P-NMR study. J Appl Physiol (1985) 64: 2333–2339. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Loenneke JP, Jo E, Wilson GJ, Zourdos MC, Kim JS. 2012. The effects of endurance, strength, and power training on muscle fiber type shifting. J Strength Cond Res 26: 1724–1729. [DOI] [PubMed] [Google Scholar]
- Wiseman RW, Beck TW, Chase PB. 1996. Effect of intracellular pH on force development depends on temperature in intact skeletal muscle from mouse. Am J Physiol 271: C878–C886. [DOI] [PubMed] [Google Scholar]
- Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO. 2007. Calcium induces increases in peroxisome proliferator-activated receptor γ coactivator-1α and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem 282: 18793–18799. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Bruton JD, Katz A, Westerblad H. 2006. Limited oxygen diffusion accelerates fatigue development in mouse skeletal muscle. J Physiol 572: 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]