Abstract
In contrast to live attenuated vaccines, which are designed to induce immunity through a time-limited bloom in systemic tissues, the microbiota is a persistent feature of body surfaces, especially the intestine. The immune responses to the microbiota are idiosyncratic depending on the niche intimacy of different taxa and generally adapt the host to avoid overgrowth and maintain mutualism rather than to eliminate the organisms of that taxon. Both the microbiota and the host have so much molecular cross talk controlling each other, that the prokaryotic and the eukaryotic spaces of the host-microbial superorganism are federal rather than sovereign. This molecular cross talk is vital for the immune system to develop its mature form. Nevertheless, the microbiota/host biomass spaces are rather well separated: The microbiota also limits colonization and penetration of pathogens through intense metabolic competition. Immune responses to those members of the microbiota mutually adapted to intimate association at mucosal surfaces have attractive potential durability, but for clinical use as persistent vehicles they would require personalization and engineered reversibility to manage the immune context and complications in individual human subjects.
Great Debates.
What are the most interesting topics likely to come up over dinner or drinks with your colleagues? Or, more importantly, what are the topics that don't come up because they are a little too controversial? In Immune Memory and Vaccines: Great Debates, Editors Rafi Ahmed and Shane Crotty have put together a collection of articles on such questions, written by thought leaders in these fields, with the freedom to talk about the issues as they see fit. This short, innovative format aims to bring a fresh perspective by encouraging authors to be opinionated, focus on what is most interesting and current, and avoid restating introductory material covered in many other reviews.
The Editors posed 13 interesting questions critical for our understanding of vaccines and immune memory to a broad group of experts in the field. In each case, several different perspectives are provided. Note that while each author knew that there were additional scientists addressing the same question, they did not know who these authors were, which ensured the independence of the opinions and perspectives expressed in each article. Our hope is that readers enjoy these articles and that they trigger many more conversations on these important topics.
The principles of vaccination are to manipulate the immune system to combat disease. This is widely and successfully applied to prevent infections, and is in development as an approach to treat tumors. Live attenuated vaccines—mostly viruses—have been massively successful in altering the epidemiology and complications of infectious disease, although there is a sting in the tail from potential revertant strains or direct complications of vaccination itself (such as oral vaccine–induced paralytic poliomyelitis). As the complications of the diseases themselves fade from the public memory in developed countries, the small risks of vaccination complications make uptake and herd immunity of the human population a challenge. Yet, this is in a setting in which we have good information that the benefits vastly outweigh the risks.
In this essay, I shall take the microbiota as meaning the stable consortia of microbes at body surfaces, and not transitory or undefined changes associated with the consumption of preparations with limited evidence for biomedical effectiveness. I shall also focus on the intestine, although the considerations are different for different surfaces. Taking the yardstick of live organisms, the permanence of the microbiota has huge durable potential for delivering health benefits. Deliberate alterations in permanent microbial colonization also need to address the potentially pervasive complications that might develop with a long lead time, with or without unforeseen genetic recombination. Whether the risks are reasonable will depend on the clinical situation: An adult patient requiring salvage treatment from tumor in relapse or treatment of intractable inflammatory bowel disease contrasts considerably with manipulating the microbiota of a healthy baby.
FEDERALISM RATHER THAN SOVEREIGNTY
In these days of Brexit (British exit) and the political suggestions of building walls along borders, let us start with biological sovereignty. I would argue that true sovereignty hardly exists in the host-microbial superorganism. Of course, at first sight, there is a fair distinction between the living spaces of the predominantly prokaryotic microbiota and those of the eukaryotic cells of the host. Yet the mutual exchange of metabolites between these eukaryotic and prokaryotic spaces (Holmes et al. 2011), which themselves have extensive mutual signaling properties, effectively provide cross-governance between the microbiota and its host. This is generally true of the consortial arrangement in higher organisms, although I focus here on mammals. The metabolic exchange is much more than a free-trade agreement: It is the promiscuous sharing of chemical directives that determine behavior of cells in the other living space (Fig. 1). The extent of this cross-governance means that almost all organ systems in the mammalian body, especially the immune system, are profoundly shaped by the presence of the microbiota (Smith et al. 2007). Compare germ-free and colonized animals: The presence of a microbiota matures the hypoplastic germ-free secondary lymphoid structures, increases myeloid output from the bone marrow, normalizes B-lymphocyte numbers and immunoglobulin (Ig) levels, and primes innate and adaptive components of mucosal immunity (Hooper et al. 2012). In animals that are born and bred in a colonized environment (and microbes colonize body surfaces in early life), there is very little ingress of live microbiota organisms to central tissues. However, there is pervasive exchange of metabolites helping the immune system to mature.
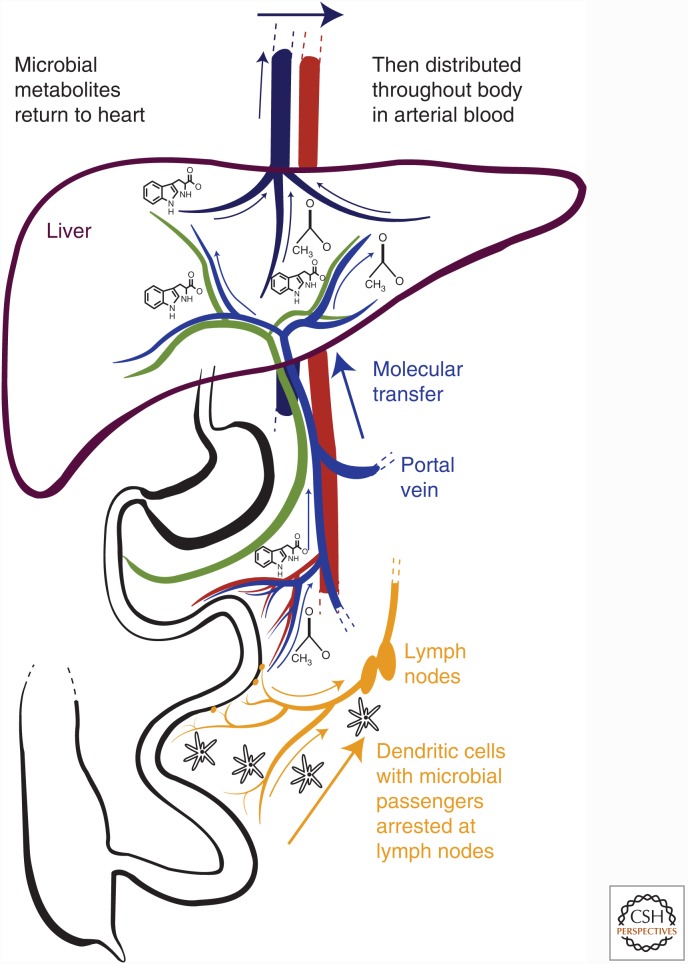
Figure 1.
Sampling of live organisms and microbial molecular products from the intestinal microbiota. Live microbes are sampled at the epithelial surface by dendritic cells that can induce responses locally in the mucosal lymphoid structures or following transit to the draining lymph nodes. Microbial molecular products are absorbed into the venous blood draining the intestine, which is a tributary of the hepatic portal vein, delivering these compounds to the liver. They are then subject to hepatic metabolism and/or are released into the hepatic vein, which returns blood to the heart for recirculation over the entire body. Pervasive molecular penetration throughout body tissues contrasts with the live lymphatic microbial sampling mechanism, because the draining lymph nodes contain most microbial mutualists within intestinal tissues.
A caveat is necessary here. Following colonization of germ-free mice, development of most maturity in organ systems, including immunity, can be recapitulated at any age. There are important exceptions, such as regulation of invariant natural killer (NK) T-cell content and (i)Treg induction, which are most effective when colonization starts during early postnatal development (Gensollen et al. 2016). This is also a time when the lymphocyte immune repertoires are very sensitive to microbial exposure. For example, the “natural” antibody repertoire in mice can be shaped by early life exposure to killed microbes. This has a later functional benefit on later protective anti-glycan responses, for example, to Streptococcus pyogenes (Kearney et al. 2015). Given that both systemic antibody isotype preference (Cahenzli et al. 2013) and the timing of mucosal antibody induction (Harris et al. 2006) are dependent on early microbial exposure, it seems likely that the early life window is a time when the developing repertoires can be shaped by the succession of postnatal colonization taxa to benefit later protective immunity to pathogens. It is also a time when the microbiota is unstable, with both immune responses and the microbiota composition being tuned by transferred maternal antibodies (Rogier et al. 2014; Koch et al. 2016). Understanding the mechanisms underlying the microbial and immunological dynamics of this process remains an important challenge, but it has massive potential to be manipulated to benefit human health.
IMMIGRATION CONTROL
Coming back to the idea of a wall, for the hypothetic eukaryotic politician promoting barriers as a means of sovereignty, one must admit that this means of separating prokaryotic and eukaryotic living spaces is rather effective. Yet the barriers are themselves a eukaryotic response to prokaryotic chemical directives. In the intestine, one example of this is Toll-like receptor (TLR)-triggered secretion of antimicrobial peptides such as RegIIIγ (Vaishnava et al. 2011). Another example is the way in which microbial metabolic exchange between a mother and her offspring matures the barrier in neonates. In experiments in which the mother is transiently colonized during pregnancy and delivers her pups germ free, even though the germ-free neonates have never been directly exposed to live microbes, the molecules of the maternal microbiota extensively reprogram neonatal epithelial gene expression (including upregulation of RegIIIγ and the mucus synthetic pathways). This means that the young animal is conditioned to avoid penetration of challenge doses of intestinal bacteria into host tissues (Gomez de Aguero et al. 2016). In other words, the presence of the microbiota is truly mutualistic, because its chemical directives include separation of the living spaces. These chemical directives also mature the immune system in terms of homeostatic lymphocyte proliferation, increased bone marrow output, and development of secondary lymphoid structures: All of these are establishing conditions for an effective immune response (Kieper et al. 2005; Balmer et al. 2014).
There is rather effective immigration control from the cumulative effect of bilateral chemical directives, high population densities of the microbiota, and metabolic specialization/competition in the prokaryotic biomass. In the early days of germ-free husbandry, it was found that axenic animal colonies were exquisitely sensitive to pathogens. Indeed, avoiding catastrophic infections in vivaria was the original motivation to create standardized microbiotas, such as that proposed by Russell Schaedler (Macpherson and McCoy 2015). This illustrates the importance of colonization resistance as a body-surface effect of protective immunity, which sets a higher threshold for the doses of infective enteric pathogens such as Salmonella and Shigella, and probably explains the clinical effectiveness of stool transplantation in patients with recurrent infections of Clostridium difficile in which repeated doses of antibiotics have failed (van Nood et al. 2013). The concept of colonization resistance is not restricted to preventing a pathogen from initially establishing itself and penetrating host tissues, as postinfective restoration of microbiota diversity is necessary to clear pathogenic bacteria (Endt et al. 2010; McDonald et al. 2015; Imhann et al. 2016).
AGITATION ACROSS FEDERAL BORDERS: THE POINTS TEST FOR DIFFERENT MICROBIAL TAXA
The very reason for the existence of an immune system is that barriers are imperfect. It is clear that the immune system has immense flexibility to generate responses to a wide range of taxa, for example, the diverse and rather distinct T-helper subset differentiation responses to different microbial taxa (Sallusto 2016). The microbiota has a correspondingly broad composition, embracing bacteria, archaea, protists, viruses, helminths, and fungi. Even those taxa that are part of the rare biosphere may have functional effects outstripping their numerical frequency, although the discussion here will be on intestinal bacteria. To appreciate the impact of the microbiota on immunity, we need to consider individual intestinal niches and how the host adapts to accept different taxa in these different physical situations.
The essential microbiological difference between a bacterial pathogen compared with a mutualist of the same species is in terms of pathogenicity islands, those relatively short stretches in the bacterial genome that encode a facility for invading or damaging host tissues and surviving host phagocytic biocidal mechanisms. The evidence comes from genetic manipulation of pathogens (abrogating pathogenicity by deleting pathogenicity island components) or genetic manipulation of the host (increasing host susceptibility to nonpathogens by deleting host genes encoding biocidal mechanisms). There is no question that when some of the pathogenic effect in bacteria is attenuated, the manipulated organism can be exploited to manipulate B- and T-lymphocyte repertoires with protective effect, making such microbes very effective immunogenic vehicles (Chatfield et al. 1989). The capacity for systemic pathogenicity (and immunogenicity) carries a metabolic cost that is rather unsuitable for persistence in the microbiota (Diard et al. 2013). So we are left with mutualists that are well adapted to existence in the prokaryotic spaces and pathogens that have adapted to bloom in the eukaryotic spaces. It is relatively easy to attenuate a pathogen to transiently immunize against the acute bloom of a pathogen, exploiting the fact that it will itself be eliminated; but this is rather different from persistently maintaining or stably controlling an immunogen in the microbiota.
There is a wide spectrum from benign behavior to proinflammatory potential in the different taxa that colonize the intestine. In contrast to the blooms of pathogens that are eliminated by pulsed systemic immune responses, there is evidence that the mucosal innate and adaptive immunity is acting more continuously to preserve mutualism with the microbiota. This is even true of the noninflammatory mutualist Bacteroides thetaiotaomicron, whose niche is restricted to the intestinal lumen or outer layers of mucus, which shows transcriptional signals in the bacteria of oxidative stress and of inflammatory networks in the mucosa in the absence of secreted immunoglobulin A (IgA) (Peterson et al. 2007). IgA also generally limits intestinal bacterial overgrowth (Wei et al. 2011). Generally, those taxa that are the most likely to generate intestinal inflammation are also the more strongly targeted by secreted Ig (Lecuyer et al. 2014; Palm et al. 2014; Bunker et al. 2015). Nevertheless, these responses to benign microbes are mainly focused on the intestinal mucosa, unless the intestinal permeability barrier is breached and the live organism reaches systemic secondary lymphoid structures (Macpherson et al. 2000; Konrad et al. 2006). Weak systemic responses may be enough to induce polyclonal expansions (Zeng et al. 2016), making pre-induced vaccine responses more durable (Pinna et al. 2009), but are likely less ideal for de novo induction of systemic protective immunity unless one were to compromise by making the organism more invasive.
At the other end of the spectrum are microbiota organisms that are certainly potentially proinflammatory, and so depend on more idiosyncratic mucosal immune responses to be mutualists. These often have rather intimate niches at or close to the epithelial surface. The particular idiosyncratic feature(s) (such as innate lymphoid cell cytokine secretion, regulatory T-cell induction, TH17 induction, interleukin [IL]-10 expression) varies according to taxon and the niche it occupies (Kullberg et al. 2006; Round et al. 2011; Sonnenberg et al. 2012; Atarashi et al. 2015). It is likely that these known adaptations for mutualism in models represent examples of a wider range of microbial taxa in the context of different host species. As with the benign microbes, mutualism depends on the immune responses that are induced, although these may now show a clear extension into systemic immunity.
The best-worked-out example of this more aggressive class within the microbiota is segmented filamentous bacteria ([SFB] Candidatus arthromitus). This organism has evolved with an exquisitely intimate niche attached to the epithelial cells of the lower small intestine. The position is ideal for SFB to harvest the many amino acids it needs from the host or diet (because its genome has dispensed with the necessary synthetic pathways). It uses special structures named “holdfasts” to anchor itself onto the epithelial layer to avoid being expelled into the large intestine where its amino acid auxotrophy would be much harder to satiate (Kuwahara et al. 2011; Sczesnak et al. 2011). In addition to provoking extremely effective IgA responses (which appear to limit overgrowth during colonization), SFB induces a remarkably specific and numerous population of mucosal TH17 cells with differentiation of the RORγt subset promoted by serum amyloid A secreted from the epithelial cells to which it is tethered, and a feedback circuit in which epithelial protective IL-22 is secreted by class 3 innate lymphoid cells (Fig. 2) (Lecuyer et al. 2014; Yang et al. 2014; Atarashi et al. 2015; Sano et al. 2015). Although this TH17 population is not directly proinflammatory, under the right conditions of major histocompatibility complex (MHC) susceptibility, the SFB TH17 subset can provide the necessary germinal center help to generate arthritis via autoantibodies (Maloy et al. 2003; Wu et al. 2010; Teng et al. 2016).
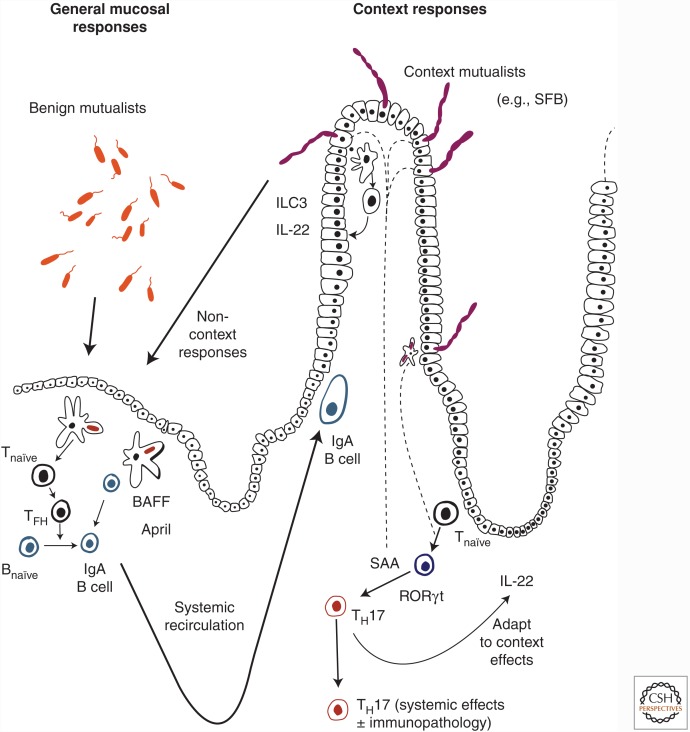
Figure 2.
Induction of immune responses by members of the intestinal microbiota. The extent and character of mucosal immune response induction by the microbiota varies according to the organism concerned. Benign noninflammatory mutualists residing in the lumen or confined to outer layers of mucus induce B- and T-cell responses within the lymphoid structures of the intestine and its draining lymph nodes. These are largely focused on the mucosa itself, because the induced lymphocytes mainly home back to the intestine after systemic recirculation through the lymphatics and the blood stream. B-cell responses may be induced with T-cell help or following direct stimulation of B cells by B-cell-activating factor of the tumor-necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL) secreted by mononuclear cells. At the other end of the spectrum, organisms with especially intimate niches induce a range of additional idiosyncratic immune responses. An example is segmented filamentous bacteria (SFB), which is responsible for generating a substantial population of specific mucosal TH17 cells, with cues from serum amyloid protein (SAA) secreted by the epithelial cells that tether the microbe in the lower small intestine. SFB also very effectively induces specific IgA, which limits overgrowth during colonization. The epithelial layer to which SFB is attached is sustained by interleukin (IL)-22 secreted by class 3 innate lymphoid cells (ILC3) through a feedback loop. Although the TH17 cells induced by SFB are noninflammatory, in the right major histocompatibility complex (MHC) background of autoimmune predisposition, they can provide help for B-cell-dependent autoimmune arthritis.
Given the local focus of most mucosal immune responses induced by the microbiota, the most promising opportunities are to use these responses to manipulate the system to dampen proinflammatory responses from its more aggressive members. In inflammatory bowel disease, one is seeking to identify the immunopathogenic microbes in specific host contexts (Maloy and Powrie 2011). For immunization, one would need to identify host contexts in which a particular microbe can be accepted or substituted with a feasible mutualistic response in that individual that is engineered to be more durable than current anti-inflammatory or immunosuppressive treatments. Provided the specificity of re-adaptation of the systemic repertoires and subsets can avoid host immunopathology or dysbiosis, context mutualists could be effective vehicles to induce systemic protective immune responses. An example of the principle is the protective immunity to malaria, which can be produced in mice by the induction of anti-gal antibodies following colonization by the pathobiont Escherichia coli O86:B7 (Yilmaz et al. 2014). The challenges of design and personalization are that we still need better understanding of some key features of the responses (such as the initiation of proinflammatory rather than homeostatic TH subsets). We also need accurate personalized a priori models of how a particular microbe can be confined to a required niche and how it will be received immunologically depending on genetics, epigenetics, and background metabolic signaling in the recipient. For safety, especially in view of the inevitability of longer-term genetic recombination, either the systems will have to be transitory or there will need to be excellent switches to eliminate aberrant responses.
PROSPECTS
I have advanced the view that there are important differences between manipulating the immune system to eliminate the bloom of an infectious pathogen (in which the immune system is trying to secure sovereignty of central tissues) and using the federalism of persistent host-microbial interactions for the same ends (when mucosal immune responses are to a large extent gardening the microbiota to maintain mutualism).
Manipulating the microbiota is in itself a multidimensional problem of community structures and dietary conditions. It seems to me that there are reasonable prospects of microbiota design to benefit general metabolism or exploiting context mutualists as transitory immunogenic vehicles. We are currently mainly following empirical approaches, which may turn out to be enough. Nevertheless, in an age of rapidly declining empiricism in biomedicine, the real challenge is to understand the microbial and host parameters well enough to have good predictive models of the outcomes of manipulations, bringing with them the opportunity to safely bioengineer a personalized microbiota for the health of the individual human host.
Footnotes
Editors: Shane Crotty and Rafi Ahmed
Additional Perspectives on Immune Memory and Vaccines: Great Debates available at www.cshperspectives.org
REFERENCES
- Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori T, et al. 2015. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163: 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer ML, Schurch CM, Saito Y, Geuking MB, Li H, Cuenca M, Kovtonyuk LV, McCoy KD, Hapfelmeier S, Ochsenbein AF, et al. 2014. Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J Immunol 193: 5273–5283. [DOI] [PubMed] [Google Scholar]
- Bunker JJ, Flynn TM, Koval JC, Shaw DG, Meisel M, McDonald BD, Ishizuka IE, Dent AL, Wilson PC, Jabri B, et al. 2015. Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity 43: 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahenzli J, Koller Y, Wyss M, Geuking MB, McCoy KD. 2013. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 14: 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield SN, Strugnell RA, Dougan G. 1989. Live Salmonella as vaccines and carriers of foreign antigenic determinants. Vaccine 7: 495–498. [DOI] [PubMed] [Google Scholar]
- Diard M, Garcia V, Maier L, Remus-Emsermann MN, Regoes RR, Ackermann M, Hardt WD. 2013. Stabilization of cooperative virulence by the expression of an avirulent phenotype. Nature 494: 353–356. [DOI] [PubMed] [Google Scholar]
- Endt K, Stecher B, Chaffron S, Slack E, Tchitchek N, Benecke A, Van Maele L, Sirard JC, Mueller AJ, Heikenwalder M, et al. 2010. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog 6: e1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensollen T, Iyer SS, Kasper DL, Blumberg RS. 2016. How colonization by microbiota in early life shapes the immune system. Science 352: 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, et al. 2016. The maternal microbiota drives early postnatal innate immune development. Science 351: 1296–1302. [DOI] [PubMed] [Google Scholar]
- Harris NL, Spoerri I, Schopfer JF, Nembrini C, Merky P, Massacand J, Urban JF Jr., Lamarre A, Burki K, Odermatt B, et al. 2006. Mechanisms of neonatal mucosal antibody protection. J Immunol 177: 6256–6262. [DOI] [PubMed] [Google Scholar]
- Holmes E, Li JV, Athanasiou T, Ashrafian H, Nicholson JK. 2011. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends Microbiol 19: 349–359. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. 2012. Interactions between the microbiota and the immune system. Science 336: 1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ, et al. 2016. Proton pump inhibitors affect the gut microbiome. Gut 65: 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney JF, Patel P, Stefanov EK, King RG. 2015. Natural antibody repertoires: Development and functional role in inhibiting allergic airway disease. Annu Rev Immunol 33: 475–504. [DOI] [PubMed] [Google Scholar]
- Kieper WC, Troy A, Burghardt JT, Ramsey C, Lee JY, Jiang HQ, Dummer W, Shen H, Cebra JJ, Surh CD. 2005. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol 174: 3158–3163. [DOI] [PubMed] [Google Scholar]
- Koch MA, Reiner GL, Lugo KA, Kreuk LS, Stanbery AG, Ansaldo E, Seher TD, Ludington WB, Barton GM. 2016. Maternal IgG and IgA antibodies dampen mucosal T helper cell responses in early life. Cell 165: 827–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad A, Cong Y, Duck W, Borlaza R, Elson CO. 2006. Tight mucosal compartmentation of the murine immune response to antigens of the enteric microbiota. Gastroenterology 130: 2050–2059. [DOI] [PubMed] [Google Scholar]
- Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, et al. 2006. IL-23 plays a key role in Helicobacter hepaticus–induced T cell-dependent colitis. J Exp Med 203: 2485–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara T, Ogura Y, Oshima K, Kurokawa K, Ooka T, Hirakawa H, Itoh T, Nakayama-Imaohji H, Ichimura M, Itoh K, et al. 2011. The lifestyle of the segmented filamentous bacterium: A non-culturable gut-associated immunostimulating microbe inferred by whole-genome sequencing. DNA Res 18: 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuyer E, Rakotobe S, Lengline-Garnier H, Lebreton C, Picard M, Juste C, Fritzen R, Eberl G, McCoy KD, Macpherson AJ, et al. 2014. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity 40: 608–620. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, McCoy KD. 2015. Standardised animal models of host microbial mutualism. Mucosal Immunol 8: 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. 2000. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288: 2222–2226. [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. 2011. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474: 298–306. [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. 2003. CD4+CD25+ TR cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med 197: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald EG, Milligan J, Frenette C, Lee TC. 2015. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med 175: 784–791. [DOI] [PubMed] [Google Scholar]
- Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, et al. 2014. Immunoglobulin a coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158: 1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DA, McNulty NP, Guruge JL, Gordon JI. 2007. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2: 328–339. [DOI] [PubMed] [Google Scholar]
- Pinna D, Corti D, Jarrossay D, Sallusto F, Lanzavecchia A. 2009. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur J Immunol 39: 1260–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogier EW, Frantz AL, Bruno ME, Wedlund L, Cohen DA, Stromberg AJ, Kaetzel CS. 2014. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci 111: 3074–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. 2011. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332: 974–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F. 2016. Heterogeneity of human CD4+ T cells against microbes. Annu Rev Immunol 34: 317–334. [DOI] [PubMed] [Google Scholar]
- Sano T, Huang W, Hall JA, Yang Y, Chen A, Gavzy SJ, Lee JY, Ziel JW, Miraldi ER, Domingos AI, et al. 2015. An IL-23R/IL-22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses. Cell 163: 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sczesnak A, Segata N, Qin X, Gevers D, Petrosino JF, Huttenhower C, Littman DR, Ivanov II. 2011. The genome of Th17 cell-inducing segmented filamentous bacteria reveals extensive auxotrophy and adaptations to the intestinal environment. Cell Host Microbe 10: 260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, McCoy KD, Macpherson AJ. 2007. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol 19: 59–69. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, et al. 2012. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 336: 1321–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng F, Klinger CN, Felix KM, Bradley CP, Wu E, Tran NL, Umesaki Y, Wu HJ. 2016. Gut microbiota drive autoimmune arthritis by promoting differentiation and migration of Peyer’s Patch T follicular helper cells. Immunity 44: 875–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. 2011. The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science 334: 255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, et al. 2013. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368: 407–415. [DOI] [PubMed] [Google Scholar]
- Wei M, Shinkura R, Doi Y, Maruya M, Fagarasan S, Honjo T. 2011. Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nat Immunol 12: 264–270. [DOI] [PubMed] [Google Scholar]
- Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. 2010. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32: 815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, et al. 2014. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature 510: 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz B, Portugal S, Tran TM, Gozzelino R, Ramos S, Gomes J, Regalado A, Cowan PJ, d’Apice AJ, Chong AS, et al. 2014. Gut microbiota elicits a protective immune response against malaria transmission. Cell 159: 1277–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, Inohara N, Nunez G. 2016. Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity 44: 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]