Abstract
Genetic studies in animals and humans indicate that gene mutations that functionally perturb transforming growth factor β (TGF-β) signaling are linked to specific hereditary vascular syndromes, including Osler–Rendu–Weber disease or hereditary hemorrhagic telangiectasia and Marfan syndrome. Disturbed TGF-β signaling can also cause nonhereditary disorders like atherosclerosis and cardiac fibrosis. Accordingly, cell culture studies using endothelial cells or smooth muscle cells (SMCs), cultured alone or together in two- or three-dimensional cell culture assays, on plastic or embedded in matrix, have shown that TGF-β has a pivotal effect on endothelial and SMC proliferation, differentiation, migration, tube formation, and sprouting. Moreover, TGF-β can stimulate endothelial-to-mesenchymal transition, a process shown to be of key importance in heart valve cushion formation and in various pathological vascular processes. Here, we discuss the roles of TGF-β in vasculogenesis, angiogenesis, and lymphangiogenesis and the deregulation of TGF-β signaling in cardiovascular diseases.
Transforming growth factor β1 (TGF-β1) is the prototype of a large family of structurally related, secreted dimeric proteins that have pleiotropic effects and play important roles in cell-to-cell signaling. Other members of this family include the closely related TGF-β2 and -β3 and more distantly related proteins like activins and inhibins, nodal proteins, and bone morphogenetic proteins (BMPs) (Hinck et al. 2016; Morikawa et al. 2016). TGF-βs regulate a large variety of cellular processes in many different cell types. Their effects are context-dependent, including the induction of proliferation, apoptosis, migration, adhesion, extracellular matrix (ECM) protein production, and cytoskeletal organization (Massagué 2012; Morikawa et al. 2016). Consequently, many TGF-β family cytokines play essential roles in embryonic development, stem cells, and cell fate determination and in adult tissue homeostasis and repair (Moustakas and Heldin 2009; Wu and Hill 2009; Itoh et al. 2014). Perturbations in the actions of TGF-β can lead to pathological conditions, including cardiovascular diseases, fibrotic disorders, and cancer (Harradine and Akhurst 2006; Ikushima and Miyazono 2010; Dooley and ten Dijke 2012; Pardali and ten Dijke 2012; Morikawa et al. 2016). Therapeutic intervention to normalize perturbed TGF-β signaling is an emerging area of intense research (Hawinkels and ten Dijke 2011; Akhurst and Hata 2012; Chang 2016).
Misregulated TGF-β signaling in humans causes vascular pathologies and cardiovascular disease such as arteriovenous malformations (AVMs), aneurysms, atherosclerosis, cardiac fibrosis, vascular remodeling of the retina (retinopathy), and valvular heart disease. Additionally, TGF-β signaling contributes to endothelial tumors like hemangiomas (Pardali et al. 2010; Akhurst and Hata 2012). The importance of the TGF-β signaling pathways in the spatial and temporal regulation of heart and blood vessel morphogenesis, as well as cardiovascular homeostasis, is evident when analyzing the phenotypes of mice deficient in components of the TGF-β signaling cascade (Goumans and Mummery 2000; Goumans et al. 2009). The multifunctional and context-dependent activities of TGF-β and its interactions with nonvascular cells (e.g., immune cells) complicate the interpretation of its in vivo roles in cardiovascular biology. In this review, we only focus on TGF-β as the role of BMP in angiogenesis is discussed elsewhere (Goumans et al. 2017). First, we discuss vascular development and TGF-β signaling, followed by the mechanisms that are at the basis of TGF-β’s control of vascular function, its effects on endothelial cells (ECs), smooth muscle cells (SMCs), and pericytes, and how a misbalance in TGF-β signaling leads to vascular dysfunction.
BLOOD AND LYMPHATIC VASCULAR NETWORK FORMATION
The Vascular System
The heart, blood, and blood vessels make up the vascular system, which supplies oxygen and nutrients to all cells of the body and removes waste products (Potente et al. 2011). This is achieved by pumping blood through a highly branched vascular network of specialized blood vessels (i.e., arteries, capillaries, and veins). Blood vessels are lined with a single layer of ECs, and stabilized by a basal lamina and a layer of connective tissue containing SMCs or pericytes. The amount of connective tissue and number of smooth muscles cells or pericytes present in the vessel wall depends on the diameter of the vessel and its function.
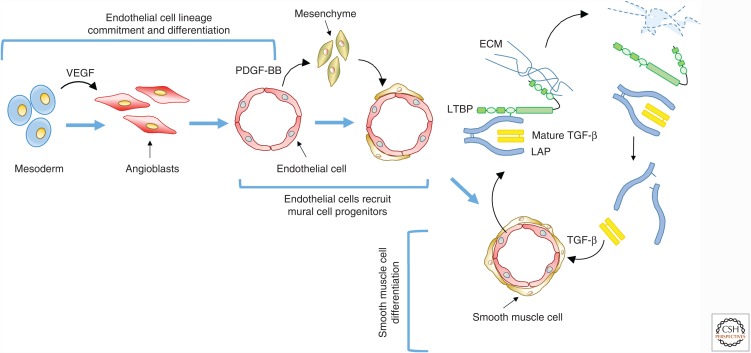
This vascular network is constructed using two highly coordinated and sequential processes, vasculogenesis and angiogenesis. During vasculogenesis (Fig. 1), mesoderm will first differentiate into proliferating EC precursors known as angioblasts. These angioblasts will differentiate into ECs that align, fuse, and gradually acquire a lumen (Ferguson et al. 2005). Vasculogenesis ends with the formation of a honeycomb-like primary vascular plexus. During angiogenesis (Fig. 1), the primary capillary plexus is remodeled into a stable hierarchical branched network, containing arteries, capillaries, and veins. Vascular endothelial growth factor (VEGF) signaling is a key pathway involved in vascular remodeling, as VEGF loosens cell–cell contacts within the newly formed capillaries and causes local degradation of the ECM. Activated ECs start to proliferate in response to VEGF or basic fibroblast growth factor (bFGF or FGF-2), and form a new sprout (Fig. 1). Loosening of cell–cell contacts can also result in fusion of capillaries to form arteries and veins.
Figure 1.
Process of vasculogenesis. Vasculogenesis starts with the differentiation and proliferation of mesodermal cells into angioblasts followed by their differentiation into endothelial cells (ECs) in response to vascular endothelial growth factor (VEGF). These ECs fuse and form a lumen in a honeycomb structure. Platelet-derived growth factor (PDGF) secreted by ECs induces recruitment of mesenchymal cells that will differentiate into smooth muscle cells (SMCs) or pericytes. When SMCs or pericytes adhere to ECs, the ECs start to produce TGF-β. TGF-β is produced as a prepropolypeptide, which is proteolytically processed, and forms a small latent TGF-β complex consisting of the mature TGF-β dimer associated with two latency-associated peptides (LAPs). The small latent TGF-β complex binds to latent transforming growth factor β binding protein (LTBP), and this complex is secreted as the large latent TGF-β complex. LTBP binds to extracellular matrix (ECM) proteins such as fibronectin. Activation of TGF-β by, for example, proteolytic release from LAP will induce growth arrest and terminal differentiation of SMCs.
The final step in vascular development is initiated by the blood circulation. In this phase, nonfunctional sprouts that were unable to fuse to patent passages are pruned. The remaining vessels are shaped and remodeled by the blood flow to suit local tissue needs (Adams and Alitalo 2007; Carmeliet and Jain 2011). VEGF is also steering vascular remodeling and pruning. Once this process is finished, TGF-β and platelet-derived growth factor (PDGF)-BB are secreted by the endothelium and stabilize the mature vascular network (Fig. 1). TGF-β stimulates the production and stabilization of the ECM and, together with PDGF-BB, attracts and regulates the differentiation of pericytes and SMCs, which surround and enclose the primitive vascular tube (Bergers and Song 2005). Finally, TGF-β promotes arterial-venous specification necessary for the establishment and enlargement of arteries and veins (Fish and Wythe 2015).
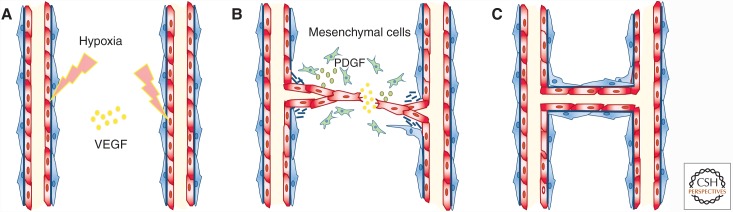
Although vasculogenesis occurs mainly during embryonic development, angiogenesis is responsible for the formation of new blood vessels after birth. Angiogenesis will result in vascularization of growing tissues and during healing, and in the formation of capillaries, the smallest blood vessels in our body (Fig. 2). The formation of new blood vessels is a tightly regulated process, governed by a balance between proangiogenic factors such as VEGF and bFGF and antiangiogenic factors such as angiostatin 1 and thrombospondin (Adams and Alitalo 2007; Carmeliet and Jain 2011; Herbert and Stainier 2011). TGF-β’s effect on blood vessel formation is context- and concentration-dependent and can be either pro- or antiangiogenic (Pardali et al. 2010). Hypoxia induces the secretion of VEGF, which in turn activates the endothelium and induces the formation of tip cells and stalk cells by binding to vascular endothelial growth factor receptor 2 (VEGFR2 or Flk-1). Conversely, the presence of VEGFR1 on the EC surface inhibits angiogenesis, partly by functioning as a VEGF ligand trap.
Figure 2.
Process of angiogenesis. (A) Angiogenesis begins when endothelial cells are activated, for example, by hypoxia. These cells proliferate and the tip cells migrate into the perivascular space toward a vascular endothelial growth factor (VEGF) gradient (B). During the resolution phase, Endothelial cells stop dividing and vascular sprouts fuse, rebuild their extracellular matrix, and attract pericytes and smooth muscle cells in a platelet-derived growth factor (PDGF)-dependent manner. (C) These latter cells will stabilize the newly formed sprout.
Angiogenesis is a multistep process (Fig. 2). The first step is a vascular activation phase when VEGF increases vascular permeability and basement membrane degradation, allowing ECs to proliferate and migrate into the extracellular space where they form new capillary sprouts. The second step is a resolution phase when the ECs cease proliferation and migration, reconstitute the basement membrane, and promote vessel maturation (Adams and Alitalo 2007; Carmeliet and Jain 2011). During vessel maturation, ECs secrete TGF-β to recruit mesenchymal cells to the perivascular space, where they differentiate into pericytes and SMCs, and form a tight, protective coat around the newly formed vessel (Armulik et al. 2011).
The Lymphatic System
The lymphatic system is formed by lymphatic capillaries and collecting lymphatic vessels. The lymphatic vasculature drains the interstitial fluid that leaks out of blood capillaries and returns it to the circulation to maintain interstitial fluid pressure. The lymphatic system is also important for the immune response by transporting white blood cells and antigen-presenting cells (Alitalo and Carmeliet 2002). Like blood vessels, lymphatic vessels are built from ECs and SMCs or pericytes (Karpanen and Alitalo 2008).
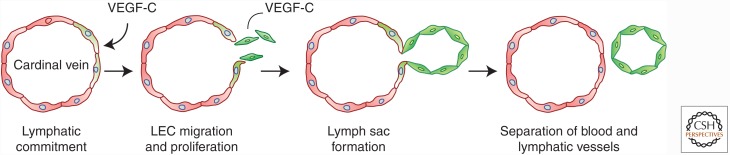
Lymphatic vessels originate from blood vessels (Fig. 3). Within the developing embryo, a subset of cardinal vein ECs starts to express lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), followed by expression of prospero homeobox transcription factor (Prox)-1. Prox-1 is a binary transcriptional switch and induces the expression of VEGFR3. Prox-1 is required for lymphangiogenesis because Prox1−/− mouse embryos do not form lymphatic endothelial cells (LECs). Prox-1 activates the expression of LEC markers and represses the expression of blood endothelial cell (BEC) markers in mature ECs (Johnson et al. 2008). The Prox-1-expressing BECs start to migrate from the cardinal vein toward VEGF-C-expressing mesenchymal cells (Karkkainen et al. 2004) and form the first lymphatic structures called the primary sac. Some LECs differentiate into lymphatic valve-forming cells, dedifferentiate into ECs with BEC-like features, or transdifferentiate into fibroblast-like cells (Yang and Oliver 2014a,b). For example, when challenged with blood flow or shear stress, LECs can change into BEC-like cells and incorporate into the blood vessel wall (Chen et al. 2012a). Postnatal lymphangiogenesis is also stimulated by LYVE-1-expressing macrophages; although macrophages may differentiate into LECs (Lee et al. 2010; Ran and Montogmery 2012), they mainly stimulate proliferation of existing LECs by inducing the secretion of growth factors, including VEGF-C or bFGF (Adams and Alitalo 2007).
Figure 3.
Process of lymphangiogenesis. Lymph vessels originate from blood vessels when and where some of the cardinal vein endothelial cells start to express lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) and prospero homeobox transcription factor (Prox)-1 (green cells). The Prox-1-expressing lymphatic endothelial cells (LECs) migrate out of the vessel, proliferate, and form the lymphatic sac, the first lymphatic structure.
TGF-β FAMILY SIGNALING
TGF-β is synthesized as an inactive precursor protein (ten Dijke and Arthur 2007; Shi et al. 2011). The TGF-β precursor contains an amino-terminal signal peptide to direct it to the endoplasmatic reticulum. After removal of the signal peptide, the remaining propeptide is cleaved by the endoprotease furin (Dubois et al. 1995) to generate a short carboxy-terminal protein, which corresponds to mature TGF-β, and a large amino-terminal propeptide called the latency-associated peptide (LAP). LAP and TGF-β remain noncovalently bound to form the small latent complex (Annes et al. 2003; Robertson et al. 2015; Robertson and Rifkin 2016). TGF-β is secreted from cells as a large latent complex consisting of a mature dimeric TGF-β molecule, LAP, and a latent transforming growth factor β binding protein (LTBP) (see Fig. 1) (Hyytiäinen et al. 2004; Shi et al. 2011). There are four LTBPs, of which LTBP-1, -3, and -4 can form covalent complexes with the small latent TGF-β complex. LTBPs can target the latent TGF-β complex to the ECM by interacting with fibrillin and other ECM components, preferentially fibronectin, through covalent transglutaminase-induced crosslinks (Hyytiäinen et al. 2004; Robertson et al. 2015; Robertson and Rifkin 2016). Targeting of the latent TGF-β complex to the ECM is important for effective TGF-β bioavailability and activation (Robertson et al. 2015; Robertson and Rifkin 2016).
To exert its effects on cells, TGF-β needs to be released from its latent complex and activated before it can bind to the signaling receptors (see Fig. 1) (Robertson and Rifkin 2016). How TGF-β is liberated from the large latent complex and activated is context- and cell type–dependent. The large latent complex is released from the microfibrils by replacement of LTBP with fibrillin-1 fragments (Chaudhry et al. 2007) or from the ECM by proteolytic cleavage by proteases like plasmin and thrombin (Annes et al. 2003; Robertson et al. 2015). Release or shedding of the large latent TGF-β complex from the ECM may also reduce the generation of active TGF-β, because TGF-β can also be activated through ECM-bound mechanisms (see below) using, for example, the αvβ6 integrin complex interacting with the ECM-bound large latent TGF-β complex (Annes et al. 2004).
Activation of latent TGF-β complexes in vitro can be achieved through mechanisms that cause protein denaturation like exposure to high temperature, extreme pH, or ionizing radiation. Although radiation treatment can induce tissue fibrosis in several organs, binding of thrombospondin-1 to LAP (Crawford et al. 1998) and binding of the integrins αvβ6 and αvβ8 to the RGD (arginine-glycine-aspartate) sequence in LAP (Sheppard 2005) are important mechanisms for in vivo activation (Shi et al. 2011). Thrombospondin activates latent TGF-β by disrupting the noncovalent interaction between LAP and the active TGF-β dimer (Schultz-Cherry et al. 1995; Ribeiro et al. 1999). Integrin-mediated activation of TGF-β involves binding of αvβ6 and αvβ8 to the RGD sequence in LAP (Sheppard 2005), which results in deformation of the latent complex by physical force generation in a LTBP-1-dependent manner, and release of active TGF-β (Annes et al. 2004).
The importance of ligand activation for TGF-β to exert its cellular responses becomes evident with the phenotypes of mice deficient for thrombospondin-1 due to Thbs1 inactivation (Crawford et al. 1998), αvβ6 (Munger et al. 1999; Aluwihare et al. 2009), or αvβ8 (Zhu et al. 2002; Aluwihare et al. 2009). These phenotypes phenocopy those of Tgfb1- and Tgfb3-deficient mice, whereas mice deficient in LTBP-4 are not able to activate latent TGF-β (Koli et al. 2004).
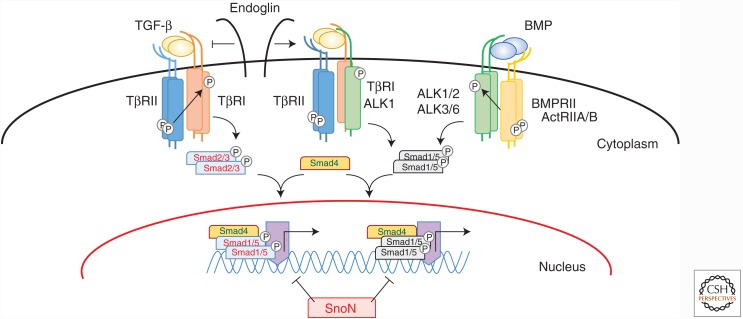
Active, dimeric TGF-β exerts its effects on cells by binding to a heteromeric receptor complex, composed of structurally related types I and II receptors, and various coreceptors (Fig. 4) (Heldin and Moustakas 2016). The TGF-β types I and II receptors (TβRI and TβRII) are single transmembrane-spanning proteins with an extracellular, cysteine-rich, ligand-binding domain and an intracellular dual specificity kinase domain, often referred to as serine-threonine kinase domain. There are seven type I receptors (i.e., ALK-1 through ALK-7), of which ALK-4 and -5 correspond to ActRIB and TβRI, respectively, and five type II receptors (i.e., TβRII, BMPRII, ActRIIA, ActRIIB, and Müllerian inhibiting substance RII). TGF-β binds to a heterotetrameric receptor complex that consists of a TβRII dimer and two type I receptors (Ehrlich et al., 2011). After TGF-β binds to TβRII, TβRI/ALK-5 is phosphorylated by the TβRII kinase on specific serine and threonine residues in the so-called glycine-serine enriched (GS)-domain (Wrana et al., 1994; Hata and Chen, 2016; Heldin and Moustakas, 2016). Upon activation, TβRI initiates intracellular signaling by phosphorylating downstream effectors. Among these, Smad transcription factors play a pivotal role in relaying the signals from the plasma membrane to the nucleus (Heldin et al. 1997; Feng and Derynck 2005; Hill 2016). TβRI phosphorylates Smad2 and Smad3 at their carboxyl termini. Phosphorylated Smad2 and Smad3 form a complex with Smad4 and translocate to the nucleus to activate or repress target gene transcription (Massagué et al. 2005; Ross and Hill 2008; Hill 2016). Activins also induce phosphorylation of Smad2 and Smad3 after binding to a complex of activin receptor IIA or IIB (ActRIIA or ActRIIB) and ALK-4. BMPs bind to heteromeric complexes of either BMP type II receptor (BMPRII), ActRIIA, or ActRIIB with ALK-1, -2, -3, or -6, which then phosphorylate Smad1, Smad5, and/or Smad8 (Heldin et al. 1997; Feng and Derynck 2005; Katagiri and Watabe 2016). Although BMP-9 and -10 bind to ALK-1 and -2 with high affinity (Brown et al. 2005; David et al. 2007), TGF-β binds to a receptor complex of a TβRII dimer and ALK-5 and -1 in cultured ECs (Goumans et al. 2003a). Besides the canonical Smad pathway, TGF-β family members can also activate non-Smad signaling pathways (Derynck and Zhang 2003; Zhang 2016), including TGF-β activated kinase 1 (TAK1)-mediated activation of p38 and c-Jun amino-terminal kinase (JNK) mitogen-activated protein (MAP) kinases (Yamaguchi et al. 1995).
Figure 4.
TGF-β signaling pathways. TGF-βs signal by binding to a specific, heteromeric complex that includes types I and II kinase receptors (TβRI/ALK-5 and TβRII, respectively). In most cells, TGF-β binds to a complex of TβRII and TβRI/ALK-5 complex, but in endothelial cells, they can also bind to a complex of TβRII and ALK-1. The coreceptor endoglin inhibits TGF-β binding to the TβRII-ALK-5 complex and promotes TGF-β binding to the TβRII-ALK-1 complex, thus activating ALK-1 signaling. In a similar manner, bone morphogenetic proteins (BMPs) also bind to complexes of two types of receptors—that is, the type I receptors ALK-1, -2, -3, or -6, and the type II receptors BMPRII, ActRIIA, or ActRIIB. Intracellular signaling through Smad activation can be divided into two pathways. In one pathway, ALK-5 phosphorylates, and thus activates, Smad2 and Smad3, and in the other one, ALK-1, -2, -3, and -6 activate Smad1, Smad5, and Smad8. These receptor-activated Smads form heteromeric complexes with the common mediator Smad, Smad4. These complexes translocate into the nucleus, where they act as transcription factor complexes and regulate the expression of specific target genes.
The inhibitory Smads, Smad6 and Smad7, repress TGF-β family signaling. They compete with R-Smads for phosphorylation or promote degradation of the R-Smads and receptors by recruiting E3 ubiquitin ligases like Smurf1 and Smurf2 (Itoh and ten Dijke 2007; de Boeck and ten Dijke 2012; Miyazawa and Miyazono 2016). Smads regulate gene transcription together with coactivators (e.g., p300 or CBP) and corepressors like c-Ski or SnoN (Massagué et al. 2005; Ross and Hill 2008; Hill 2016). SnoN represses the Smad-mediated transcriptional activity by disrupting the functional heteromeric complexes of Smad2 and/or Smad3 with Smad4 (Stroschein et al. 1999). Upon TGF-β stimulation, SnoN is rapidly degraded in a Smad3-dependent manner. Already after 2 h, the expression of SnoN is markedly increased and, in a negative-feedback loop, terminates TGF-β signaling (Stroschein et al. 1999).
In the TGF-β pathway, endoglin and betaglycan, also known as the TβRIII receptors, have been characterized as accessory receptors. They do not display intrinsic enzymatic activity but, instead, regulate the access of TGF-β ligands to the signaling receptors (Fig. 4) (ten Dijke et al. 2008; Bernabéu et al. 2009; Bilandzic and Stenvers 2011). These two coreceptors possess TGF-β binding sites in their extracellular domains, and their intracellular domains are rich in serine and threonine residues of which specific residues are phosphorylated by TβRI and TβRII (Koleva et al. 2006). Betaglycan must be present at the cell surface for TGF-β2 to bind to TβRII (Lin et al. 1995). Endoglin and betaglycan have also been reported to enhance BMP signaling of defined BMP family members (David et al. 2007; Kirkbride et al. 2008).
Endoglin exists in two variants arising by alternative splicing, a long form and a short form (L- and S-endoglin, respectively). L-endoglin has a cytoplasmic domain of 47 residues and is the predominant isoform. S-endoglin contains a cytoplasmic tail of only 14 amino acids (Bellón et al. 1993; Pérez-Gómez et al. 2005). Both L- and S-endoglin are able to bind ligand (Bellón et al. 1993), but they differ in their phosphorylation levels (Lastres et al. 1994) and their capacity to regulate certain TGF-β-dependent responses (Lastres et al. 1996).
TGF-β SIGNALING IN ECs
TGF-β has pleiotropic effects on ECs. TGF-β inhibits or promotes angiogenesis depending on several factors like the origin of the EC, ligand concentrations, serum components, the type of exogenously provided ECM, treatment duration, cellular density, and combination of TGF-β receptors expressed at the cell surface. ECs express the more widely distributed TβRI/ALK-5 at their cell surface, but also express ALK-1 to which TGF-β can bind with low affinity. Furthermore, the ALK-5 and -1 pathways have different effects on the expression and signaling of VEGF (Liu et al. 2009; Shao et al. 2009), which together with differential binding of TGF-β to these two type I receptors, may explain the biphasic effect of TGF-β on neovascularization, being either angiogenic, thus stimulating new vessel formation, or antiangiogenic, that is, inhibiting EC proliferation and migration and stimulating maturation of the vessel (Oh et al. 2000; Goumans et al. 2002).
The antiangiogenic effect of TGF-β is mainly mediated by TGF-β signaling through TβRII and ALK-5 (Goumans et al. 2003a). Overexpression of a constitutively active ALK-5 kinase inhibits the proliferation and migration of ECs and induces the expression of plasminogen activator inhibitor 1 (PAI-1) (Goumans et al. 2002). Conversely, inhibition of the ALK-5 kinase with the small molecule SB-431542 stimulates EC proliferation and sheet formation of mouse embryonic ECs in culture (Watabe et al. 2003) and blood vessel formation in an ex vivo fetal mouse metatarsal assay (Liu et al. 2009).
The antiangiogenic properties of this signaling pathway are confirmed in vivo (Table 1). Mice carrying null mutations in the genes encoding TGF-β1 (Dickson et al. 1995; Bonyadi et al. 1997), TβRII (Oshima et al. 1996), or TβRI/ALK-5 (Larsson et al. 2001) and mice expressing a dominant-negative TβRII mutant in the extraembryonic mesoderm of the yolk sac (Goumans et al. 1999) all die at around embryonic day (E)10.5 because of impaired remodeling of the yolk sac vascular plexus, which prevents the formation of a robust network of vessels. Furthermore, these embryos show reduced ECM production, which results in loosely attached layers in the vessel wall. Interestingly, endothelial-specific deletion of TβRII and TβRI recapitulates the phenotype of Tgfbr2−/− and Tgfbr1−/− mice, respectively, suggesting that the primary cause of the vascular phenotype is a loss of endothelial TGF-β signaling and, consequently, an impairment in SMC recruitment and differentiation (Carvalho et al. 2007). These studies, both in cell culture and in vivo, show that TGF-β signaling through TβRII and ALK-5 keeps the ECs in a quiescent state and is required for vascular network maturation.
Table 1.
Genetic defects in the cardiovascular (CV) system
| Protein (gene name) | Age of death | CV phenotype, animal model | Human genetic CV disorder | References |
|---|---|---|---|---|
| TGF-β1 (TGFB1) | E8.5–adult | Owing to gene modifier, ranging from embryonic lethal with (yolk sac) vascular defects to postnatal lethality, caused by autoimmune disease | No CV malformations | Letterio et al. 1994; Goumans and Mummery 2000; Tang et al. 2005 |
| TGF-β2 (TGFB2) | P0 | Defect in cardiac septation and valve remodeling | Loeys–Dietz syndrome type 4; syndromic TAA disorder; Kawasaki disease | Sanford et al. 1997; Azhar et al. 2011; Shimizu et al. 2011; Lindsay et al. 2012; Kruithof et al. 2013; Leutermann et al. 2014 |
| TGF-β3 (TGFB3) | P0 | Defect in pulmonary development and cleft palate. No CV disease | Unknown | Kaartinen et al. 1995; Proetzel et al. 1995 |
| BMP-9 (BMP9) | Viable | Defects in lymphatic drainage, retina vascularization, and valve maturation | Vascular anomaly similar to HHT | Ricard et al. 2012; Levet et al. 2013; Wooderchak-Donahue et al. 2013 |
| TβRII (TGFBR2) | E10.5 | Abnormal development of yolk sac vasculature and capillary vessel formation in the embryo | Loeys–Dietz syndrome types 1 and 2; MFS | Oshima et al. 1996; Loeys et al. 2005, 2006; Stheneur et al. 2008 |
| BMPRII (BMPR2) | E6.5–E9.5 | Defect in mesoderm induction; heterogenic: pulmonary hypertension | PAH; HHT | Beppu et al. 2000, 2004; Lane et al. 2000; Soubrier et al. 2013 |
| TβRI/ ALK-5 (TGFBR1) | E10.5 | Abnormal angiogenesis, impaired EC migration, and fibronectin production | Loeys–Dietz syndrome type 1 | Larsson et al. 2001; Loeys et al. 2005, 2006 |
| ALK-1 (ACVRL1) | E11.5 | Abnormal angiogenesis, impaired differentiation, and SMC recruitment | HHT-2; PAH | Johnson et al. 1995; Oh et al. 2000; Urness et al. 2000; Fujiwara et al. 2008; Soubrier et al. 2013 |
| Endoglin (ENG) | E10.5–11.5 | Angiogenesis defect, arrested remodeling and SMC development, cardiac defects; heterogenic: HHT | HHT-1 | McAllister et al. 1994; Bourdeau et al. 1999; Li et al. 1999; Arthur et al. 2000 |
| TβRIII/ betaglycan (TGFBR3) | E16.5–P0 | Defect in compaction of ventricular wall and cardiac septation | Unknown | Stenvers et al. 2003 |
| Smad1 (SMAD1) | E9.5 | No allantois formed; disorganized vessels | Unknown | Lechleider et al. 2001 |
| Smad3 (SMAD3) | Viable | Accelerated wound healing; aortic aneurysm | Aneurysm-osteoarthritis syndrome | Ashcroft et al. 1999; van de Laar et al. 2011; van der Linde et al. 2012; Tan et al. 2013; Wischmeijer et al. 2013 |
| Smad4 (SMAD4) | <E7.5 | Gastrulation defect, defect in visceral endoderm differentiation | Myhre syndrome, HHT | Sirard et al. 1998; Yang et al. 1998; Piccolo et al. 2014 |
| Smad5 (SMAD5) | E9.5–10.5 | Defect in angiogenesis, ectopic vascularization | No CV syndrome | Chang et al. 1999, 2000; Yang et al. 1999 |
| Smad6 (SMAD6) | Cardiovascular abnormalities, defect in endocardial cushion formation | BAV | Galvin et al. 2000 | |
| Fibrillin-1 (FBN1) | P0 | Aortic aneurysm and rupture, impaired pulmonary function. | MFS1 | Carta et al. 2006 |
| Fibrillin-2 (FBN2) | Viable | No CV defects | No CV syndrome | Arteaga-Solis et al. 2011 |
| Fibrillin-1/2 (FBN1/2) | E14.5–16.5 | Defects in fiber formation of aortic wall, aortic aneurysms | No CV syndrome | Carta et al. 2006 |
| LTBP-1 (LTBP1) | P0 | Persistent truncus arteriosus and aortic arch defects | No CV syndrome | Todorovic et al. 2007 |
| LTBP-3 (LTBP3) | Thoracic aneurism | No CV syndrome | Zilberberg et al. 2015 | |
| LTBP-4 (LTBP4) | P9-14 | Pulmonary emphysema and cardiomyopathy | ARCL1C | Bultmann-Mellin et al. 2015 |
| LTBP-4S | Adult | Cardiomyopathy | ARCL1C | Sterner-Kock et al. 2002 |
| Thrombo-spondin-1 (THBS1) | 4 wk | Cerebral hemorrhages | No CV syndrome | Crawford et al. 1998 |
TAA, Thoracic aortic aneurysm; HHT, hereditary hemorrhagic telangiectasia; PAH, pulmonary arterial hypertension; EC, endothelial cell; SMCs, smooth muscle cells; BAV, bicuspid aortic valve; MFS, Marfan syndrome; ARCL1C, autosomal-recessive cutis laxa type IC.
The angiogenic capacity of TGF-β results from TGF-β binding to the complex of TβRII with ALK-1 and endoglin, which stimulates EC proliferation, migration, and tube formation (Goumans et al. 2003b). Adenoviral expression of constitutively active ALK-1 stimulates the proliferation of ECs and the formation of endothelial tube-like structures (Goumans et al. 2002; Wu et al. 2006). ALK-1 is also present in EC caveolae, in which it interacts with caveolin-1. This results in enhanced ALK-1 signaling, whereas TGF-β signaling through ALK-5 and Smad2 and Smad3 activation is inhibited (Santibanez et al. 2008). In a mouse model for pancreatic tumors, heterozygosity of the gene encoding for ALK-1 results in a reduction of vascular density within the tumor (Cunha et al. 2010). These cell culture and in vivo findings suggest that ALK-1 signaling is proangiogenic and maintains ECs in an activated state.
Endothelial-specific deletion of Acvrl1/Alk1 in mice shows that ALK-1 is crucial for EC proliferation and the establishment of an arterial identity during development, whereas ALK-1 controls vessel stabilization and integrity during adult life (Tual-Chalot et al. 2014). Expression of a constitutively active ALK-1 in ECs inhibits cell proliferation, migration, and adhesion, indicative of the maturation phase of angiogenesis (Lamouille et al. 2002). Furthermore, silencing ALK-1 expression in cultured ECs results in enhanced vascular sprouting and loss of ephrinB2 (Kim et al. 2012). ALK-1-deficient ECs show enhanced migration and sprout formation in response to angiogenic stimuli in cell culture (Choi et al. 2013). Finally, mouse embryos deficient for ALK-1 expression display dilated large vessels and delayed SMC differentiation and migration. Alk-1−/− embryos show excessive fusion of capillary networks and the major arteries and veins, thus giving rise to AVMs (Oh et al. 2000; Urness et al. 2000; Park et al. 2008), suggesting a role for ALK-1 in vessel maturation. The contradictory findings on the role of ALK-1 in TGF-β-stimulated angiogenesis suggest a delicate balance between the pro- and antiangiogenic effects of TGF-β. This balance must be unraveled before we can fully understand how TGF-β signaling affects angiogenesis during development and disease.
Endoglin is highly expressed in ECs and plays an important role in regulating the balance between the pro- and antiangiogenic responses of TGF-β. Endoglin enhances TGF-β signaling through ALK-1 and Smad1 and Smad5 activation and inhibits TGF-β signaling through ALK-5 and Smad2 and Smad3 phosphorylation (Lebrin et al. 2004; Pomeraniec et al. 2015). Endoglin-deficient mice die around E11.5, and both the yolk sac and the embryonic vasculature are affected (Table 1) (Li et al. 1999; Arthur et al. 2000; Bourdeau et al. 2000b), suggesting a proangiogenic role of endoglin. Eng−/− mouse embryos develop enlarged and weak vessels and at E9.5 lack SMCs around the major vessels in the embryo (Li et al. 1999). This defect in SMC development precedes the defect in endothelial remodeling. However, defective TGF-β signaling in ECs reduces the endogenous levels of TGF-β1 protein and impairs the recruitment and differentiation of the SMC layer (Carvalho et al. 2004). Furthermore, Eng−/− mouse embryonic stem cells can differentiate into ECs, whereas endoglin deficiency impairs VEGF-induced angiogenesis (Liu et al. 2014). Finally, endoglin contributes to both shear-induced collateral artery growth and ischemia-induced angiogenesis in a mouse hind limb ischemia model, suggesting a role for endoglin in stimulating both angiogenesis and arteriogenesis (Seghers et al. 2012).
SnoN inhibits decapentaplegic (DPP)- and activin-induced vein formation in the Drosophila wing model (Ramel et al. 2007). Introducing a mutant form of SnoN, which is unable to bind Smads and abolishes the ability of SnoN to repress TGF-β signaling, in the original mouse Skil locus that encodes for SnoN results in embryonic lethality because of cardiovascular defects. Although vasculogenesis does occur, angiogenesis is impaired in both the yolk sac and the embryo proper (Pan et al. 2009; Zhu et al. 2013). Furthermore, the embryos expressing the mutant SnoN develop AVMs, a typical feature of Alk1−/− embryos, and show reduced expression of the arterial marker ephrinB2. ECs isolated from these SnoN mutant embryos show an increased expression of PAI-1, which is encoded by a TβRI/ALK-5-induced Smad3 target gene and represses EC migration (Zhu et al. 2013). The SnoN mutation reduces the expression of the inhibitor of differentiation 1 (Id1), encoded by an ALK-1-induced Smad1 and/or Smad5 target gene, and reduces the ability of ALK-1 to form complexes with Smad1 and Smad5 after TGF-β or BMP-9 stimulation. In contrast, no difference in ALK-5-induced Smad2 and Smad3 complex formation is observed between wild-type and SnoN mutant cells (Zhu et al. 2013). These results suggest that SnoN prevents functional Smad interaction at targeted promoters.
Similar to the biphasic effects reported for TGF-β (Pepper et al. 1993; Goumans et al. 2002), BMP-9 also regulates EC proliferation and differentiation in a context-dependent manner. BMP-9 inhibits EC proliferation at high concentrations (David et al. 2007; Scharpfenecker et al. 2007) but stimulates proliferation of multiple EC types at low concentrations (Suzuki et al. 2010). The underlying mechanism for this differential response is not clear. One explanation might be the presence of BMP-10, because both BMP-9 and -10 are expressed at significant levels in human and mouse sera (Chen et al. 2013) and are found to be critical for postnatal retinal vascular remodeling (Ricard et al. 2012). Although BMP-9 is dispensable during embryonic and neonatal vascular development (Chen et al. 2013), early postnatal lymphatic development is disrupted on genetic deletion, antibody suppression, or when an ALK-1-Fc ligand trap is used to inhibit both BMP-9 and -10. This defect in development of the lymphatic microvessels and maturation of the lymphatic plexus is similar to that observed as a result of silencing ALK-1 expression in mice (Niessen et al. 2010). Analyses of retinal vascular differentiation showed that endothelial-specific silencing of either Eng or Alk1 causes a delay in vascular remodeling of the retinal capillary plexus (Mahmoud et al. 2010; Tual-Chalot et al. 2014). Treating mice with a ligand trap for BMP-9 and -10 (ALK-1-Fc) or for BMP-10 (anti-BMP-10 antibody) in a Bmp9-deficient background revealed that BMP-10 can substitute for BMP-9 in retinal angiogenesis. This apparent substitution may be explained partly by enhanced Notch signaling, and partly by decreased expression of the proangiogenic factor apelin, which activates the G-protein coupled receptor apelin receptor (APLNR, alias APJ) (Ricard et al. 2012).
Lymphatic Endothelium
TGF-β signaling has a dual effect on LECs. TGF-β prevents differentiation of LECs by inhibiting the expression of Prox-1 and LYVE-1 in cultured LECs (Oka et al. 2008). In a mouse model of pancreatic ductal adenocarcinoma, inhibition of TGF-β signaling using a TβRI kinase inhibitor results in enhanced lymphangiogenesis (James et al. 2013). LEC-specific deletion of Tgfbr2 or Tgfbr1 in vivo using a Prox-1-specific Cre-driver (Prox1-CreERT2; Tgfbr2f/f and Prox1-CreERT2; Tgfbr1f/f) results in severe reduction of lymphatic vessel sprouting and remodeling and inhibits LEC proliferation (James et al. 2013). Stimulation of LECs with BMP-9 reduces the number of LECs (Yoshimatsu et al. 2013). Furthermore, systemic intraperitoneal administration by injection of the BMP-9 ligand trap ALK-1-Fc into neonatal mice at P1, P3, and P5 disturbs postnatal lymphatic vessel development in the retina, tail, and skin (Niessen et al. 2010). Alk1- or Bmp9-deficient mice develop enlarged lymphatic vessels (Yoshimatsu et al. 2013), again highlighting the importance of this signal transduction pathway for lymphatic vessel maturation and lymphatic valve formation.
BMP-9 signaling through ALK-1 was also shown to convert ECs from a LEC phenotype to a BEC phenotype (Yoshimatsu et al. 2013). The loss of LEC-specific markers like Prox-1 coincides with the activation of several genes known to be involved in lymphatic valve formation, such as Foxc2 and Nrp1 (encoding neuropilin-1), and maturation of the lymphatic vessels (Levet et al. 2013). Bmp9 inactivation results in enlarged lymphatic-collecting vessels as a result of an increase in number of LECs in the lymphatic wall (Levet et al. 2013). Finally, BMP-9 prevents tumor lymphangiogenesis and inhibits lymphangiogenesis in a chronic aseptic peritonitis mouse model (Yoshimatsu et al. 2013). This result shows that TGF-β family signaling is not essential for lymphatic system function under physiological conditions. However, it remains to be elucidated how both TGF-β signaling through ALK-5 and BMP-9 signaling through ALK-1 inhibit LEC proliferation, decrease Prox-1 expression, and reprogram LECs to become BECs.
Mural Cells and Interplay with ECs
In addition to its effects on ECs, TGF-β also has potent, context-dependent effects on pericytes and SMCs. TGF-β stimulates SMC proliferation by inducing the expression of growth factors, including PDGF; however, at high TGF-β concentrations, this response is inhibited (Battegay et al. 1990). Increased levels of Smad3 can switch TGF-β from an inhibitor of SMC proliferation to an activator of SMC proliferation. TGF-β-induced growth inhibition, causing a G0/G1 arrest, requires the activity of p38 MAP kinase (Seay et al. 2005). Intimal hyperplasia, caused by SMC proliferation, is induced by activation of p38 MAP kinase, the extracellular signal-regulated kinase (Erk) MAP kinases, and PI3K (phosphatidylinositol-3 kinase)-Akt (Suwanabol et al. 2012) signaling. Furthermore, increased VEGF expression enhances intimal hyperplasia because VEGF functions as an autocrine inhibitor of SMC apoptosis (Shi et al. 2014). When SMCs are deprived of serum, TGF-β can both inhibit and promote apoptosis. Again, the effect of TGF-β appears to depend on the cell physiological context, including cell–ECM interactions (Hishikawa et al. 1999; Pollman et al. 1999). Additionally, TGF-β was found to inhibit SMC migration by inducing N-cadherin expression, which mediates RhoA activation and modulates the actin cytoskeleton (Nuessle et al. 2011).
TGF-β stimulates SMC differentiation, which is apparent by the induction of α-SMA (α-smooth muscle actin, gene name ACTA2), calponin1, and SMC1a (structural maintenance of chromosomes 1A) expression. The effects of TGF-β on the expression of SMC-specific genes require phosphorylation of Smad2 or Smad3 (Chen et al. 2003; Hu et al. 2003; Wang et al. 2012) but also depend on CArG-binding transcription factors such as serum response factor (SRF) and myocardin (Owens et al. 2004). SRF and myocardin physically interact with Smad3 and synergistically transactivate the promoters of TAGLN (i.e., the gene encoding SM22α) and ACTA2 in a Smad-binding element (SBE)-dependent but CArG box-independent manner (Qiu et al. 2003, 2005). TGF-β can induce pluripotent embryonic stem cells to differentiate into SMCs (Sinha et al. 2004). TGF-β-induced Smad2, Smad3, and p38 MAP kinase activation are required for SMC differentiation (Chen and Lechleider 2004; Deaton et al. 2005) in a RhoA-dependent manner (Chen et al. 2006). Inhibition of RhoA blocks TGF-β-induced SMC contractility (Chen et al. 2006). The ability of TGF-β to induce stem cells to differentiate into SMCs is, in part, mediated by the induced expression of the Notch ligand, Jagged (Kurpinski et al. 2010). The Notch pathway and the TGF-β pathway also cooperate in promoting mesenchymal stem cell differentiation. The canonical Notch effector, C-promoter binding factor-1 (CBF1), interacts with phosphorylated Smad2 and Smad3 to activate SMC promoters (Darland and D’Amore 2001; Tang et al. 2012).
Interactions between ECs and surrounding mural cells are essential for vascular development (Fig. 1). In elegant, three-dimensional, coculture studies, it was shown that latent TGF-β deposited in the ECM is activated when ECs interact with multipotent 10T1/2 mesenchymal cells. Activated TGF-β then stimulates the differentiation of 10T1/2 cells into SMCs or pericytes, and stabilizes the endothelial, capillary-like structures (Ding et al. 2004). Together with the induction of the expression of the serine protease furin that cleaves the TGF-β precursor, shear stress on ECs stimulates the formation of active TGF-β, which enhances TGF-β signaling and, consequently, the effects of TGF-β on ECs and SMCs (Negishi et al. 2001). Shear stress also enhances TGF-β signaling by inducing the expression of TGF-β1 and -β3 in ECs (Egorova et al. 2011; Walshe et al. 2013).
TGF-β signaling controls the differentiation and function of SMCs. Deletion of Smad4 in SMCs, or inhibition of TGF-β signaling using specific pharmacological inhibitors or small-interfering RNAs against Smad2 or Smad3, decreases SMC proliferation, migration, and expression of SMC-specific gene markers and contractile proteins like α-SMA, SM22α, calponin, and myosin heavy chain 11 (MYH11, also known as SMMHC), whereas inhibition of BMP signaling only affects cell migration (Mao et al. 2012). Targeted Smad4 inactivation in ECs shows that Smad4 is required for cerebral vascular integrity. By cooperating with Notch, Smad4 mediates activation of N-cadherin expression, and contributes to stabilizing the interactions of ECs with pericytes (Li et al. 2011).
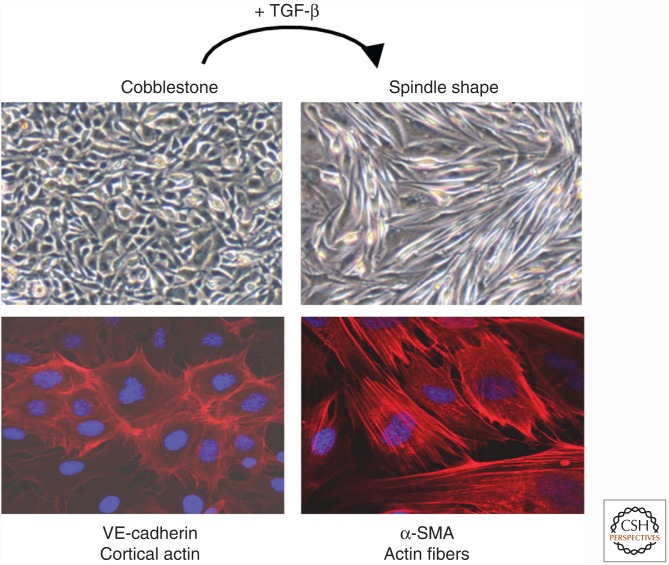
Endothelial-to-Mesenchymal Transition
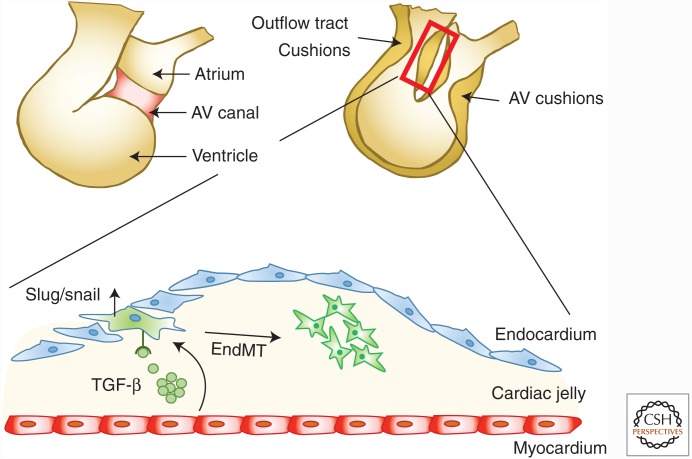
ECs lining the inside of blood vessels and lymphatic vessels can undergo a transition into mesenchymal cells, a process known as endothelial-to-mesenchymal transition (EndMT) (Goumans et al. 2008; Kovacic et al. 2012; Medici and Kalluri 2012). In three-dimensional and in vivo models, ECs undergo EndMT when stimulated with TGF-β (Fig. 5). During EndMT, the ECs lose cell–cell contact, delaminate from the endothelium, and invade and migrate into the underlying tissue, thereby displaying a mesenchymal phenotype. EndMT is characterized by the loss of EC markers, like platelet/endothelial cell adhesion molecule-1 (PECAM1, also known as CD31) and vascular endothelial cadherin (VE-cadherin), and the expression of mesenchymal cell markers, including α-SMA, vimentin, procollagen I, and fibroblast-specific protein 1 (FSP1, also known as S100A4). Thus, EndMT may contribute to the generation of fibrotic myofibroblasts in vivo. In addition, the concomitant loss of functional ECs may also lead to capillary rarefaction (a reduction in capillary density), thus causing tissue ischemia, a potent driver of the fibrotic process. EndMT was first described during embryonic cushion formation (Fig. 6); in that scenario, cells detach from the endocardial layer, acquire a mesenchymal phenotype, and migrate into the cardiac jelly where they ultimately form the cardiac valves (Markwald et al. 1977; Kruithof et al. 2012). EndMT has been implicated in a wide variety of organ pathologies, including cardiac fibrosis, kidney fibrosis, pulmonary fibrosis, and cancer (Kalluri and Weinberg 2009).
Figure 5.
Endothelial-to-mesenchymal transition. When stimulated with TGF-β, endothelial cells undergo EndMT. They lose their cobble-stone morphology (upper panels, bright field), reduce the expression of endothelial cell markers such as platelet/endothelial cell adhesion molecule-1 (PECAM1), and start to express mesenchymal markers such as α-smooth muscle actin (α-SMA). Cortical actin (in red) is reorganized into stress fibers. The nucleus is stained with DAPI in blue.
Figure 6.
Cardiac cushion formation. The developmental formation of cardiac cushions involves endothelial-to-mesenchymal transition (EndMT). The endothelial cells lose their cobblestone morphology, adopt a mesenchymal phenotype, and invade the cardiac jelly. AV, Arteriovenous.
Abundant examples show that TGF-β family signaling is a major molecular mediator in the pathological activation of EndMT (Goumans et al. 2008; Kovacic et al. 2012). In human disease, EndMT is observed during pathological processes in the heart that involve fibrosis of ischemic areas (Zeisberg et al. 2007). EndMT is also implicated in the pathology of fibrodysplasia ossificans progressiva (FOP), a rare genetic disorder, in which acute inflammation induces heterotopic ossification of soft tissues and the formation of ectopic skeletal structures (Medici and Olsen 2012). The fibroblast growth factor (FGF)-induced microRNA (miR) let-7 (miR-let-7) can suppress TGF-β signaling by reducing the stability of TβRI mRNA, thereby preventing the induction of EndMT (Chen et al. 2012b). TGF-β inhibition also promotes the generation and maintenance of phenotypically stable, embryonic stem cell–derived ECs (James et al. 2010). Although inhibition of TGF-β signaling, by repressing Smad3, contributes to the ability of Akt to maintain stable embryonic stem cell–derived ECs (Israely et al. 2014), TGF-β rapidly induces endothelial expression of miR-21. miR-21 facilitates EndMT through a PTEN/Akt-dependent pathway, as shown by a reduction in PECAM1 and endothelial nitric oxide synthase (eNOS) (Kumarswamy et al. 2012). miR-21 expression is also induced in cardiac ECs during pressure overload–induced cardiac remodeling and fibrosis, a process well known to involve TGF-β signaling (Zeisberg et al. 2007). In animals treated with transaortic constriction to produce cardiac pressure overload, a fraction of the ECs coexpresses endothelial and fibroblast markers, which is strongly inhibited by an antagomir against miR-21 (Kumarswamy et al. 2012). Finally, TGF-β induces miR-155 expression in ECs. miR-155 stimulates EndMT by repressing the Rho kinase activity (Bijkerk et al. 2012).
EndMT also occurs in the vasculature in the brain of patients with cerebral cavernous malformations (Maddaluno et al. 2013). Patients with this genetic disorder have enlarged, irregular blood vessels, which often give rise to cerebral hemorrhage. Endothelial-specific deletion of cerebral cavernous malformation protein 1 (CCM1; also known as KRTI1), one of three CCM-encoding genes, recapitulates the disease phenotype. In this context, EndMT is apparent by the disorganized VE-cadherin staining, and increased expression of the mesenchymal markers, N-cadherin, Slug, Id1, and α-SMA results from increased TGF-β and/or BMP signaling. Moreover, in mice with endothelial-specific Ccm1-inactivation, inhibition of the TGF-β pathway, by treating the animals with LY-364947 and SB-431542, reduces vascular malformations and prevents vascular leakage (Maddaluno et al. 2013). The development of cerebral cavernous malformations in the endothelial-specific Ccm1-deficient mouse is also reduced by silencing Klf4 expression. Apparently, KLF4 is required in TGF-β- or BMP-induced EndMT of CCM1-deficient ECs (Cuttano et al. 2015).
EndMT has also been observed in vascular injury. Lineage tracing in a vein graft model revealed that endothelial-derived mesenchymal cells comprise the majority of the neointima (Cooley et al. 2014). Biomechanical injury to the luminal ECs initiates a TGF-β signaling cascade, which results in maladaptive EndMT. ECs transdifferentiate into proliferating smooth muscle-like cells within the neointima in a TGF-β-Smad2/Smad3-Slug-dependent manner (Cooley et al. 2014). Aberrant neointima formation can undermine the longevity of coronary bypasses and contribute to the pathogenesis of cardiovascular disease by narrowing the vessel diameter. Patients that undergo percutaneous angioplasty often show massive neointimal formations. Angioplasty usually involves inflating a balloon to open the artery and allow for blood flow. Stents or scaffolds may be placed at the site of the blockage to hold the artery open. To prevent neointima formation, a drug-eluting stent may be used, eluting a compound that will inhibit EndMT or SMC proliferation. For example, inhibiting TGF-β signaling by placing gene silencer pyrrole-imidazole polyamide eluting stents, which target the activator protein (AP)-1 binding site in the TGFB1 promoter (Yao et al. 2009), or anti-endoglin antibody-eluting stents (Cui et al. 2014), may reduce or prevent pathological neointimal hyperplasia.
ECs that line the blood vessels can sense changes in hemodynamics exerted by blood flow regulation. Shear stress induces the expression of the Krüppel-like transcription factors KLF2 (Dekker et al. 2006) and KLF4, which results in quiescent ECs. Conversely, oscillating flow will not induce expression of these factors; instead, an inflammatory profile is activated in these ECs. Like an antenna, the endothelial primary cilium, a specialized, membrane-covered, rod-like protrusion, is capable of sensing, transducing, and amplifying information about the direction, viscosity, and velocity of the flow (Egorova et al. 2012; Praetorius 2015). ECs are devoid of cilia in areas of high shear stress and are ciliated in areas of oscillating flow. High shear stress and nonciliated ECs coincide with areas of EndMT, and these ECs release growth factors, including TGF-β1 and -β3 (Egorova et al. 2011). Thus, the absence of a cilium facilitates TGF-β-dependent EndMT in embryonic ECs. Moreover, EndMT was also found to depend on the expression of KLF4 (Egorova et al. 2011; Cuttano et al. 2015). Shear-induced KLF2 expression induces the expression of Smad7, which subsequently inactivates TGF-β-induced Smad signaling in human umbilical vein ECs (Boon et al. 2007). Shear-induced changes in mechanical forces at the blood–endothelial interface can also lead to morphological changes of the EC monolayer and rearrangement of the endothelial cytoskeleton at the onset of atherogenesis; these changes depend on activation of TGF-β, KLF2, and EndMT (Boon et al. 2007; Walshe et al. 2013).
Transdifferentiation of LECs into fibroblast cells is also stimulated by TGF-β. bFGF is required for maintaining the differentiated LEC identity by inhibiting TGF-β-induced EndMT. bFGF depletion synergizes with TGF-β to induce EndMT and results in increased α-SMA expression in LECs (Ichise et al. 2014). TGF-β-induced EndMT also depends on Notch signaling. Although BECs can be immortalized and maintained with an endothelial morphology, immortalized LECs lose their EC markers and express α-SMA (Ichise et al. 2014). TGF-β signaling might be required for lymphatic regeneration and function in adulthood; however, endothelial-specific deletion of Smad2 does not impair developmental lymphangiogenesis (Itoh et al. 2012).
TGF-β SIGNALING IN VALVE DEVELOPMENT AND DISEASE
Cardiac valve formation is essential for proper function of the four-chambered heart. Septal malformations, such as prolapses in bicuspid valves and mitral valves, are the most common congenital heart defects, and affect 1%–2% of the population (Michelena et al. 2015). During heart development, when the cardiac chambers appear, valves form in the atrioventricular canal, which separates the atria and ventricles, and in the outflow tract, which connects the ventricles to the aortic sac and ensure proper blood flow (Kruithof et al. 2012). The endocardial cushions are the primordial valves and, during the initial stages of cardiac valve formation, endocardial cells overlying the cushions undergo EndMT. They lose cell–cell contact, differentiate into mesenchymal cells, and migrate into the cardiac jelly, which is the ECM between the endocardium and myocardium and is rich in collagen, proteoglycans, and hyaluronan (Fig. 6).
TGF-β signaling represents one of three recognized signaling pathways that play key roles in cushion formation, in which it promotes EndMT of endocardial cells and their invasion as mesenchymal cells into the cardiac cushions (Garside et al. 2013). The developmental expression patterns suggest that TGF-β1 and -β2 promote EndMT (Akhurst et al. 1990; Molin et al. 2003). At E8, TGF-β1 is expressed in the endocardium and, when EndMT takes place, TGF-β1 expression becomes restricted to the atrioventricular canal endocardial cells. TGF-β1 expression remains restricted to valve endocardial cells until shortly after birth. TGF-β2 is expressed in atrioventricular myocardial and endocardial cells that flank the cushions (Akhurst et al. 1990; Molin et al. 2003). In contrast, TGF-β3 is expressed in the cushion mesenchyme after EndMT, and its expression increases as valve development proceeds after birth, suggesting that it may play a role in postnatal valve remodeling and function (Camenisch et al. 2002).
In several mouse studies, genes for TGF-β signaling components were inactivated, either in all tissues or in specific tissues, to understand their function during valve formation. Because TGF-β1 can cross the placental membranes, only Tgfb1-deficient embryos born from Tgfb1−/− mothers are thought to not be developmentally exposed to TGF-β1. These embryos show severe heart defects, including disorganized atrioventricular valves (Letterio et al. 1994). Embryos deficient in TGF-β2 expression show numerous defects that indicate that TGF-β2 is critical for cardiac valve and septal development. Tgfb2−/− embryos frequently show incomplete ventricular septation of the heart and, at E14.5, have enlarged, hypercellular cardiac cushions (Sanford et al. 1997; Bartram et al. 2001), suggesting that TGF-β2 is involved in the initiation and termination of EndMT (Sanford et al. 1997; Azhar et al. 2009, 2011). The valves in Tgfb2−/− mice at E18.5 display defective remodeling and differentiation (Azhar et al. 2011), which might indicate additional roles for TGF-β2. Tgfb3−/− mice do not have valve defects (Azhar et al. 2003); however, TGF-β3 has been implicated in epithelial-to-mesenchymal transition in the developing palate and lung (Kaartinen et al. 1995), as well as during chicken valvulogenesis (Boyer et al. 1999), and complementary activities of TGF-β1 may have masked a function for TGF-β3 in EndMT. Endothelial-specific inactivation of Tgfbr1 using Cre-mediated recombination driven by the Tie2 promoter leads to formation of severely hypoplastic atrioventricular cushions at E10.5. The lack of mesenchymal cells migrating into the cushions is consistent with a crucial role for TGF-β signaling in EndMT progression (Sridurongrit et al. 2008). Endothelial-specific inactivation of Tgfbr2 results in embryonic death at E11.5, but EndMT occurs normally in the atrioventricular cushions. Although mesenchymal cells are detected in the inferior cardiac cushion, their proliferation is impaired (Jiao et al. 2006). Inducible, Cre-mediated inactivation of Tgfbr2 in VE-cadherin-expressing ECs at E11.5 results in a ventricular septal defect because of failure of cushion fusion (Robson et al. 2010).
LTBP-1 also stimulates cardiac EndMT. Ltbp1−/− mice show hypoplastic cushions and enlarged valves as a result of attenuated EMT (Todorovic et al. 2011). Furthermore, an LTBP-1 antibody inhibits EndMT of mouse atrioventricular explants in three-dimensional collagen culture assays (Nakajima et al. 1997). LTBP-1 is made as a short (LTBP-1S) and long (LTBP-1L) isoform, and LTBP-1L binds to the ECM more efficiently than LTBP-1S (Olofsson et al. 1995). Lack of either LTBP-1L (Todorovic et al. 2007) or both LTBP-1 isoforms (Horiguchi et al. 2015) during embryonic development leads to hypoplastic endocardial cushions as a result of delayed EndMT in early valve development and hyperplastic mitral and tricuspid valves because of prolonged EndMT in late valvulogenesis. The thickening of the valves is accompanied by reduced TGF-β activity in the valvular interstitial cells (VICs) (Todorovic et al. 2007), revealing an important in vivo role for LTBP-1 as an extracellular regulator of TGF-β activity.
Targeted inactivation of either the Eng gene, encoding the TGF-β coreceptor endoglin, or Tgfbr3, encoding the coreceptor betaglycan, results in embryonic death caused by cardiovascular defects. In vivo lineage tracing shows that endoglin is required for efficient EndMT and cushion development (Nomura-Kitabayashi et al. 2009). Endoglin-deficient embryos show hypocellular cardiac cushions, consistent with a role for endoglin in EndMT (Li et al. 1999; Arthur et al. 2000; Bourdeau et al. 2000a). Haplotype analysis revealed a relationship between bicuspid aortic valves, pathological fusion, EndMT, and endoglin expression (Wooten et al. 2010). Betaglycan is also expressed in the endocardium during cushion formation (Stenvers et al. 2003), and Tgfbr3−/− mice die just before birth because of a thin myocardial wall and poorly developed septa (Compton et al. 2007).
The leaflets of the aortic valve are highly specialized structures, consisting mostly of VICs and ECM (Hinton and Yutzey 2011). VICs maintain valve tissue homeostasis by regulating ECM biosynthesis and transdifferentiating into osteoblast-like cells (Taylor et al. 2003; Wang et al. 2014c). Because TGF-β is a potent inducer of ECM deposition and remodeling, it is tempting to speculate that a causal relationship exists between aberrant TGF-β activation, dysfunctional flow, and changes in valve function. Indeed, in syndromes like Marfan syndrome (MFS) (Judge et al. 2011) and Williams syndrome (Hinton et al. 2010), atrioventricular valves frequently undergo pathological changes associated with impaired TGF-β signaling, VIC activation, increased proliferation, and abnormal reorganization and production of ECM and proteoglycans. These changes may induce mitral valve leaflet thickening and elongation, which can result in a prolapsed valve. However, perturbed TGF-β signaling is associated with syndromic and nonsyndromic valvulopathies. Myxomatous mitral valve disease is associated with impaired TGF-β signaling, VIC activation, proliferation, leaflet thickening, fibrosis, matrix remodeling, and leaflet prolapse. Activation of TGF-β signaling is seen as a major contributor to this pathology (Hagler et al. 2013; Rizzo et al. 2015).
The involvement of TGF-β and TβRI signaling in heart valve integrity may complicate the development of cancer treatments, based on inhibition of the TβRI kinase activity with a small molecule. Indeed, inhibition of TβRI function results in heart valve lesions, including hemorrhage, inflammation, degeneration, and interstitial cell activation and proliferation (Anderton et al. 2011). TGF-β signaling may be involved in the continued remodeling of the adult valve (Walker et al. 2004) as a result of ongoing mechanical stress caused by valve opening and closing during each heart cycle; inhibiting TβRI activity may therefore disturb this process. However, the TβRI kinase inhibitor LY2157299 does not show cardiac toxicity and thus represents an interesting candidate to treat patients with cancer (Kovacs et al. 2015). In a mouse model of MFS and Loeys–Dietz syndrome (LDS) (Habashi et al. 2006; Gallo et al. 2014), losartan, an angiotensin-II type 1 (AT1) receptor blocker, inhibits TGF-β-induced Smad2 and Erk phosphorylation and reduces the EndMT-driven fibrotic response. Losartan prevents mitral valve lengthening and thickening, which are typically caused by changes in hemodynamics and/or cytokine production after myocardial infarction (MI), and does so by inhibiting EndMT in cardiac mitral valve ECs. In cultured mitral valve ECs, losartan prevents EndMT by inhibiting TGF-β-induced Erk MAP kinase pathway activation (Wylie-Sears et al. 2014).
Aortic valve stenosis is characterized by valve fibrosis and calcification. This aortic valve narrowing causes high shear stress across the valves, which activates latent TGF-β that is released by platelets. In turn, TGF-β release induces EndMT (Mahler et al. 2013), which causes further narrowing of the valves. Although valvular interstitial and ECs show activation of Smad2 and Smad3, as well as Erk MAP kinase signaling (Wang et al. 2014b), leukocytes only show Smad2 and Smad3 activation. In mice, the stenotic phenotype is reverted when floxed Tgfb1 is excised in platelets by Cre recombinase expressed from the promoter of the Pf4 gene (Wang et al. 2014b), suggesting that platelet-derived TGF-β1 contributes to aortic valve stenosis.
TGF-β SIGNALING IN HEREDITARY VASCULAR DISEASES
Hereditary Hemorrhagic Telangiectasia
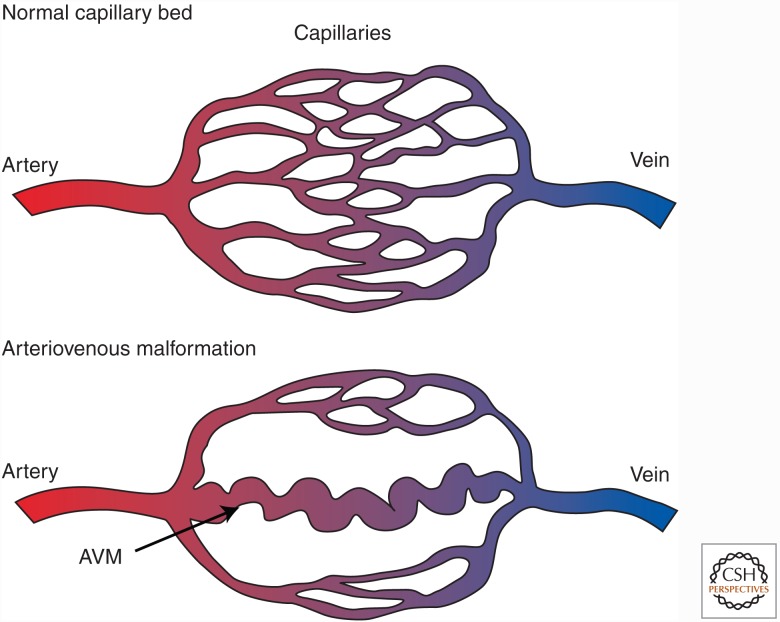
The TGF-β and BMP coreceptor endoglin and the type I receptor ALK-1 are highly expressed by ECs. The involvement of ALK-1 and endoglin in TGF-β-regulated angiogenic responses is evident from the inherited vascular dysplasia in humans, named hereditary hemorrhagic telangiectasia (HHT) or Osler–Rendu–Weber syndrome (García de Vinuesa et al. 2016; Morrell et al. 2016; Goumans et al. 2017). HHT is an autosomal-dominant disorder that affects approximately 1 in 5000 people and arises from mutations in the genes that encode endoglin (ENG), ALK-1 (ACVRL1), or Smad4 (SMAD4). HHT is characterized by many telangiectasias (i.e., red spots that emerge from vascular abnormalities in connections between arteries and veins). In small arteries and veins, these spots are associated with loss of capillary beds. Larger arteries and veins develop AVMs (Fig. 7), which are predominantly found in the lungs, liver, gastrointestinal (GI) tract, and brain (Abdalla and Letarte 2006). These fragile vessels are prone to severe bleeding. HHT typically manifests as recurrent nosebleeds (epistaxis), which are difficult to treat and severely affect quality of life. In more severe cases, HHT may result in ischemic injury and stroke, which require major clinical attention.
Figure 7.
Arteriovenous malformations (AVMs) in hereditary hemorrhagic telangiectasia (HHT). One hallmark of HHT is the development of an AVM. In the AVM, the capillary bed is lost, and the artery drains directly into the vein via a tortuous, weak vessel, which is prone to rupture.
HHT is divided into four subtypes, HHT type 1 (HHT-1) to HHT-4. These subtypes are caused, respectively, by mutations in ENG encoding endoglin (McAllister et al. 1994), ACVRL1 encoding ALK-1 (Johnson et al. 1995), chromosome 5 (Cole et al. 2005), and chromosome 7 (Bayrak-Toydemir et al. 2006). SMAD4 mutations are linked to a combined syndrome with characteristics of juvenile polyposis and HHT (Gallione et al. 2006, 2010). Additionally, BMP9 mutations are linked to a vascular anomaly that phenotypically overlaps with HHT (Wooderchak-Donahue et al. 2013).
To date, the genetic underpinnings of HHT and the clinical phenotype points to roles of ALK-1 and endoglin in vascular development and vascular integrity. Nevertheless, the specific cellular and molecular mechanisms remain to be clearly understood. Ongoing clinical trials are evaluating antiangiogenesis agents in patients with HHT. These agents include bevacizumab, a neutralizing antibody against VEGF, which inhibits HHT-associated pathologies (Epperla and Hocking 2015), and thalidomide, which inhibits bleeding and other vascular malformations associated with HHT (Lebrin et al. 2010), although not all patients benefit from this treatment (Hosman et al. 2015).
Endothelial-specific Alk1 inactivation in mice results in vascular malformations, including systemic enlargement of arteriovenous connections, GI bleeding, and all the pathological features of HHT-2 (Park et al. 2008). Although these mice lack ALK-1, AVMs do not develop, suggesting the requirement of a second hit. VEGF-stimulated angiogenesis triggers the development of AVMs in endothelial-specific Alk1 mice, both in the GI tract (Park et al. 2008) and in the brain (Chen et al. 2014). Furthermore, angiogenic stimulation after wounding and increased expression of VEGF gives rise to AVMs. Conversely, VEGF blockade prevents AVM formation and normalizes established arteriovenous shunts in ALK-1-deficient mice (Han et al. 2014).
Although treatment with anti-VEGF antibodies is beneficial in patients with HHT-1, different mechanisms are at the basis of HHT-1 and -2. Mice heterozygous for Eng show dilated, fragile blood vessels, which resemble the clinical symptoms observed in HHT-1 (Arthur et al. 2000; Bourdeau et al. 2000b, 2001; Sorensen et al. 2003). In addition, Eng heterozygous mice show dysregulated microvascular density in the lungs, associated with increased thrombospondin-1 expression. In contrast, mice heterozygous for Alk1 inactivation, thus mimicking HHT-2, show secondary right ventricular hypertrophy, associated with increased expression of angiopoietin-2 (Ardelean et al. 2014). Anti-VEGF treatment normalizes the pulmonary microvascularization anomaly, and attenuates the right ventricular hypertrophy in this mouse model (Ardelean et al. 2014; Ardelean and Letarte 2015).
Impaired mural coverage of the vasculature is one cause for fragile vessels in mice heterozygous for Eng. Interestingly, treatment of these mice with thalidomide results in increased expression of PDGF-B, which will attract mural cells and stimulate their differentiation into pericytes and SMCs, resulting in vessel coverage and rescue of vessel wall defects (Lebrin et al. 2010). In humans, thalidomide treatment of a small group of patients with HHT-1 reduced the severity and frequency of nosebleeds and attenuated bleeding GI angiodysplasia (Alam et al. 2011; Franchini et al. 2013). However, thalidomide is thrombogenic (Penaloza et al. 2011), and might stimulate the development of peripheral neuropathy. Therefore, safer thalidomide homologs are needed for treating patients with HHT.
Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH) is a life-threatening disease characterized by progressive remodeling of the pulmonary vasculature because of abnormal proliferation of pulmonary endothelial and SMCs. The resulting increase in pulmonary vascular resistance will ultimately lead to the development of right heart failure (Morrell et al. 2016). The hereditary form of this disease is mostly linked to mutations in the coding region of BMPRII, although mutations in ENG, ACVRL1/ALK1, and BMP9 have also been reported. The role of BMPRII in arterial hypertension is shown by the increased susceptibility of mice heterozygous for Bmpr2 to experimentally induced pulmonary hypertension (Beppu et al. 2004; Song et al. 2005, 2008). Cell culture studies have shown that pulmonary ECs and SMCs from PAH patients have reduced levels of Smad1 and Smad5 activation. TGF-β1 increases the proliferation of PAH SMCs compared with control SMCs, and elevated Smad2 activation is found in plexiform lesions of PAH patients (Gore et al. 2014). Therefore, both reduced BMP signaling, as well as elevated TGF-β signaling, contribute to the pathogenesis of PAH.
New treatment modalities are now focusing on either activating BMP signaling or inhibiting the TGF-β pathway. High-throughput screening revealed that the immunosuppressive drug tacrolimus (also known as FK506) stimulates BMP signaling by forming a complex with FKBP12. Treating human pulmonary microvascular ECs with a low dose of tacrolimus releases FKBP12 from the BMP type I receptor and increases the level of Smad1 and Smad5 activation (Spiekerkoetter et al. 2013). Tacrolimus was shown to prevent the development of PAH in endothelial deficient Bmpr2−/− mice, as well as in rats treated with monocrotalin to injure pulmonary ECs. A few PAH patients have been successfully treated with a low dose of tacrolimus (TransformPAH trial; Spiekerkoetter et al. 2015) and have been free from hospitalization ever since. Enhancing BMP signaling by administration of BMP-9 to mice carrying one allele encoding a human BMPRII mutant that has been associated with PAH prevents spontaneous development of PAH (Long et al. 2015). Additionally, treating rats with experimentally induced PAH using BMP-9 also reverses their pulmonary hypertension. These elegant studies show that restoring BMP signaling provides a potential treatment strategy for PAH. To find treatment options to inhibit TGF-β signaling, prostacyclin was found to reduce the TGF-β-induced Smad3 activation and inhibit proliferation of PAH-mouse pulmonary artery SMCs in culture (Ogo et al. 2013), and in vivo, monocrotalin-treated mice show a reduction in disease progression when treated with prostacyclin.
Aortic Aneurysms and Dissection: Lessons Learned from MFS and LDS
The aortic aneurysm is a cardiovascular disorder with a high mortality rate due to the risk of aortic dissection and sudden rupture. TGF-β stimulates the formation of aortic aneurysm, apparent from elevated levels of nuclear Smad2 in vascular tissue from patients that experienced a thoracic aortic aneurysm (TAA) (Gomez et al. 2009). Inherited forms of TAA can be autosomal-dominant or autosomal-recessive. Autosomal-dominant TAAs include MFS, which is caused by mutations in the gene encoding the microfibrillar glycoprotein, fibrillin-1 (FBN1) (Dietz et al. 1991), and LDS, which is linked to mutations in TGFB2 (Lindsay et al. 2012), TGFB3 (Rienhoff et al. 2013), or the TGFBR1 or TGFBR2 genes encoding the two TGF-β receptors, TβRI and TβRII (Loeys et al. 2005). Autosomal-recessive TAA is found in cutis laxa, a disease caused by mutations in the genes encoding fibulin-4 (Renard et al. 2010) or LTBP-4 (Callewaert et al. 2013). Furthermore, aortic aneurisms have been associated with mutations in SMAD3 and SKI, which encode a repressor of TGF-β signaling. Mutations in Smad3 that disturb Smad3 protein function also lead to the aneurysms-osteoarthritis syndrome, a form of TAA with tortuosity of the arterial tree and early onset of osteoarthritis (Regalado et al. 2011; van de Laar et al. 2012). Mutations in the R-Smad binding domain of c-Ski hampers the formation of a Smad3-c-Ski complex, results in enhanced TGF-β signaling, and causes Shprintzen–Goldberg syndrome (SGS) (Carmignac et al. 2012; Doyle et al. 2012; Schepers et al. 2015). Finally, mutations in LTBP-4 and TGF-β3 have been associated with abdominal aortic aneurysm progression (Thompson et al. 2010).
MFS has served as a paradigm for understanding the pathogenesis of TAAs and aortic dissections. Fibrillin-1 is an important component of the microfibrils in elastic fibers; these fibers provide stiffness and elasticity to dynamic structures like blood vessels. Mutant fibrillin-1, which is defective in microfibril assembly and function, increases the aortic wall susceptibility to dissection and rupture (Marque et al. 2001; Ramirez and Dietz 2007). In addition to its function in connective tissues, fibrillin-1 contains multiple LTBP binding sites with conserved 8-cysteine motifs (Chaudhry et al. 2007), which allows it to sequester TGF-β (ten Dijke and Arthur 2007; Bolar et al. 2012; Doyle et al. 2012). Nonfunctional fibrillin-1, like the one found in MFS, results in release of the latent TGF-β complex from the ECM, pronounced TGF-β activation, and elevated TGF-β-induced Smad2 activation (Neptune et al. 2003; Habashi et al. 2006). Impaired binding of LTBP-3, when present in the latent TGF-β complex that associates with fibrillin-1, causes enhanced TGF-β signaling in MFS (Zilderberg et al. 2015). MFS mice deficient for Ltbp3 show improved survival and no aortic aneurysms and rupture.
Neutralizing anti-TGF-β antibodies administered to a mouse model of MFS heterozygous for an Fbn1 allele encoding fibrillin-1 with the cysteine substitution, Cys1039→Gly (C1039G) (i.e., Fbn1C1039G/+ mice) inhibit the increased TGF-β signaling associated with the pathology of MFS, apparent by its level of Smad2 activation, and the development of aortic root aneurysm (Neptune et al. 2003). Moreover, aortic growth is inhibited in the MFS mouse, when Erk or JNK MAP kinase activation is inhibited (Holm et al. 2011). Thus, inhibiting either the canonical pathway or the Erk and JNK MAPK pathways, although not TGF-β specific, may be beneficial for treating MFS. Similar protection against aortic aneurisms is also achieved by treating the MFS mouse with losartan (Habashi et al. 2006). Blocking angiotensin-II signaling reduces the expression of TGF-β ligands and receptors (Fukuda et al. 2000); this effect is apparent from the decreased Smad2 phosphorylation (Fukuda et al. 2000). Losartan also attenuates non-Smad signaling through inhibition of the Erk MAP kinase pathway (Habashi et al. 2011; Holm et al. 2011). This beneficial effect of losartan on aortic dilatation is supported by the results of a phase III clinical trial in patients with MFS (Groenink et al. 2013). Finally, doxycycline, an anti-inflammatory agent and matrix metalloproteinase (MMP) inhibitor, also reduces aortic root dilation in MFS mice by inhibiting TGF-β-induced MMP activation and MMP-induced release of TGF-β (Xiong et al. 2008; Lindeman et al. 2009), but not by reducing inflammation (Chung et al. 2008; Franken et al. 2014).
It is still unclear whether excessive or diminished TGF-β signaling is the primary cause for aortic root aneurysms because systemic abrogation of TGF-β signaling by treating mice with a neutralizing anti-TGF-β1 antibody promotes angiotensin-II-induced abdominal aortic aneurysm formation (Wang et al. 2010). Furthermore, some TGFBR2, SMAD3, and SKI mutations found in MFS, LDS, or TAA, which cause aneurysmal disorders (Loeys et al. 2006; Horbelt et al. 2010; Gallo et al. 2014), lead to decreased TGF-β-induced Smad2 and Smad3 phosphorylation. Conditional inactivation of Tgfbr2 in postnatal SMCs using Myh11-CreERT2 (Li et al. 2014) or Acta2-Cre (Yang et al. 2016) induces aortic aneurisms and dissection as a result of enhanced elastin degradation, and accelerates disease progression in the murine model for MFS. Loss of Tgfbr2 reduces Smad2 phosphorylation in SMCs but increases Erk MAPK phosphorylation. This was unexpected because these pathologies are typically observed in patients with MFS and linked to increased TGF-β signaling. Smad4 deletion in SMCs using Acta2-Cre results in the development of TAA and abdominal aortic aneurysm, and blood vessel rupture between weeks 3 and 10 after birth because of enhanced elastin degradation and enhanced SDF-1α-stimulated macrophage infiltration (Yang et al. 2016). In contrast to loss of Tgfbr2, Smad4-deficient aortic walls show elevated activation of Smad2, Smad3, and JNK and enhanced TGF-β3 and CTGF expression. The LDS mouse model, which carries LDS-associated missense mutations in either Tgfbr1 or Tgfbr2, does recapitulate the vascular phenotype of LDS (Gallo et al. 2014).
The aortic wall in TAA can be recognized by the characteristic degeneration of the medial layer, which is caused by decreased expression of contractile proteins and collagens (Crosas-molist et al. 2015). The loss of contractile SMCs, which leads to greater cellular and ECM stiffness and vessel wall rigidity, is a TGF-β-dependent process and is seen as a major contributor to the pathogenesis of aortic aneurysms. These conditions result in SMC apoptosis and, eventually, vessel dissection. miR-29b levels are increased in the SMCs of the ascending aorta of Fbn1C1039G/+ MFS mice, and treatment with losartan or TGF-β inhibitors decreases miR-29b expression, thus inhibiting SMC apoptosis and stimulating ECM production (Merk et al. 2012). Furthermore, delivery of a locked nucleic acid (LNA) that targets and inhibits the activity of miR-29b to MFS mice prevents aneurysm formation and reduces aortic wall apoptosis (Merk et al. 2012).
Familial TAA can also result from loss of TβRI or TβRII signaling, when no other features of MFS or LDS are apparent (Milewicz et al. 1996; Pannu et al. 2005; Tran-Fadulu et al. 2009; Inamoto et al. 2010; Lerner-Ellis et al. 2014). Additionally, increased TGF-β signaling is also observed in TAA aortic walls of patients with mutations in ACTA2, encoding α-SMA or MYH11, two genes involved in SMC contraction. This result suggests that reduced vascular contractility might weaken the aortic wall and make it susceptible to aneurysm formation (Renard et al. 2013a). Dysregulated TGF-β signaling is also associated with a vulnerable aortic wall in patients with mitral valve disease (Renard et al. 2013b) or bicuspid aortic valves (Padang et al. 2013; Grewal et al. 2014a,b). Reduced TGF-β signaling in the vessel wall of patients with bicuspid aortic valves may be a result of the observed elevated expression of LTPB-3, and increased generation of large latent TGF-β complexes (Paloschi et al. 2015).
TGF-β SIGNALING IN ATHEROSCLEROSIS
Atherosclerosis is a progressive, chronic disease, characterized by plaque formation in the vascular wall. Atherosclerosis causes gradual luminal narrowing or, when a plaque ruptures, an acute thrombotic occlusion (Libby et al. 2011). Stable plaques are primarily composed of collagen and SMCs, and unstable plaques are primarily composed of macrophages and a large lipid core, covered with a thin fibrous cap. Unstable lesions are prone to rupture, which exposes the lipid core to the blood (Bentzon et al. 2014). The lipid core is rich in tissue factor and other strong procoagulants, which promote the propagation of a thrombus. Clinical symptoms caused by a thrombotic event include cerebrovascular accidents, aneurysms, or an MI (Bentzon et al. 2014). Atherosclerosis arises from an injury in the vessel wall, and TGF-β most likely modulates the fibrotic and inflammatory components of the lesion. TGF-β is produced by vascular cells and inflammatory cells, but there is some controversy in the literature as to the precise function of TGF-β in atherosclerosis (Grainger 2004; Toma and McCaffrey 2012).
Although TGF-β signaling may participate in the development of atherosclerosis, several studies have shown that it promotes a stable lesion phenotype by stimulating SMC differentiation and preventing the switch from contractile to proliferative SMCs (Gomez and Owens 2012). The expression of TGF-β1 is higher in stable lesions than in unstable lesions, and human atherosclerotic plaques with elevated TGF-β1 expression correlate with advanced atherosclerosis (Panutsopulos et al. 2005; Herder et al. 2012). Loss of TGF-β signaling in atherosclerotic human coronary arteries coincides with enhanced FGF signaling and loss of SMC contractile markers (Chen et al. 2016). Other studies show an inverse relationship between serum TGF-β1 levels and the development of atherosclerosis; that is, low levels of circulating TGF-β associate with unstable plaques (Bobik et al. 1999; Kalinina et al. 2004; Bot et al. 2009). Moreover, inducible overexpression of TGF-β1 in cardiomyocytes, which increases the levels of TGF-β1 in the plasma, limits plaque growth and induces plaque stabilization (Frutkin et al. 2009). These results suggest that TGF-β released from the cardiomyocytes is antiatherogenic (Tedgui and Mallat 2001; Hering et al. 2002). Furthermore, in ApoE−/− mice, which lack apolipoprotein E and represent an animal model of atherosclerosis, inhibition of TGF-β activity by intraperitoneal administration of recombinant, soluble TβRII (Lutgens et al. 2002) or an anti-TGF-β1 antibody (Tedgui and Mallat 2001) twice or once weekly, respectively, promotes atherogenic changes in the vessel wall. Specifically, the inflammatory response increases, and the lesions show an unstable phenotype, characterized by loss of SMC markers, low amounts of fibrosis, increased numbers of inflammatory cells, and hemorrhage within the plaque.
Disruption of TGF-β signaling, specifically in T cells (Gojova et al. 2003; Robertson et al. 2003) or dendritic cells (Lievens et al. 2013), accelerates the lesion progression and promotes the development of an unstable plaque phenotype because of the presence of abundant inflammatory cells and a parallel reduction in plaque fibrosis. Furthermore, increased expression of TGF-β1 in macrophage reduces the development of atherosclerotic lesions in ApoE-deficient mice and stabilizes existing plaques (Reifenberg et al. 2012). Transplantation of Smad7−/− T cells in hypercholesterolemic Ldlr−/− mice that lack the low density lipoprotein receptor results in larger, but more stable, atherosclerotic lesions with a large collagen-rich cap (Gistera et al. 2013). Increased Smad3 expression in cardiovascular tissues resulting from gene delivery using an adeno-associated virus vector inhibits atherogenesis and enhances the TH2 response without stimulating fibrosis (Zhu et al. 2014).
Endoglin may be involved in the as yet unresolved role of TGF-β in atherosclerosis. Human aortic plaque SMCs have elevated levels of endoglin, which supports a role for endoglin in vascular wall integrity (Conley et al. 2000; Bot et al. 2009). In addition, elevated soluble endoglin levels are found in patients with coronary artery disease (Seghers et al. 2012), and changes in soluble endoglin levels in the plasma after an MI have predictive value for acute mortality in this patient group (Cruz-Gonzalez et al. 2008). Elevated expression of both the long and short forms of endoglin was found in atherosclerotic lesions. Interestingly, the long form of endoglin has an athero-protective effect by inducing eNOS expression, and both the short form of endoglin and soluble endoglin promote atherogenesis by inhibiting eNOS expression and TGF-β signaling (Jang and Choi 2014).
Finally, although some features are common to the development of calcified valves and atherosclerosis, current atherosclerosis medications have not prevented progression of calcification. The different responses to treatment may partly be explained by differences in flow; the coronary artery supports laminar blood flow, and valves support pulsatile shear flow on the ventricular side and reciprocal shear flow on the aortic side (Rajamannan et al. 2011; Gould et al. 2013).
TGF-β SIGNALING IN CARDIAC FIBROSIS
Cardiac fibrosis is a hallmark of the heart’s pathological remodeling response to mechanical or biochemical stresse, like hypertension, pressure overload, cardiac inflammation, or MI (Hill and Olson 2008; Burchfield et al. 2013). The two types of cardiac fibrosis are reactive interstitial fibrosis, when fibrosis develops without significant cardiomyocyte loss, and reparative or replacement fibrosis, when a fibrotic scar forms in response to MI (Krenning et al. 2010; Kong et al. 2014). Cardiac fibrosis is characterized by the induction of expression of profibrotic growth factors, like TGF-β, and by the formation of cardiac fibroblasts into myofibroblasts. Activated fibroblasts differentiate into myofibroblasts, which increases their ability to produce ECM proteins. This change leads to increased myocardial stiffness and, ultimately, cardiac dysfunction and heart failure (Souders et al. 2012; Sullivan and Black 2013). The fibrotic process is driven primarily by local myocardial increases in TGF-β (Goumans et al. 2008; Creemers and Pinto 2011; Dobaczewski et al. 2011). For example, TGF-β1 expression increases during the development of cardiac hypertrophy (Ruwhof and van der Laarse 2000), dilated cardiomyopathy (Khan and Sheppard 2006), and aortic stenosis (Beaumont et al. 2014), and after an MI (de Sousa Lopes et al. 2003; Frantz et al. 2008).
Genetic mouse models have provided evidence for the roles of different components in the TGF-β signaling pathway in myocardial stiffening and heart failure development. Cardiomyocyte hypertrophy and interstitial fibrosis are enhanced in the hearts of mice that have elevated circulating TGF-β1 as a result of TGF-β1 overexpression in the liver (Rosenkranz et al. 2002). These changes result from reduced expression of interstitial collagenases (Seeland et al. 2002) and are inhibited by treatment with the AT1-receptor inhibitor, telmisartan (Seeland et al. 2009). In Tgfb1−/− mice (Brooks and Conrad 2000), or in wild-type mice treated with a neutralizing anti-TGF-β antibody (Okada et al. 2005; Ellmers et al. 2008), reduced TGF-β levels prevent collagen accumulation after pressure overload and attenuate diastolic dysfunction. Inhibition of TGF-β signaling by overexpressing a soluble TβRII (Okada et al. 2005) or a dominant-negative TβRII (Lucas et al. 2010) decreases cardiac fibrosis and improves cardiac function. However, although it reduces collagen deposition after pressure overload, expression of a dominant-negative TβRII also results in increased left ventricular dilatation and diastolic dysfunction (Okada et al. 2005; Lucas et al. 2010). In contrast, inhibiting the TβRI kinase activity by treating mice with an orally active pharmacological ALK-5 inhibitor reduces cardiac fibrosis and improves cardiac output (Engebretsen et al. 2014). Finally, the TGF-β coreceptor, endoglin, is also involved in the development of cardiac fibrosis. Endoglin expression increases during angiotensin-II-induced cardiac fibrosis, and treatment with soluble endoglin reduces cardiac fibrosis and attenuates the development of heart failure after aortic bending (Kapur et al. 2012). Interestingly, overexpression of miR-208a in the left ventricle increases endoglin levels and induces cardiac hypertrophy during pressure overload; these effects can be inhibited by treatment with the angiotensin converting enzyme inhibitor atorvastatin or with an antagomir of miR-208a (Wang et al. 2014a). In addition, Eng+/− mice show reduced fibrosis and death rate after right ventricular pressure overload (Kapur et al. 2014).
Both Smad and non-Smad pathways are involved in the pathological remodeling of the heart at the transcriptional level. Smad3 deficiency lowers interstitial fibrosis by reducing the deposition of collagen after left ventricular pressure overload (Divakaran et al. 2009). Smad3-deficient fibroblasts show less myofibroblast transformation and less collagen production in response to TGF-β (Dobaczewski et al. 2010). Moreover, fibrotic scar remodeling after MI is also reduced. In addition, Smad7−/− mice show enhanced myocardial fibrosis and remodeling during angiotensin-II-induced cardiac hypertrophy (Wei et al. 2013). Finally, TAK1 is activated by myocardial pressure overload, and activated TAK1 induces cardiac hypertrophy and severe systolic dysfunction (Zhang et al. 2000).
Much effort has been invested into developing new treatment modalities for cardiac fibrosis. miRs have received increasing attention, and it has become clear that TGF-β can mediate cardiac fibrosis through the control of miR expression. miR-29b protects against angiotensin-II-induced cardiac fibrosis by inhibiting increases in TGF-β1 levels (Zhang et al. 2014), and miR-378 inhibits TGF-β1 expression during cardiac remodeling, which reduces fibrosis (Nagalingam et al. 2014). Additionally, miR-34a regulates fibrosis by inhibiting Smad4 expression (Huang et al. 2014). Finally, miR-15 reduces the expression of TβRI and several other components of the TGF-β pathway, and increased miR-15 expression protects against ventricular pressure-induced cardiac fibrosis (Tijsen et al. 2014). The antifibrotic miRs act by inhibiting TGF-β protein expression, and several antifibrotic drugs, like resveratrol, inhibit fibrosis by interfering with the TGF-β1-Smad3 pathway (Chen et al. 2015).
CONCLUDING REMARKS
Initial studies on angiogenesis have focused mainly on either tyrosine kinase receptor ligands, like VEGF, angiopoietins, FGF, and PDGF, or angiostatin and endostatin inhibitors, like plasminogen fragments and collagen XVIII fragments. Analyzing the different TGF-β- and TGF-β-receptor-deficient mice showed the importance of TGF-β in yolk sac angiogenesis (Dickson et al. 1995; Oshima et al. 1996; Goumans et al. 1999). However, the possible role of TGF-β has remained neglected by the cardiovascular research community; because many of its functions go beyond vascular biology, its effects are highly context-dependent, and its pathways are thought to be insufficiently defined and complex. Nevertheless, an increasing number of cardiovascular diseases have been linked to mutated or inappropriately expressed TGF-β signaling components (ten Dijke and Arthur 2007). Conditional inactivation of genes expressing TGF-β signaling components in endothelial or mural cells produced informative vascular phenotypes (Jakobsson and van Meeteren 2013). Cell culture studies with three-dimensional (co)culture systems have identified the key interactions between TGF-β and other angiogenic regulators. Those findings provide a mechanistic basis for the multifunctional behavior of TGF-β. Furthermore, TGF-β was found to play important roles in the identity and fate of (vascular) stem cells (Watabe et al. 2003). As a result, TGF-β has taken center stage in vascular research, along with other angiogenic regulators.
Based on this detailed insight into the molecular and cellular mechanisms underlying the TGF-β effects, agents have been developed that selectively inhibit TGF-β, including neutralizing anti-TGF-β antibodies, receptor ligand traps, and small-molecule kinase inhibitors. Exciting progress has been made toward gaining regulatory approval of these agents for treating cardiovascular diseases caused by perturbed TGF-β signaling. For example, losartan has been tested as a treatment to counteract overactive TGF-β signaling in MFS (Möberg et al. 2012; Groenink et al. 2013), neutralizing anti-TGF-β antibodies may potentially inhibit vein graft stenosis (Cooley et al. 2014), and neutralizing anti-ALK-1 antibodies and ALK-1-Fc ligand traps may inhibit tumor angiogenesis (Cunha et al. 2010, 2015; Hu-Lowe et al. 2011; van Meeteren et al. 2011; Vecchia et al. 2013; Necchi et al. 2014; Hawinkels et al. 2016). Because the TGF-β effects are strongly context dependent and can affect many different cell types, inhibition of any pathological response may be accompanied by on-target side effects (Akhurst and Hata 2012). Potential side effects may become a complication in the clinical advancement of TGF-β signaling modulators; careful patient selection and monitoring during treatment are warranted.
Animal models that recapitulate diseases related to perturbed TGF-β signaling are of key importance for the preclinical testing of new drugs. However, with the advent of induced pluripotent stem (iPS) cell technology, cells from patients with cardiovascular disease can be induced to differentiate into specific vascular cells, allowing for the use of these cells for mechanistic studies and drug screening purposes (Bellin et al. 2012; Orlova et al. 2014; Sanchez Alvarado and Yamanaka 2014). Moreover, recently developed gene editing technology, such as the CRIPR-Cas9 system (Hsu et al. 2014), can be combined with iPS cells to design rescue experiments in which specific genetic alteration(s) in patient-derived cell lines can be corrected with gene editing. Furthermore, isogenic cell lines from embryonic stem cells can be created with specific genetic changes observed in cardiovascular diseases, and these cells can then be differentiated into specific vascular cell types to examine effects on differentiation and function. These technologies will promote the development of new therapeutic modalities for diseases with perturbed TGF-β signaling.
ACKNOWLEDGMENTS
Studies in our laboratory on the role of TGF-β receptor signaling in cardiovascular diseases were supported by the Netherlands CardioVascular Research Initiative, the Dutch Heart Foundation, the Dutch Federation of University Medical Centers, the Netherlands Organization for Health Research and Development, the Royal Netherlands Academy of Science, the LeDucq foundation, and a TOP grant from the Netherlands Organization for Scientific Research (ZonMW-NWO).
Footnotes
Editors: Rik Derynck and Kohei Miyazono
Additional Perspectives on The Biology of the TGF-β Family available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Abdalla SA, Letarte M. 2006. Hereditary haemorrhagic telangiectasia: Current views on genetics and mechanisms of disease. J Med Genet 43: 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH, Alitalo K. 2007. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Res Mol Cell Biol 8: 464–478. [DOI] [PubMed] [Google Scholar]
- Akhurst RJ, Hata A. 2012. Targeting the TGF-β signalling pathway in disease. Nat Rev Drug Discov 11: 790–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhurst RJ, Lehnert SA, Faissner A, Duffie E. 1990. TGF-β in murine morphogenetic processes: The early embryo and cardiogenesis. Development 108: 645–656. [DOI] [PubMed] [Google Scholar]
- Alam MA, Sami S, Babu S. 2011. Successful treatment of bleeding gastro-intestinal angiodysplasia in hereditary haemorrhagic telangiectasia with thalidomide. BMJ Case Rep 2011: pii: bcr0820114585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K, Carmeliet P. 2002. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 1: 219–227. [DOI] [PubMed] [Google Scholar]
- Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, Horan GS, Violette SM, Munger JS. 2009. Mice that lack activity of αvβ6- and αvβ8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci 122: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton MJ, Mellor HR, Bell A, Sadler C, Pass M, Powell S, Steele SJ, Roberts RR, Heier A. 2011. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol Pathol 39: 916–924. [DOI] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. 2003. Making sense of latent TGF-β activation. J Cell Sci 116: 217–224. [DOI] [PubMed] [Google Scholar]
- Annes JP, Chen Y, Munger JS, Rifkin DB. 2004. Integrin αVβ6-mediated activation of latent TGF-β requires the latent TGF-β binding protein-1. J Cell Biol 165: 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardelean DS, Letarte M. 2015. Anti-angiogenic therapeutic strategies in hereditary hemorrhagic telangiectasia. Front Genet 6: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardelean DS, Jerkic M, Yin M, Peter M, Ngan B, Kerbel RS, Foster FS, Letarte M. 2014. Endoglin and activin receptor-like kinase 1 heterozygous mice have a distinct pulmonary and hepatic angiogenic profile and response to anti-VEGF treatment. Angiogenesis 17: 129–146. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Betsholtz C. 2011. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21: 193–215. [DOI] [PubMed] [Google Scholar]
- Arteaga-Solis E, Sui-Arteaga L, Kim M, Schaffler MB, Jepsen KJ, Pleshko N, Ramirez F. 2011. Material and mechanical properties of bones deficient for fibrillin-1 or fibrillin-2 microfibrils. Matrix Biol 30: 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, Charlton R, Parums DV, Jowett T, Marchuk DA, et al. 2000. Endoglin, an ancillary TGF-β receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol 217: 42–53. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, et al. 1999. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol 1: 260–266. [DOI] [PubMed] [Google Scholar]
- Azhar M, Schultz Jel J, Grupp I, Dorn GW 2nd, Meneton P, Molin DG, Gittenberger-de Groot AC, Doetschman T. 2003. Transforming growth factor β in cardiovascular development and function. Cytokine Growth Factor Rev 14: 391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar M, Runyan RB, Gard C, Sanford LP, Miller ML, Andringa A, Pawlowski S, Rajan S, Doetschman T. 2009. Ligand-specific function of transforming growth factor β in epithelial-mesenchymal transition in heart development. Dev Dyn 238: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar M, Brown K, Gard C, Chen H, Rajan S, Elliott DA, Stevens MV, Camenisch TD, Conway SJ, Doetschman T. 2011. Transforming growth factor β-2 is required for valve remodeling during heart development. Dev Dyn 240: 2127–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartram U, Molin DG, Wisse LJ, Mohamad A, Sanford LP, Doetschman T, Speer CP, Poelmann RE, Gittenberger-de Groot AC. 2001. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-β2-knockout mice. Circulation 103: 2745–2752. [DOI] [PubMed] [Google Scholar]
- Battegay EJ, Raines EW, Seifert RA, Bowen-Pope DF, Ross R. 1990. TGF-β induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell 63: 515–524. [DOI] [PubMed] [Google Scholar]
- Bayrak-Toydemir P, McDonald J, Akarsu N, Toydemir RM, Calderon F, Tuncali T, Tang W, Miller F, Mao R. 2006. A fourth locus for hereditary hemorrhagic telangiectasia maps to chromosome 7. Am J Med Genet A 140: 2155–2162. [DOI] [PubMed] [Google Scholar]
- Beaumont J, Lopez B, Hermida N, Schroen B, San Jose G, Heymans S, Valencia F, Gomez-Doblas JJ, De Teresa E, Diez J, et al. 2014. microRNA-122 down-regulation may play a role in severe myocardial fibrosis in human aortic stenosis through TGF-β1 up-regulation. Clin Sci 126: 497–506. [DOI] [PubMed] [Google Scholar]
- Bellin M, Marchetto MC, Gage FH, Mummery CL. 2012. Induced pluripotent stem cells: The new patient? Nat Rev Mol Cell Biol 13: 713–726. [DOI] [PubMed] [Google Scholar]
- Bellón T, Corbí A, Lastres P, Calés C, Cebrián M, Vera S, Cheifetz S, Massagué J, Letarte M, Bernabéu C. 1993. Identification and expression of two forms of the human transforming growth factor-β-binding protein endoglin with distinct cytoplasmic regions. Eur J Immunol 23: 2340–2345. [DOI] [PubMed] [Google Scholar]
- Bentzon JF, Otsuka F, Virmani R, Falk E. 2014. Mechanisms of plaque formation and rupture. Circ Res 114: 1852–1866. [DOI] [PubMed] [Google Scholar]
- Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. 2000. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol 221: 249–258. [DOI] [PubMed] [Google Scholar]
- Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E, Bloch KD. 2004. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L1241–L1247. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song S. 2005. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 7: 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabéu C, Lopez-Novoa JM, Quintanilla M. 2009. The emerging role of TGF-β superfamily coreceptors in cancer. Bioch Biophys Acta 1792: 954–973. [DOI] [PubMed] [Google Scholar]
- Bijkerk R, de Bruin RG, van Solingen C, Duijs JM, Kobayashi K, van der Veer EP, ten Dijke P, Rabelink TJ, Goumans MJ, van Zonneveld AJ. 2012. MicroRNA-155 functions as a negative regulator of RhoA signaling in TGF-β-induced endothelial to mesenchymal transition. MicroRNA 1: 2–10. [DOI] [PubMed] [Google Scholar]
- Bilandzic M, Stenvers KL. 2011. Betaglycan: A multifunctional accessory. Mol Cell Endocrinol 339: 180–189. [DOI] [PubMed] [Google Scholar]
- Bobik A, Agrotis A, Kanellakis P, Dilley R, Krushinsky A, Smirnov V, Tararak E, Condron M, Kostolias G. 1999. Distinct patterns of transforming growth factor-β isoform and receptor expression in human atherosclerotic lesions. Colocalization implicates TGF-β in fibrofatty lesion development. Circulation 99: 2883–2891. [DOI] [PubMed] [Google Scholar]
- Bolar N, Van Laer L, Loeys BL. 2012. Marfan syndrome: From gene to therapy. Curr Opin Pediatr 24: 498–504. [DOI] [PubMed] [Google Scholar]
- Bonyadi M, Rusholme SA, Cousins FM, Su HC, Biron CA, Farrall M, Akhurst RJ. 1997. Mapping of a major genetic modifier of embryonic lethality in TGF-β1 knockout mice. Nat Genet 15: 207–211. [DOI] [PubMed] [Google Scholar]
- Boon RA, Fledderus JO, Volger OL, van Wanrooij EJ, Pardali E, Weesie F, Kuiper J, Pannekoek H, ten Dijke P, Horrevoets AJ. 2007. KLF2 suppresses TGF-β signaling in endothelium through induction of Smad7 and inhibition of AP-1. Arterioscler Thromb Vasc Biol 27: 532–539. [DOI] [PubMed] [Google Scholar]
- Bot PT, Hoefer IE, Sluijter JP, van Vliet P, Smits AM, Lebrin F, Moll F, de Vries JP, Doevendans P, Piek JJ, et al. 2009. Increased expression of the transforming growth factor-β signaling pathway, endoglin, and early growth response-1 in stable plaques. Stroke 40: 439–447. [DOI] [PubMed] [Google Scholar]
- Bourdeau A, Dumont DJ, Letarte M. 1999. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest 104: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau A, Cymerman U, Paquet ME, Meschino W, McKinnon WC, Guttmacher AE, Becker L, Letarte M. 2000a. Endoglin expression is reduced in normal vessels but still detectable in arteriovenous malformations of patients with hereditary hemorrhagic telangiectasia type 1. Am J Pathol 156: 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau A, Faughnan ME, Letarte M. 2000b. Endoglin-deficient mice, a unique model to study hereditary hemorrhagic telangiectasia. Trends Cardiovasc Med 10: 279–285. [DOI] [PubMed] [Google Scholar]
- Bourdeau A, Faughnan ME, McDonald ML, Paterson AD, Wanless IR, Letarte M. 2001. Potential role of modifier genes influencing transforming growth factor-β1 levels in the development of vascular defects in endoglin heterozygous mice with hereditary hemorrhagic telangiectasia. Am J Pathol 158: 2011–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB. 1999. TGF-β2 and TGF-β3 have separate and sequential activities during epithelial–mesenchymal cell transformation in the embryonic heart. Dev Biol 208: 530–545. [DOI] [PubMed] [Google Scholar]
- Brooks WW, Conrad CH. 2000. Myocardial fibrosis in transforming growth factor β1 heterozygous mice. J Mol Cell Cardiol 32: 187–195. [DOI] [PubMed] [Google Scholar]
- Brown MA, Zhao Q, Baker KA, Naik C, Chen C, Pukac L, Singh M, Tsareva T, Parice Y, Mahoney A, et al. 2005. Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J Biol Chem 280: 25111–25118. [DOI] [PubMed] [Google Scholar]
- Bultmann-Mellin I, Conradi A, Maul AC, Dinger K, Wempe F, Wohl AP, Imhof T, Wunderlich FT, Bunck AC, Nakamura T, et al. 2015. Modeling autosomal recessive cutis laxa type 1C in mice reveals distinct functions for Ltbp-4 isoforms. Dis Model Mech 8: 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchfield JS, Xie M, Hill JA. 2013. Pathological ventricular remodeling: Mechanisms: Part 1 of 2. Circulation 128: 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert B, Su CT, Van Damme T, Vlummens P, Malfait F, Vanakker O, Schulz B, Mac Neal M, Davis EC, Lee JG, et al. 2013. Comprehensive clinical and molecular analysis of 12 families with type 1 recessive cutis laxa. Hum Mutat 34: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. 2002. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med 8: 850–855. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. 2011. Molecular mechanisms and clinical applications of angiogenesis. Nature 473: 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignac V, Thevenon J, Adès L, Callewaert B, Julia S, Thauvin-Robinet C, Gueneau L, Courcet JB, Lopez E, Holman K, et al. 2012. In-frame mutations in exon 1 of SKI cause dominant Shprintzen–Goldberg syndrome. Am J Hum Genet 91: 950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta L, Pereira L, Arteaga-Solis E, Lee-Arteaga SY, Lenart B, Starcher B, Merkel CA, Sukoyan M, Kerkis A, Hazeki N, et al. 2006. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J Biol Chem 281: 8016–8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho RL, Jonker L, Goumans MJ, Larsson J, Bouwman P, Karlsson S, ten Dijke P, Arthur HM, Mummery CL. 2004. Defective paracrine signalling by TGF-β in yolk sac vasculature of endoglin mutant mice: A paradigm for hereditary haemorrhagic telangiectasia. Development 131: 6237–6247. [DOI] [PubMed] [Google Scholar]
- Carvalho RL, Itoh F, Goumans MJ, Lebrin F, Kato M, Takahashi S, Ema M, Itoh S, van Rooijen M, Bertolino P, et al. 2007. Compensatory signalling induced in the yolk sac vasculature by deletion of TGF-β receptors in mice. J Cell Sci 120: 4269–4277. [DOI] [PubMed] [Google Scholar]
- *.Chang C. 2016. Agonists and antagonists of TGF-β family ligands. Cold Spring Harb Perspect Biol 8: a021923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Zwijsen A, Vogel H, Huylebroeck D, Matzuk MM. 2000. Smad5 is essential for left-right asymmetry in mice. Dev Biol 219: 71–78. [DOI] [PubMed] [Google Scholar]
- Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, Zwijsen A. 1999. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development 126: 1631–1642. [DOI] [PubMed] [Google Scholar]
- Chaudhry SS, Cain SA, Morgan A, Dallas SL, Shuttleworth CA, Kielty CM. 2007. Fibrillin-1 regulates the bioavailability of TGF-β1. J Cell Biol 176: 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Kulik M, Lechleider RJ. 2003. Smad proteins regulate transcriptional induction of the SM22α gene by TGF-β. Nucleic Acids Res 31: 1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Lechleider RJ, 2004. Transforming growth factor-β-induced differentiation of smooth muscle from a neural crest stem cell line. Circ Res 94: 1195–1202. [DOI] [PubMed] [Google Scholar]
- Chen S, Crawford M, Day RM, Briones VR, Leader JE, Jose PA, Lechleider RJ. 2006. RhoA modulates Smad signaling during transforming growth factor-β-induced smooth muscle differentiation. J Biol Chem 281: 1765–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Bertozzi C, Zou Z, Yuan L, Lee JS, Lu M, Stachelek SJ, Srinivasan S, Guo L, Vicente A, et al. 2012a. Blood flow reprograms lymphatic vessels to blood vessels. J Clin Invest 122: 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, Ali R, Medina PP, Yu J, Slack FJ, et al. 2012b. FGF regulates TGF-β signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep 2: 1684–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Brady Ridgway J, Sai T, Lai J, Warming S, Chen H, Roose-Girma M, Zhang G, Shou W, Yan M. 2013. Context-dependent signaling defines roles of BMP9 and BMP10 in embryonic and postnatal development. Proc Nat Acad Sci 110: 11887–11892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Sun Z, Han Z, Jun K, Camus M, Wankhede M, Mao L, Arnold T, Young WL, Su H. 2014. De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke 45: 900–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Li J, Liu J, Li N, Wang S, Liu H, Zeng M, Zhang Y, Bu P. 2015. Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGF-β/Smad3 pathway. Am J Physiol Heart Circ Physiol 308: H424–H434. [DOI] [PubMed] [Google Scholar]
- Chen PY, Qin L, Li G, Tellides G, Simons M. 2016. Smooth muscle FGF/TGF-β cross talk regulates atherosclerosis progression. EMBO Mol Med 8: 712–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EJ, Kim YH, Choe SW, Tak YG, Garrido-Martin EM, Chang M, Lee YJ, Oh SP. 2013. Enhanced responses to angiogenic cues underlie the pathogenesis of hereditary hemorrhagic telangiectasia 2. PLoS ONE 8: e63138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AW, Yang HH, Radomski MW, van Breemen C. 2008. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ Res 102: e73–e85. [DOI] [PubMed] [Google Scholar]
- Cole SG, Begbie ME, Wallace GM, Shovlin CL. 2005. A new locus for hereditary haemorrhagic telangiectasia (HHT3) maps to chromosome 5. J Med Genet 42: 577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton LA, Potash DA, Brown CB, Barnett JV. 2007. Coronary vessel development is dependent on the type III transforming growth factor-β receptor. Circ Res 101: 784–791. [DOI] [PubMed] [Google Scholar]
- Conley BA, Smith JD, Guerrero-Esteo M, Bernabéu C, Vary CP. 2000. Endoglin, a TGF-β receptor-associated protein, is expressed by smooth muscle cells in human atherosclerotic plaques. Atherosclerosis 153: 323–335. [DOI] [PubMed] [Google Scholar]
- Cooley BC, Nevado J, Mellad J, Yang D, St Hilaire C, Negro A, Fang F, Chen G, San H, Walts AD, et al. 2014. TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci Transl Med 6: 227ra234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. 1998. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell 93: 1159–1170. [DOI] [PubMed] [Google Scholar]
- Creemers EE, Pinto YM. 2011. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc Res 89: 265–272. [DOI] [PubMed] [Google Scholar]
- Crosas-Molist E, Meirelles T, López-Luque J, Serra-Peinado C, Selva J, Caja L, Del Blanco DG, Uriarte JJ, Bertran E, Mendizábal Y, et al. 2015. Vascular smooth muscle cell phenotypic changes in patients with Marfan syndrome. Arterioscler Thromb Vasc Biol 35: 960–972. [DOI] [PubMed] [Google Scholar]
- Cruz-Gonzalez I, Pabon P, Rodriguez-Barbero A, Martin-Moreiras J, Pericacho M, Sanchez PL, Ramirez V, Sanchez-Ledesma M, Martin-Herrero F, Jimenez-Candil J, et al. 2008. Identification of serum endoglin as a novel prognostic marker after acute myocardial infarction. J Cell Mol Med 12: 955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Liu JH, Song XT, Ma GL, Du BJ, Lv SZ, Meng LJ, Gao QS, Li K. 2014. A novel stent coated with antibodies to endoglin inhibits neointimal formation of porcine coronary arteries. Biomed Res Int 2014: 428619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha SI, Pardali E, Thorikay M, Anderberg C, Hawinkels L, Goumans MJ, Seehra J, Heldin CH, ten Dijke P, Pietras K. 2010. Genetic and pharmacological targeting of activin receptor-like kinase 1 impairs tumor growth and angiogenesis. J Exp Med 207: 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha SI, Bocci M, Lövrot J, Eleftheriou N, Roswall P, Cordero E, Lindström L, Bartoschek M, Haller BK, Pearsall RS, et al. 2015. Endothelial ALK1 is a therapeutic target to block metastatic dissemination of breast cancer. Cancer Res 75: 2445–2456. [DOI] [PubMed] [Google Scholar]
- Cuttano R, Rudini N, Bravi L, Corada M, Giampietro C, Papa E, Morini MF, Maddaluno L, Baeyens N, Adams RH, et al. 2015. KLF4 is a key determinant in the development and progression of cerebral cavernous malformations. EMBO Mol Med 8: 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland DC, D’Amore PA. 2001. TGF-β is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis 4: 11–20. [DOI] [PubMed] [Google Scholar]
- David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. 2007. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 in endothelial cells. Blood 109: 1953–1961. [DOI] [PubMed] [Google Scholar]
- Deaton RA, Su C, Valencia TG, Grant SR. 2005. Transforming growth factor-β1-induced expression of smooth muscle marker genes involves activation of PKN and p38 MAPK. J Biol Chem 280: 31172–31181. [DOI] [PubMed] [Google Scholar]
- de Boeck M, ten Dijke. 2012. Key role for ubiquitin protein modification in TGF-β signal transduction. Ups J Med Sci 117: 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker RJ, Boon RA, Rondaij MG, Kragt A, Volger OL, Elderkamp YW, Meijers JC, Voorberg J, Pannekoek H, Horrevoets AJ. 2006. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood 107: 4354–4363. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. 2003. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425: 577–584. [DOI] [PubMed] [Google Scholar]
- de Sousa Lopes SM, Carvalho RL, van den Driesche S, Goumans MJ, ten Dijke P, Mummery CL. 2003. Distribution of phosphorylated Smad2 identifies target tissues of TGF-β ligands in mouse development. Gene Expr Patterns 3: 355–360. [DOI] [PubMed] [Google Scholar]
- Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. 1995. Defective haematopoiesis and vasculogenesis in transforming growth factor-β1 knock out mice. Development 121: 1845–1854. [DOI] [PubMed] [Google Scholar]
- Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, et al. 1991. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 352: 337–339. [DOI] [PubMed] [Google Scholar]
- Ding R, Darland DC, Parmacek MS, D’Amore PA. 2004. Endothelial-mesenchymal interactions in vitro reveal molecular mechanisms of smooth muscle/pericyte differentiation. Stem Cells Dev 13: 509–520. [DOI] [PubMed] [Google Scholar]
- Divakaran V, Adrogue J, Ishiyama M, Entman ML, Haudek S, Sivasubramanian N, Mann DL. 2009. Adaptive and maladptive effects of SMAD3 signaling in the adult heart after hemodynamic pressure overloading. Cir Heart Fail 2: 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG. 2010. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res 107: 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobaczewski M, Chen W, Frangogiannis NG. 2011. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol 51: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley S, ten Dijke P. 2012. TGF-β in progression of liver disease. Cell Tissue Res 347: 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Gerber EE, Dietz HC. 2012. Matrix-dependent perturbation of TGF-β signaling and disease. FEBS Lett 586: 2003–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois CM, Laprise MH, Blanchette F, Gentry LE, Leduc R. 1995. Processing of transforming growth factor β1 precursor by human furin convertase. J Biol Chem 270: 10618–10624. [DOI] [PubMed] [Google Scholar]
- Egorova AD, Khedoe PP, Goumans MJ, Yoder BK, Nauli SM, ten Dijke P, Poelmann RE, Hierck BP. 2011. Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ Res 108: 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova AD, van der Heiden K, Poelmann RE, Hierck BP. 2012. Primary cilia as biomechanical sensors in regulating endothelial function. Differentiation 83: S56–S61. [DOI] [PubMed] [Google Scholar]
- Ehrlich M, Horbelt D, Marom B, Knaus P, Henis YI. 2011. Homomeric and heteromeric complexes among TGF-β and BMP receptors and their roles in signaling. Cell Signal 23: 1424–1432. [DOI] [PubMed] [Google Scholar]
- Ellmers LJ, Scott NJ, Medicherla S, Pilbrow AP, Bridgman PG, Yandle TG, Richards AM, Protter AA, Cameron VA. 2008. Transforming growth factor-β blockade down-regulates the renin-angiotensin system and modifies cardiac remodeling after myocardial infarction. Endocrinology 149: 5828–5834. [DOI] [PubMed] [Google Scholar]
- Engebretsen KV, Skardal K, Bjornstad S, Marstein HS, Skrbic B, Sjaastad I, Christensen G, Bjornstad JL, Tonnessen T. 2014. Attenuated development of cardiac fibrosis in left ventricular pressure overload by SM16, an orally active inhibitor of ALK5. J Mol Cell Cardiol 76: 148–157. [DOI] [PubMed] [Google Scholar]
- Epperla N, Hocking W. 2015. Blessing for the bleeder: Bevacizumab in hereditary hemorrhagic telangiectasia. Clin Med Res 13: 32–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng XH, Derynck R. 2005. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol 21: 659–693. [DOI] [PubMed] [Google Scholar]
- Ferguson JE III, Kelley RW, Patterson C. 2005. Mechanisms of endothelial differentiation in embryonic vasculogenesis. Arterioscler Thromb Vasc Biol 25: 2246–2254. [DOI] [PubMed] [Google Scholar]
- Fish JE, Wythe JD. 2015. The molecular regulation of arteriovenous specification and maintenance. Dev Dyn 244: 391–409. [DOI] [PubMed] [Google Scholar]
- Franchini M, Frattini F, Crestani S, Bonfanti C. 2013. Novel treatments for epistaxis in hereditary hemorrhagic telangiectasia: A systematic review of the clinical experience with thalidomide. J Thromb Thrombolysis 36: 355–357. [DOI] [PubMed] [Google Scholar]
- Franken R, Hibender S, den Hartog W, Radonic T, de Vries CJ, Zwinderman AH, Groenink M, Mulder BJ, de Waard V. 2014. No beneficial effect of general and specific anti-inflammatory therapies on aortic dilatation in marfan mice. PLoS ONE 9: e107221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz S, Hu K, Adamek A, Wolf J, Sallam A, Maier SK, Lonning S, Ling H, Ertl G, Bauersachs J. 2008. Transforming growth factor-β inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol 103: 485–492. [DOI] [PubMed] [Google Scholar]
- Frutkin AD, Otsuka G, Stempien-Otero A, Sesti C, Du L, Jaffe M, Dichek HL, Pennington CJ, Edwards DR, Nieves-Cintron M, et al. 2009. TGF-β1 limits plaque growth, stabilizes plaque structure, and prevents aortic dilation in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol 29: 1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M, Yagi H, Matsuoka R, Akimoto K, Furutani M, Imamura S, Uehara R, Nakayama T, Takao A, Nakazawa M, Saji T. 2008. Implications of mutations of activin receptor-like kinase 1 gene (ALK1) in addition to bone morphogenetic protein receptor II gene (BMPR2) in children with pulmonary arterial hypertension. Circ J 72: 127–133. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Hu WY, Kubo A, Kishioka H, Satoh C, Soma M, Izumi Y, Kanmatsuse K. 2000. Angiotensin II upregulates transforming growth factor-β type I receptor on rat vascular smooth muscle cells. Am J Hypertens 13: 191–198. [DOI] [PubMed] [Google Scholar]
- Gallione CJ, Richards JA, Letteboer TG, Rushlow D, Prigoda NL, Leedom TP, Ganguly A, Castells A, Ploos van Amstel JK, Westermann CJ, et al. 2006. SMAD4 mutations found in unselected HHT patients. J Med Genet 43: 793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallione C, Aylsworth AS, Beis J, Berk T, Bernhardt B, Clark RD, Clericuzio C, Danesino C, Drautz J, Fahl J, et al. 2010. Overlapping spectra of SMAD4 mutations in juvenile polyposis JP and JP-HHT syndrome. Am J Med Genet A 152A: 333–339. [DOI] [PubMed] [Google Scholar]
- Gallo EM, Loch DC, Habashi JP, Calderon JF, Chen Y, Bedja D, van Erp C, Gerber EE, Parker SJ, Sauls K, et al. 2014. Angiotensin II-dependent TGF-β signaling contributes to Loeys–Dietz syndrome vascular pathogenesis. J Clin Invest 124: 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA Jr, et al. 2000. A role for Smad6 in development and homeostasis of the cardiovascular system. Nat Genet 24: 171–174. [DOI] [PubMed] [Google Scholar]
- García de Vinuesa A, Abdelilah-Seyfried S, Knaus P, Zwijsen A, Bailly S. 2016. BMP signaling in vascular biology and dysfunction. Cytokine Growth Factor Rev 27: 65–79. [DOI] [PubMed] [Google Scholar]
- Garside VC, Chang AC, Karsan A, Hoodless PA. 2013. Co-ordinating Notch, BMP, and TGF-β signaling during heart valve development. Cell Mol Life Sci 70: 2899–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gistera A, Robertson AK, Andersson J, Ketelhuth DF, Ovchinnikova O, Nilsson SK, Lundberg AM, Li MO, Flavell RA, Hansson GK. 2013. Transforming growth factor-β signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci Transl Med 5: 196ra100. [DOI] [PubMed] [Google Scholar]
- Gojova A, Brun V, Esposito B, Cottrez F, Gourdy P, Ardouin P, Tedgui A, Mallat Z, Groux H. 2003. Specific abrogation of transforming growth factor-β signaling in T cells alters atherosclerotic lesion size and composition in mice. Blood 102: 4052–4058. [DOI] [PubMed] [Google Scholar]
- Gomez D, Owens GK. 2012. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res 95: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez D, Al Haj Zen A, Borges LF, Philippe M, Gutierrez PS, Jondeau G, Michel JB, Vranckx R. 2009. Syndromic and non-syndromic aneurysms of the human ascending aorta share activation of the Smad2 pathway. J Pathol 218: 131–142. [DOI] [PubMed] [Google Scholar]
- Gore B, Izikki M, Mercier O, Dewachter L, Fadel E, Humbert M, Dartevelle P, Simonneau G, Naeije R, Lebrin F, et al. 2014. Key role of the endothelial TGF-β/ALK1/endoglin signaling pathway in humans and rodents pulmonary hypertension. PLoS ONE 9: e100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould ST, Srigunapalan S, Simmons CA, Anseth KS. 2013. Hemodynamic and cellular response feedback in calcific aortic valve disease. Circ Res 113: 186–197. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Mummery CL. 2000. Functional analysis of the TGF-β receptor/Smad pathway through gene ablation in mice. Int J Dev Biol 44: 253–265. [PubMed] [Google Scholar]
- Goumans MJ, Zwijsen A, van Rooijen MA, Huylebroeck D, Roelen BA, Mummery CL. 1999. Transforming growth factor-β signalling in extraembryonic mesoderm is required for yolk sac vasculogenesis in mice. Development 126: 3473–3483. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. 2002. Balancing the activation state of the endothelium via two distinct TGF-β type I receptors. EMBO J 21: 1743–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumans MJ, Lebrin F, Valdimarsdottir G. 2003a. Controlling the angiogenic switch: A balance between two distinct TGF-β receptor signaling pathways. Trends Cardiovasc Med 13: 301–307. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. 2003b. Activin receptor-like kinase ALK1 is an antagonistic mediator of lateral TGF-β/ALK5 signaling. Mol Cell 12: 817–828. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, van Zonneveld AJ, ten Dijke P. 2008. Transforming growth factor-β-induced endothelial-to-mesenchymal transition: A switch to cardiac fibrosis? Trends Cardiovasc Med 18: 293–298. [DOI] [PubMed] [Google Scholar]
- Goumans M-J, Liu Z, ten Dijke P. 2009. TGF-β signaling in vascular biology and dysfunction. Cell Res 19: 116–127. [DOI] [PubMed] [Google Scholar]
- *.Goumans M-J, Zwijsen A, ten Dijke P, Bailly S. 2017. Bone morphogenetic proteins in vascular homeostasis and disease. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger DJ. 2004. Transforming growth factor-β and atherosclerosis: So far, so good for the protective cytokine hypothesis. Arterioscler Thromb Vasc Biol 24: 399–404. [DOI] [PubMed] [Google Scholar]
- Grewal N, Gittenberger-de Groot AC, DeRuiter MC, Klautz RJ, Poelmann RE, Duim S, Lindeman JH, Koenraadt WM, Jongbloed MR, Mohamed SA, et al. 2014a. Bicuspid aortic valve: Phosphorylation of c-Kit and downstream targets are prognostic for future aortopathy. Eur J Cardiothorac Surg 46: 831–839. [DOI] [PubMed] [Google Scholar]
- Grewal N, Gittenberger-de Groot AC, Poelmann RE, Klautz RJ, Lindeman JH, Goumans MJ, Palmen M, Mohamed S.A, Sievers HH, Bogers AJ, et al. 2014b. Ascending aorta dilation in association with bicuspid aortic valve: A maturation defect of the aortic wall. J Thorac Cardiovasc Surg 148: 1583–1590. [DOI] [PubMed] [Google Scholar]
- Groenink M, den Hartog AW, Franken R, Radonic T, de Waard V, Timmermans J, Scholte AJ, van den Berg MP, Spijkerboer AM, Marquering HA, et al. 2013. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: A randomized controlled trial. Eur Heart J 34: 3491–3500. [DOI] [PubMed] [Google Scholar]
- Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, et al. 2006. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312: 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC. 2011. Angiotensin-II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science 332: 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler MA, Hadley TM, Zhang H, Mehra K, Roos CM, Schaff HV, Suri RM, Miller JD. 2013. TGF-β signalling and reactive oxygen species drive fibrosis and matrix remodelling in myxomatous mitral valves. Cardiovasc Res 99: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Choe SW, Kim YH, Acharya AP, Keselowsky BG, Sorg BS, Lee YJ, Oh SP. 2014. VEGF neutralization can prevent and normalize arteriovenous malformations in an animal model for hereditary hemorrhagic telangiectasia 2. Angiogenesis 17: 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harradine KA, Akhurst RJ. 2006. Mutations of TGF-β signaling molecules in human disease. Ann Med 38: 403–414. [DOI] [PubMed] [Google Scholar]
- *.Hata A, Chen Y-G. 2016. TGF-β signaling from receptors to Smads. Cold Spring Harb Perspect Biol 8: a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawinkels LJ, ten Dijke P. 2011. Exploring anti-TGF-β therapies in cancer and fibrosis. Growth Factors 29: 140–152. [DOI] [PubMed] [Google Scholar]
- Hawinkels LJ, García de Vinuesa A, Paauwe M, Kruithof-de Julio M, Wiercinska E, Pardali E, Mezzanotte L, Keereweer S, Braumuller TM, Heijkants RC, et al. 2016. Activin receptor-like kinase 1 ligand trap reduces microvascular density and improves chemotherapy efficiency to various solid tumors. Clin Cancer Res 22: 96–106. [DOI] [PubMed] [Google Scholar]
- *.Heldin CH, Moustakas A. 2016. Signaling receptors for TGF-β family members. Cold Spring Harb Perspect Biol 8: a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. 1997. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390: 465–471. [DOI] [PubMed] [Google Scholar]
- Herbert SP, Stainier DY. 2011. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol 12: 551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herder C, Peeters W, Zierer A, de Kleijn DP, Moll FL, Karakas M, Roden M, Meisinger C, Thorand B, Pasterkamp G, et al. 2012. TGF-β1 content in atherosclerotic plaques, TGF-β1 serum concentrations and incident coronary events. Eur J Clin Invest 42: 329–337. [DOI] [PubMed] [Google Scholar]
- Hering S, Jost C, Schulz H, Hellmich B, Schatz H, Pfeiffer H. 2002. Circulating transforming growth factor-β1 (TGF-β1) is elevated by extensive exercise. Eur J Appl Physiol 86: 406–410. [DOI] [PubMed] [Google Scholar]
- *.Hill CS. 2016. Transcriptional control by the SMADs. Cold Spring Harb Perspect Biol 8: a022079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA, Olson EN. 2008. Cardiac plasticity. N Engl J Med 358: 1370–1380. [DOI] [PubMed] [Google Scholar]
- *.Hinck AP, Mueller TD, Springer TA. 2016. Structural biology and evolution of the TGF-β family. Cold Spring Harb Perspect Biol 8: a022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton RB, Yutzey KE. 2011. Heart valve structure and function in development and disease. Annu Rev Physiol 73: 29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton RB, Adelman-Brown J, Witt S, Krishnamurthy VK, Osinska H, Sakthivel B, James JF, Li DY, Narmoneva DA, Mecham RP, et al. 2010. Elastin haploinsufficiency results in progressive aortic valve malformation and latent valve disease in a mouse model. Circ Res 107: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishikawa K, Nakaki T, Fujii T. 1999. Transforming growth factor-β1 induces apoptosis via connective tissue growth factor in human aortic smooth muscle cells. Eur J Pharmacol 385: 287–290. [DOI] [PubMed] [Google Scholar]
- Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, et al. 2011. Noncanonical TGF-β signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 332: 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horbelt D, Guo G, Robinson PN, Knaus P. 2010. Quantitative analysis of TGFBR2 mutations in Marfan-syndrome-related disorders suggests a correlation between phenotypic severity and Smad signaling activity. J Cell Sci 123: 4340–4350. [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Todorovic V, Hadjiolova K, Weiskirchen R, Rifkin DB. 2015. Abrogation of both short and long forms of latent transforming growth factor-β binding protein-1 causes defective cardiovascular development and is perinatally lethal. Matrix Biol 43: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosman A, Westermann CJ, Snijder R, Disch F, Mummery CL, Mager JJ. 2015. Follow-up of Thalidomide treatment in patients with Hereditary Haemorrhagic Telangiectasia. Rhinology 53: 340–344. [DOI] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F. 2014. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157: 1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Wu Z, Phan SH. 2003. Smad3 mediates transforming growth factor-β-induced α-smooth muscle actin expression. Am J Respir Cell Mol Biol 29: 397–404. [DOI] [PubMed] [Google Scholar]
- Huang Y, Qi Y, Du JQ, Zhang DF. 2014. MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4. Expert Opin Ther Targets 18: 1355–1365. [DOI] [PubMed] [Google Scholar]
- Hu-Lowe DD, Chen E, Zhang L, Watson KD, Mancuso P, Lappin P, Wickman G, Chen JH, Wang J, Jiang X, et al. 2011. Targeting activin receptor-like kinase 1 inhibits angiogenesis and tumorigenesis through a mechanism of action complementary to anti-VEGF therapies. Cancer Res 71: 1362–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytiäinen M, Penttinen C, Keski-Oja J. 2004. Latent TGF-β binding proteins: Extracellular matrix association and roles in TGF-β activation. Crit Rev Clin Lab Sci 41: 233–264. [DOI] [PubMed] [Google Scholar]
- Ichise T, Yoshida N, Ichise H. 2014. FGF2-induced Ras-MAPK signalling maintains lymphatic endothelial cell identity by upregulating endothelial-cell-specific gene expression and suppressing TGF-β signalling through Smad2. J Cell Sci 127: 845–857. [DOI] [PubMed] [Google Scholar]
- Ikushima H, Miyazono K. 2010. TGF-β signalling: A complex web in cancer progression. Nat Rev Cancer 10, 415–424. [DOI] [PubMed] [Google Scholar]
- Inamoto S, Kwartler CS, Lafont AL, Liang YY, Fadulu VT, Duraisamy S, Willing M, Estrera A, Safi H, Hannibal MC, et al. 2010. TGFBR2 mutations alter smooth muscle cell phenotype and predispose to thoracic aortic aneurysms and dissections. Cardiovasc Res 88: 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israely E, Ginsberg M, Nolan D, Ding BS, James D, Elemento O, Rafii S, Rabbany SY. 2014. Akt suppression of TGF-β signaling contributes to the maintenance of vascular identity in embryonic stem cell-derived endothelial cells. Stem Cells 32: 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S, ten Dijke P. 2007. Negative regulation of TGF-β receptor/Smad signal transduction. Curr Opin Cell Biol 19: 176–184. [DOI] [PubMed] [Google Scholar]
- Itoh F, Itoh S, Adachi T, Ichikawa K, Matsumura Y, Takagi T, Festing M, Watanabe T, Weinstein M, Karlsson S, et al. 2012. Smad2/Smad3 in endothelium is indispensable for vascular stability via S1PR1 and N-cadherin expressions. Blood 119: 5320–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh F, Watabe T, Miyazono K. 2014. Roles of TGF-β family signals in the fate determination of pluripotent stem cells. Semin Cell Dev Biol 32C: 98–106. [DOI] [PubMed] [Google Scholar]
- Jakobsson L, van Meeteren LA. 2013. Transforming growth factor-β family members in regulation of vascular function: In the light of vascular conditional knockouts. Exp Cell Res 319: 1264–1270. [DOI] [PubMed] [Google Scholar]
- James D, Nam HS, Seandel M, Nolan D, Janovitz T, Tomishima M, Studer L, Lee G, Lyden D, Benezra R, et al. 2010. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGF-β inhibition is Id1 dependent. Nat Biotech 28: 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JM, Nalbandian A, Mukouyama YS. 2013. TGF-β signaling is required for sprouting lymphangiogenesis during lymphatic network development in the skin. Development 140: 3903–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YS, Choi IH. 2014. Contrasting roles of different endoglin forms in atherosclerosis. Immune Netw 14: 237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao K, Langworthy M, Batts L, Brown CB, Moses HL, Baldwin HS. 2006. TGF-β signaling is required for atrioventricular cushion mesenchyme remodeling during in vivo cardiac development. Development 133: 4585–4593. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Berg JN, Gallione CJ, McAllister KA, Warner JP, Helmbold EA, Markel DS, Jackson CE, Porteous ME, Marchuk DA. 1995. A second locus for hereditary hemorrhagic telangiectasia maps to chromosome 12. Genome Res 5: 21–28. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. 2008. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev 22: 3282–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge DP, Markwald RR, Hagege AA, Levine RA. 2011. Translational research on the mitral valve: From developmental mechanisms to new therapies. J Cardiovasc Transl Res 4: 699–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. 1995. Abnormal lung development and cleft palate in mice lacking TGF-β3 indicates defects of epithelial-mesenchymal interaction. Nat Genet 11: 415–421. [DOI] [PubMed] [Google Scholar]
- Kalinina N, Agrotis A, Antropova Y, Ilyinskaya O, Smirnov V, Tararak E, Bobik A. 2004. Smad expression in human atherosclerotic lesions: Evidence for impaired TGF-β/Smad signaling in smooth muscle cells of fibrofatty lesions. Arterioscler Thromb Vasc Biol 24: 1391–1396. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. 2009. The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur NK, Wilson S, Yunis AA, Qiao X, Mackey E, Paruchuri V, Baker C, Aronovitz MJ, Karumanchi SA, Letarte M, et al. 2012. Reduced endoglin activity limits cardiac fibrosis and improves survival in heart failure. Circulation 125: 2728–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur NK, Qiao X, Paruchuri V, Mackey EE, Daly GH, Ughreja K, Morine KJ, Levine J, Aronovitz MJ, Hill NS, et al. 2014. Reducing endoglin activity limits calcineurin and TRPC-6 expression and improves survival in a mouse model of right ventricular pressure overload. J Am Heart Assoc 3: e000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, et al. 2004. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5: 74–80. [DOI] [PubMed] [Google Scholar]
- Karpanen T, Alitalo K. 2008. Molecular biology and pathology of lymphangiogenesis. Ann Rev Pathol 3: 367–397. [DOI] [PubMed] [Google Scholar]
- *.Katagiri T, Watabe T. 2016. Bone morphogenetic proteins. Cold Spring Harb Perspect Biol 8: a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R, Sheppard R. 2006. Fibrosis in heart disease: Understanding the role of transforming growth factor-β in cardiomyopathy, valvular disease and arrhythmia. Immunology 118: 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Peacock MR, George SC, Hughes CC. 2012. BMP9 induces EphrinB2 expression in endothelial cells through an Alk1-BMPRII/ActRII-ID1/ID3-dependent pathway: Implications for hereditary hemorrhagic telangiectasia type II. Angiogenesis 15: 497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkbride KC, Townsend TA, Bruinsma MW, Barnett JV, Blobe GC. 2008. Bone morphogenetic proteins signal through the transforming growth factor-β type III receptor. J Biol Chem 283: 7628–7637. [DOI] [PubMed] [Google Scholar]
- Koleva RI, Conley BA, Romero D, Riley KS, Marto JA, Lux A, Vary CP. 2006. Endoglin structure and function: Determinants of endoglin phosphorylation by transforming growth factor-β receptors. J Biol Chem 281: 25110–25123. [DOI] [PubMed] [Google Scholar]
- Koli K, Wempe F, Sterner-Kock A, Kantola A, Komor M, Hofmann WK, von Melchner H, Keski-Oja J. 2004. Disruption of LTBP-4 function reduces TGF-β activation and enhances BMP-4 signaling in the lung. J Cell Biol 167: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong P, Christia P, Frangogiannis NG. 2014. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 71: 549–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. 2012. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: From cardiovascular development to disease. Circulation 125: 1795–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs RJ, Maldonado G, Azaro A, Fernandez MS, Romero FL, Sepulveda-Sanchez JM, Corretti M, Carducci M, Dolan M, Gueorguieva I, et al. 2015. Cardiac safety of TGF-β receptor I kinase inhibitor LY2157299 monohydrate in cancer patients in a first-in-human dose study. Cardiovasc Toxicol 15: 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenning G, Zeisberg EM, Kalluri R. 2010. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol 225: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruithof BP, Duim SN, Moerkamp AT, Goumans MJ. 2012. TGF-β and BMP signaling in cardiac cushion formation: Lessons from mice and chicken. Differentiation 84: 89–102. [DOI] [PubMed] [Google Scholar]
- Kruithof BP, Kruithof-De-Julio M, Poelmann RE, Gittenberger-De-Groot AC, Gaussin V, Goumans MJ. 2013. Remodeling of the myocardium in early trabeculation and cardiac valve formation; a role for TGF-β2. Int J Dev Biol 57: 853–863. [DOI] [PubMed] [Google Scholar]
- Kumarswamy R, Volkmann I, Jazbutyte V, Dangwal S, Park DH, Thum T. 2012. Transforming growth factor-β-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler Thromb Vasc Biol 32: 361–369. [DOI] [PubMed] [Google Scholar]
- Kurpinski K, Lam H, Chu J, Wang A, Kim A, Tsay E, Agrawal S, Schaffer DV, Li S. 2010. Transforming growth factor-β and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells 28: 734–742. [DOI] [PubMed] [Google Scholar]
- Lamouille S, Mallet C, Feige JJ, Bailly S. 2002. Activin receptor-like kinase 1 is implicated in the maturation phase of angiogenesis. Blood 100: 4495–4501. [DOI] [PubMed] [Google Scholar]
- Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA III, Loyd JE, Nichols WC, Trembath RC. 2000. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat Genet 26: 81–84. [DOI] [PubMed] [Google Scholar]
- Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, Xu X, ten Dijke P, Mummery CL, Karlsson S. 2001. Abnormal angiogenesis but intact hematopoietic potential in TGF-β type I receptor–deficient mice. EMBO J 20: 1663–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastres P, Martín-Perez J, Langa C, Bernabéu C. 1994. Phosphorylation of the human-transforming-growth-factor-β-binding protein endoglin. Biochem J 301: 765–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastres P, Letamendía A, Zhang H, Rius C, Almendro N, Raab U, López LA, Langa C, Fabra A, Letarte M, et al. 1996. Endoglin modulates cellular responses to TGF-β1. J Cell Biol 133: 1109–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P. 2004. Endoglin promotes endothelial cell proliferation and TGF-β/ALK1 signal transduction. EMBO J 23: 4018–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrin F, Srun S, Raymond K, Martin S, van den Brink S, Freitas C, Breant C, Mathivet T, Larrivee B, Thomas JL, et al. 2010. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med 16: 420–428. [DOI] [PubMed] [Google Scholar]
- Lechleider RJ, Ryan JL, Garrett L, Eng C, Deng C, Wynshaw-Boris A, Roberts AB. 2001. Targeted mutagenesis of Smad1 reveals an essential role in chorioallantoic fusion. Dev Biol 240: 157–167. [DOI] [PubMed] [Google Scholar]
- Lee JY, Park C, Cho YP, Lee E, Kim H, Kim P, Yun SH, Yoon YS. 2010. Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation 122: 1413–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner-Ellis JP, Aldubayan SH, Hernandez AL, Kelly MA, Stuenkel AJ, Walsh J, Joshi VA. 2014. The spectrum of FBN1, TGFβR1, TGFβR2 and ACTA2 variants in 594 individuals with suspected Marfan syndrome, Loeys–Dietz syndrome or thoracic aortic aneurysms and dissections (TAAD). Mol Genet Metab 112: 171–176. [DOI] [PubMed] [Google Scholar]
- Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB. 1994. Maternal rescue of transforming growth factor-β 1 null mice. Science 264: 1936–1938. [DOI] [PubMed] [Google Scholar]
- Leutermann R, Sheikhzadeh S, Brockstädt L, Rybczynski M, van Rahden V, Kutsche K, von Kodolitsch Y, Rosenberger G. 2014. A 1-bp duplication in TGFβ2 in three family members with a syndromic form of thoracic aortic aneurysm. Eur J Hum Genet 22: 944–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levet S, Ciais D, Merdzhanova G, Mallet C, Zimmers TA, Lee SJ, Navarro FP, Texier I, Feige JJ, Bailly S, et al. 2013. Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation. Blood 122: 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. 1999. Defective angiogenesis in mice lacking endoglin. Science 284: 1534–1537. [DOI] [PubMed] [Google Scholar]
- Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F, Han H, Meng A, Wang Y, Yang X. 2011. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell 20: 291–302. [DOI] [PubMed] [Google Scholar]
- Li W, Li Q, Jiao Y, Qin L, Ali R, Zhou J, Ferruzzi J, Kim RW, Geirsson A, Dietz HC, et al. 2014. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J Clin Invest 124: 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. 2011. Progress and challenges in translating the biology of atherosclerosis. Nature 473: 317–325. [DOI] [PubMed] [Google Scholar]
- Lievens D, Habets KL, Robertson AK, Laouar Y, Winkels H, Rademakers T, Beckers L, Wijnands E, Boon L, Mosaheb M, et al. 2013. Abrogated transforming growth factor-β receptor II (TGFβRII) signalling in dendritic cells promotes immune reactivity of T cells resulting in enhanced atherosclerosis. Eur Heart J 34: 3717–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Moustakas A, Knaus P, Wells RG, Henis YI, Lodish HF. 1995. The soluble exoplasmic domain of the type II transforming growth factor TGF-β receptor. A heterogeneously glycosylated protein with high affinity and selectivity for TGF-β ligands. J Biol Chem 270: 2747–2754. [DOI] [PubMed] [Google Scholar]
- Lindeman JH, Abdul-Hussien H, van Bockel JH, Wolterbeek R, Kleemann R. 2009. Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: Doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation 119: 2209–2216. [DOI] [PubMed] [Google Scholar]
- Lindsay ME, Schepers D, Bolar NA, Doyle JJ, Gallo E, Fert-Bober J, Kempers MJ, Fishman EK, Chen Y, Myers L, et al. 2012. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet 44: 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Kobayashi K, van Dinther M, van Heiningen SH, Valdimarsdottir G, van Laar T, Scharpfenecker M, Lowik CW, Goumans MJ, ten Dijke P, et al. 2009. VEGF and inhibitors of TGF-β type-I receptor kinase synergistically promote blood-vessel formation by inducing α5-integrin expression. J Cell Sci 122: 3294–3302. [DOI] [PubMed] [Google Scholar]
- Liu Z, Lebrin F, Maring JA, van den Driesche S, van der Brink S, van Dinther M, Thorikay M, Martin S, Kobayashi K, Hawinkels LJ, et al. 2014. Endoglin is dispensable for vasculogenesis, but required for vascular endothelial growth factor-induced angiogenesis. PLoS ONE 9: e86273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, et al. 2005. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 37: 275–281. [DOI] [PubMed] [Google Scholar]
- Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, De Backer JF, Oswald GL, Symoens S, Manouvrier S, et al. 2006. Aneurysm syndromes caused by mutations in the TGF-β receptor. N Engl J Med 355: 788–798. [DOI] [PubMed] [Google Scholar]
- Long L, Ormiston ML, Yang X, Southwood M, Gräf S, Machado RD, Mueller M, Kinzel B, Yung LM, Wilkinson JM, et al. 2015. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med 21: 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA, Zhang Y, Li P, Gong K, Miller AP, Hassan E, Hage F, Xing D, Wells B, Oparil S, et al. 2010. Inhibition of transforming growth factor-β signaling induces left ventricular dilation and dysfunction in the pressure-overloaded heart. Am J Physiol Heart Circ Physiol 298: H424–H432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgens E, Gijbels M, Smook M, Heeringa P, Gotwals P, Koteliansky VE, Daemen MJ. 2002. Transforming growth factor-β mediates balance between inflammation and fibrosis during plaque progression. Arterioscler Thromb Vasc Biol 22: 975–982. [DOI] [PubMed] [Google Scholar]
- Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Papa E, Boulday G, et al. 2013. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 498: 492–496. [DOI] [PubMed] [Google Scholar]
- Mahler GJ, Farrar EJ, Butcher JT. 2013. Inflammatory cytokines promote mesenchymal transformation in embryonic and adult valve endothelial cells. Arterioscler Thromb Vasc Biol 33: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud M, Allinson KR, Zhai Z, Oakenfull R, Ghandi P, Adams RH, Fruttiger M, Arthur HM. 2010. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ Res 106: 1425–1433. [DOI] [PubMed] [Google Scholar]
- Mao X, Debenedittis P, Sun Y, Chen J, Yuan K, Jiao K, Chen Y. 2012. Vascular smooth muscle cell Smad4 gene is important for mouse vascular development. Arterioscler Thromb Vasc Biol 32: 2171–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwald RR, Fitzharris TP, Manasek FJ. 1977. Structural development of endocardial cushions. Am J Anat 148: 85–119. [DOI] [PubMed] [Google Scholar]
- Marque V, Kieffer P, Gayraud B, Lartaud-Idjouadiene I, Ramirez F, Atkinson J. 2001. Aortic wall mechanics and composition in a transgenic mouse model of Marfan syndrome. Arterioscler Thromb Vasc Biol 21: 1184–1189. [DOI] [PubMed] [Google Scholar]
- Massagué J. 2012. TGF-β signalling in context. Nat Rev Mol Cell Biol 13: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J, Seoane J, Wotton D. 2005. Smad transcription factors. Genes Dev 19: 2783–2810. [DOI] [PubMed] [Google Scholar]
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, et al. 1994. Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 8: 345–351. [DOI] [PubMed] [Google Scholar]
- Medici D, Kalluri R. 2012. Endothelial-mesenchymal transition and its contribution to the emergence of stem cell phenotype. Semin Cancer Biol 22: 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici D, Olsen BR. 2012. The role of endothelial-mesenchymal transition in heterotopic ossification. J Bone Miner Res 27: 1619–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merk DR, Chin JT, Dake BA, Maegdefessel L, Miller MO, Kimura N, Tsao PS, Iosef C, Berry GJ, Mohr FW, et al. 2012. miR-29b participates in early aneurysm development in Marfan syndrome. Circ Res 110: 312–324. [DOI] [PubMed] [Google Scholar]
- Michelena HI, Corte AD, Prakash SK, Milewicz DM, Evangelista A, Enriquez-Sarano M. 2015. Bicuspid aortic valve aortopathy in adults: Incidence, etiology, and clinical significance. Int J Cardiol 15: 400–407. [DOI] [PubMed] [Google Scholar]
- Milewicz DM, Michael K, Fisher N, Coselli JS, Markello T, Biddinger A. 1996. Fibrillin-1 (FBN1) mutations in patients with thoracic aortic aneurysms. Circulation 94: 2708–2711. [DOI] [PubMed] [Google Scholar]
- *.Miyazawa K, Miyazono K. 2017. Regulation of TGF-β family signaling by inhibitory Smads. Cold Spring Harb Perspect Biol 9: a022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möberg K, De Nobele S, Devos D, Goetghebeur E, Segers P, Trachet B, Vervaet C, Renard M, Coucke P, Loeys B, et al. 2012. The Ghent Marfan Trial—A randomized, double-blind placebo controlled trial with losartan in Marfan patients treated with β-blockers. Int J Cardiol 157: 354–358. [DOI] [PubMed] [Google Scholar]
- Molin DG, Bartram U, Van der Heiden K, Van Iperen L, Speer CP, Hierck BP, Poelmann RE, Gittenberger-de-Groot AC. 2003. Expression patterns of TGF-β1-3 associate with myocardialisation of the outflow tract and the development of the epicardium and the fibrous heart skeleton. Dev Dyn 227: 431–444. [DOI] [PubMed] [Google Scholar]
- *.Morikawa M, Derynck R, Miyazono K. 2016. TGF-β and the TGF-β family: Context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol 8: a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell NW, Bloch DB, ten Dijke P, Goumans MJ, Hata A, Smith J, Yu PB, Bloch KD. 2016. Targeting BMP signalling in cardiovascular disease and anaemia. Nat Rev Cardiol 13: 106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. 2009. The regulation of TGF-β signal transduction. Development 136: 3699–3714. [DOI] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. 1999. The integrin α v β 6 binds and activates latent TGF β 1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328. [DOI] [PubMed] [Google Scholar]
- Nagalingam RS, Sundaresan NR, Noor M, Gupta MP, Solaro RJ, Gupta M. 2014. Deficiency of cardiomyocyte-specific microRNA-378 contributes to the development of cardiac fibrosis involving a transforming growth factor β (TGF-β1)-dependent paracrine mechanism. J Biol Chem 289: 27199–27214. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nakajima Y, Miyazono K, Kato M, Takase M, Yamagishi T, Nakamura H. 1997. Extracellular fibrillar structure of latent TGF β binding protein-1: Role in TGF β-dependent endothelial-mesenchymal transformation during endocardial cushion tissue formation in mouse embryonic heart. J Cell Biol 136: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necchi A, Giannatempo P, Mariani L, Fare E, Raggi D, Pennati M, Zaffaroni N, Crippa F, Marchiano A, Nicolai N, et al. 2014. PF-03446962, a fully-human monoclonal antibody against transforming growth-factor-β (TGF-β) receptor ALK1, in pre-treated patients with urothelial cancer: An open label, single-group, phase 2 trial. Invest New Drugs 32: 555–560. [DOI] [PubMed] [Google Scholar]
- Negishi M, Lu D, Zhang YQ, Sawada Y, Sasaki T, Kayo T, Ando J, Izumi T, Kurabayashi M, Kojima I, et al. 2001. Upregulatory expression of furin and transforming growth factor-β by fluid shear stress in vascular endothelial cells. Arterioscler Thromb Vasc Biol 21: 785–790. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. 2003. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411. [DOI] [PubMed] [Google Scholar]
- Niessen K, Zhang G, Ridgway JB, Chen H, Yan M. 2010. ALK1 signaling regulates early postnatal lymphatic vessel development. Blood 115: 1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura-Kitabayashi A, Phoon CK, Kishigami S, Rosenthal J, Yamauchi Y, Abe K, Yamamura K, Samtani R, Lo CW, Mishina Y. 2009. Outflow tract cushions perform a critical valve-like function in the early embryonic heart requiring BMPRIA-mediated signaling in cardiac neural crest. Am J Physiol Heart Circ Physiol 297: H1617–H1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuessle JM, Giehl K, Herzog R, Stracke S, Menke A. 2011. TGFβ1 suppresses vascular smooth muscle cell motility by expression of N-cadherin. Biol Chem 392: 461–474. [DOI] [PubMed] [Google Scholar]
- Ogo T, Chowdhury HM, Yang J, Long L, Li X, Torres Cleuren YN, Morrell NW, Schermuly RT, Trembath RC, Nasim MT. 2013. Inhibition of overactive TGF-β signaling by prostacyclin analogs in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 48: 733–741. [DOI] [PubMed] [Google Scholar]
- Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, et al. 2000. Activin receptor-like kinase 1 modulates transforming growth factor-β1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci 97: 2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka M, Iwata C, Suzuki HI, Kiyono K, Morishita Y, Watabe T, Komuro A, Kano MR, Miyazono K. 2008. Inhibition of endogenous TGF-β signaling enhances lymphangiogenesis. Blood 111: 4571–4579. [DOI] [PubMed] [Google Scholar]
- Okada H, Takemura G, Kosai K, Li Y, Takahashi T, Esaki M, Yuge K, Miyata S, Maruyama R, Mikami A, et al. 2005. Postinfarction gene therapy against transforming growth factor-β signal modulates infarct tissue dynamics and attenuates left ventricular remodeling and heart failure. Circulation 111: 2430–2437. [DOI] [PubMed] [Google Scholar]
- Olofsson A, Ichijo H, Morén A, ten Dijke P, Miyazono K, Heldin CH. 1995. Efficient association of an amino-terminally extended form of human latent TGF-β binding protein with the extracellular matrix. J Biol Chem 270: 31294–31297. [DOI] [PubMed] [Google Scholar]
- Orlova VV, Drabsch Y, Freund C, Petrus-Reurer S, van den Hil FE, Muenthaisong S, ten Dijke P, Mummery CL. 2014. Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts. Arterioscler Thromb Vasc Biol 34: 177–186. [DOI] [PubMed] [Google Scholar]
- Oshima M, Oshima H, Taketo MM. 1996. TGF-β receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol 179: 297–302. [DOI] [PubMed] [Google Scholar]
- Owens GK1, Kumar MS, Wamhoff BR. 2004. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801. [DOI] [PubMed] [Google Scholar]
- Padang R, Bannon PG, Jeremy R, Richmond DR, Semsarian C, Vallely M, Wilson M, Yan TD. 2013. The genetic and molecular basis of bicuspid aortic valve associated thoracic aortopathy: A link to phenotype heterogeneity. Ann Cardiothorac Surg 2: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloschi V, Gådin JR, Khan S, Björck HM, Du L, Maleki S, Roy J, Lindeman JH, Mohamed SA, Tsuda T, et al. 2015. Aneurysm development in patients with a bicuspid aortic valve is not associated with transforming growth factor-β activation. Arterioscler Thromb Vasc Biol 35: 973–980. [DOI] [PubMed] [Google Scholar]
- Pan D, Zhu Q, Luo K. 2009. SnoN functions as a tumour suppressor by inducing premature senescence. EMBO J 28: 3500–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannu H, Fadulu VT, Chang J, Lafont A, Hasham SN, Sparks E, Giampietro PF, Zaleski C, Estrera AL, Safi HJ, et al. 2005. Mutations in transforming growth factor-β receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation 112: 513–520. [DOI] [PubMed] [Google Scholar]
- Panutsopulos D, Papalambros E, Sigala F, Zafiropoulos A, Arvanitis DL, Spandidos DA. 2005. Protein and mRNA expression levels of VEGF-A and TGF-β1 in different types of human coronary atherosclerotic lesions. Int J Mol Med 15: 603–610. [PubMed] [Google Scholar]
- Pardali E, ten Dijke P. 2012. TGF-β signaling and cardiovascular diseases. Int J Biol Sci 8: 195–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardali E, Goumans MJ, ten Dijke P. 2010. Signaling by members of the TGF-β family in vascular morphogenesis and disease. Trends Cell Biol 20: 556–567. [DOI] [PubMed] [Google Scholar]
- Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, Park A, Wu X, Kaartinen V, Roman BL, et al. 2008. ALK5- and TGFβR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood 111: 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penaloza A, Vekemans MC, Lambert C, Hermans C. 2011. Deep vein thrombosis induced by thalidomide to control epistaxis secondary to hereditary haemorrhagic telangiectasia. Blood Coagul Fibrinolysis 22: 616–618. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Vassalli JD, Orci L, Montesano R. 1993. Biphasic effect of transforming growth factor-β1 on in vitro angiogenesis. Exp Cell Res 204: 356–363. [DOI] [PubMed] [Google Scholar]
- Pérez-Gómez E, Eleno N, López-Novoa JM, Ramirez JR, Velasco B, Letarte M, Bernabéu C, Quintanilla M. 2005. Characterization of murine S-endoglin isoform and its effects on tumor development. Oncogene 24: 4450–4461. [DOI] [PubMed] [Google Scholar]
- Piccolo P, Mithbaokar P, Sabatino V, Tolmie J, Melis D, Schiaffino MC, Filocamo M, Andria G, Brunetti-Pierri N. 2014. SMAD4 mutations causing Myhre syndrome result in disorganization of extracellular matrix improved by losartan. Eur J Hum Genet 22: 988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollman MJ, Naumovski L, Gibbons GH. 1999. Vascular cell apoptosis: Cell type-specific modulation by transforming growth factor-β1 in endothelial cells versus smooth muscle cells. Circulation 99: 2019–2026. [DOI] [PubMed] [Google Scholar]
- Pomeraniec L, Hector-Greene M, Ehrlich M, Blobe GC, Henis YI. 2015. Regulation of TGF-β receptor hetero-oligomerization and signaling by endoglin. Mol Biol Cell 26: 3117–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. 2011. Basic and therapeutic aspects of angiogenesis. Cell 146: 873–887. [DOI] [PubMed] [Google Scholar]
- Praetorius HA. 2015. The primary cilium as sensor of fluid flow: New building blocks to the model. A review in the theme: Cell signaling: Proteins, pathways and mechanisms. Am J Physiol Cell Physiol 308: C198–C208. [DOI] [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T. 1995. Transforming growth factor-β3 is required for secondary palate fusion. Nat Genet 11: 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu P, Feng XH, Li L. 2003. Interaction of Smad3 and SRF-associated complex mediates TGF-β1 signals to regulate SM22 transcription during myofibroblast differentiation. J Mol Cell Cardiol 35: 1407–1420. [DOI] [PubMed] [Google Scholar]
- Qiu P, Ritchie RP, Fu Z, Cao D, Cumming J, Miano JM, Wang DZ, Li HJ, Li L. 2005. Myocardin enhances Smad3-mediated transforming growth factor-β1 signaling in a CArG box-independent manner: Smad-binding element is an important cis element for SM22α transcription in vivo. Circ Res 97: 983–991. [DOI] [PubMed] [Google Scholar]
- Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, et al. 2011. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation 124: 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel MC, Emery CM, Foulger R, Goberdhan DC, van den Heuvel M, Wilson C. 2007. Drosophila SnoN modulates growth and patterning by antagonizing TGF-β signalling. Mech Dev 124: 304–317. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Dietz HC. 2007. Marfan syndrome: From molecular pathogenesis to clinical treatment. Curr Opin Genet Dev 17: 252–258. [DOI] [PubMed] [Google Scholar]
- Ran S, Montgomery KE. 2012. Macrophage-mediated lymphangiogenesis: The emerging role of macrophages as lymphatic endothelial progenitors. Cancer 4: 618–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regalado ES, Guo DC, Villamizar C, Avidan N, Gilchrist D, McGillivray B, Clarke L, Bernier F, Santos-Cortez RL, Leal SM, et al. 2011. Exome sequencing identifies SMAD3 mutations as a cause of familial thoracic aortic aneurysm and dissection with intracranial and other arterial aneurysms. Circ Res 109: 680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifenberg K, Cheng F, Orning C, Crain J, Kupper I, Wiese E, Protschka M, Blessing M, Lackner KJ, Torzewski M. 2012. Overexpression of TGF-β1 in macrophages reduces and stabilizes atherosclerotic plaques in ApoE-deficient mice. PLoS ONE 7: e40990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard M, Holm T, Veith R, Callewaert BL, Ades LC, Baspinar O, Pickart A, Dasouki M, Hoyer J, Rauch A, et al. 2010. Altered TGF-β signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur J Hum Genet 18: 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard M, Callewaert B, Baetens M, Campens L, MacDermot K, Fryns JP, Bonduelle M, Dietz HC, Gaspar I.M, Cavaco D, et al. 2013a. Novel MYH11 and ACTA2 mutations reveal a role for enhanced TGF-β signaling in FTAAD. Int J Cardiol 165: 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard M, Callewaert B, Malfait F, Campens L, Sharif S, del Campo M, Valenzuela I, McWilliam C, Coucke P, De Paepe A, et al. 2013b. Thoracic aortic-aneurysm and dissection in association with significant mitral valve disease caused by mutations in TGFβ2. Int J Cardiol 165: 584–587. [DOI] [PubMed] [Google Scholar]
- Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. 1999. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-β. J Biol Chem 274: 13586–13593. [DOI] [PubMed] [Google Scholar]
- Ricard N, Ciais D, Levet S, Subileau M, Mallet C, Zimmers TA, Lee SJ, Bidart M, Feige JJ, Bailly S. 2012. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood 119: 6162–6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienhoff HY Jr, Yeo CY, Morissette R, Khrebtukova I, Melnick J, Luo S, Leng N, Kim YJ, Schroth G, Westwick J, et al. 2013. A mutation in TGFβ3 associated with a syndrome of low muscle mass, growth retardation distal arthrogryposis and clinical features overlapping with Marfan and Loeys–Dietz syndrome. Am J Med Genet A 161A: 2040–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo S, Basso C, Lazzarini E, Celeghin R, Paolin A, Gerosa G, Valente M, Thiene G, Pilichou K. 2015. TGF-β1 pathway activation and adherens junction molecular pattern in nonsyndromic mitral valve prolapse. Cardiovasc Pathol 24: 359–367. [DOI] [PubMed] [Google Scholar]
- Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. 2003. Disruption of TGF-β signaling in T cells accelerates atherosclerosis. J Clin Invest 112: 1342–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB. 2015. Latent TGF-β-binding proteins. Matrix Biol 47: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Robertson IB, Rifkin DB. 2016. Regulation of the bioavailability of TGF-β and TGF-β-related proteins. Cold Spring Harb Perspect Biol 8: a021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson A, Allinson KR, Anderson RH, Henderson DJ, Arthur HM. 2010. The TGF-β type II receptor plays a critical role in the endothelial cells during cardiac development. Dev Dyn 239: 2435–2442. [DOI] [PubMed] [Google Scholar]
- Rosenkranz S, Flesch M, Amann K, Haeuseler C, Kilter H, Seeland U, Schluter KD, Bohm M. 2002. Alterations of β-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-β1. Am J Physiol Heart Circ Physiol 283: H1253–H1262. [DOI] [PubMed] [Google Scholar]
- Ross S, Hill CS. 2008. How the Smads regulate transcription. Int J Biochem Cell 2008: 383–408. [DOI] [PubMed] [Google Scholar]
- Ruwhof C, van der Laarse A. 2000. Mechanical stress-induced cardiac hypertrophy: Mechanisms and signal transduction pathways. Cardiovasc Res 47: 23–37. [DOI] [PubMed] [Google Scholar]
- Sanchez Alvarado A, Yamanaka S. 2014. Rethinking differentiation: Stem cells, regeneration, and plasticity. Cell 157: 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. 1997. TGF-β2 knockout mice have multiple developmental defects that are non-overlapping with other TGF-β knockout phenotypes. Development 124: 2659–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santibanez JF, Blanco FJ, Garrido-Martin EM, Sanz-Rodriguez F, del Pozo MA, Bernabéu C. 2008. Caveolin-1 interacts and cooperates with the transforming growth factor-β type I receptor ALK1 in endothelial caveolae. Cardiovasc Res 77: 791–799. [DOI] [PubMed] [Google Scholar]
- Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, Lowik CW, ten Dijke P. 2007. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci 120: 964–972. [DOI] [PubMed] [Google Scholar]
- Schepers D, Doyle AJ, Oswald G, Sparks E, Myers L, Willems PJ, Mansour S, Simpson MA, Frysira H, Maat-Kievit A, et al. 2015. The SMAD-binding domain of SKI: A hotspot for de novo mutations causing Shprintzen–Goldberg syndrome. Eur J Hum Genet 23: 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE. 1995. Regulation of transforming growth factor-β activation by discrete sequences of thrombospondin 1. J Biol Chem 270: 7304–7310. [DOI] [PubMed] [Google Scholar]
- Seay U, Sedding D, Krick S, Hecker M, Seeger W, Eickelberg O. 2005. Transforming growth factor-β-dependent growth inhibition in primary vascular smooth muscle cells is p38-dependent. J Pharmacol Exp Ther 315: 1005–1012. [DOI] [PubMed] [Google Scholar]
- Seeland U, Haeuseler C, Hinrichs R, Rosenkranz S, Pfitzner T, Scharffetter-Kochanek K, Bohm M. 2002. Myocardial fibrosis in transforming growth factor-β1 (TGF-β1) transgenic mice is associated with inhibition of interstitial collagenase. Eur J Clin Invest 32: 295–303. [DOI] [PubMed] [Google Scholar]
- Seeland U, Schaffer A, Selejan S, Hohl M, Reil JC, Muller P, Rosenkranz S, Bohm M. 2009. Effects of AT1- and β-adrenergic receptor antagonists on TGF-β1-induced fibrosis in transgenic mice. Eur J Clin Invest 39: 851–859. [DOI] [PubMed] [Google Scholar]
- Seghers L, de Vries MR, Pardali E, Hoefer IE, Hierck BP, ten Dijke P, Goumans MJ, Quax PH. 2012. Shear induced collateral artery growth modulated by endoglin but not by ALK1. J Cell Mol Med 16: 2440–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao ES, Lin L, Yao Y, Boström KI. 2009. Expression of vascular endothelial growth factor is coordinately regulated by the activin-like kinase receptors 1 and 5 in endothelial cells. Blood 114: 2197–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D. 2005. Integrin-mediated activation of latent transforming growth factor-β. Cancer Metastasis Rev 24: 395–402. [DOI] [PubMed] [Google Scholar]
- Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. 2011. Latent TGF-β structure and activation. Nature 474: 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Guo LW, Seedial SM, Si Y, Wang B, Takayama T, Suwanabol PA, Ghosh S, DiRenzo D, Liu B, et al. 2014. TGF-β/Smad3 inhibit vascular smooth muscle cell apoptosis through an autocrine signaling mechanism involving VEGF-A. Cell Death Dis 5: e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu C, Jain S, Davila S, Hibberd ML, Lin KO, Molkara D, Frazer JR, Sun S, Baker AL, Newburger JW, et al. 2011. Transforming growth factor-β signaling pathway in patients with Kawasaki disease. Circ Cardiovasc Genet 4: 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Hoofnagle MH, Kingston PA, McCanna ME, Owens GK. 2004. Transforming growth factor-β1 signaling contributes to development of smooth muscle cells from embryonic stem cells. Am J Physiol Cell Physiol 287: C1560–C1568. [DOI] [PubMed] [Google Scholar]
- Sirard C, de la Pompa JL, Elia A, Itie A, Mirtsos C, Cheung A, Hahn S, Wakeham A, Schwartz L, Kern SE, et al. 1998. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev 12: 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Jones JE, Beppu H, Keaney JF Jr, Loscalzo J, Zhang YY. 2005. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation 112: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Coleman L, Shi J, Beppu H, Sato K, Walsh K, Loscalzo J, Zhang YY. 2008. Inflammation, endothelial injury, and persistent pulmonary hypertension in heterozygous BMPR2-mutant mice. Am J Physiol Heart Circ Physiol 295: H677–H690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen LK, Brooke BS, Li DY, Urness LD. 2003. Loss of distinct arterial and venous boundaries in mice lacking endoglin, a vascular-specific TGF-β coreceptor. Dev Biol 261: 235–250. [DOI] [PubMed] [Google Scholar]
- Soubrier F, Chung WK, Machado R, Grünig E, Aldred M, Geraci M, Loyd JE, Elliott CG, Trembath RC, Newman JH, et al. 2013. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 62: D13–D21. [DOI] [PubMed] [Google Scholar]
- Souders CA, Borg TK, Banerjee I, Baudino TA. 2012. Pressure overload induces early morphological changes in the heart. Am J Pathol 181: 1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R, et al. 2013. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest 123: 3600–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiekerkoetter E, Sung YK, Sudheendra D, Bill M, Aldred MA, van de Veerdonk MC, Vonk Noordegraaf A, Long-Boyle J, Dash R, Yang PC, et al. 2015. Low-dose FK506 (Tacrolimus) in endstage pulmonary arterial hypertension. Am J Respir Crit Care Med 192: 254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridurongrit S, Larsson J, Schwartz R, Ruiz-Lozano P, Kaartinen V. 2008. Signaling via the TGF-β type I receptor Alk5 in heart development. Dev Biol 322: 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvers KL, Tursky ML, Harder KW, Kountouri N, Amatayakul-Chantler S, Grail D, Small C, Weinberg RA, Sizeland AM, Zhu HJ. 2003. Heart and liver defects and reduced transforming growth factor β2 sensitivity in transforming growth factor β type III receptor–deficient embryos. Mol Cell Biol 23: 4371–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner-Kock A, Thorey IS, Koli K, Wempe F, Otte J, Bangsow T, Kuhlmeier K, Kirchner T, Jin S, Keski-Oja J, et al. 2002. Disruption of the gene encoding the latent transforming growth factor-β binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev 16: 2264–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stheneur C, Collod-Béroud G, Faivre L, Gouya L, Sultan G, Le Parc JM, Moura B, Attias D, et al. 2008. Identification of 23 TGFβR2 and 6 TGFβR1 gene mutations and genotype-phenotype investigations in 457 patients with Marfan syndrome type I and II, Loeys–Dietz syndrome and related disorders. Hum Mutat 29: E284–E295. [DOI] [PubMed] [Google Scholar]
- Stroschein SL, Wang W, Zhou S, Zhou Q, Luo K. 1999. Negative feedback regulation of TGF-β signaling by the SnoN oncoprotein. Science 286: 771–774. [DOI] [PubMed] [Google Scholar]
- Sullivan KE, Black LD. 2013. The role of cardiac fibroblasts in extracellular matrix-mediated signaling during normal and pathological cardiac development. J Biomech Eng 135: 71001. [DOI] [PubMed] [Google Scholar]
- Suwanabol PA, Seedial SM, Shi X, Zhang F, Yamanouchi D, Roenneburg D, Liu B, Kent KC. 2012. Transforming growth factor-β increases vascular smooth muscle cell proliferation through the Smad3 and extracellular signal-regulated kinase mitogen-activated protein kinases pathways. J Vasc Surg 56: 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Ohga N, Morishita Y, Hida K, Miyazono K, Watabe T. 2010. BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J Cell Sci 123: 1684–1692. [DOI] [PubMed] [Google Scholar]
- Tan CK, Tan EH, Luo B, Huang CL, Loo JS, Choong C, Tan NS. 2013. SMAD3 deficiency promotes inflammatory aortic aneurysms in angiotensin II–infused mice via activation of iNOS. J Am Heart Assoc 2: e000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Lee KS, Yang H, Logan DW, Wang S, McKinnon ML, Holt LJ, Condie A, Luu MT, Akhurst RJ. 2005. Epistatic interactions between modifier genes confer strain-specific redundancy for Tgfβ1 in developmental angiogenesis. Genomics 85: 60–70. [DOI] [PubMed] [Google Scholar]
- Tang Y, Boucher JM, Liaw L. 2012. Histone deacetylase activity selectively regulates notch-mediated smooth muscle differentiation in human vascular cells. J Am Heart Assoc 1: e000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PM, Batten P, Brand NJ, Thomas PS, Yacoub MH. 2003. The cardiac valve interstitial cell. Int J Biochem Cell Biol 35: 113–118. [DOI] [PubMed] [Google Scholar]
- Tedgui A, Mallat Z. 2001. Anti-inflammatory mechanisms in the vascular wall. Circ Res 88: 877–887. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Arthur HM. 2007. Extracellular control of TGF-β signalling in vascular development and disease. Nat Rev Mol Cell Biol 8: 857–869. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Goumans MJ, Pardali E. 2008. Endoglin in angiogenesis and vascular diseases. Angiogenesis 11: 79–89. [DOI] [PubMed] [Google Scholar]
- Thompson AR, Cooper JA, Jones GT, Drenos F, van Bockxmeer FM, Biros E, Walker PJ, van Rij AM, Golledge J, Norman PE, et al. 2010. Assessment of the association between genetic polymorphisms in transforming growth factor-β, and its binding protein (LTBP), and the presence, and expansion, of abdominal aortic aneurysm. Atherosclerosis 209: 367–373. [DOI] [PubMed] [Google Scholar]
- Tijsen AJ, van der Made I, van den Hoogenhof MM, Wijnen WJ, van Deel ED, de Groot NE, Alekseev S, Fluiter K, Schroen B, Goumans MJ, et al. 2014. The microRNA-15 family inhibits the TGF-β-pathway in the heart. Cardiovasc Res 104: 61–71. [DOI] [PubMed] [Google Scholar]
- Todorovic V, Frendewey D, Gutstein DE, Chen Y, Freyer L, Finnegan E, Liu F, Murphy A, Valenzuela D, Yancopoulos G, et al. 2007. Long form of latent TGF-β binding protein 1 (Ltbp1L) is essential for cardiac outflow tract septation and remodeling. Development 134: 3723–3732. [DOI] [PubMed] [Google Scholar]
- Todorovic V, Finnegan E, Freyer L, Zilberberg L, Ota M, Rifkin DB. 2011. Long form of latent TGF-β binding protein 1 (LTBP-1L) regulates cardiac valve development. Dev Dyn 240: 176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma I, McCaffrey TA. 2012. Transforming growth factor-β and atherosclerosis: Interwoven atherogenic and atheroprotective aspects. Cell Tissue Res 347: 155–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Fadulu V, Pannu H, Kim DH, Vick GW III, Lonsford CM, Lafont AL, Boccalandro C, Smart S, Peterson KL, Hain JZ, et al. 2009. Analysis of multigenerational families with thoracic aortic aneurysms and dissections due to TGFBR1 or TGFBR2 mutations. J Med Genet 46: 607–613. [DOI] [PubMed] [Google Scholar]
- Tual-Chalot S, Allinson KR, Fruttiger M, Arthur HM. 2013. Whole mount immunofluorescent staining of the neonatal mouse retina to investigate angiogenesis in vivo. J Vis Exp 77: e50546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tual-Chalot S, Mahmoud M, Allinson KR, Redgrave RE, Zhai Z, Oh SP, Fruttiger M, Arthur HM. 2014. Endothelial depletion of Acvrl1 in mice leads to arteriovenous malformations associated with reduced endoglin expression. PLoS ONE 9: e98646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urness LD, Sorensen LK, Li DY. 2000. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet 26: 328–331. [DOI] [PubMed] [Google Scholar]
- van de Laar IM, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JM, Hoedemaekers YM, Willemsen R, Severokmem LA, Venselaar H, et al. 2011. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet 43: 121–126. [DOI] [PubMed] [Google Scholar]
- van de Laar IM, van der Linde D, Oei EH, Bos PK, Bessems JH, Bierma-Zeinstra SM, van Meer BL, Pals G, Oldenburg RA, Bekkers JA, et al. 2012. Phenotypic spectrum of the SMAD3-related aneurysms-osteoarthritis syndrome. J Med Genet 49: 47–57. [DOI] [PubMed] [Google Scholar]
- van der Linde D, van de Laar IM, Bertoli-Avella AM, Oldenburg RA, Bekkers JA, Mattace-Raso FU, van den Meiracker AH, Moelker A, van Kooten F, Frohn-Mulder IM, et al. 2012. Aggressive cardiovascular phenotype of aneurysms-osteoarthritis syndrome caused by pathogenic SMAD3 variants. J Am Coll Cardiol 60: 397–403. [DOI] [PubMed] [Google Scholar]
- van Meeteren LA, Goumans MJ, ten Dijke P. 2011. TGF-β receptor signaling pathways in angiogenesis; emerging targets for anti-angiogenesis therapy. Curr Pharm Biotechnol 12: 2108–2120. [DOI] [PubMed] [Google Scholar]
- Vecchia L, Olivieri C, Scotti C. 2013. Activin Receptor-like kinase 1: A novel anti-angiogenesis target from TGF-β family. Mini Rev Med Chem 13: 1398–1406. [DOI] [PubMed] [Google Scholar]
- Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA. 2004. Valvular myofibroblast activation by transforming growth factor β: Implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res 95: 253–260. [DOI] [PubMed] [Google Scholar]
- Walshe TE, dela Paz NG, D’Amore PA. 2013. The role of shear-induced transforming growth factor-β signaling in the endothelium. Arterioscler Thromb Vasc Biol 33: 2608–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadière C, Rénia L, et al. 2010. TGF-β activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest 120: 422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Ren GD, Zhou Z, Xu Y, Qin T, Yu RF, Zhang TC. 2012. Cooperation of myocardin and Smad2 in inducing differentiation of mesenchymal stem cells into smooth muscle cells. IUBMB Life 64: 331–339. [DOI] [PubMed] [Google Scholar]
- Wang BW, Wu GJ, Cheng WP, Shyu KG. 2014a. MicroRNA-208a increases myocardial fibrosis via endoglin in volume overloading heart. PLoS ONE 9: e84188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Vootukuri S, Meyer A, Ahamed J, Coller BS. 2014b. Association between shear stress and platelet-derived transforming growth factor-β1 release and activation in animal models of aortic valve stenosis. Arterioscler Thromb Vasc Biol 34: 1924–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Leinwand LA, Anseth KS. 2014c. Cardiac valve cells and their microenvironment––Insights from in vitro studies. Nat Rev Cardiol 11: 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe T, Nishihara A, Mishima K, Yamashita J, Shimizu K, Miyazawa K, Nishikawa S, Miyazono K. 2003. TGF-β receptor kinase inhibitor enhances growth and integrity of embryonic stem cell-derived endothelial cells. J Cell Biol 163: 1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LH, Huang XR, Zhang Y, Li YQ, Chen HY, Heuchel R, Yan BP, Yu CM, Lan HY. 2013. Deficiency of Smad7 enhances cardiac remodeling induced by angiotensin II infusion in a mouse model of hypertension. PLoS ONE 8: e70195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischmeijer A, Van Laer L, Tortora G, Bolar NA, Van Camp G, Fransen E, Peeters N, di Bartolomeo R, Pancini D, Gargiulo G, et al. 2013. Thoracic aortic aneurysm in infancy in aneurysms-osteoarthritis syndrome due to a novel SMAD3 mutation: Further delineation of the phenotype. Am J Med Genet A 161A: 1028–1035. [DOI] [PubMed] [Google Scholar]
- Wooderchak-Donahue WL, McDonald J, O’Fallon B, Upton PD, Li W, Roman BL, Young S, Plant P, Fulop GT, Langa C, et al. 2013. BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am J Hum Genet 93: 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten EC, Iyer LK, Montefusco MC, Hedgepeth AK, Payne DD, Kapur NK, Housman DE, Mendelsohn ME, Huggins GS. 2010. Application of gene network analysis techniques identifies AXIN1/PDIA2 and endoglin haplotypes associated with bicuspid aortic valve. PLoS ONE 5: e8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. 1994. Mechanism of activation of the TGF-β receptor. Nature 370: 341–347. [DOI] [PubMed] [Google Scholar]
- Wu MY, Hill CS. 2009. TGF-β superfamily signaling in embryonic development and homeostasis. Dev Cell 16: 329–343. [DOI] [PubMed] [Google Scholar]
- Wu X, Ma J, Han JD, Wang N, Chen YG. 2006. Distinct regulation of gene expression in human endothelial cells by TGF-β and its receptors. Microvasc Res 71: 12–19. [DOI] [PubMed] [Google Scholar]
- Wylie-Sears J, Levine RA, Bischoff J. 2014. Losartan inhibits endothelial-to-mesenchymal transformation in mitral valve endothelial cells by blocking transforming growth factor-β-induced phosphorylation of ERK. Biochem Biophys Res Commun 446: 870–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Knispel RA, Dietz HC, Ramirez F, Baxter BT. 2008. Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J Vasc Surg 47: 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. 1995. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science 270: 2008–2011. [DOI] [PubMed] [Google Scholar]
- Yang Y, Oliver G. 2014a. Development of the mammalian lymphatic vasculature. J Clin Invest 124: 888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Oliver G. 2014b. Transcriptional control of lymphatic endothelial cell type specification. Adv Anat Embryol Cell Biol 214: 5–22. [DOI] [PubMed] [Google Scholar]
- Yang X, Li C, Xu X, Deng C. 1998. The tumor suppressor SMAD4/DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc Natl Acad Sci 95: 3667–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Castilla LH, Xu X, Li C, Gotay J, Weinstein M, Liu PP, Deng CX. 1999. Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development 126: 1571–1580. [DOI] [PubMed] [Google Scholar]
- Yang P, Schmit BM, Fu C, DeSart K, Oh SP, Berceli SA, Jiang Z. 2016. Smooth muscle cell-specific Tgfbr1 deficiency promotes aortic aneurysm formation by stimulating multiple signaling events. Sci Rep 6: 35444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao EH, Fukuda N, Ueno T, Matsuda H, Nagase H, Matsumoto Y, Sugiyama H, Matsumoto K. 2009. A pyrrole-imidazole polyamide targeting transforming growth factor-β1 inhibits restenosis and preserves endothelialization in the injured artery. Cardiovasc Res 81: 797–804. [DOI] [PubMed] [Google Scholar]
- Yoshimatsu Y, Lee YG, Akatsu Y, Taguchi L, Suzuki HI, Cunha SI, Maruyama K, Suzuki Y, Yamazaki T, Katsura A, et al. 2013. Bone morphogenetic protein-9 inhibits lymphatic vessel formation via activin receptor-like kinase 1 during development and cancer progression. Proc Natl Acad Sci 110: 18940–18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, et al. 2007. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961. [DOI] [PubMed] [Google Scholar]
- *.Zhang YE. 2016. Non-Smad signaling pathways of the TGF-β family. Cold Spring Harb Perspect Biol 8: a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Gaussin V, Taffet GE, Belaguli NS, Yamada M, Schwartz RJ, Michael LH, Overbeek PA, Schneider MD. 2000. TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat Med 6: 556–563. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huang XR, Wei LH, Chung AC, Yu CM, Lan HY. 2014. miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling. Mol Ther 22: 974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Hou S, Chen J, Zhang J, Lin F, Ju R, Cheng X, Ma X, Song Y, Zhang Y, et al. 2016. Smad4 deficiency in smooth muscle cells initiates the formation of aortic aneurysm. Circ Res 118: 388–399. [DOI] [PubMed] [Google Scholar]
- Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. 2002. β8 integrins are required for vascular morphogenesis in mouse embryos. Development 129: 2891–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Kim YH, Wang D, Oh SP, Luo K. 2013. SnoN facilitates ALK1-Smad1/5 signaling during embryonic angiogenesis. J Cell Biol 202: 937–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Cao M, Figueroa JA, Cobos E, Uretsky BF, Chiriva-Internati M, Hermonat PL. 2014. AAV2/8-hSMAD3 gene delivery attenuates aortic atherogenesis, enhances Th2 response without fibrosis in LDLR-KO mice on high cholesterol diet. J Trans Med 12: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberberg L, Phoon CK, Robertson I, Dabovic B, Ramirez F, Rifkin DB. 2015. Genetic analysis of the contribution of LTBP-3 to thoracic aneurysm in Marfan syndrome. Proc Natl Acad Sci 112: 14012–14017. [DOI] [PMC free article] [PubMed] [Google Scholar]