Abstract
Transmissible spongiform encephalopathies are infectious neurodegenerative diseases caused by the conversion of prion protein (PrP) into a self-replicating conformation that spreads via templated conversion of natively folded PrP molecules within or between cells. Recent studies provide compelling evidence that prion-like behavior is a general property of most protein aggregates associated with neurodegenerative diseases. Many of these disorders are associated with spontaneous protein aggregation, but genetic mutations can increase the aggregation propensity of specific proteins, including expansion of polyglutamine (polyQ) tracts, which is causative of nine inherited neurodegenerative diseases. Aggregates formed by polyQ-expanded huntingtin (Htt) in Huntington’s disease can transfer between cells and seed the aggregation of cytoplasmic wild-type Htt in a prion-like manner. Additionally, prion-like properties of glutamine-rich proteins underlie nonpathological processes in yeast and higher eukaryotes. Here, we review current evidence supporting prion-like characteristics of polyQ and glutamine-rich proteins.
The hypothesis that proteins could behave as agents of infection was first proposed in the late 1960s when it was observed that material capable of transmitting the neurodegenerative disease scrapie in sheep and goats was resistant to treatment with heat, formaldehyde, and ultraviolet radiation (Alper et al. 1967; Pattison and Jones 1967). The infectious agent responsible for spreading scrapie was termed a prion, but it was not until the early 1980s that the major protein component in these prions was isolated and named prion protein (PrP) (Bolton et al. 1982; McKinley et al. 1983). The term prion was later extended to include proteins that mediate the inheritance of altered phenotypes in yeast and other fungi (Wickner 1994; Patino et al. 1996; Coustou et al. 1997), with the recognition that prion behavior describes the ability of any protein to adopt alternative, nonnative conformations, at least one of which is self-replicating and leads to the formation of a stable amyloid fold. This understanding of the mechanism of prion formation allows expansion of the prion concept to include any protein that assembles into nuclei that drive the formation of thermodynamically stable, ordered fibrillar aggregates by monomer addition to fibril ends and that can fragment to generate new fibril ends (Prusiner 2013). The extraordinary stability of these fibrils ensures that once formed, nuclei will continue to grow even if the amyloidogenic state is exceedingly rare (Eisenberg and Jucker 2012). The discovery that even well-folded, globular proteins have the capacity to access an amyloidogenic state under native conditions (Fändrich and Dobson 2002; Chiti and Dobson 2009) indicates that the prion concept is likely far more general than originally believed and can be applied to a large set of structurally diverse proteins.
PROTEIN HOMEOSTASIS AND AGGREGATION
To function, newly synthesized polypeptides must fold properly, assemble with their binding partners, and be targeted to their appropriate subcellular destinations (Braselmann et al. 2013). In the cell, most nascent polypeptides achieve their mature conformation through the assistance of molecular chaperones, a class of proteins that bind transiently to unfolded or partially folded polypeptides and promote their transition to the native state (Kim et al. 2013). Chaperone networks maintain protein homeostasis and respond to changes in environmental conditions or gene expression programs that increase the burden of unfolded or misfolded proteins in the cell (Smith et al. 2015). Traditionally, protein folding has been viewed as a biased search for a single native state defined by the lowest free-energy conformation (Powers et al. 2009). However, most proteins display conformational flexibility in their native states that is required for their function. In fact, a substantial fraction of proteins or protein domains lack any stable three-dimensional structure and are known as intrinsically disordered proteins (IDPs) or intrinsically disordered regions (IDRs) (Wright and Dyson 2015). IDPs and IDRs are enriched in charged and polar residues and show low sequence complexity, which prevents them from spontaneously folding into a single native state. Because of their inherently unstructured nature, IDPs and proteins containing IDRs often play key roles in protein interaction and signaling networks by facilitating interactions with diverse protein partners. For example, IDPs play roles as linkers that allow dynamic movement of adjacent, covalently attached protein regions or in facilitating interactions with multiple protein partners (Das et al. 2015).
Cellular stress, genetic mutations, or a decline in cellular proteostasis capacity can lead to the accumulation of misfolded proteins (Hipp et al. 2014) and, in many cases, their self-assembly into highly ordered fibrils or spines that show characteristic X-ray diffraction patterns reflecting an underlying organization of β-strands stacked one on top of another perpendicular to the fiber axis (Eisenberg and Jucker 2012). The extensive hydrogen-bonding network between these cross-β-sheets, in-register packing of side chains, and iterative long-range order contribute to the extraordinary thermodynamic stability of these conformers (Makin et al. 2005; Nelson et al. 2005; Tsemekhman et al. 2007). Amyloid formation is biphasic and characterized by an initial lag period, during which an oligomeric nucleus forms and is followed by a period of exponential growth when monomers are recruited onto the ends of a growing fibril (Arosio et al. 2015). Most neurodegenerative diseases, including prion diseases, are associated with the deposition of amyloid, initially formed by a specific protein in each disorder, in the brain (Prusiner 2013). The majority of these disease-associated proteins are IDPs or contain IDRs (Cleveland et al. 1977; Bennett et al. 2002; Mukrasch et al. 2009; Fauvet et al. 2012), suggesting that their inherent metastability facilitates aggregation in pathological states.
The relative contribution of heterogeneous oligomeric species and stable amyloid to cytotoxicity and whether sequestration of misfolded proteins into an aggregate serves a neuroprotective function are matters of intense debate (Mucke et al. 2000; Stephan et al. 2001; Walsh et al. 2002; Arrasate et al. 2004; Ko et al. 2008; Winner et al. 2011; Pieri et al. 2012; Barmada et al. 2014). In prion diseases, PrP aggregation and recruitment of monomeric protein into amyloid underlie disease transmission (Prusiner 2013), indicating that aggregates can transmit toxicity by propagating between individual cells or even organisms. However, the mechanism(s) by which protein aggregation causes cell death is not well understood. Formation of protein aggregates is closely correlated with neuronal dysfunction and death and could drive toxicity by two possible mechanisms: first, by sequestering soluble monomeric proteins, preventing them from performing their normal cellular function, and second, by perturbing cellular protein homeostasis (Powers et al. 2009; Holmes et al. 2014). A unique feature of amyloid fibrils is their frangibility; fragmentation accelerates polymerization by creating new fibril ends for continued recruitment of monomers (Gillam and MacPhee 2013). Breakage events could also contribute to prion-like spread of amyloid by creating smaller fibrillar nuclei capable of moving between cells. Because neurodegenerative disorders (e.g., Parkinson’s disease [PD], Alzheimer’s disease [AD], Huntington’s disease [HD], and amyotrophic lateral sclerosis [ALS]) are characterized by the conversion of normal cellular proteins into amyloid, it has been postulated that transmissible amyloids could underlie the stereotypical spread of disease pathology through the brain (Braak and Braak 1991; Braak et al. 2004; Brettschneider et al. 2013). This hypothesis is supported by the finding that amyloid associated with the most common neurodegenerative diseases can transmit between cells in culture and in the brains of animal models of these diseases (Desplats et al. 2009; Frost et al. 2009; Ren et al. 2009; Munch et al. 2011; Volpicelli-Daley et al. 2011; de Calignon et al. 2012; Lasagna-Reeves et al. 2012; Liu et al. 2012b; Luk et al. 2012; Trevino et al. 2012; Clavaguera et al. 2013; Costanzo et al. 2013; Holmes et al. 2013; Nonaka et al. 2013; Ahmed et al. 2014; Pecho-Vrieseling et al. 2014; Pearce et al. 2015). Together, these studies provide evidence for a common prion-like component in neurodegenerative disease progression.
POLYGLUTAMINE (POLYQ)- AND GLUTAMINE (Q)-RICH DOMAINS
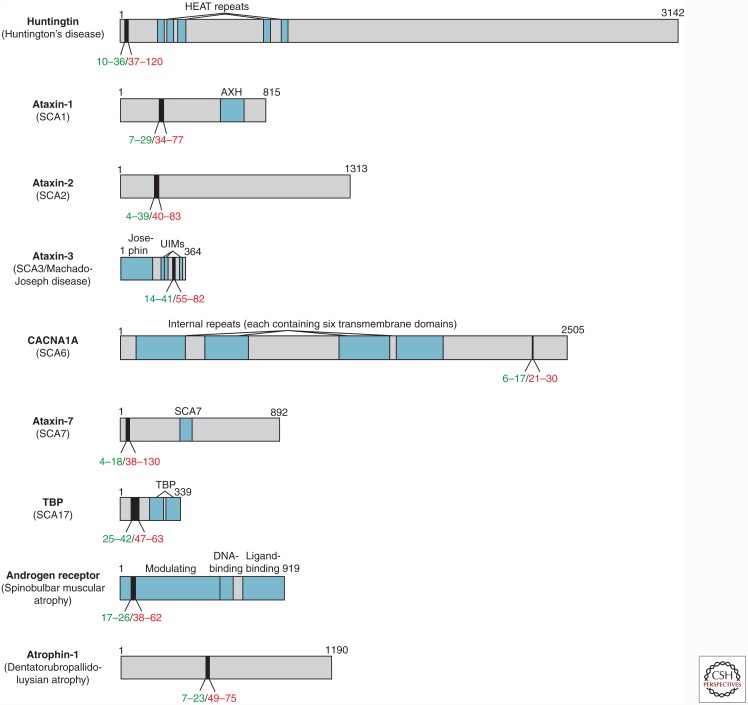
Unlike most cases of the major neurodegenerative diseases (AD, PD, and ALS) that occur spontaneously, certain neurodegenerative disorders are exclusively caused by dominantly inherited genetic mutations that produce proteins that make up the major component of the pathogenic aggregates detected in these diseases. Nine of these genetic disorders are caused by the inheritance of a mutation that expands a CAG repeat region in a coding region of a particular gene (Everett and Wood 2004). CAG repeat expansion results in the expression of proteins with tracts of uninterrupted glutamine residues (polyQ) that are longer than in the wild-type (WT) protein (Fig. 1) and increases the aggregation propensity of these proteins (Fig. 2A,B) (Chen et al. 2002a). CAG repeat regions are unstable DNA elements that tend to increase in length (particularly in the male germline) through successive generations, a phenomenon known as genetic anticipation (Duyao et al. 1993). CAG repeat length is inversely correlated with age of disease onset, with extremely long repeats causing disease manifestation in children (Chen et al. 2002b; Langbehn et al. 2010).
Figure 1.
Polyglutamine (PolyQ)-containing proteins implicated in inherited neurodegenerative diseases. The domain organizations of nine proteins associated with inherited neurodegenerative diseases caused by polyQ expansion are shown. PolyQ tracts are shown in black, with reported ranges for wild-type (WT) (green) and mutant (red) lengths indicated underneath. HEAT, Repeats found in huntingtin, elongation factor 3, protein phosphatase 2A, and TOR1; SCA, spinocerebellar ataxia; AXH, ataxin-1 and HMG box-containing protein 1 domain; UIM, ubiquitin interacting motif; CACNA1A, Cav2.1 P/Q voltage-dependent calcium channel; SCA7, ataxin-7 atypical zinc finger; TBP, TATA box-binding protein.
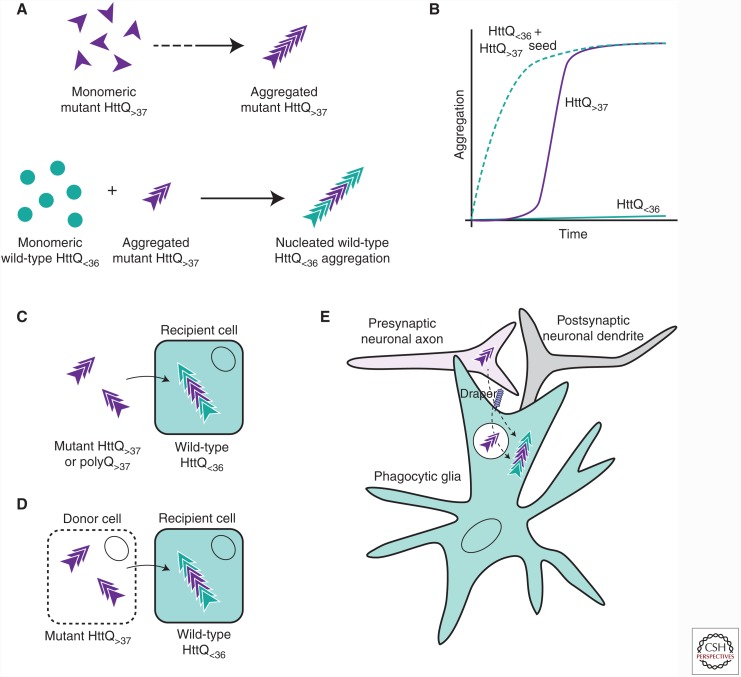
Figure 2.
Prion-like transmission of mutant huntingtin (Htt) and polyQ aggregates. (A) Monomeric mutant Htt proteins containing a polyQ tract of greater than 37 glutamines will self-assemble into amyloid when a critical concentration has been achieved (top). Monomeric WT Htt proteins containing 36 or fewer glutamines do not form aggregates unless nucleated by a preformed Htt aggregate seed (bottom). (B) Aggregation kinetics for mutant Htt (purple line), WT Htt (green line), and WT Htt after addition of an aggregate mutant Htt seed (dotted green line). (C) Purified mutant Htt or polyQ aggregates nucleate the aggregation of WT Htt expressed in the cytoplasm of cultured mammalian cells. (D) Mutant Htt aggregates can transfer between individual co-cultured cells, before or after cell lysis (indicated by dotted cell membrane), and nucleate the aggregation of cytoplasmic WT Htt proteins in recipient cells. (E) In a Drosophila model of Huntington’s disease (HD), mutant Htt aggregates formed in presynaptic olfactory receptor neurons transfer to neighboring phagocytic glia and nucleate the aggregation of cytoplasmic WT Htt in a process that requires the phagocytic receptor, Draper, and its associated engulfment machinery. Whether cytoplasmic entry of neuronal Htt aggregates occurs at the plasma membrane during phagocytic engulfment or after formation of a sealed nascent phagosome is not yet clear.
PolyQ tract expansion causes the proteins responsible for these inherited neurodegenerative diseases to adopt alternative aggregation-prone conformations within some neuronal populations. PolyQ expansion above a critical threshold dramatically increases the aggregation propensity of these proteins in vitro and in vivo (Scherzinger et al. 1997; Huynh et al. 2000; Yoshizawa et al. 2001). However, other regions in these proteins can also influence aggregation kinetics, including regions directly flanking the polyQ stretch located in exon 1 of the huntingtin (Htt) gene product (Steffan et al. 2004; Bhattacharyya et al. 2006; Subramaniam et al. 2009; O’Rourke et al. 2013), the gene that is mutated in HD (Huntington’s Disease Collaborative Research Group 1993). The exact mechanism by which polyQ tract expansion enhances aggregation of these proteins is not fully understood but involves exacerbation of the intrinsic disorder of glutamine-rich sequences and stabilization of the steric zipper core structure of amyloid fibers by glutamine side chains that provide additional hydrogen-bonding surfaces (Thakur et al. 2009; Eisenberg and Jucker 2012; Labbadia and Morimoto 2013).
PolyQ tracts appear in more than 60 proteins encoded by the human genome, most of which are not associated with inherited disease, suggesting that this protein domain has a conserved function (Butland et al. 2007). PolyQ tracts and their flanking regions have been reported to mediate protein–protein interactions with other polyQ-containing proteins (Fiumara et al. 2010; Schaefer et al. 2012), and polyQ proteins are overrepresented in protein networks related to nuclear functions, including transcriptional regulation, chromatin maintenance, and RNA binding (Schaefer et al. 2012). A common feature of polyQ-containing and glutamine-rich proteins is the capacity to convert into a prionogenic form, and these self-replicating conformations have been described to mediate pathogenic and nonpathogenic processes in yeast and higher eukaryotes (Shorter and Lindquist 2005).
NONPATHOGENIC Q- AND Q/N-RICH PRIONS
In the budding yeast Saccharomyces cerevisiae, more than 25 proteins form naturally occurring prions that are heritable between mother and daughter cells (Wickner 1994; True and Lindquist 2000). The protein domain that drives amyloidogenesis is known as a prion-forming domain (PFD) and is often intrinsically disordered and rich in glutamine and asparagine residues (Q/N-rich) (Ross et al. 2004). Yeast prion proteins follow similar aggregation kinetics to mammalian prions with a lag phase during which one or more monomers undergo conversion into a prion state, followed by rapid polymerization into fibrils once a stable nucleus of sufficient size forms (Thual et al. 1999; Chen et al. 2002b). In yeast, prions mediate the non-Mendelian inheritance of traits acquired by mother cells in response to changed environmental conditions (e.g., stress), generating diverse, persistent phenotypes in otherwise isogenic populations of yeast cells (Ter-Avanesyan et al. 1994; Wickner 1994; Patino et al. 1996; True and Lindquist 2000; Halfmann et al. 2012). Adding to this diversity, fibrillar forms of the same prion protein can vary widely in morphological, biochemical, and functional characteristics (Krishnan and Lindquist 2005; Toyama et al. 2007). The existence of such prion “strains” suggests that subtle changes in structure can translate into phenotypic variations among prion populations.
In higher eukaryotes, Q-rich prion proteins can also play roles in nonpathogenic processes. In snails and flies, the synaptic protein CPEB/Orb2 can convert into an amyloidogenic form to recruit monomeric forms of the same protein in a process that is involved in the formation of long-term memories (Si et al. 2010; Majumdar et al. 2012). Formation of CPEB/Orb2 amyloid is required for the maintenance of learned behavior beyond 48 h in snails (Si et al. 2010) and beyond 24 h in flies (Majumdar et al. 2012), suggesting that this self-perpetuating form of CPEB/Orb2 allows certain synaptic phenotypes to persist for extended periods of time. This prion-like behavior of CPEB/Orb2 is stabilized by its N-terminal Q-rich PFD (Fiumara et al. 2010; Si et al. 2010; Majumdar et al. 2012) and is an example of amyloid that has evolved to be functional and physiologically relevant.
PATHOGENIC POLYQ PRIONS
In most neurodegenerative diseases, WT proteins aggregate spontaneously in susceptible neurons, and prion-like processes are then proposed to promote disease progression by spreading the aggregation phenotype to naïve cells. However, in autosomal dominantly inherited disorders such as HD, the mutant aggregation-prone protein is expressed in every neuron throughout life. How then could prion-like transfer of aggregates between individual cells contribute to the pathogenesis of these inherited diseases? The cellular concentration at which mutant polyQ proteins aggregate and the rate at which aggregates are cleared from the nucleus and cytoplasm are related to a cell’s capacity to maintain protein homeostasis and likely vary depending on the specific neuronal subtype (Tsvetkov et al. 2013) and activity (Prahlad and Morimoto 2011). Thus, prion-like transfer of polyQ aggregates could serve to accelerate the progression of disease pathology from more susceptible to less susceptible neurons within an individual brain.
Expression of pathogenic polyQ proteins in yeast, worm, fly, and mouse models recapitulates many aspects of neurodegenerative disease, including cell death, mitochondrial dysfunction, impaired synaptic activity, reactive gliosis, and disrupted protein homeostasis (Mangiarini et al. 1996; Scherzinger et al. 1997; Klement et al. 1998; Warrick et al. 1998; Marsh et al. 2000; Meriin et al. 2002; Morley et al. 2002; Yu et al. 2003; Bennett et al. 2007; Figiel et al. 2012; Tsou et al. 2015). These genetic models have been used in numerous screens to identify modifiers of polyQ disease toxicity. Factors that have been identified to influence the aggregation state or toxicity associated with pathogenic polyQ protein expression include genes involved in promoting protein folding, suppressing aggregation and degradation (most notably, chaperones) (Krobitsch and Lindquist 2000; Bilen and Bonini 2007; Zhang et al. 2010), transcription (Ren et al. 2011; Liu et al. 2012a; Yamanaka et al. 2014), and signal transduction (Giorgini et al. 2005; Doumanis et al. 2009; Schulte et al. 2011). Interestingly, in yeast, expression of a polyQ-expanded Htt exon 1 fragment induces the conversion of yeast prion proteins (e.g., Sup35 and Rnq1) into their self-propagating state (Goehler et al. 2010), suggesting that pathogenic polyQ expression affects global protein homeostasis or potentially promotes cross-seeding of other Q-rich proteins, in which nonidentical side chains hydrogen bond within the amyloid core, termed a heterosteric zipper (Eisenberg and Jucker 2012). More recent screens for modifiers of polyQ-induced toxicity in yeast uncovered a surprisingly strong bias for Q- and N-rich proteins as suppressors (Kayatekin et al. 2014; Ripaud et al. 2014), suggesting that interactions between polyQ and Q/N-rich proteins lead to disrupted protein homeostasis or to cross-seeded amyloid formation.
Prion-like properties of aggregated forms of mutant Htt exon 1 or pure polyQ have now been reported in several model systems, suggesting that this behavior can be attributed to protein aggregates associated with all polyQ diseases. Early in vitro studies showed that purified polyQ peptides of subpathogenic lengths could be induced to aggregate upon introduction of preformed Htt aggregate seeds in a classic amyloidogenic process (Fig. 2A,B) (Preisinger et al. 1999; Chen et al. 2001). Fluorescently labeled aggregates formed from synthetic polyQ peptides were later shown by confocal microscopy to be internalized by cultured mammalian cells, and the aggregates could be delivered to the nucleus by fusion of a positively charged nuclear localization sequence to the N terminus of the polyQ peptides (Yang et al. 2002), suggesting that these aggregates are able to gain access to the nuclear import machinery within the cytoplasm. The ability of extracellular polyQ or recombinant Htt exon 1 fragments to gain access to the cell cytoplasm was established by our laboratory in a study using a cytoplasmic WT Htt exon 1 reporter (Fig. 2C) (Ren et al. 2009). Because WT Htt can only aggregate upon physically encountering an aggregated Htt seed (Chen et al. 2001), changes in the solubility of this WT Htt reporter reflect the ability of exogenous aggregates to enter the cell cytoplasm. When purified aggregates derived from synthetic polyQ peptides of pathogenic length or recombinant mutant Htt proteins were added to cultured cells, the aggregates were visible within the interior of the cell and induced the conversion of soluble, WT Htt from its normally diffuse state to an aggregated form in the cell cytoplasm, indicating that the exogenous aggregates directly nucleated WT Htt aggregation (Ren et al. 2009). The internalized aggregates co-localized with cytoplasmic markers (e.g., hsp70, ubiquitin, and proteasomal subunits), confirming that the aggregates accumulated in the cytoplasmic rather than in an endolysosomal compartment of the cell. The nucleated aggregation of WT Htt was strictly homotypic, as it could not be induced by fibrillar aggregates formed by either Aβ or Sup35. Aggregated WT Htt is able to persist throughout many generations in cell culture, indicating that the nucleated aggregation of WT Htt proteins is a stable, heritable trait in dividing cells. In addition, cell-to-cell transfer of Htt aggregates was shown in experiments in which WT Htt could be converted into an aggregated state by co-cultured cells expressing aggregated, mutant Htt proteins. This intercellular transmission was enhanced by selective lysis of mutant Htt-expressing cells in the co-culture, suggesting that cell death may contribute to the transfer of aggregates between individual cells (Fig. 2D).
The ability of extracellular polyQ and/or Htt aggregates to gain access to the cytoplasm of intact cells has now been observed in a variety of cultured cell lines and primary cell types (Herrera et al. 2011; Trevino et al. 2012; Costanzo et al. 2013; Ruiz-Arlandis et al. 2015), suggesting that the existence of cellular pathways that provide a route for aggregate entry are common to many different cells. However, the mechanistic details of how large aggregates breach a membrane bilayer to enter the cell’s cytoplasm remain largely enigmatic. Our laboratory has shown that aggregate charge and proteins exposed on the cell surface contribute to the efficiency of internalization of purified Htt or polyQ aggregates by cultured cells (Trevino et al. 2012), suggesting that interactions with proteins on the cell surface play an important role in this process. In one study using a mouse catecholaminergic neuronal cell line, physical contact was required for the transfer of Htt aggregates between co-cultured cells (Costanzo et al. 2013). In this same study, tunneling nanotubes, membrane-bound structures that physically connect two cell cytoplasms, were enhanced in cells expressing mutant Htt, and some of these nanotubes were found to contain Htt aggregates, suggesting that they may provide a route for intercellular aggregate movement between cultured neuronal cells.
Endocytosis has been proposed to mediate uptake of extracellular aggregates formed by α-synuclein (Hansen et al. 2011), MAP-tau (Holmes et al. 2013), and mutant SOD1 (Munch et al. 2011) in models of PD, AD, and ALS, respectively. Interestingly, macropinocytosis mediated by cell-surface heparan sulfate proteoglycans was required for entry of tau and α-synuclein aggregates into mouse neural precursor cells, but mutant Htt aggregate internalization by the same cell type was not perturbed by inhibiting this pathway, suggesting that Htt aggregates utilize an alternative receptor and/or entry mechanism from that used by tau and α-synuclein (Holmes et al. 2013). Another recent report showed that inhibitors that block clathrin-dependent endocytosis reduced Htt exon 1 aggregate uptake by undifferentiated and differentiated Neuro2A neuroblastoma cells (Ruiz-Arlandis et al. 2015), suggesting that some endocytic pathways can facilitate transfer of polyQ aggregates to the cytoplasm or that cell type–specific features are important in delivering diverse protein aggregates to the cytoplasmic compartment.
PATHOGENIC POLYQ PRION TRANSMISSION IN VIVO
We currently have very little understanding of how protein aggregates associated with any neurodegenerative disease are able to transfer between cells in vivo. In PD and HD patients, fetal neuronal grafts were found to acquire α-synuclein and Htt aggregates, respectively, over a period of ∼10 yr (Kordower et al. 2008; Li et al. 2008; Cicchetti et al. 2014), suggesting host-to-graft transfer of the protein aggregates. However, unlike Lewy bodies in PD patients, Htt aggregates were only detected in the extracellular space surrounding grafted cells in HD patient brains (Cicchetti et al. 2014). Thus, whether host-derived aggregates are capable of entering the cytoplasm of grafted neurons remains unclear. A recent report from our laboratory showed that in Drosophila brains, mutant Htt aggregates generated in neurons can transfer into neighboring glia, inducing the aggregation of WT Htt expressed in the glial cytoplasm (Pearce et al. 2015). This process requires the glial scavenger receptor, Draper, and downstream phagocytic engulfment machinery, indicating that phagocytosed neuronal Htt aggregates can escape from the phagolysosomal compartment and gain entry into the glial cytoplasm (Fig. 2E). This study showed for the first time that phagocytic glia are capable of eliminating potentially toxic Htt aggregates from neurons, but this seemingly beneficial process can simultaneously facilitate the prion-like spread of aggregates to other cell cytoplasms in an intact brain.
The brain regions that first display aggregate pathology differ in each neurodegenerative disease, suggesting that certain neuronal and glial subtypes are selectively vulnerable to aggregation and/or to aggregation-induced toxicity of a particular protein. The patterns of pathology spread throughout the brain are also unique but highly stereotypical as each of these diseases progresses over time. Evidence from staging of human disease brains (Braak and Braak 1991; Braak et al. 2004) and focal injection of fibrils into brains of mouse models of PD and tauopathies (Clavaguera et al. 2009; Liu et al. 2012b; Luk et al. 2012; Iba et al. 2013) suggests that aggregate pathology spreads between anatomically connected regions of the brain. Thus, certain neurons and/or synaptic connections might be especially vulnerable to aggregate transmission, providing a route for propagation between distant brain regions. Imaging studies performed in HD patients have indicated that cortical thinning occurs before symptomatic disease, followed later by widespread cortical and striatal degeneration, suggesting a hierarchical spread of aggregates from the cortex to the striatum in HD (Deng et al. 2004; Rosas et al. 2008). For a review of the mechanisms of pathogenesis in HD, see Jimenez-Sanchez et al. (2016).
Non-cell-autonomous mechanisms of aggregate-related toxicity have been described in animal models of other polyQ diseases. Purkinje neuron degeneration occurred in a mouse model of SCA7 in which mutant, polyQ-expanded ataxin-7 was expressed in all neurons but excluded from Purkinje neurons (Garden et al. 2002). In another SCA7 mouse model, Bergmann glia degeneration was observed after mutant ataxin-7 expression was selectively silenced in this cell type but maintained in surrounding cerebellar neurons (Furrer et al. 2011). These findings indicate that ataxin-7 aggregation can produce toxic effects in adjacent neurons or glia in the cerebellum, despite the absence of ataxin-7 expression in those cells. A similar finding has been described for aggregates formed by a version of mutant SOD1 that is implicated in ∼10% of cases of familial ALS. Selective inactivation of mutant SOD1 in microglia in a mouse model of ALS dramatically slowed disease progression (Boillee et al. 2006), suggesting that microglial mutant SOD1 can negatively impact the survival of motor neurons. These data point to a role for non-cell-autonomous processes in the propagation of cytopathology. Although the agents of such non-cell-autonomous processes could be prions, other types of cell-to-cell signaling events and even neuronal activity could also contribute to disease spread within an affected brain, and careful study will be necessary to unequivocally establish a role for prion-like mechanisms in each case.
CONCLUDING REMARKS AND UNANSWERED QUESTIONS
Protein aggregation is intimately tied to the pathogenesis of neurodegenerative diseases, but a detailed mechanism for how the presence of protein aggregates or the process of protein aggregation causes neurotoxicity remains unclear. The hypothesis that prion-like processes are responsible for the spread of pathology in nearly all neurodegenerative diseases is gaining momentum, with increasing evidence demonstrating that protein aggregates can transmit between individual cells in vitro and in vivo. Still, many questions remain about how the propagation of protein aggregates influences disease progression and/or severity. What toxic effects do protein aggregation and the spread of protein aggregates have on neurons and glia? What is the molecular nature of the aggregate species that is transmissible in the brain? What roles do extracellular intermediates play in the spread of aggregates in the brain? How do protein aggregates cross biological membranes to access the cytoplasmic compartment? How do glial cells, some types of which are mobilized in response to neuronal damage in the brain, contribute to aggregate propagation?
Therapeutic approaches to treat polyQ and other dominantly inherited neurodegenerative diseases so far have been directed at reducing the concentration of the aggregation-prone mutant gene product by delivery of gene silencing or antisense reagents to affected tissues (Yu et al. 2014). Accumulating evidence indicates that prion-like transmission of protein aggregates may be a common contributing feature to many, if not all, neurodegenerative diseases, raising the possibility that nucleus formation or intercellular transmission could be promising therapeutic targets. Gaining a better understanding of the fundamental molecular mechanisms that pathogenic protein aggregates use to transmit between neurons and/or glia will offer novel and invaluable opportunities for the development of rational strategies that effectively combat these devastating diseases.
ACKNOWLEDGMENTS
We thank Dr. Annemieke van der Goot for critical reading of the manuscript and the members of the Kopito laboratory for many helpful discussions. We apologize for any references that were omitted because of space restrictions.
Footnotes
Editor: Stanley B. Prusiner
Additional Perspectives on Prion Diseases available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Ahmed Z, Cooper J, Murray TK, Garn K, McNaughton E, Clarke H, Parhizkar S, Ward MA, Cavallini A, Jackson S, et al. 2014. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: The pattern of spread is determined by connectivity, not proximity. Acta Neuropathol 127: 667–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper T, Cramp WA, Haig DA, Clarke MC. 1967. Does the agent of scrapie replicate without nucleic acid? Nature 214: 764–766. [DOI] [PubMed] [Google Scholar]
- Arosio P, Knowles TP, Linse S. 2015. On the lag phase in amyloid fibril formation. Phys Chem Chem Phys 17: 7606–7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. 2004. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431: 805–810. [DOI] [PubMed] [Google Scholar]
- Barmada SJ, Serio A, Arjun A, Bilican B, Daub A, Ando DM, Tsvetkov A, Pleiss M, Li X, Peisach D, et al. 2014. Autophagy induction enhances TDP43 turnover and survival in neuronal ALS models. Nat Chem Biol 10: 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MJ, Huey-Tubman KE, Herr AB, West AP Jr, Ross SA, Bjorkman PJ. 2002. A linear lattice model for polyglutamine in CAG-expansion diseases. Proc Natl Acad Sci 99: 11634–11639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett EJ, Shaler TA, Woodman B, Ryu KY, Zaitseva TS, Becker CH, Bates GP, Schulman H, Kopito RR. 2007. Global changes to the ubiquitin system in Huntington’s disease. Nature 448: 704–708. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, Thakur AK, Chellgren VM, Thiagarajan G, Williams AD, Chellgren BW, Creamer TP, Wetzel R. 2006. Oligoproline effects on polyglutamine conformation and aggregation. J Mol Biol 355: 524–535. [DOI] [PubMed] [Google Scholar]
- Bilen J, Bonini NM. 2007. Genome-wide screen for modifiers of ataxin-3 neurodegeneration in Drosophila. PLoS Genet 3: 1950–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. 2006. Onset and progression in inherited ALS determined by motor neurons and microglia. Science 312: 1389–1392. [DOI] [PubMed] [Google Scholar]
- Bolton DC, McKinley MP, Prusiner SB. 1982. Identification of a protein that purifies with the scrapie prion. Science 218: 1309–1311. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82: 239–259. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. 2004. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318: 121–134. [DOI] [PubMed] [Google Scholar]
- Braselmann E, Chaney JL, Clark PL. 2013. Folding the proteome. Trends Biochem Sci 38: 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, et al. 2013. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 74: 20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butland SL, Devon RS, Huang Y, Mead CL, Meynert AM, Neal SJ, Lee SS, Wilkinson A, Yang GS, Yuen MM, et al. 2007. CAG-encoded polyglutamine length polymorphism in the human genome. BMC Genomics 8: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Berthelier V, Yang W, Wetzel R. 2001. Polyglutamine aggregation behavior in vitro supports a recruitment mechanism of cytotoxicity. J Mol Biol 311: 173–182. [DOI] [PubMed] [Google Scholar]
- Chen S, Berthelier V, Hamilton JB, O’Nuallain B, Wetzel R. 2002a. Amyloid-like features of polyglutamine aggregates and their assembly kinetics. Biochemistry 41: 7391–7399. [DOI] [PubMed] [Google Scholar]
- Chen S, Ferrone FA, Wetzel R. 2002b. Huntington’s disease age-of-onset linked to polyglutamine aggregation nucleation. Proc Natl Acad Sci 99: 11884–11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. 2009. Amyloid formation by globular proteins under native conditions. Nat Chem Biol 5: 15–22. [DOI] [PubMed] [Google Scholar]
- Cicchetti F, Lacroix S, Cisbani G, Vallières N, Saint-Pierre M, St-Amour I, Tolouei R, Skepper J, Hauser R, Mantovani D, et al. 2014. Mutant huntingtin is present in neuronal grafts in Huntington’s disease patients. Ann Neurol 76: 31–42. [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, et al. 2009. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11: 909–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M, et al. 2013. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci 110: 9535–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland DW, Hwo SY, Kirschner MW. 1977. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol 116: 227–247. [DOI] [PubMed] [Google Scholar]
- Costanzo M, Abounit S, Marzo L, Danckaert A, Chamoun Z, Roux P, Zurzolo C. 2013. Transfer of polyglutamine aggregates in neuronal cells occurs in tunneling nanotubes. J Cell Sci 126: 3678–3685. [DOI] [PubMed] [Google Scholar]
- Coustou V, Deleu C, Saupe S, Begueret J. 1997. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc Natl Acad Sci 94: 9773–9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das RK, Ruff KM, Pappu RV. 2015. Relating sequence encoded information to form and function of intrinsically disordered proteins. Curr Opin Struct Biol 32: 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, et al. 2012. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73: 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng YP, Albin RL, Penney JB, Young AB, Anderson KD, Reiner A. 2004. Differential loss of striatal projection systems in Huntington’s disease: A quantitative immunohistochemical study. J Chem Neuroanat 27: 143–164. [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. 2009. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc Natl Acad Sci 106: 13010–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumanis J, Wada K, Kino Y, Moore AW, Nukina N. 2009. RNAi screening in Drosophila cells identifies new modifiers of mutant huntingtin aggregation. PLoS ONE 4: e7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyao M, Ambrose C, Myers R, Novelletto A, Persichetti F, Frontali M, Folstein S, Ross C, Franz M, Abbott M, et al. 1993. Trinucleotide repeat length instability and age of onset in Huntington’s disease. Nat Genet 4: 387–392. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Jucker M. 2012. The amyloid state of proteins in human diseases. Cell 148: 1188–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett CM, Wood NW. 2004. Trinucleotide repeats and neurodegenerative disease. Brain 127: 2385–2405. [DOI] [PubMed] [Google Scholar]
- Fändrich M, Dobson CM. 2002. The behaviour of polyamino acids reveals an inverse side chain effect in amyloid structure formation. EMBO J 21: 5682–5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvet B, Mbefo MK, Fares MB, Desobry C, Michael S, Ardah MT, Tsika E, Coune P, Prudent M, Lion N, et al. 2012. α-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem 287: 15345–15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figiel M, Szlachcic WJ, Switonski PM, Gabka A, Krzyzosiak WJ. 2012. Mouse models of polyglutamine diseases: Review and data table. Part I. Mol Neurobiol 46: 393–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumara F, Fioriti L, Kandel ER, Hendrickson WA. 2010. Essential role of coiled coils for aggregation and activity of Q/N-rich prions and PolyQ proteins. Cell 143: 1121–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI. 2009. Propagation of Tau misfolding from the outside to the inside of a cell. J Biol Chem 284: 12845–12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furrer SA, Mohanachandran MS, Waldherr SM, Chang C, Damian VA, Sopher BL, Garden GA, La Spada AR. 2011. Spinocerebellar ataxia type 7 cerebellar disease requires the coordinated action of mutant ataxin-7 in neurons and glia, and displays non-cell-autonomous Bergmann glia degeneration. J Neurosci 31: 16269–16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Libby RT, Fu YH, Kinoshita Y, Huang J, Possin DE, Smith AC, Martinez RA, Fine GC, Grote SK, et al. 2002. Polyglutamine-expanded ataxin-7 promotes non-cell-autonomous purkinje cell degeneration and displays proteolytic cleavage in ataxic transgenic mice. J Neurosci 22: 4897–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam JE, MacPhee CE. 2013. Modelling amyloid fibril formation kinetics: Mechanisms of nucleation and growth. J Phys Condens Matter 25: 373101. [DOI] [PubMed] [Google Scholar]
- Giorgini F, Guidetti P, Nguyen Q, Bennett SC, Muchowski PJ. 2005. A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease. Nat Genet 37: 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler H, Dröge A, Lurz R, Schnoegl S, Chernoff YO, Wanker EE. 2010. Pathogenic polyglutamine tracts are potent inducers of spontaneous Sup35 and Rnq1 amyloidogenesis. PLoS ONE 5: e9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann R, Jarosz DF, Jones SK, Chang A, Lancaster AK, Lindquist S. 2012. Prions are a common mechanism for phenotypic inheritance in wild yeasts. Nature 482: 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergström AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, et al. 2011. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest 121: 715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera F, Tenreiro S, Miller-Fleming L, Outeiro TF. 2011. Visualization of cell-to-cell transmission of mutant huntingtin oligomers. PLoS Curr 3: Rrn1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp MS, Park SH, Hartl FU. 2014. Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol 24: 506–514. [DOI] [PubMed] [Google Scholar]
- Holmes BB, DeVos SL, Kfoury N, Li M, Jacks R, Yanamandra K, Ouidja MO, Brodsky FM, Marasa J, Bagchi DP, et al. 2013. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci 110: E3138–E3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes WM, Klaips CL, Serio TR. 2014. Defining the limits: Protein aggregation and toxicity in vivo. Crit Rev Biochem Mol Biol 49: 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington’s Disease Collaborative Research Group. 1993. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72: 971–983. [DOI] [PubMed] [Google Scholar]
- Huynh DP, Figueroa K, Hoang N, Pulst SM. 2000. Nuclear localization or inclusion body formation of ataxin-2 are not necessary for SCA2 pathogenesis in mouse or human. Nat Genet 26: 44–50. [DOI] [PubMed] [Google Scholar]
- Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM. 2013. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J Neurosci 33: 1024–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Jimenez-Sanchez M, Licitra F, Underwood BR, Rubinsztein DC. 2016. Huntington’s disease: Mechanisms of pathogenesis and therapeutic strategies. Cold Spring Harb Perspect Med 10.1101/cshperspect.a024240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayatekin C, Matlack KE, Hesse WR, Guan Y, Chakrabortee S, Russ J, Wanker EE, Shah JV, Lindquist S. 2014. Prion-like proteins sequester and suppress the toxicity of huntingtin exon 1. Proc Natl Acad Sci 111: 12085–12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. 2013. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem 82: 323–355. [DOI] [PubMed] [Google Scholar]
- Klement IA, Skinner PJ, Kaytor MD, Yi H, Hersch SM, Clark HB, Zoghbi HY, Orr HT. 1998. Ataxin-1 nuclear localization and aggregation: Role in polyglutamine-induced disease in SCA1 transgenic mice. Cell 95: 41–53. [DOI] [PubMed] [Google Scholar]
- Ko LW, Ko HH, Lin WL, Kulathingal JG, Yen SH. 2008. Aggregates assembled from overexpression of wild-type α-synuclein are not toxic to human neuronal cells. J Neuropathol Exp Neurol 67: 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. 2008. Lewy body–like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14: 504–506. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Lindquist SL. 2005. Structural insights into a yeast prion illuminate nucleation and strain diversity. Nature 435: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krobitsch S, Lindquist S. 2000. Aggregation of huntingtin in yeast varies with the length of the polyglutamine expansion and the expression of chaperone proteins. Proc Natl Acad Sci 97: 1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbadia J, Morimoto RI. 2013. Huntington’s disease: Underlying molecular mechanisms and emerging concepts. Trends Biochem Sci 38: 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbehn DR, Hayden M, Paulsen JS; PREDICT-HD Investigators of the Huntington Study Group. 2010. CAG-repeat length and the age of onset in Huntington disease (HD): A review and validation study of statistical approaches. Am J Med Genet Part B 153B: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, Kiritoshi T, Neugebauer V, Jackson GR, Kayed R. 2012. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci Rep 2: 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Björklund A, et al. 2008. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 14: 501–503. [DOI] [PubMed] [Google Scholar]
- Liu CR, Chang CR, Chern Y, Wang TH, Hsieh WC, Shen WC, Chang CY, Chu IC, Deng N, Cohen SN, et al. 2012a. Spt4 is selectively required for transcription of extended trinucleotide repeats. Cell 148: 690–701. [DOI] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. 2012b. Trans-synaptic spread of tau pathology in vivo. PLoS ONE 7: e31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. 2012. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338: 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Cesario WC, White-Grindley E, Jiang H, Ren F, Khan MR, Li L, Choi EM, Kannan K, Guo F, et al. 2012. Critical role of amyloid-like oligomers of Drosophila Orb2 in the persistence of memory. Cell 148: 515–529. [DOI] [PubMed] [Google Scholar]
- Makin OS, Atkins E, Sikorski P, Johansson J, Serpell LC. 2005. Molecular basis for amyloid fibril formation and stability. Proc Natl Acad Sci 102: 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, et al. 1996. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87: 493–506. [DOI] [PubMed] [Google Scholar]
- Marsh JL, Walker H, Theisen H, Zhu YZ, Fielder T, Purcell J, Thompson LM. 2000. Expanded polyglutamine peptides alone are intrinsically cytotoxic and cause neurodegeneration in Drosophila. Hum Mol Genet 9: 13–25. [DOI] [PubMed] [Google Scholar]
- McKinley MP, Bolton DC, Prusiner SB. 1983. A protease-resistant protein is a structural component of the scrapie prion. Cell 35: 57–62. [DOI] [PubMed] [Google Scholar]
- Meriin AB, Zhang X, He X, Newnam GP, Chernoff YO, Sherman MY. 2002. Huntington toxicity in yeast model depends on polyglutamine aggregation mediated by a prion-like protein Rnq1. J Cell Biol 157: 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JF, Brignull HR, Weyers JJ, Morimoto RI. 2002. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci 99: 10417–10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. 2000. High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: Synaptotoxicity without plaque formation. J Neurosci 20: 4050–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukrasch MD, Bibow S, Korukottu J, Jeganathan S, Biernat J, Griesinger C, Mandelkow E, Zweckstetter M. 2009. Structural polymorphism of 441-residue tau at single residue resolution. PLoS Biol 7: e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch C, O’Brien J, Bertolotti A. 2011. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc Natl Acad Sci 108: 3548–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, Grothe R, Eisenberg D. 2005. Structure of the cross-β spine of amyloid-like fibrils. Nature 435: 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka T, Masuda-Suzukake M, Arai T, Hasegawa Y, Akatsu H, Obi T, Yoshida M, Murayama S, Mann DM, Akiyama H, et al. 2013. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep 4: 124–134. [DOI] [PubMed] [Google Scholar]
- O’Rourke JG, Gareau JR, Ochaba J, Song W, Raskó T, Reverter D, Lee J, Monteys AM, Pallos J, Mee L, et al. 2013. SUMO-2 and PIAS1 modulate insoluble mutant huntingtin protein accumulation. Cell Rep 4: 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino MM, Liu JJ, Glover JR, Lindquist S. 1996. Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science 273: 622–626. [DOI] [PubMed] [Google Scholar]
- Pattison IH, Jones KM. 1967. The possible nature of the transmissible agent of scrapie. Vet Rec 80: 2–9. [DOI] [PubMed] [Google Scholar]
- Pearce MM, Spartz EJ, Hong W, Luo L, Kopito RR. 2015. Prion-like transmission of neuronal huntingtin aggregates to phagocytic glia in the Drosophila brain. Nat Commun 6: 6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecho-Vrieseling E, Rieker C, Fuchs S, Bleckmann D, Esposito MS, Botta P, Goldstein C, Bernhard M, Galimberti I, Müller M, et al. 2014. Transneuronal propagation of mutant huntingtin contributes to non-cell autonomous pathology in neurons. Nat Neurosci 17: 1064–1072. [DOI] [PubMed] [Google Scholar]
- Pieri L, Madiona K, Bousset L, Melki R. 2012. Fibrillar α-synuclein and huntingtin exon 1 assemblies are toxic to the cells. Biophys J 102: 2894–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. 2009. Biological and chemical approaches to diseases of proteostasis deficiency. Ann Rev Biochem 78: 959–991. [DOI] [PubMed] [Google Scholar]
- Prahlad V, Morimoto RI. 2011. Neuronal circuitry regulates the response of Caenorhabditis elegans to misfolded proteins. Proc Natl Acad Sci 108: 14204–14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisinger E, Jordan BM, Kazantsev A, Housman D. 1999. Evidence for a recruitment and sequestration mechanism in Huntington’s disease. Philos Trans R Soc Lond B Biol Sci 354: 1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. 2013. Biology and genetics of prions causing neurodegeneration. Annu Rev Genet 47: 601–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. 2009. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol 11: 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Jegga AG, Zhang M, Deng J, Liu J, Gordon CB, Aronow BJ, Lu LJ, Zhang B, Ma J. 2011. A Drosophila model of the neurodegenerative disease SCA17 reveals a role of RBP-J/Su(H) in modulating the pathological outcome. Hum Mol Genet 20: 3424–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripaud L, Chumakova V, Antonin M, Hastie AR, Pinkert S, Korner R, Ruff KM, Pappu RV, Hornburg D, Mann M, et al. 2014. Overexpression of Q-rich prion-like proteins suppresses polyQ cytotoxicity and alters the polyQ interactome. Proc Natl Acad Sci 111: 18219–18224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Salat DH, Lee SY, Zaleta AK, Pappu V, Fischl B, Greve D, Hevelone N, Hersch SM. 2008. Cerebral cortex and the clinical expression of Huntington’s disease: Complexity and heterogeneity. Brain 131: 1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ED, Baxa U, Wickner RB. 2004. Scrambled prion domains form prions and amyloid. Mol Cell Biol 24: 7206–7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Arlandis G, Pieri L, Bousset L, Melki R. 2015. Binding, internalization and fate of Huntingtin Exon1 fibrillar assemblies in mitotic and non-mitotic neuroblastoma cells. Neuropathol Appl Neurobiol 42: 137–152. [DOI] [PubMed] [Google Scholar]
- Schaefer MH, Wanker EE, Andrade-Navarro MA. 2012. Evolution and function of CAG/polyglutamine repeats in protein–protein interaction networks. Nucleic Acids Res 40: 4273–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E, Lurz R, Turmaine M, Mangiarini L, Hollenbach B, Hasenbank R, Bates GP, Davies SW, Lehrach H, Wanker EE. 1997. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell 90: 549–558. [DOI] [PubMed] [Google Scholar]
- Schulte J, Sepp KJ, Wu C, Hong P, Littleton JT. 2011. High-content chemical and RNAi screens for suppressors of neurotoxicity in a Huntington’s disease model. PLoS ONE 6: e23841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J, Lindquist S. 2005. Prions as adaptive conduits of memory and inheritance. Nat Rev Genet 6: 435–450. [DOI] [PubMed] [Google Scholar]
- Si K, Choi YB, White-Grindley E, Majumdar A, Kandel ER. 2010. Aplysia CPEB can form prion-like multimers in sensory neurons that contribute to long-term facilitation. Cell 140: 421–435. [DOI] [PubMed] [Google Scholar]
- Smith HL, Li W, Cheetham ME. 2015. Molecular chaperones and neuronal proteostasis. Semin Cell Dev Biol 40: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan JS, Agrawal N, Pallos J, Rockabrand E, Trotman LC, Slepko N, Illes K, Lukacsovich T, Zhu YZ, Cattaneo E, et al. 2004. SUMO modification of Huntingtin and Huntington’s disease pathology. Science 304: 100–104. [DOI] [PubMed] [Google Scholar]
- Stephan A, Laroche S, Davis S. 2001. Generation of aggregated β-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J Neurosci 21: 5703–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Sixt KM, Barrow R, Snyder SH. 2009. Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science 324: 1327–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter-Avanesyan MD, Dagkesamanskaya AR, Kushnirov VV, Smirnov VN. 1994. The SUP35 omnipotent suppressor gene is involved in the maintenance of the non-Mendelian determinant [psi+] in the yeast Saccharomyces cerevisiae. Genetics 137: 671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur AK, Jayaraman M, Mishra R, Thakur M, Chellgren VM, Byeon IJ, Anjum DH, Kodali R, Creamer TP, Conway JF, et al. 2009. Polyglutamine disruption of the huntingtin exon 1 N terminus triggers a complex aggregation mechanism. Nat Struct Mol Biol 16: 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thual C, Komar AA, Bousset L, Fernandez-Bellot E, Cullin C, Melki R. 1999. Structural characterization of Saccharomyces cerevisiae prion-like protein Ure2. J Biol Chem 274: 13666–13674. [DOI] [PubMed] [Google Scholar]
- Toyama BH, Kelly MJ, Gross JD, Weissman JS. 2007. The structural basis of yeast prion strain variants. Nature 449: 233–237. [DOI] [PubMed] [Google Scholar]
- Trevino RS, Lauckner JE, Sourigues Y, Pearce MM, Bousset L, Melki R, Kopito RR. 2012. Fibrillar structure and charge determine the interaction of polyglutamine protein aggregates with the cell surface. J Biol Chem 287: 29722–29728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True HL, Lindquist SL. 2000. A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature 407: 477–483. [DOI] [PubMed] [Google Scholar]
- Tsemekhman K, Goldschmidt L, Eisenberg D, Baker D. 2007. Cooperative hydrogen bonding in amyloid formation. Protein Sci 16: 761–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou WL, Hosking RR, Burr AA, Sutton JR, Ouyang M, Du X, Gomez CM, Todi SV. 2015. DnaJ-1 and karyopherin α3 suppress degeneration in a new Drosophila model of spinocerebellar ataxia type 6. Hum Mol Genet 24: 4385–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkov AS, Arrasate M, Barmada S, Ando DM, Sharma P, Shaby BA, Finkbeiner S. 2013. Proteostasis of polyglutamine varies among neurons and predicts neurodegeneration. Nat Chem Biol 9: 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. 2011. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72: 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. 2002. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416: 535–539. [DOI] [PubMed] [Google Scholar]
- Warrick JM, Paulson HL, Gray-Board GL, Bui QT, Fischbeck KH, Pittman RN, Bonini NM. 1998. Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell 93: 939–949. [DOI] [PubMed] [Google Scholar]
- Wickner RB. 1994. [URE3] as an altered URE2 protein: Evidence for a prion analog in Saccharomyces cerevisiae. Science 264: 566–569. [DOI] [PubMed] [Google Scholar]
- Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, et al. 2011. In vivo demonstration that α-synuclein oligomers are toxic. Proc Natl Acad Sci 108: 4194–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PE, Dyson HJ. 2015. Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol 16: 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Wong HK, Tosaki A, Bauer PO, Wada K, Kurosawa M, Shimogori T, Hattori N, Nukina N. 2014. Large-scale RNA interference screening in mammalian cells identifies novel regulators of mutant huntingtin aggregation. PLoS ONE 9: e93891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Dunlap JR, Andrews RB, Wetzel R. 2002. Aggregated polyglutamine peptides delivered to nuclei are toxic to mammalian cells. Hum Mol Genet 11: 2905–2917. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T, Yoshida H, Shoji S. 2001. Differential susceptibility of cultured cell lines to aggregate formation and cell death produced by the truncated Machado-Joseph disease gene product with an expanded polyglutamine stretch. Brain Res Bull 56: 349–352. [DOI] [PubMed] [Google Scholar]
- Yu ZX, Li SH, Evans J, Pillarisetti A, Li H, Li XJ. 2003. Mutant huntingtin causes context-dependent neurodegeneration in mice with Huntington’s disease. J Neurosci 23: 2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Liang Y, Palacino J, Difiglia M, Lu B. 2014. Drugging unconventional targets: Insights from Huntington’s disease. Trends Pharmacol Sci 35: 53–62. [DOI] [PubMed] [Google Scholar]
- Zhang S, Binari R, Zhou R, Perrimon N. 2010. A genomewide RNA interference screen for modifiers of aggregates formation by mutant Huntingtin in Drosophila. Genetics 184: 1165–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]