Abstract
Deficiencies in pancreatic β-cell mass contribute to both type 1 and type 2 diabetes. We investigated the role of the glucose-regulated protein (GRP) 94, an endoplasmic reticulum protein abundantly expressed in the pancreatic acini and islets, in β-cell development, survival, and function. We used a conditional knockout (KO) mouse in which the GRP94 gene, Hsp90b1, was specifically deleted in pancreatic and duodenal homeobox 1 (Pdx1)–expressing cells. These Hsp90b1 flox/flox;Pdx1Cre KO mice exhibited pancreatic hypoplasia at embryonic day (E) 16.5 to E18.5 and had significantly reduced β-cell mass at 4 weeks after birth. Further mechanistic studies showed that deletion of GRP94 reduced β-cell proliferation with increased cell apoptosis in both Pdx1+ endocrine progenitor cells and differentiated β cells. Although Hsp90b1 flox/flox;Pdx1Cre KO mice remained euglycemic at 8 weeks of age, they exhibited impaired glucose tolerance. In aggregate, these findings indicate that GRP94 is an essential regulator of pancreatic β-cell development, mass, and function.
We studied the role of GRP94 in conditional KO mice in which the GRP94 gene was depleted in Pdx1+ cells and found GRP94 is an essential regulator of pancreatic β-cell development, mass, and function.
Destruction of pancreatic β-cell mass is a hallmark of both type 1 diabetes and type 2 diabetes (T2D) (1). In type 1 diabetes, β-cell death is mainly mediated by autoreactive T cells (2). In T2D, β-cell death is caused by excessive insulin demand and stress under insulin resistance conditions (3). Recent genomic studies have shown that most genes associated with increased susceptibility to diabetes are also involved in the regulation of β-cell growth and function during embryonic and fetal periods (4–7). β-Cell proliferation and neogenesis during the neonatal period are critical for generation of a sufficient pancreatic β-cell mass/reserve and have a profound impact on long-term protection against T2D (7). Therefore, elucidation of cellular signals that regulate β-cell mass, survival, and function may allow improvement of therapeutic strategies for diabetes through preservation and expansion of β-cell mass and enhancement of β-cell function.
Endoplasmic reticulum (ER) is responsible for proper protein synthesis, folding, trafficking, and secretion of proteins destined to the secretory pathway. When a protein cannot be folded efficiently, misfolded or unfolded proteins accumulate in the ER lumen, leading to the induction of the unfolded protein response (UPR). UPR reduces protein translation, accelerates degradation of misfolded proteins, and increases chaperone availability to maintain ER homeostasis. UPR functions through inositol-requiring protein 1, protein kinase RNA-like ER kinase, and activated transcriptional factor 6 (8). Severe and prolonged UPR will lead to cell dysfunction and death. In β cells under acute and chronic stresses in which excessive nutrients such as glucose, arginine, and lipids are present, the demand for insulin secretion is increased. The UPR response can be activated to compensate for the increased demand. However, prolonged unresolved UPR can engage the apoptotic pathway, leading to β-cell apoptosis. It has been shown that mutations in genes required for ER function may lead to β-cell failure and early onset of diabetes in animal models and in humans (9, 10), underscoring the important roles of proper UPR regulation.
Glucose-regulated protein (GRP) 94 is the most abundant protein in the ER lumen. GRP94 contributes to ER quality control via chaperoning the folding of proteins, interacting with other components of the ER protein-folding machinery, participating in calcium storage, and assisting in the targeting of misfolded proteins for ER-associated degradation (11). GRP94 shares similar biochemical features with other heat shock protein (HSP) 90s, including its domain structure and ATPase activity, but also has calcium-binding ability (12–14). Homozygous knockout (KO) of GRP94 leads to embryonic lethality at E10.5 in mice (15). The interactions of GRP94 with its clients are dependent on cochaperones and are different from other ER chaperons. Known clients of GRP94 include immunoglobulin heavy and light chains, insulinlike proteins, insulinlike growth factor (IGF) I and II, integrins, and toll-like receptors, all of which depend on GRP94 for their maturation (16–22). GRP94 (and orthologs) is essential for the development of plants, fruit flies, and mice (19, 23–25). Specifically, in each organism, GRP94 is required during a specific developmental stage. However, the requirement for GRP94 does not correlate with the onset of its protein expression as it is often synthesized prior to demand but occurs when a particular client needs it in a cell- and tissue type–dependent manner (19, 26–28).
In this study, we assessed the role of GRP94 in the regulation of pancreatic β-cell development, mass, and function using a mouse strain in which the GRP94 gene was deleted in Pdx1+ pancreatic progenitors and Pdx1+ Ins+ β cells. These studies show a novel and essential role of GRP94 in pancreatic β cells and identify GRP94 deficiency as a likely contributing factor in the pathogenesis of diabetes.
Material and Methods
Mice and genotyping
The mouse strain that contained a floxed GRP94 allele (Hsp90b1 flox/flox/GRP94flox/flox) was described previously (22, 29). The Pdx1Cre line [B6.FVB-Tg(pdx1-cre)6Tuv/J] and R26R line [B6.129S4-Gt(ROSA)26Sortm1sor/J] were purchased from the Jackson Laboratory (Bar Harbor, ME). Only male mice were analyzed in this study. All animal studies have been approved by the Animal Care and Use Committee at the Medical University of South Carolina.
Generation of conditional KO mice and transgenic mice
Pancreatic progenitor cell (Pdx1+)–specific GRP94 conditional KO mice were generated by breeding the Pdx1Cre mouse strain with the GRP94flox/flox mice. We also crossed the Pdx1Cre;GRP94flox/+ mice with the R26R;GRP94flox/+ mice (30). The Cre-mediated recombination was then assessed by X-gal staining (31). Pdx1Cre;R26R mice revealed robust LacZ staining in the pancreas.
Lentiviral infection of GRP94 short hairpin RNA
To generate GRP94 lentivirus, 293T cells were cotransfected with GRP94 short hairpin RNA (shRNA), Δ8.9, and VSV-G plasmids at a ratio of 2:2:1. Forty-eight hours later, lentivirus was collected, and cells were transduced by spin infection at 1800 g for 1.5 hours at 32°C. Knockdown of GRP94 in insulinoma βTC3 cells was achieved by two to three rounds of shRNA (against position 119 of GRP94) transduction on consecutive days. On day 8, knockdown (KD) efficiency was determined by intracellular staining or Western blotting for GRP94 using anti-GRP94 9G-10 antibody.
Western blot
Total membrane proteins were prepared using the Mem-Per plus membrane protein extraction kit (89842; Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions. Total proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to polyvinylidine difluoride membranes, and incubated with primary antibodies against GRP94 (ADI-SPA-850-F; Enzo Life Sciences, Farmingdale, NY), GRP78 (sc-13968; Santa Cruz Biotechnology, Santa Cruz, CA), Glut2 (NBP2-22218; Novus, Littleton, CO), and β-actin (SC-47778; Santa Cruz Biotechnology), and followed by the horseradish peroxidase–conjugated secondary antibody (Cell Signaling Technology, Danvers, MA). Signals were visualized using an ECL detection kit (34096; Thermo Fisher Scientific). Relative expression of genes was quantified using ImageJ software (National Institutes of Health).
Real-time polymerase chain reaction analysis
RNA was extracted from pancreas tissues and reverse transcribed into complementary DNA using a real-time polymerase chain reaction (RT-PCR) kit. Advanced Universal SYBR Green Supermix was used in a quantitative RT-PCR in a CFX384 RT-PCR Detection System (Bio-Rad, Hercules, CA) to determine messenger RNA (mRNA) expression levels of Pdx1, Nkx6.1, Nkx.2.2, NeuroD1, Mafa, Ngn3, insulin, glucagon, somatostatin, Ins1, Ins2, and Glut2. Fold changes in gene expression normalized to β-actin or Pdx1 expression were plotted and compared between groups.
Intraperitoneal glucose tolerance test and insulin assay
Intraperitoneal glucose tolerance tests (IPGTTs) were performed on overnight fasted animals by injecting glucose (2 mg/kg) as described previously (32). Plasma insulin levels were measured using a mouse insulin enzyme-linked immunosorbent assay kit (80-INSMS-E01; ALPCO Diagnostics, Salem, NH).
Electron microscopy
Pancreases were pelleted and fixed in 2% phosphate-buffered glutaraldehyde and 2% aqueous osmium tetroxide (Sigma, St. Louis, MO) and then dehydrated in 50% to 100% ethanol. The dehydrant was removed using the intermediate fluid, propylene oxide (20401; EMS, Hatfield, PA). The pellets were infiltrated with a 1:1 solution of propylene oxide and EMbed 812 (EMS). The infiltration was continued using a 1:2 solution of propylene oxide and EMbed 812 overnight. The pellets were embedded in EMbed 812 the following day and polymerized in a 60°C oven for 48 hours. Preliminary 0.5-μm sections were cut, stained with toluidine blue (22050; EMS), and examined under a light microscope.
Immunohistochemistry, immunofluorescence, and terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling assay
Pancreatic tissues were processed as previously described (33). In brief, mouse pancreases were dissected and fixed in 4% formaldehyde at 4°C for 12 hours before embedding in paraffin. Mouse pancreas 5-μm sections were deparaffinized, rehydrated, and incubated overnight at 4°C with anti-insulin (Thermo Scientific, Waltham, MA), glucagon (Abcam, Cambridge, MA), somatostatin (Abcam), Pdx1 (Abcam), E-cadherin (BD Biosciences, San Jose, CA), phospho-histone H3 (pHH3) (EMD Millipore, Burlington, MA), GRP94 (Enzo), and Amylase (Sigma) antibodies followed by fluorescein isothiocyanate–, cyanine dye 3–, or cyanine dye 5–conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). Slides were mounted with Vectashield with 4′6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). β-Cell apoptosis for mouse sections or primary islets cultured on ECM dishes was analyzed by the terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) technique according to the manufacturer’s instructions (In Situ Cell Death Detection Kit, TMR red; Roche, Indianapolis, IN) and double stained for Pdx1. Fluorescence was analyzed using a Zeiss Axio Imager M2 microscope (Carl Zeiss, Inc., Oberkochen, Germany) microscope and images were acquired using ImageJ. The frequency of TUNEL was presented as the percentage of stained cells among the Pdx1+ cells. Pancreas area quantitative analyses were performed by an Olympus BX40 on images taken by an Olympus microscopy image system (Olympus, Waltham, MA).
Morphometric analysis of islet/β-cell mass
To measure β- and α-cell mass, pancreases were removed from mice, weighed, embedded in paraffin, and then sectioned continuously. Sections were collected at 200-μm intervals throughout the entire pancreas. About 10 sections were collected and stained for each antibody. Immunostaining of insulin+ (β) and glucagon+ (α) cells was performed using the anti-insulin and antiglucagon antibodies, followed by fluorescein-conjugated secondary antibodies (34). Pancreatic tissue area and insulin- or glucagon-positive area were determined by computer-assisted measurements using a Zeiss Axio Imager M2 microscope (Carl Zeiss, Inc.), and images were acquired using ImageJ software. The number of islets (insulin-positive aggregates ≥25 µm in diameter) were scored and used to calculate islet density (number of islets per square centimeter of tissue). Mean percentage of β-cell fraction per pancreas was calculated as the ratio of insulin-positive to whole pancreatic tissue area. β-Cell mass was obtained by multiplying the ratio of total insulin-positive area to total pancreatic area with the pancreas weight. α-Cell mass was obtained by multiplying the ratio of total glucagon-positive area to total pancreatic area with the pancreas weight. Morphometric β-cell and islet characterizations were obtained from analyses of at least 100 islets per mouse.
X-gal staining
Embryos were fixed in 4% paraformaldehyde for 1 hour. After washing with phosphate-buffered saline, the embryos were immersed in permeabilization buffer (0.02% deoxycholate, 0.01% NP-40 in phosphate-buffered saline). Embryos were stained with X-gal solution as described (31).
Statistical analyses
Results are expressed as the mean ± standard deviation of the mean of multiple independent experiments, as indicated in figure legends. Statistical analyses were carried out by two-tailed Student t test or analysis of variance. P < 0.05 was denoted as significant.
Results
Generation of GRP94 conditional KO mice in which the GRP94 gene was deleted in Pdx1+ cells
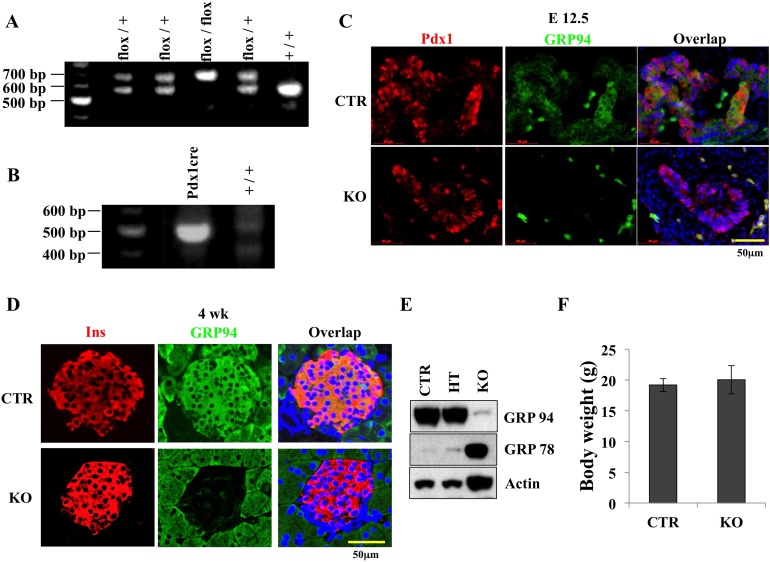
To assess the role of GRP94 in pancreatic β-cell development, we generated pancreatic progenitor cell-specific GRP94 KO mice (Hsp90b1 flox/flox;Pdx1Cre) by breeding a Pdx1Cre transgenic mouse strain with the published GRP94flox/flox mice (22, 29) (Fig. 1A and 1B). Ablation of the GRP94 gene was confirmed by immunofluorescent assays in Pdx1+ cells at embryonic day (E) 12.5 (Fig. 1C) and in β cells in 4-week-old pancreases (Fig. 1D). Western blot (WB) analysis showed that islets from KO mice had only about 5% as much GRP94 protein expression as control [CTR (Pdx1Cre;GRP94+/+)] islets (Fig. 1E). It is likely that the remnant GRP94 protein was derived from incomplete Hsp90b1 gene deletion in pancreatic cells or from non–β cells in the islets.
Figure 1.
Generation of GRP94 conditional KO mice. (A) Identification of GRP94 genotypes of mice used in this study using primers specific for GRP94. GRP94 conditional KO (Pdx1Cre;GRP94flox/flox), conditional heterozygote (HT) (Pdx1Cre;GRP94flox/+), and CTR (Pdx1Cre;GRP94+/+). (B) Identification of Cre recombinase genotype using specific primers. (C) Immunohistochemical staining of GRP94 (green) and Pdx1 (red) in KO and CTR mice at E12.5 (bottom). Scale bar = 50 μm. (D) Immunohistochemical staining of GRP94 (green) and insulin (Ins) (red) in KO and CTR in 4-week-old mice. Nuclei are stained blue. Scale bar = 50 μm. (E) WB analysis of GRP94 protein expression in islets harvested from KO mice. (F) Body weights of CTR and KO mice at 8 weeks of age (n = 4 in each group).
All conditional KO mice were viable and fertile. KO mice had similar body weights as CTR and heterozygote (Pdx1Cre;GRP94flox/+) littermates (Fig. 1F). At 8 weeks of age, <10% of KO mice showed higher fasting blood glucose (∼200 mg/dL) compared with CTRs (average 100 mg/dL; data not shown). At 18 weeks of age, three of 35 male KO mice were hyperglycemic (blood glucose >300 mg/dL). Heterozygote mice exhibited similar GRP94 expression and similar phenotypes as controls; therefore, only CTR mice were used as controls in the subsequent studies.
Ablation of GRP94 in Pdx1+ cells causes pancreatic hypoplasia with reduced β-cell numbers during embryonic development
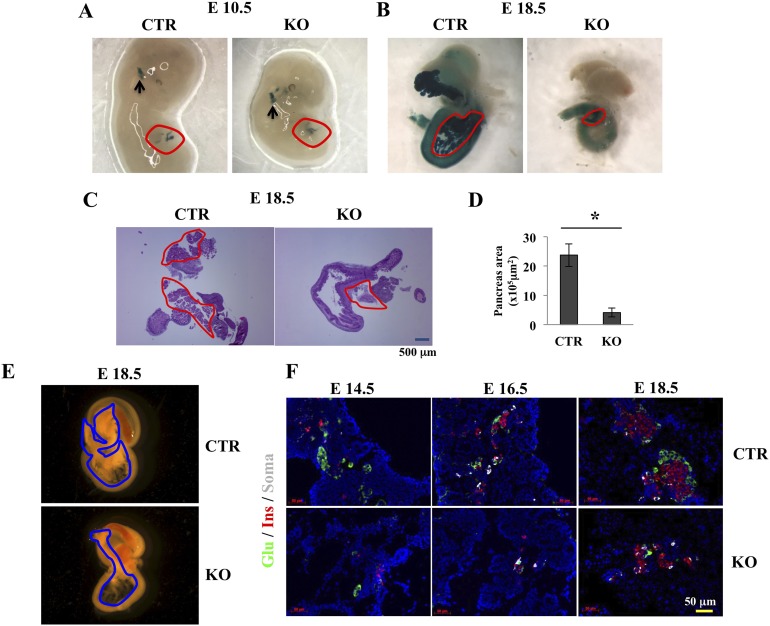
To determine the impact of GRP94/Hsp90b1 inactivation in pancreatic development, pancreases from Pdx1Cre;GRP94+/+;R26R reporter mice and Pdx1Cre;GRP94flox/flox;R26R KO mice were collected at E10.5, E12.5, and E18.5. X-gal staining of whole embryo or pancreas together with intestine was performed to help visualize the pancreatic tissue. We observed uniform X-gal labeling in the pancreatic epithelium, indicating high efficiency of the Pdx1-driven Cre recombination (Fig. 2A and 2B), although we cannot conclude whether the recombination was completed or not by only LacZ staining. We further performed immunohistochemical staining for GRP94 and amylase in pancreatic tissue sections collected at E16.5 from CTR or GRP94 KO mice. GRP94 expression was observed in some acinar cells in the KO mice (Supplemental Fig. 1 (4.7MB, tif) ), suggesting incomplete deletion of GRP94 in acinar cells.
Figure 2.
Deletion of GRP94 in Pdx1+ cells leads to pancreas hypoplasia and reduced β-cell numbers during embryonic development. (A, B) Expression of LacZ (dark blue) in CTR and KO embryos at (A) E10.5 and (B) E18.5. Circled areas in A and B indicate pancreatic tissues, and black arrows in A point to LacZ expression in brain. (C) Hematoxylin and eosin staining of pancreas sections from CTR and KO mice. (D) Quantification of pancreatic area in CTR and KO mice at day 18.5. (E) Micrographs of pancreases and connected intestine from CTR and KO mice. (F) Representative immunohistochemical staining of glucagon (Glu) for α cells (green), insulin (Ins) for β cells (red), and somatostatin (Soma) for δ cells (gray) in pancreases from CTR and KO mice at E14.5, E16.5, and E18.5. Nuclei are stained blue. Scale bar = 50 μm. *P < 0.05, Student t test.
No differences in pancreas size were observed at E10.5 (Fig. 2A) or at E12.5 between pancreases from CTR and KO mice (not shown). In contrast, markedly smaller pancreases were observed at both E16.5 (not shown) and E18.5 in KO mice compared with CTR mice (Fig. 2B, 2C, and 2E). A normal pancreas includes the ventral and dorsal lobes. However, in the KO mice, the two parts were often indistinguishable, and the pancreatic area was significantly reduced in the KO mice at E18.5 (Fig. 2B, 2C, and 2E). These results indicate that GRP94 was required for pancreas development during the embryonic stage. Of note, as observed in other Pdx1Cre transgenic mice (35), X-gal staining was also observed in brain tissues of both CTR and KO mice because they both carry the cre recombinase transgene (Fig. 2A).
To further assess the role of GRP94 on endocrine cell development, we investigated the numbers of α, β, and δ cells in pancreases of CTR and KO mice at E14.5, E16.5, and E18.5 in serial pancreatic sections. Immunofluorescence staining of different endocrine cell markers targeting insulin (β cells), somatostatin (δ cells), and glucagon (α cells) showed a dramatic difference in the distribution patterns of endocrine cells between CTR and KO mice as early as E14.5. The differences were more pronounced at later time points (E16.5 and E18.5) as reduced numbers of α, β, and δ were observed in KO mice (Fig. 2F). At E18.5, α and β cells in the CTR pancreas had migrated and formed islets of Langerhans, as represented by a typical structure in which insulin-positive β cells cluster in the core with glucagon-positive α cells at the periphery. By contrast, α and β cells remained scattered in the KO pancreas throughout development (Fig. 2F). Taken together, these results suggest that GRP94 deletion during embryonic development led to reduced numbers of endocrine cells and disrupted islet structure.
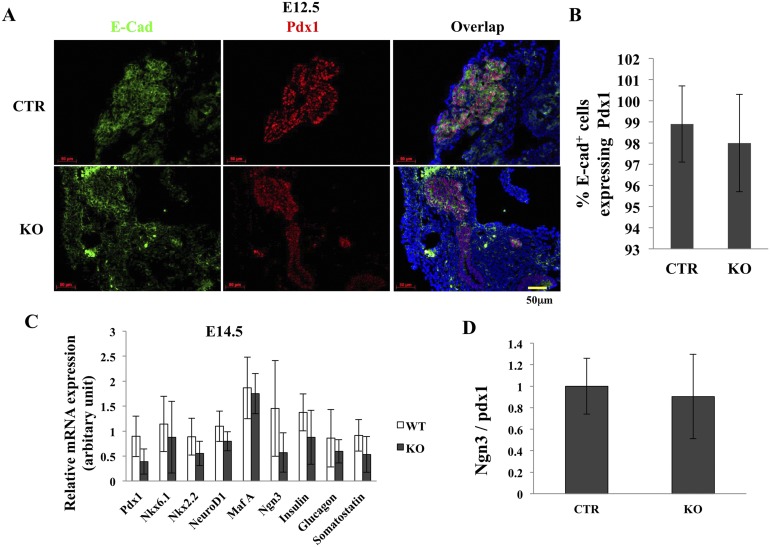
Impact of GRP94 depletion in Pdx1+ cells at E12.5
The presence of appropriate numbers of progenitor cells at the appropriate time is critical for pancreas development and formation of sufficient β-cell mass (36). Pdx1+ progenitor cells appear at E8.5 to E12.5. After that, Pdx1 expression becomes higher in the endocrine lineage and lower in the exocrine lineage. To determine the underlying cause of pancreatic hypoplasia in GRP94KO mice, we counted the number of Pdx1+ cells in pancreases from CTR and KO mice at E12.5. Immunohistochemical analysis using anti-Pdx1 and anti–E-cadherin antibodies indicated that cells from KO and CTR mice exhibited normal expression patterns and had similar percentages of Pdx1+ cells among pancreatic cells (Fig. 3A and 3B). Furthermore, pancreas from E14.5 (Fig. 3C) and E16.5 (data not shown) KO and CTR mice exhibited a similar amount of mRNA expression of the transcription factors Pdx1, Nkx6.1, Nkx2.2, NeuroD1, Ngn3, MafA, insulin, glucagon, and somatostatin. In addition, KO and CTR mice exhibited no differences in the Ngn3/Pdx1 mRNA ratio (Fig. 3D), suggesting that loss of GRP94 did not affect the ability of Pdx1+ cells to express these markers of endocrine differentiation. Because mutant buds may have more mesenchyme tissue, we also used Pdx1 expression as an endogenous marker to compare expression patterns of the above transcriptional factors. Again, no differences in gene expression have been observed (data not shown). It thus seems that GRP94 did not alter the differentiation behaviors of the progenitors.
Figure 3.
Pancreas endocrine differentiation in CTR and KO mice. (A) Immunohistochemical staining of pancreas from CTR and KO mice using anti–E-cadherin (green) and anti-Pdx1 (red) antibodies. Nuclei are stained blue. Scale bar = 50 μm. (B) Percentages of Pdx1+ cells/E-cadherin+ cells in pancreas from CTR and KO mice. (C) Quantitative RT-PCR analysis of expression of endocrine differentiation-related genes in CTR and KO mice at E14.5. (D) Quantitative RT-PCR analysis of Ngn3/Pdx1 mRNA ratio in CTR and KO mice at E14.5. Data in (C) and (D) were normalized to β-actin expression. Scale bar = 50 μm. At least four embryos from each genotype were analyzed.
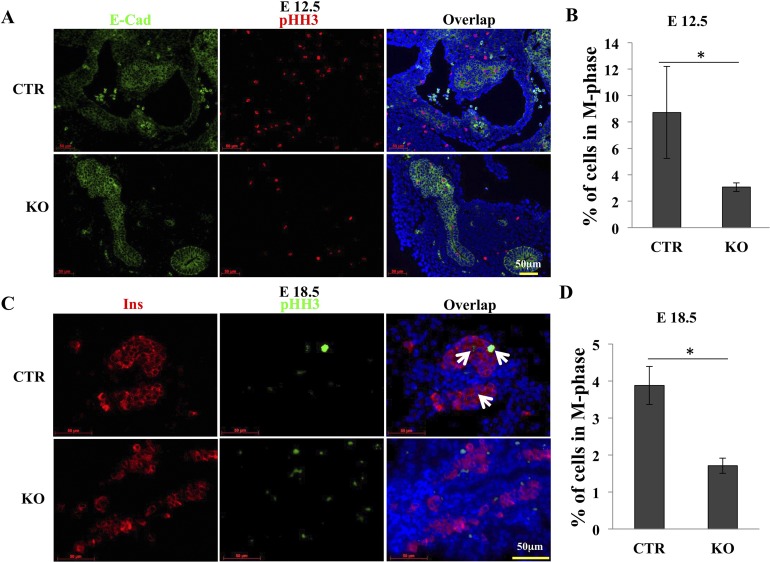
Depletion of GRP94 reduces cell proliferation
To determine the mechanistic effects of GRP94, we measured cell proliferation in CTR and KO islets. The percentage of mitotic cells in Pdx1+ cells was identified by costaining with pHH3 in pancreas tissue sections. At E12.5, we found an average of 65% reduction of E-cad+pHH3+ cells in KO pancreas compared with CTR pancreas (Fig. 4A and 4B), indicating that GRP94 expression was needed for proliferation of pancreatic progenitor cells. Furthermore, at E18.5, we found a 56% reduction in Ins+pHH3+cells in KO pancreas compared with CTR pancreas (Fig. 4C and 4D), indicating that GRP94 expression was needed for pancreatic β-cell proliferation.
Figure 4.
Deletion of GRP94 reduces proliferation of Pdx1+ cells at E12.5. (A) Representative immunohistochemical staining of pancreases from CTR (n = 4) and KO (n = 5) embryos at E12.5 using anti–E-cadherin (green) and anti-pHH3 (red) antibodies. Nuclei are stained blue. Scale bar = 50 μm. (B) Percentage of pHH3+ cells in Pdx1+ cells. (C) Representative immunohistochemical staining of pancreases from CTR (n = 4) and KO (n = 4) embryos at E18.5 using anti-insulin (Ins; red) and anti-pHH3 (green) antibodies. Nuclei are stained blue. Arrows point to pHH3+Ins+ cells. Scale bar = 50 μm. (D) Percentage of pHH3+ cells in Ins+ cells.
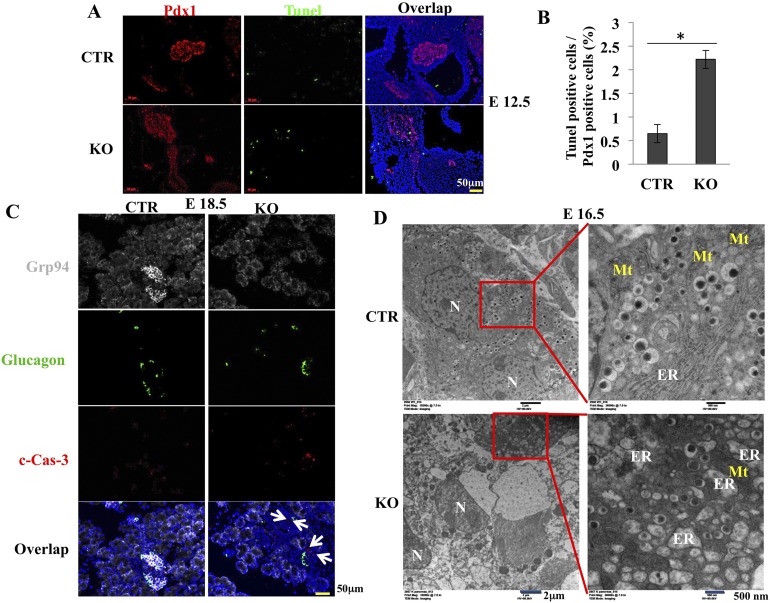
Increased apoptosis may also contribute to reduced β-cell mass. We measured apoptosis of Pdx1+ cells using the TUNEL assay. There was an average 3.4-fold increase in TUNEL+ apoptotic cells among Pdx1+ cells in KO pancreas compared with CTR pancreas at E12.5 (Fig. 5A and 5B). Cell death was further confirmed by immunohistochemical staining of cleaved caspase-3 on frozen sections of E18.5 embryos and in pancreas at 4 weeks after birth. We found significantly increased cleaved caspase-3+ cells in islets but not in glucagon+ cells at E18.5 (Fig. 5C) and 4 weeks after birth (Supplemental Fig. 2 (4.5MB, tif) ). Pancreatic β-cell ultrastructure was further examined by transmission electronic microscopy. In developing pancreas, insulin-producing cells emerge at E10.5 (37). By E16.5, most CTR β cells have accumulated large numbers of insulin granules. We next compared the morphology of ER by using method described by Tao et al. (38). In contrast to the normal cisternae structure of the rough ER in CTR β cells, distended ER was observed in most β cells in the KO mice at E12.5 (data not shown). By E16.5, extremely distended ER was observed in most KO β cells (Fig. 5D). Collectively, these results suggest that both decreased proliferation and increased apoptosis contributed to the reduction of pancreatic progenitor cells and mature β cells in the GRP94 KO pancreas.
Figure 5.
Deletion of GRP94 leads to more cell death and ER structural changes in KO mice compared with CTR. (A) Immunohistochemical staining and (B) quantification of TUNEL+ (green) cells among Pdx1+ (red) cells in embryos from CTR (n = 4) and KO (n = 5) mice at E12.5. Nuclei are stained blue. Scale bar = 50 μm. (C) Cleaved caspase-3 (c-Cas-3) staining in pancreatic islets from CTR (n =3) and KO (n = 3) mice at E18.5. GRP94 (gray), glucagon (green), and c-Cas-3 (red). Nuclei are stained blue. White arrows point to c-Cas-3+ cells. Scale bar = 50 μm. (D) Transmission electron microscopy of pancreatic β cells from CTR and KO mice at E16.5. Islets are identified by the presence of insulin-containing granules with a typical electron-dense core, readily seen at higher magnification (right panels). Mt, mitochondria; N, nucleus. Scale bar: left = 2 μm and right = 500 nm. *P < 0.05, Student t test.
GRP94 KO mice display reduced pancreatic β-cell mass at 4 weeks after birth
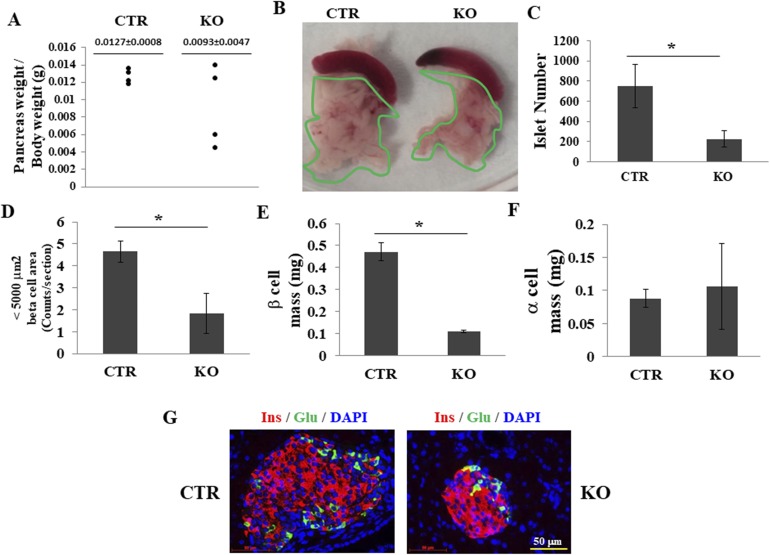
A dramatic expansion of the endocrine pancreas is initiated before birth and lasts until 2 to 3 weeks (39) or even a month after birth (40). We tested whether depletion of GRP94 affected pancreas size and pancreatic β-cell mass after birth. At 4 weeks of age, no significant difference in pancreas weight/body weight was observed between CTR and KO mice (Fig. 6A and 6B). However, islet numbers (Fig. 6C) and the numbers of small islets (determined from β-cell area) (Fig. 6D) were both dramatically decreased in the KO mice. Next, we measured β- and α-cell mass in serial pancreatic sections to determine whether deletion of GRP94 affected the phenotype and architecture of the islet clusters in the KO mice (Fig. 6G). Broad morphometric measurements showed that β-cell mass was reduced by ∼75% in the GRP94 KO mice (Fig. 6E), whereas α-cell mass was unaffected (Fig. 6F), suggesting that ablation of GRP94 further reduced pancreatic β-cell mass after birth.
Figure 6.
Deletion of GRP94 leads to reduced pancreatic β-cell mass at 4 weeks of age. (A) Pancreas weight/body weight in CTR (n = 4) and KO (n = 4) mice. (B) Images of pancreases from CTR and KO mice. (C) Total islet number from CTR and KO mice. (D) Numbers of small islets (<5000 μm2) in CTR and KO mice. (E) β-Cell mass in CTR and KO mice. (F) α-Cell mass in CTR and KO mice. At least five mice in each group were analyzed. *P < 0.05, Student t test. (G) Representative fluorescent images of pancreatic sections from 4-week-old CTR and KO mice stained for glucagon (green) and insulin (red).
GRP94 KO show impaired glucose disposal after IPGTT
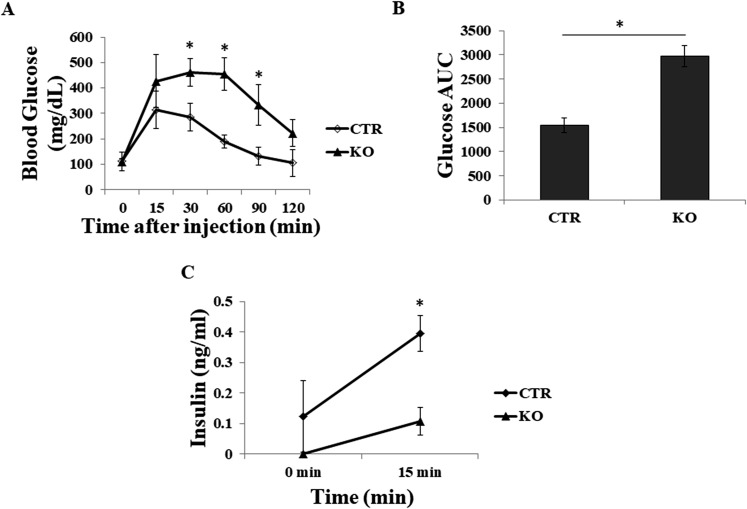
We further assessed the effects of GRP94 depletion on β-cell function using IPGTT in KO and CTR mice at 8 weeks of age. Compared with CTR mice, KO mice exhibited impaired glucose disposal as manifested by markedly elevated blood glucose levels at most time points measured (Fig. 7A) and increased area under the curve (Fig. 7B). KO mice also exhibited reduced plasma insulin levels before and at 15 minutes after glucose challenge (Fig. 7C). These data provide evidence that deletion of GRP94 led to impaired islet insulin secretion and function in the KO mice.
Figure 7.
GRP94 KO mice exhibit impaired glucose tolerance during IPGTT. (A) IPGTT of CTR (n = 5) and KO (n = 5) mice. (B) Areas under the curve (AUC) after glucose injection in CTR and KO mice. (C) Serum insulin levels before (0 minutes) and 15 minutes after glucose injection. *P < 0.05, Student t test. DAPI, 4′6-diamidino-2-phenylindole; Glu, glucagon; Ins, insulin.
GRP94 in β-cell function
Despite the significant reduction in islet numbers (Fig. 6C), adult KO mice showed normal fasting glucose levels or response during the glucose tolerance test (Fig. 7A and 7B). These results were not surprising in consideration of the fact that, in most rodent models, overt diabetes occurs only upon loss of >80% of the total pancreatic β-cell number (41). To further determine the role of GRP94 in insulin secretion in β cells, we generated GRP94 KD cells using an insulinoma cell line, βTC3, by transfection with shRNA for GRP94. Control cells were infected with scrambled shRNA. We found that GRP94 KD cells showed increased insulin content (Supplemental Fig. 3A (2.1MB, tif) ) as well as more basal and stimulated insulin secretion (Supplemental Fig. 3B (2.1MB, tif) ) after treatment with high glucose (16.7 mM). RT-PCR analysis showed that GRP94 KD cells had increased mRNA expression of Ins1, Ins2, and Glut2 (Supplemental Fig. 3C and 3D (2.1MB, tif) ). Increased Glut2 protein expression was further confirmed in membrane and cytosolic fractions of GRP94 KD cells by WB analysis (Supplemental Fig. 3E (2.1MB, tif) ). These data suggest that GRP94 is essential in regulating β-cell function.
Discussion
Insufficient β-cell mass and β-cell dysfunction contribute to diabetes (42). Genetic studies suggest that most genes associated with increased susceptibility to diabetes are also involved in the regulation of β-cell growth and function during embryonic and fetal periods. GRP94 is one of the most abundant proteins in pancreas and islets, yet its roles in pancreatic β-cell development, survival, and function remain largely unknown. Here, we successfully depleted the GRP94 gene in pancreatic progenitor cells and mature β cells using Pdx1Cre and GRP94 floxed transgenic mice. We found that GRP94 is essential for pancreatic β-cell development, proliferation, and function. Deletion of GRP94 led to pancreatic hypotrophy during the embryonic stage, reduced β-cell mass, and impaired insulin secretion after birth. Our studies suggest that reduced cell proliferation and increased apoptosis might have contributed to defects in β-cell development in the GRP94 conditional KO mice. Our results document a novel and essential role of GRP94 in pancreatic β cells and identify GRP94 deficiency as a contributing factor in the pathogenesis of diabetes. Our findings suggest that a strategy to restore GRP94 function may be of therapeutic benefit in diabetes.
Development of mouse pancreas starts as two epithelial buds appearing on the ventral and dorsal surface of the posterior foregut endoderm at E8.5 to E9.0 (43). The buds then form a ductal tree consisting mainly of epithelial or pancreatic cords. At E13.5 to E14.5, a secondary transition includes intensive epithelial cell proliferation and initiation of differentiation. By E16.5, exocrine acinar cells separate from the central ducts, and endocrine cells begin to cluster as isletlike structures. Islet cells continue to proliferate and to undergo remodeling until 3 weeks after birth. Pdx1 is expressed in a population of cells in the pancreatic endoderm, and Pdx1+ cells give rise to the entire pancreas, including exocrine, endocrine, and duct cells (44). When Cre-mediated recombination is driven by the Pdx1 promoter during embryonic development, all endodermal lineages of the developing pancreas, including acinar cells, duct cells, β cells, and other endocrine cells, are affected, but after birth, Pdx1-driven Cre-mediated recombination is restricted to β cells (45). Although Pdx1+ cells appear in pancreas from E8.5 to E9.5 (46), we did not observe dramatic changes in pancreas sizes at E10.5 or E12.5 in the KO mice compared with controls, and significant pancreatic hypoplasia was observed only until E18.5. However, defects in pancreas development in the KO mice might have been initiated as early as E8.5 when Pdx1+ progenitor cells first appeared in the endoderm, eventually leading to a 75% reduction in pancreas volume at E18.5. Although deletion is widespread at E12.5, the acinar cells surrounding the adult islets are GRP94 positive, indicating incomplete recombination within the exocrine lineage. These results are consistent with a previous publication studying the role of another ER homeostasis regulator, X-box binding protein 1, in which X-box binding protein 1 from adult acinar cells caused their rapid death, followed by complete repopulation by cells that did not undergo deletion (47), and similar phenomena were observed in the pancreas-specific deletion of β-catenin with Pdx1-cre mice (48). It is reasonable to speculate that GRP94 is more important for the exocrine than the endocrine cells; therefore, exocrine cells are rapidly replaced by nondeleted cells. However, further studies out of the scope of this study are needed to test this hypothesis.
In contrast, GRP94 was depleted in most of the β cells and therefore led to increased β-cell death and reduced β-cell mass during the embryonic stages as well as after birth. However, because islets/β cells comprise only 1% of the pancreas volume (49), with other cell types within the pancreas still expressing GRP94 and surviving, no differences on total pancreas weights would be observed at 4 weeks after birth between CTR and KO mice. Therefore, our observation of pancreatic hypoplasia during mouse embryonic development suggests that GRP94 is required for the development of all endodermal lineages during the embryonic period. Mice can lose up to 80% of islet mass and still remain normoglycemic (41), which may explain why GRP94 KO mice that had lost >70% of their β-cell mass at 4 weeks of age remained normoglycemic at 8 weeks of age, although they showed impaired glucose tolerance and reduced insulin secretion when challenged by IPGTT.
Although we observed increased insulin secretion in GRP94 KD β cells compared with wild-type cells in vitro, GRP94 KO mice at 8 weeks of age showed reduced insulin secretion after stimulation with glucose. The discrepancy may be caused by reduced β-cell mass in the KO mice: even GRP94 KO β cells secrete more insulin on a per cell basis, and the total amount of insulin secretion was still low in GRP94 KO mice at 8 weeks of age when a dramatic loss of β-cell mass occurred. Unfortunately, we could not obtain a sufficient number of islets from the adult GRP94 KO mice to measure insulin secretion or content to confirm it.
It is worth noting that Cre gene expression is not only found in pancreatic β cells but also seen in nutrient-sensing neurons in the Pdx1Cre mice (35). Therefore, there is a possibility that GRP94 deletion in Pdx1+ neurons also may have affected pancreatic and β-cell development in our study. However, because β-cell differentiation starts during embryonic development and lasts until weeks after birth (50), our results could not distinguish the relative importance of GRP94 to β-cell growth before or after birth. This question might be addressed using tamoxifen-inducible conditional KO mice in which GRP94 can be depleted in terminally differentiated β cells.
There are other limitations with our study. For example, because Pdx1 expression levels showed dynamic changes in the differentiation of each cell type, it is hard to compare our results with studies using other Pdx1Cre mice. Further studies using different Cre lines may be needed to better interpret the phenotype observed (44, 45, 51, 52). For example, Ins1creERT mice could be used to generate Ins1creGRP94 KO mice (53). The Ins1creERT mouse model permits gene depletion to be induced by tamoxifen administration specifically in pancreatic β cells. Gene depletion can be achieved in up to 92% of β cells by treatment with 200 mg/kg tamoxifen (31, 53). We are currently pursuing further studies using this mouse model to more fully elucidate the role of GRP94 on β-cell growth and function.
As reported in other studies, compensatory mechanisms might exist in GRP94-depleted cells. For example, in some circumstances, the other ER stress protein, GRP78, has been shown to partially compensate for loss of GRP94 (15, 54). We have found that expression of the GRP78 protein was increased in GRP94 KO islets (not shown), which suggests the possibility that GRP78 might have compensated for the loss of GRP94 in Pdx1+ cells. If true, remnant β cells would likely have persisted within the pancreas in KO mice long after GRP94 depletion.
GRP94 functions mainly through particular clients depending on cell and tissue types (19, 26–28, 55). This may be true for pancreatic β cells as well. That is to say, GRP94 might modulate maturation of membrane or secretory “clients” in β cells that are critical for β-cell survival and function. However, there is no evidence regarding the clients through which GRP94 exerts its function in β cells. Potential targets are integrins, IGF-I, and IGF-II, as observed in the pancreas or other tissues (19, 55, 56). Further studies are needed to answer this question.
Acknowledgments
We thank Drs. Charles Murtaugh and Michael Kern for technical support.
Financial Support: This work was made possible by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grants DK097544, DK099696, and 1R01DK105183 (to H. Wang) and DK107412 (to H. Wu).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog No. | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|
| GRP94 | Grp94 monoclonal antibody (9G10) | Enzo Life Sciences, ADI-SPA-850-F | Rat; monoclonal | 1:2000 (WB), 1:100 (IHC) | AB_777179 |
| PDX1 | Anti-PDX1 antibody | Abcam, ab47267 | Rabbit; polyclonal | 1:2000 (IHC) | AB_777179 |
| Insulin | Insulin antibody | Thermo Fisher Scientific, PA1-26938 | Guinea pig; polyclonal antibody | 1:100 (IHC) | AB_794668 |
| HSPA5 | GRP 78 (H-129) antibody | Santa Cruz Biotechnology, sc-13968 | Rabbit; polyclonal | 1:1000 (WB) | AB_2119991 |
| Glucagon | Mouse antiglucagon antibody | Abcam, ab82270 | Mouse; monoclonal | 1:2000 (IHC) | AB_1658481 |
| Somatostatin | Rabbit antisomatostatin antibody | Abcam, ab64053 | Rabbit; polyclonal | 1:300 (IHC) | AB_1143012 |
| β-Actin | β-Actin antibody (C4) | Santa Cruz Biotechnology, SC-47778 | Mouse; monoclonal | 1:2000 (IHC) | AB_2714189 |
| E-cadherin | Purified mouse anti–E-cadherin antibody | BD Bioscience, 610181 | Mouse; monoclonal | 1:100 (IHC) | AB_397580 |
| Histone H3 | Rabbit anti–histone H3, phospho (Ser10) antibody | Cell Signaling Technology, 3377s | Rabbit; monoclonal | 1:200 (IHC) | AB_1549592 |
| Cleaved caspase-3 (Asp175) | Cleaved caspase-3 (Asp175) antibody | Cell Signaling Technology, 9661s | Rabbit; polyclonal | 1:200 (IHC) | AB_2341188 |
| Glut2 | Glucose transporter type 2 antibody | Novus, NBP2-22218 | Rabbit; polyclonal | 1:1000 (WB) | AB_2335858 |
Abbreviations: IHC, immunohistochemistry; RRID, Research Resource Identifier.
Footnotes
- CTR
- control
- E
- embryonic day
- ER
- endoplasmic reticulum
- GRP
- glucose-regulated protein
- HSP
- heat shock protein
- IGF
- insulinlike growth factor
- IPGTT
- intraperitoneal glucose tolerance test
- KD
- knockdown
- KO
- knockout
- mRNA
- messenger RNA
- Pdx1
- pancreatic and duodenal homeobox 1
- pHH3
- phospho-histone H3
- RT-PCR
- real-time polymerase chain reaction
- shRNA
- short hairpin RNA
- T2D
- type 2 diabetes
- TUNEL
- terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling
- UPR
- unfolded protein response
- WB
- Western blot.
References
- 1.Weir GC, Bonner-Weir S. Islet β cell mass in diabetes and how it relates to function, birth, and death. Ann N Y Acad Sci. 2013;1281(1):92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham KL, Sutherland RM, Mannering SI, Zhao Y, Chee J, Krishnamurthy B, Thomas HE, Lew AM, Kay TW. Pathogenic mechanisms in type 1 diabetes: the islet is both target and driver of disease. Rev Diabet Stud. 2012;9(4):148–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, Reinecke M. Mechanisms of beta-cell death in type 2 diabetes. Diabetes. 2005;54(Suppl 2):S108–S113. [DOI] [PubMed] [Google Scholar]
- 4.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445(7130):881–885. [DOI] [PubMed] [Google Scholar]
- 5.Stankov K, Benc D, Draskovic D. Genetic and epigenetic factors in etiology of diabetes mellitus type 1. Pediatrics. 2013;132(6):1112–1122. [DOI] [PubMed] [Google Scholar]
- 6.Prasad RB, Groop L. Genetics of type 2 diabetes-pitfalls and possibilities. Genes (Basel). 2015;6(4):87–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newbern D, Freemark M. Placental hormones and the control of maternal metabolism and fetal growth. Curr Opin Endocrinol Diabetes Obes. 2011;18(6):409–416. [DOI] [PubMed] [Google Scholar]
- 8.Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol Metab. 2011;22(7):266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165–1176. [DOI] [PubMed] [Google Scholar]
- 10.Lee AH, Heidtman K, Hotamisligil GS, Glimcher LH. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci USA. 2011;108(21):8885–8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eletto D, Dersh D, Argon Y. GRP94 in ER quality control and stress responses. Semin Cell Dev Biol. 2010;21(5):479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van PN, Peter F, Söling HD. Four intracisternal calcium-binding glycoproteins from rat liver microsomes with high affinity for calcium: no indication for calsequestrin-like proteins in inositol 1,4,5-trisphosphate-sensitive calcium sequestering rat liver vesicles. J Biol Chem. 1989;264(29):17494–17501. [PubMed] [Google Scholar]
- 13.Frey S, Leskovar A, Reinstein J, Buchner J. The ATPase cycle of the endoplasmic chaperone Grp94. J Biol Chem. 2007;282(49):35612–35620. [DOI] [PubMed] [Google Scholar]
- 14.Chen B, Zhong D, Monteiro A. Comparative genomics and evolution of the HSP90 family of genes across all kingdoms of organisms. BMC Genomics. 2006;7(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao C, Wang M, Luo B, Wey S, Dong D, Wesselschmidt R, Rawlings S, Lee AS. Targeted mutation of the mouse Grp94 gene disrupts development and perturbs endoplasmic reticulum stress signaling. PLoS One. 2010;5(5):e10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrimpe-Rutledge AC, Fontès G, Gritsenko MA, Norbeck AD, Anderson DJ, Waters KM, Adkins JN, Smith RD, Poitout V, Metz TO. Discovery of novel glucose-regulated proteins in isolated human pancreatic islets using LC-MS/MS-based proteomics. J Proteome Res. 2012;11(7):3520–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lansford B, Haas GG Jr, DeBault LE, Wolf DP. Effect of sperm-associated antibodies on the acrosomal status of human sperm. J Androl. 1990;11(6):532–538. [PubMed] [Google Scholar]
- 18.Melnick J, Dul JL, Argon Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994;370(6488):373–375. [DOI] [PubMed] [Google Scholar]
- 19.Wanderling S, Simen BB, Ostrovsky O, Ahmed NT, Vogen SM, Gidalevitz T, Argon Y. GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion. Mol Biol Cell. 2007;18(10):3764–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostrovsky O, Ahmed NT, Argon Y. The chaperone activity of GRP94 toward insulin-like growth factor II is necessary for the stress response to serum deprivation. Mol Biol Cell. 2009;20(6):1855–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S, Hong F, Gewirth D, Guo B, Liu B, Li Z. The molecular chaperone gp96/GRP94 interacts with Toll-like receptors and integrins via its C-terminal hydrophobic domain. J Biol Chem. 2012;287(9):6735–6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staron M, Yang Y, Liu B, Li J, Shen Y, Zúñiga-Pflücker JC, Aguila HL, Goldschneider I, Li Z. gp96, an endoplasmic reticulum master chaperone for integrins and Toll-like receptors, selectively regulates early T and B lymphopoiesis. Blood. 2009;115(12):2380–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maynard JC, Pham T, Zheng T, Jockheck-Clark A, Rankin HB, Newgard CB, Spana EP, Nicchitta CV. Gp93, the Drosophila GRP94 ortholog, is required for gut epithelial homeostasis and nutrient assimilation-coupled growth control. Dev Biol. 2010;339(2):295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baviskar SN, Shields MS. RNAi silenced Dd-grp94 (Dictyostelium discoideum glucose-regulated protein 94 kDa) cell lines in Dictyostelium exhibit marked reduction in growth rate and delay in development. Gene Expr. 2011;15(2):75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishiguro S, Watanabe Y, Ito N, Nonaka H, Takeda N, Sakai T, Kanaya H, Okada K. SHEPHERD is the Arabidopsis GRP94 responsible for the formation of functional CLAVATA proteins. EMBO J. 2002;21(5):898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staron M, Wu S, Hong F, Stojanovic A, Du X, Bona R, Liu B, Li Z. Heat-shock protein gp96/grp94 is an essential chaperone for the platelet glycoprotein Ib-IX-V complex. Blood. 2011;117(26):7136–7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randow F, Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol. 2001;3(10):891–896. [DOI] [PubMed] [Google Scholar]
- 28.Liu B, Li Z. Endoplasmic reticulum HSP90b1 (gp96, grp94) optimizes B-cell function via chaperoning integrin and TLR but not immunoglobulin. Blood. 2008;112(4):1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrançois L, Li Z. Heat shock protein gp96 is a master chaperone for Toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26(2):215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21(1):70–71. [DOI] [PubMed] [Google Scholar]
- 31.Tamarina NA, Roe MW, Philipson L. Characterization of mice expressing Ins1 gene promoter driven CreERT recombinase for conditional gene deletion in pancreatic β-cells. Islets. 2014;6(1):e27685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Sun Z, Gou W, Adams DB, Cui W, Morgan KA, Strange C, Wang H. α-1 antitrypsin enhances islet engraftment by suppression of instant blood-mediated inflammatory reaction. Diabetes. 2017;66(4):970–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shu L, Sauter NS, Schulthess FT, Matveyenko AV, Oberholzer J, Maedler K. Transcription factor 7-like 2 regulates beta-cell survival and function in human pancreatic islets. Diabetes. 2007;57(3):645–653. [DOI] [PubMed] [Google Scholar]
- 34.Gu Y, Lindner J, Kumar A, Yuan W, Magnuson MA. Rictor/mTORC2 is essential for maintaining a balance between beta-cell proliferation and cell size. Diabetes. 2011;60(3):827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song J, Xu Y, Hu X, Choi B, Tong Q. Brain expression of Cre recombinase driven by pancreas-specific promoters. Genesis. 2010;48(11):628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murtaugh LC. Pancreas and beta-cell development: from the actual to the possible. Development. 2006;134(3):427–438. [DOI] [PubMed] [Google Scholar]
- 37.Teitelman G, Alpert S, Polak JM, Martinez A, Hanahan D. Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development. 1993;118(4):1031–1039. [DOI] [PubMed] [Google Scholar]
- 38.Tao J, Zhu M, Wang H, Afelik S, Vasievich MP, Chen XW, Zhu G, Jensen J, Ginsburg D, Zhang B. SEC23B is required for the maintenance of murine professional secretory tissues. Proc Natl Acad Sci USA. 2012;109(29):E2001–E2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes. 1995;44(3):249–256. [DOI] [PubMed] [Google Scholar]
- 40.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997;138(4):1736–1741. [DOI] [PubMed] [Google Scholar]
- 41.Bonner-Weir S, Trent DF, Weir GC. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983;71(6):1544–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho JH, Kim JW, Shin JA, Shin J, Yoon KH. β-Cell mass in people with type 2 diabetes. J Diabetes Investig. 2010;2(1):6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jørgensen MC, Ahnfelt-Rønne J, Hald J, Madsen OD, Serup P, Hecksher-Sørensen J. An illustrated review of early pancreas development in the mouse. Endocr Rev. 2007;28(6):685–705. [DOI] [PubMed] [Google Scholar]
- 44.Gannon M, Herrera PL, Wright CV. Mosaic Cre-mediated recombination in pancreas using the pdx-1 enhancer/promoter. Genesis. 2000;26(2):143–144. [DOI] [PubMed] [Google Scholar]
- 45.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129(10):2447–2457. [DOI] [PubMed] [Google Scholar]
- 46.Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22(24):3435–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hess DA, Humphrey SE, Ishibashi J, Damsz B, Lee AH, Glimcher LH, Konieczny SF. Extensive pancreas regeneration following acinar-specific disruption of Xbp1 in mice. Gastroenterology. 2011;141(4):1463–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dessimoz J, Bonnard C, Huelsken J, Grapin-Botton A. Pancreas-specific deletion of beta-catenin reveals Wnt-dependent and Wnt-independent functions during development. Curr Biol. 2005;15(18):1677–1683. [DOI] [PubMed] [Google Scholar]
- 49.Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev. 2001;15(2):111–127. [DOI] [PubMed] [Google Scholar]
- 50.Borowiak M. The new generation of beta-cells: replication, stem cell differentiation, and the role of small molecules. Rev Diabet Stud. 2010;7(2):93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–450. [DOI] [PubMed] [Google Scholar]
- 52.Magnuson MA, Osipovich AB. Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab. 2013;18(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasegawa Y, Daitoku Y, Mizuno S, Tanimoto Y, Mizuno-Iijima S, Matsuo M, Kajiwara N, Ema M, Oishi H, Miwa Y, Mekada K, Yoshiki A, Takahashi S, Sugiyama F, Yagami K. Generation and characterization of Ins1-cre-driver C57BL/6N for exclusive pancreatic beta cell-specific Cre-loxP recombination. Exp Anim. 2014;63(2):183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu G, Lee AS. Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J Cell Physiol. 2015;230(7):1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barton ER, Park S, James JK, Makarewich CA, Philippou A, Eletto D, Lei H, Brisson B, Ostrovsky O, Li Z, Argon Y. Deletion of muscle GRP94 impairs both muscle and body growth by inhibiting local IGF production. FASEB J. 2012;26(9):3691–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cirulli V, Beattie GM, Klier G, Ellisman M, Ricordi C, Quaranta V, Frasier F, Ishii JK, Hayek A, Salomon DR. Expression and function of alpha(v)beta(3) and alpha(v)beta(5) integrins in the developing pancreas: roles in the adhesion and migration of putative endocrine progenitor cells. J Cell Biol. 2000;150(6):1445–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]