Abstract
The reliance of the immune system on constitutive microbial stimulation support the idea that both responsiveness to vaccines and vaccine design need to be considered in the context of host–microbiota interactions. Manipulation of microbe function or composition via diet alteration or microbiota engraftment may soon become a viable approach to control immunity and, as such, vaccine responses. Learning from our endogenous original adjuvants could be critical in overcoming the enormous hurdle of vaccine design against the numerous pathogens that cause chronic infection. Going forward, rationally designed vaccines that take advantage of the inherent adjuvant properties of the microbiota could have a major impact on the prevention of disease.
Great Debates.
What are the most interesting topics likely to come up over dinner or drinks with your colleagues? Or, more importantly, what are the topics that don't come up because they are a little too controversial? In Immune Memory and Vaccines: Great Debates, Editors Rafi Ahmed and Shane Crotty have put together a collection of articles on such questions, written by thought leaders in these fields, with the freedom to talk about the issues as they see fit. This short, innovative format aims to bring a fresh perspective by encouraging authors to be opinionated, focus on what is most interesting and current, and avoid restating introductory material covered in many other reviews.
The Editors posed 13 interesting questions critical for our understanding of vaccines and immune memory to a broad group of experts in the field. In each case, several different perspectives are provided. Note that while each author knew that there were additional scientists addressing the same question, they did not know who these authors were, which ensured the independence of the opinions and perspectives expressed in each article. Our hope is that readers enjoy these articles and that they trigger many more conversations on these important topics.
Humans are inhabited by a diverse commensal microbiota that consists of bacteria, fungi, viruses, and eukaryotic species (Ley et al. 2006). This relationship between host and commensals has evolved over millennia and is beneficial to both parties. For example, commensals are provided a nutrient-rich environment to inhabit, whereas the host gains energy-providing metabolites that would otherwise be unavailable (Tremaroli and Backhed 2012). However, in recent years it has become increasingly clear that the benefits derived by the host from commensals extend far beyond those that are metabolic, with the microbiota shown to be critical in many aspects of human physiology, health, and disease (Brestoff and Artis 2013; Belkaid and Hand 2014). Of relevance to the present discussion, the microbiota has been shown to play a central role in ensuring that cells of the immune system are appropriately regulated at steady state and in the context of infectious challenges (Honda and Littman 2016). The constitutive involvement of the microbiota in the regulation and function of the immune system supports the idea that vaccine design and trials should be approached in the context of the constant dialogue between microbes and the host, an area of research that has been given little attention to date (Ferreira et al. 2010; Valdez et al. 2014). In this review, we will highlight recent findings supporting the idea of a role for the microbiota in controlling vaccine responses before discussing the potential mechanisms of action and opportunities to harness such knowledge to increase vaccine efficacy.
INFLUENCE OF COMMENSAL MICROBIOTA ON THE IMMUNE SYSTEM AND RESPONSES TO INFECTION
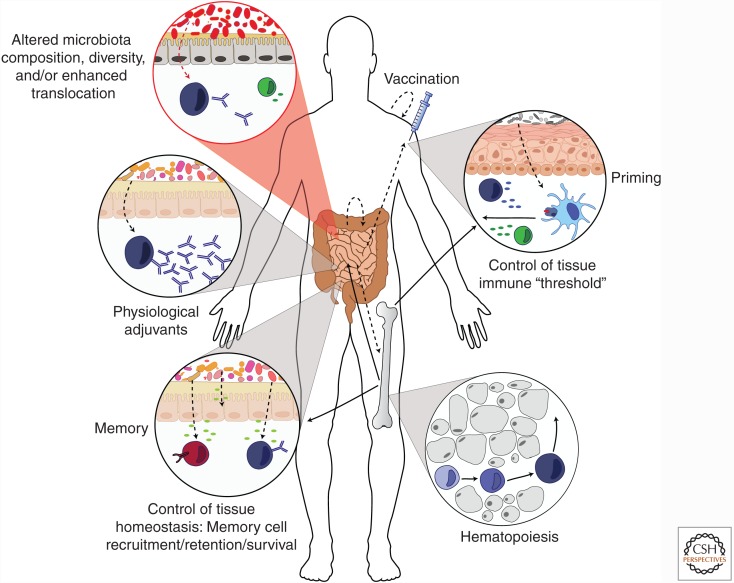
The majority of infections occur at peripheral tissues such as the gut, skin, or lung. These sites contain a complex microbiota, as well as an intricate network of immune cells that are positioned to rapidly respond to invading pathogens (Sheridan and Lefrancois 2011). At these sites, the microbiota has been shown to control diverse aspects of immunity including the development of the immune system, the calibration of innate responses, and the induction of both effector and regulatory responses (Fig. 1) (Mazmanian et al. 2005; Belkaid and Hand 2014; Belkaid and Segre 2014; Trama et al. 2014; Jeffries et al. 2016; Kim et al. 2016; Zeng et al. 2016). As such, the microbiota represents a powerful adjuvant of the immune system that is able to promote adaptive responses against a wide range of bacterial, viral, fungal, and parasitic infections (Brandl et al. 2007; Hall et al. 2008; Ivanov et al. 2009; Zeng et al. 2016). Microbiota control of the immune system occurs via numerous mechanisms, including the generation of metabolites that activate lymphocytes or antigen-presenting cells, the activation of the inflammasome in innate cell populations, and by directly acting on epithelial cells (Belkaid and Hand 2014; Kim et al. 2016). In addition to its direct impact at barrier sites, the microbiota also influences the quality and amplitude of immune responses at distal sites (Belkaid and Hand 2014). One such example of systemic control is mediated by constitutive antibody responses to the microbiota that not only provide protection against gut microbiota translocation but also against infections with unrelated pathogenic bacteria via recognition of conserved outer-membrane molecules (Zeng et al. 2016). In this context, commensal-specific antibodies have been shown to also cross-react with HIV-1 (Trama et al. 2014; Jeffries et al. 2016), supporting the idea that preexisting commensal-specific antibodies could influence early responses (positively in the context of neutralization or negatively via enhanced infectivity) to a diverse range of microbes. How the preexistence of microbiota-specific antibodies controls the quality of vaccine responses and in particular those using “live” organisms remains to be established.
Figure 1.
Influence of commensals on vaccine responses. Commensal bacteria influence the immune response in a number of ways, with these effects mediated either locally or from distal sites. Such influences include the promotion of hematopoiesis in bone marrow progenitor cells, which can then migrate throughout the body. In peripheral tissues such as the skin and gut, the microbiota can regulate the “immune threshold” of innate cells such as antigen-presenting cells or epithelial cells, allowing for rapid and efficient responses on activation. During a response to vaccination, commensals can directly promote the function of the adaptive immune system, such as by providing a source of physiological adjuvants in the gut that promote the production of antibody by B cells. Following the peak of a response, commensals may also be involved in creating an environmental niche within tissues that allow for the recruitment, development, and survival of long-lived memory cells. In addition (red circle), an altered relationship with the microbiota (e.g., decrease in microbial diversity or increase microbial translocation) of the gut microflora in settings of malnutrition and/or chronic infections could result in heightened systemic inflammation leading to blunted immune responses to vaccines. Solid lines, immune cell movement; dotted lines, microbiota effect.
Regardless of the mechanisms involved, a consistent theme across numerous studies is that commensals influence the immune system in a way that the threshold required to respond to infection is lowered, thereby allowing for more rapid and efficient responses to pathogens. Such reliance of the immune system on constitutive microbial stimulation indicates that both responsiveness to vaccines and vaccine design need to be considered in the context of host–microbiota interactions.
INFLUENCE OF COMMENSAL MICROBIOTA ON VACCINE EFFICACY: MICROBIOTA STRATIFICATION AS A MEANS TO PREDICT VACCINE EFFICACY?
Vaccination has drastically lowered the incidence rate of infections with pathogens such as polio and measles. However, there are no commercially available vaccines that currently exist for numerous life-threatening diseases caused by infections such as tuberculosis, AIDS, or malaria (Holmgren and Czerkinsky 2005). Whether the microbiota can be harnessed to improve the efficacy of vaccination against these pathogens remains an open question. A possible link between the microbiota and vaccine efficacy was first shown in a model of oral vaccination using heat-labile enterotoxin of enterotoxigenic Escherichia coli as adjuvant (LT R192G/L211A) (Hall et al. 2008; Norton et al. 2011). In this setting, depletion of the gut microbiota was associated with profoundly depressed Th1 and Th17 responses to the antigen (Hall et al. 2008). Similarly, optimal antibody responses to the seasonal trivalent influenza vaccine (TIV), as well as to the polio vaccine (IPOL) required the presence of gut commensals (Oh et al. 2014). Recent studies have suggested that the gut microbiota could influence vaccine efficacy in human and nonhuman primates (Valdez et al. 2014). For instance, a study using Cynomolgus macaques supports the idea that a stable and more diverse gut microbiota correlates with a better response to vaccination and subsequent immunity (Seekatz et al. 2013). This finding is consistent with another study involving a small human cohort, in which the majority of individuals that responded to vaccination had greater community richness and diversity among their gut microbiota (Eloe-Fadrosh et al. 2013). However, we cannot exclude the possibility that, as for many other microbiota-related observations, a lack of microbial diversity may be a “red herring” and in fact results from underlying immune defects in the less responsive groups. Nonetheless, although these studies remain limited in number and in scope, their findings raise some intriguing possibilities. In the context of future vaccine trials, it may be important to stratify individuals based on their microbiota profile and, more importantly, microbiota metabolism as well as host-genetic influences. Such lines of research should provide insight into the link between host-genetic variation in shaping both immunity and the composition of the human microbiome (Goodrich et al. 2004; Blekhman et al. 2015) and provide a starting point toward understanding factors that predict vaccine success.
RESIDENT COMMENSAL MICROFLORA IN ALTERING THE TISSUE ENVIRONMENT TO PROMOTE THE FORMATION AND SURVIVAL OF MEMORY CELLS
Because virtually all aspects of the immune system, ranging from hematopoiesis to lymphocyte function, can be controlled by the microbiota, the response to vaccination, as well as the intensity of the response and memory formation, are likely controlled directly or indirectly by these microbial partners (Fig. 1). Of interest, the tonic action of the microbiota on tissue immunity could promote the formation of a niche within the tissue microenvironment that results in enhanced recruitment, survival, and maturation of both T and B cells. For instance, an absence of commensals is associated with decreased amounts of T-cell chemoattractants as well as the survival factors interleukin (IL)-7 and IL-15 (Vonarbourg et al. 2010; Fink et al. 2012; Jiang et al. 2013). Another systemic control mediated by the microbiota occurs via the manipulation of the metabolic landscape. For instance, fatty acids that are regulated by gut commensals are also important in the development, survival, and function of memory T cells (Martin et al. 2007; Pearce et al. 2009; van der Windt et al. 2013; Cui et al. 2015). Furthermore, microbiota-dependent fermentation of plant-derived polysaccharides into short chain fatty acids also promotes the differentiation of B cells into plasma cells (Kim et al. 2016). Thus, it is conceivable that the commensal microbiota could be exploited to create an environment better equipped to not only attract memory cells but also to promote a metabolic niche compatible with enhanced memory formation and survival.
HOST-MICROBIOTA DYSREGULATION AS AN UNDERLYING CAUSE OF VACCINE FAILURE?
The role of the commensal microbiota could be particularly important when considering vaccine-induced immune responses in low- to middle-income countries, which are typically some of the worst affected by infection (Valdez et al. 2014). Studies on the effectiveness of vaccination in these areas have consistently shown blunted responses when compared to that of high-income countries. These defects are particularly striking when considering oral vaccines. For instance, oral vaccines for Rotavirus, Poliomyelitis, Vibrio cholerae, and Shigella administered to children in low- to middle-income countries often fail to protect with the same degree of efficacy as in high-income countries (Hallander et al. 2002; Grassly et al. 2009; Jiang et al. 2010; Levine 2010; Valdez et al. 2014). Several interrelated conditions including persistent infections, gut inflammation, malnutrition, and environmental enteropathy have been proposed to contribute to this important public health issue (Levine 2010; Korpe and Petri 2012). Aberrant relationships with the microbiota under these extreme settings could also contribute to these defects. Indeed, children from defined low-income areas of the world have a distinct gut microbiota compared to those in high-income countries with the “malnourished microbiota” enriched in microbes with invasive and/or inflammatory properties (Yatsunenko et al. 2012). Another phenomenon contributing to microbiota-induced vaccine deficiencies could be associated with immune defects imposed by defined infections and sustained by the microbiota. For instance, acute infections can have dramatic and long-term consequences for tissue-specific immunity via tissue remodeling, a process that can be sustained by the microbiota in the gut (Fonseca et al. 2015). Chronic exposure to pathogens and malnutrition is also associated with a phenomenon referred to as “leaky gut,” a setting leading to increased systemic microbial translocation and subsequent inflammation (Brenchley and Douek 2012; Valdez et al. 2014). Such a heightened inflammatory tone caused by both invasive microbes and enhanced microbial translocation may play a negative role in the proper orchestration of adaptive immune responses to vaccines and preclude the establishment of a healthy memory pool (Fig. 1). While resolving the environmental challenges underlying immune suppression in parts of the world deprived of basic infrastructure remains the priority, ongoing work currently exploring the microbiota of these vulnerable populations (Humphrey 2009) may allow for the development of interventions aimed at tackling defined classes of microbes, metabolites, or pathogens to improve vaccine efficacy.
LEARNING FROM THE ENDOGENOUS ADJUVANTS: THE COMMENSAL MICROBIOTA AND THEIR PRODUCTS AS A SOURCE OF PHYSIOLOGICAL ADJUVANTS?
A critical aspect in vaccine design is that the antigen administered is able to induce a potent immune response. However, antigens administered alone are not immunogenic, and so must be coupled to an immunostimulatory component, referred to as an adjuvant. The choice of an appropriate adjuvant is critical, as it can dramatically impact the long-term protective effects of the vaccine (Galli et al. 2009). A concern and major challenge going forward for the design of future vaccines is adjuvant safety (Mutsch et al. 2004). This may provide a unique opportunity to exploit the resident commensal microbiota and their products, which have been shown to possess natural adjuvant properties (Fig. 1). For example, the DNA of commensals is critical in regulating effector T-cell responses in the gut (Hall et al. 2008). Optimal antibody responses to the seasonal influenza TIV vaccine as well as to the IPOL polio vaccine require the presence of gut commensals (Oh et al. 2014). Specifically, the presence of E. coli species expressing flagellin were essential, with this component able to directly promote plasma cell differentiation as well as stimulate lymph node macrophages to produce plasma cell growth factors (Oh et al. 2014). It therefore appears that defined commensals and their products can promote the development of vaccine-induced immunity. While the identification of novel molecular determinants and the underlying mechanisms of microbiota–host interactions remains in its infancy, such lines of investigation appear promising. Indeed, because the microbiota has coevolved with its host to finely tune the unique requirements of the gut, these microbes may provide highly adapted tissue-specific adjuvants. Uncovering these pathways and the microbiota-derived molecules (Donia and Fischbach 2015; Medema and Fischbach 2015) involved in these processes may allow for the development of novel classes of adjuvants capable of boosting local immunity while preserving tissue homeostasis.
FUTURE PERSPECTIVES
Manipulation of microbe function or composition via diet alteration or microbiota engraftment may soon become a viable approach to control immunity and, as such, vaccine responses. This is not only true for the gut microbiota but also for all barrier tissues. For instance, at sites such as the skin or lung, which are characterized by low microbial biomass, subtle alterations in defined nutrients (Scharschmidt and Fischbach 2013) may have a dramatic impact on the microbiota composition. Going forward, rationally designed vaccines that take advantage of the inherent adjuvant properties of the microbiota could have a major impact on the prevention of disease. To the initial question “Can the microbiota be exploited to improve the efficacy of vaccines?” the answer is that learning from our endogenous original adjuvants could be critical in overcoming the enormous hurdle of vaccine design against the numerous microbes that cause chronic infections.
ACKNOWLEDGMENTS
This work is supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases (NIAID).
Footnotes
Editors: Shane Crotty and Rafi Ahmed
Additional Perspectives on Immune Memory and Vaccines: Great Debates available at www.cshperspectives.org
REFERENCES
- Belkaid Y, Hand TW. 2014. Role of the microbiota in immunity and inflammation. Cell 157: 121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Segre JA. 2014. Dialogue between skin microbiota and immunity. Science 346: 954–959. [DOI] [PubMed] [Google Scholar]
- Blekhman R, Goodrich JK, Huang K, Sun Q, Bukowski R, Bell JT, Spector TD, Keinan A, Ley RE, Gevers D, et al. 2015. Host genetic variation impacts microbiome composition across human body sites. Genome Biol 16: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. 2007. MyD88-mediated signals induce the bactericidal lectin RegIII γ and protect mice against intestinal Listeria monocytogenes infection. J Exp Med 204: 1891–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Douek DC. 2012. Microbial translocation across the GI tract. Annu Rev Immunol 30: 149–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff JR, Artis D. 2013. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol 14: 676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Staron MM, Gray SM, Ho PC, Amezquita RA, Wu J, Kaech SM. 2015. IL-7-induced glycerol transport and TAG synthesis promotes memory CD8+ T cell longevity. Cell 161: 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donia MS, Fischbach MA. 2015. HUMAN MICROBIOTA. Small molecules from the human microbiota. Science 349: 1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloe-Fadrosh EA, McArthur MA, Seekatz AM, Drabek EF, Rasko DA, Sztein MB, Fraser CM. 2013. Impact of oral typhoid vaccination on the human gut microbiota and correlations with S. Typhi-specific immunological responses. PLoS ONE 8: e62026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira RB, Antunes LC, Finlay BB. 2010. Should the human microbiome be considered when developing vaccines? PLoS Pathog 6: e1001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink LN, Metzdorff SB, Zeuthen LH, Nellemann C, Kristensen MB, Licht TR, Frøkiær H. 2012. Establishment of tolerance to commensal bacteria requires a complex microbiota and is accompanied by decreased intestinal chemokine expression. Am J Physiol Gastrointest Liver Physiol 302: G55–G65. [DOI] [PubMed] [Google Scholar]
- Fonseca DM, Hand TW, Han SJ, Gerner MY, Glatman Zaretsky A, Byrd AL, Harrison OJ, Ortiz AM, Quinones M, Trinchieri G, et al. 2015. Microbiota-dependent sequelae of acute infection compromise tissue-specific immunity. Cell 163: 354–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, Malzone C, Castellino F, Gentile C, McNally T, et al. 2009. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci 106: 7962–7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, et al. 2004. Human genetics shape the gut microbiome. Cell 159: 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassly NC, Jafari H, Bahl S, Durrani S, Wenger J, Sutter RW, Aylward RB. 2009. Mucosal immunity after vaccination with monovalent and trivalent oral poliovirus vaccine in India. J Infect Dis 200: 794–801. [DOI] [PubMed] [Google Scholar]
- Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. 2008. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity 29: 637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallander HO, Paniagua M, Espinoza F, Askelöf P, Corrales E, Ringman M, Storsaeter J. 2002. Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine 21: 138–145. [DOI] [PubMed] [Google Scholar]
- Holmgren J, Czerkinsky C. 2005. Mucosal immunity and vaccines. Nat Med 11: S45–S53. [DOI] [PubMed] [Google Scholar]
- Honda K, Littman DR. 2016. The microbiota in adaptive immune homeostasis and disease. Nature 535: 75–84. [DOI] [PubMed] [Google Scholar]
- Humphrey JH. 2009. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 374: 1032–1035. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139: 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries TL Jr, Sacha CR, Pollara J, Himes J, Jaeger FH, Dennison SM, McGuire E, Kunz E, Eudailey JA, Trama AM, et al. 2016. The function and affinity maturation of HIV-1 gp120-specific monoclonal antibodies derived from colostral B cells. Mucosal Immunol 9: 414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang V, Jiang B, Tate J, Parashar UD, Patel MM. 2010. Performance of rotavirus vaccines in developed and developing countries. Hum Vaccin 6: 532–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Wang X, Zeng B, Liu L, Tardivel A, Wei H, Han J, MacDonald HR, Tschopp J, Tian Z, et al. 2013. Recognition of gut microbiota by NOD2 is essential for the homeostasis of intestinal intraepithelial lymphocytes. J Exp Med 210: 2465–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Qie Y, Park J, Kim CH. 2016. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 20: 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpe PS, Petri WA Jr. 2012. Environmental enteropathy: Critical implications of a poorly understood condition. Trends Mol Med 18: 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MM. 2010. Immunogenicity and efficacy of oral vaccines in developing countries: Lessons from a live cholera vaccine. BMC Biol 8: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. 2006. Microbial ecology: Human gut microbes associated with obesity. Nature 444: 1022–1023. [DOI] [PubMed] [Google Scholar]
- Martin FP, Dumas ME, Wang Y, Legido-Quigley C, Yap IK, Tang H, Zirah S, Murphy GM, Cloarec O, Lindon JC, et al. 2007. A top-down systems biology view of microbiome–mammalian metabolic interactions in a mouse model. Mol Syst Biol 3: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122: 107–118. [DOI] [PubMed] [Google Scholar]
- Medema MH, Fischbach MA. 2015. Computational approaches to natural product discovery. Nat Chem Biol 11: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R. 2004. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med 350: 896–903. [DOI] [PubMed] [Google Scholar]
- Norton EB, Lawson LB, Freytag LC, Clements JD. 2011. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin Vaccine Immunol 18: 546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JZ, Ravindran R, Chassaing B, Carvalho FA, Maddur MS, Bower M, Hakimpour P, Gill KP, Nakaya HI, Yarovinsky F, et al. 2014. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity 41: 478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. 2009. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharschmidt TC, Fischbach MA. 2013. What lives on our skin: Ecology, genomics and therapeutic opportunities of the skin microbiome. Drug Discov Today 10: e83–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seekatz AM, Panda A, Rasko DA, Toapanta FR, Eloe-Fadrosh EA, Khan AQ, Liu Z, Shipley ST, Detolla LJ, Sztein MB, et al. 2013. Differential response of the cynomolgus macaque gut microbiota to Shigella infection. PLoS ONE 8: e64212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan BS, Lefrancois L. 2011. Regional and mucosal memory T cells. Nat Immunol 12: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trama AM, Moody MA, Alam SM, Jaeger FH, Lockwood B, Parks R, Lloyd KE, Stolarchuk C, Scearce R, Foulger A, et al. 2014. HIV-1 envelope gp41 antibodies can originate from terminal ileum B cells that share cross-reactivity with commensal bacteria. Cell Host Microbe 16: 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaroli V, Backhed F. 2012. Functional interactions between the gut microbiota and host metabolism. Nature 489: 242–249. [DOI] [PubMed] [Google Scholar]
- Valdez Y, Brown EM, Finlay BB. 2014. Influence of the microbiota on vaccine effectiveness. Trends Immunol 35: 526–537. [DOI] [PubMed] [Google Scholar]
- van der Windt GJ, O'Sullivan D, Everts B, Huang SC, Buck MD, Curtis JD, Chang CH, Smith AM, Ai T, Faubert B, et al. 2013. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc Natl Acad Sci 110: 14336–14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Hölscher C, et al. 2010. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt+ innate lymphocytes. Immunity 33: 736–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, et al. 2012. Human gut microbiome viewed across age and geography. Nature 486: 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, Inohara N, Núñnez G. 2016. Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity 44: 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]