Abstract
Humans are ubiquitously exposed to many phthalates, a class of endocrine-disrupting chemicals commonly used in many consumer goods, and diet, especially fatty food, is presumed to be a major source of exposure. Here, we use a rat model of human prenatal exposure to investigate the potential interactive effects of an environmentally relevant mixture of phthalates and a maternal high-fat diet (HFD). From gestation through postnatal day (P)10, dams consumed the mixture of phthalates (0, 200, or 1000 μg/kg/d) and were fed a control diet or HFD. In males, perinatal exposure to the mixture of phthalates decreased prepubertal body weight and, in a dose-specific manner, periadolescent social play behavior. A dose-specific effect from phthalates with HFD was also seen in increased time alone in females during social play. HFD resulted in dams consuming more calories, having greater gestational weight gain, and licking and nursing their pups more, such that an early postnatal HFD generally increased pup body weight. There also was a tendency for increased oxidative stress markers at P10 within the medial prefrontal cortex of males exposed to the relatively high dose of phthalates and HFD. Effects on gene expression were inconsistent at P10 and P90 in both the medial prefrontal cortex and hypothalamus. Overall, this study demonstrates that phthalates and a maternal HFD only rarely interacted, except in oxidative stress markers in males. Additionally, perinatal exposure to an environmentally relevant mixture of phthalates can have a modest, but lasting, impact on social behaviors in both males and females.
A perinatal maternal high-fat diet increased maternal care while simultaneous exposure to a mixture of phthalates resulted in a modest dose-dependent decrease in adolescent social play in males.
Endocrine disruptors pose major health concerns in our industrialized society (1). One particular group of endocrine-disrupting chemicals, known as phthalates, come in a variety of forms with different properties and applications. Exposure to these phthalates is facilitated through their ubiquitous use as a plasticizer or solvent in consumer goods, including personal care products, plastics, cleaning materials, pharmaceuticals, clothing, and building materials. Because phthalates are not covalently bound to the polyvinyl chloride plastics in which they are primarily used, direct contact is not the only source of exposure as phthalates can readily leak into products or evaporate into the environment. Consequently, exposure can occur through ingestion, inhalation, and even dermal contact with contaminated air (2).
Interestingly, diet is presumed to be the main source of exposure to some phthalates due to environmental contamination during production, processing, and packaging (3, 4). Moreover, fatty foods such as oils, dairy, meat, and fish contain the highest level of phthalates, which is of concern as calorically dense and high-fat foods are readily available in the developed world (5). Also, given that phthalates and high-fat diets (HFDs) can separately increase oxidative stress and inflammation (6, 7), it is important to study them together to examine the potential for interactive effects.
Aside from the oxidative stress and inflammatory effects, phthalates can have antiandrogenic, estrogenic, and antiestrogenic activity (8) as well as the ability to suppress the synthesis of steroidogenic enzymes (9, 10). Moreover, phthalates can have such wide-ranging effects as suppressing calcium signaling in nicotinic receptors (11), weakly antagonizing cannabinoid receptors (12), and interfering with thyroid (13), IGF-1 (14), and insulin signaling (15, 16).
Phthalates readily cross the placenta (17), which makes the gestational period a particularly vulnerable window for these endocrine- and metabolic-disrupting effects. Additionally, considering that hormones influence the development of the brain, it is not surprising that there are studies associating concentrations of urinary phthalate metabolites during pregnancy with adverse outcomes in children’s social (18, 19) and executive function (20–22).
Likewise, in rodents, perinatal exposure to a single phthalate [di-(2-ethylhexyl) phthalate (DEHP), diisobutyl phthalate (DiBP), or diethyl phthalate (DEP)] has been shown to affect cognitive behavior (23–30). However, much less is known about the effect of phthalates on maternal and social behaviors in rodents. This is particularly important considering that maternal care is known to have a long-term impact on the offspring’s behavior (31) including social behaviors (32), and may mediate the observed behavioral effects of perinatal phthalate exposure in offspring.
To date, DEHP is the most frequently studied phthalate. One study using mice has explored the effects of perinatal exposure to DEHP on these behaviors and found no differences in maternal care, but in both males and females, the higher doses generally affected social interactions in juvenile mice (33). These findings suggest that perinatal exposure to DEHP can affect social behaviors independent of maternal behavior. However, humans are exposed to a variety of phthalates as well as what is often an HFD, which is independently known to influence maternal behaviors (34, 35), as well as periadolescent social behaviors in rodents (36).
Therefore, the purpose of this study is to examine the effects of an HFD and an environmentally relevant mixture of phthalates using a rat model of human prenatal exposure. Because the prenatal period through postnatal day (P)10 in rats roughly corresponds to prenatal brain development in humans, exposure of the dams occurred at this time. Maternal behavior was assessed during this time, and later social play was quantified during the periadolescent period. Unlike mice (37), rats are known for their extensive engagement in social play, which is well-characterized and a standard model for studying neurodevelopment disorders (38). Furthermore, social play is observed more frequently in males (39), affected by perinatal hormone exposure (40), and known to peak during periadolescence (41). At the end of the phthalate and diet exposure, the medial prefrontal cortex (mPFC), an area of the cortex that is involved in both cognitive and social behaviors, was assayed for oxidative stress proteins and the expression of genes related to oxidative stress, inflammation, apoptosis, social behaviors, endocrine receptors, and steroid synthesis. To our knowledge, no studies have investigated the effects of early metabolic programming from an HFD on the gene expression of steroidogenic enzymes and hormone receptors in the mPFC. The expression of genes related to social behaviors was also examined in the hypothalamus. Both the mPFC and hypothalamus are known to be sensitive to endocrine disruptors (42–44).
Materials and Methods
Subjects
Male (n = 60) and female (n = 60) Long-Evans hooded rats were obtained from Harlan Laboratories (Indianapolis, IN) at approximately 3 months of age and housed for at least 2 weeks prior to being paired for breeding in five cohorts. They were housed in same-sex pairs on a 12-hour light/dark cycle with food and water available ad libitum. To reduce exposure to endocrine-disrupting chemicals, all animals were housed in bisphenol A–free polysulfone cages, fed a low-phytoestrogen food (Harlan 2020X; Teklad Diets, Madison, WI), and hydrated with reverse osmosis-filtered water in glass bottles. All procedures were approved by the University of Illinois Institutional Care and Use Committee and adhere to the National Institutes of Health guidelines on the ethical use of animals.
For breeding, animals were placed in suspended wire-bottom cages where the presence of sperm plugs was inspected daily. When a sperm plug was detected, the day was recorded as gestational day (GD) 0 and the dams were removed, housed individually, and assigned to one of six groups (n = 11, 10, 10, 9, 11, and 10, respectively): 0 µg phthalates/kg body weight with a control diet (CON) (n = 11) or an HFD (n = 10), 200 µg phthalates/kg with a CON (n = 10) or an HFD (n = 9), or 1000 µg phthalates/kg with a CON (n = 11) or an HFD (n = 10). One 200 µg/kg phthalate–exposed CON-fed litter was dropped after birth due to infanticide. One pup of each sex was used for each measure, or the average of a sex within a litter was used as the unit of analysis. When the litter was not balanced for sex, preference was given to the social behavior endpoints, as opposed to being euthanized for P10 tissue collection.
From GD0 through P10, dams were fed ad libitum either a CON (15.8% kcal fat; D10012G) or HFD (45% kcal fat; D12451) obtained from Research Diets Inc. (New Brunswick, NJ). On GD 0 and 1, the dams were given half a cookie (Newman’s Own organic alphabet cookie, vanilla flavor) with tocopherol-stripped corn oil pipetted onto it for acclimatization. Starting on GD2 through P10, dams readily consumed half a cookie overlaid with the daily dose of phthalate mixture at their corresponding concentration. It is worth mentioning that phthalate exposure can also occur through lactation in rats (45). Furthermore, this method of oral administration is presumably not stressful and similar to the major route of human exposure to phthalates. Pups were weaned on P25 and pair-housed with similarly aged animals of same sex, exposure, and diet.
Phthalate mixture
The phthalate mixture was derived from back-calculating exposures based on the urinary metabolites of pregnant women in the Champaign-Urbana community (unpublished data), which approximates the US population levels (46). The phthalate mixture was comprised of 35% DEP, 21% DEHP, 15% dibutyl phthalate, 15% diisononyl phthalate (DiNP), 8% DiBP, and 5% benzyl butyl phthalate. The mixture was prepared by suspending 0, 0.6, or 3 mg phthalates/mL tocopherol-stripped corn oil to ensure equivalent volume (1 µL/3 g body weight) in administering the respective 0- (control), 200-, or 1000-µg phthalates/kg doses. These doses are relatively low within the rodent literature, and based on the body surface area normalization method (47), which is prescribed by the US Food and Drug Administration, a 200- and 1000-μg/kg dose in rats is equivalent to a human dose of 32.43 and 162.16 μg/kg, respectively. These doses align within the range of the estimated daily intakes of humans (3) and, interestingly, are even below some of the tolerable daily intakes of some governing organizations (48). However, there are limitations to translating these doses of phthalates from rats to the human population due to the lack of information on interspecies differences in the toxicokinetics and their associated dose-response relationships.
Gestational and developmental indices
The amount of food eaten was recorded to compare the caloric intake between the groups from GD0 to P10. Additionally, body weight of the dams and pups, as well as the size and sex ratio of the litters, were documented. However, on the day of birth, designated as P0, the litters were not perturbed, and thus the body weight, number, anogenital distance, and sex of the pups were not determined until P1. An anogenital index was also calculated in two ways, either by dividing anogenital distance by body weight or by using body weight as a covariate. After measurements were taken on P1, each litter was culled to 10 pups to control for litter size and sex ratio. In addition to the measurements collected on P1, pup body weight was also recorded on P10, P25, and P90. Furthermore, the day of pubertal onset for each pup was determined by peripheral markers (i.e., preputial separation in males and vaginal opening in females). All the developmental measurements within a sex of a specific litter were averaged to avoid overrepresentation of any given litter.
Maternal behavior
From P3 to P10, dams and their litters were observed under red light at the start of the dark cycle when they are most active. The observer was blind to exposure group and recorded an observation every 3 minutes for a total of 30 observations per day. The maternal behavior categories were nursing, licking pups, nest reorganization (i.e., retrieving pups, nest building), and away from the nest (i.e., laying down, eating, self-grooming, etc.). Note, behavioral categories were not mutually exclusive, such that licking often coincided with nursing. When these behaviors occurred simultaneously, they were recorded in both behavioral categories. Maternal care was collectively defined by any nursing, licking, retrieving, and nest-reorganizing behaviors.
Gene expression
When adequate pairs were available, one male and female from each litter were collected at P10 within hours after the last exposure for tissue collection intended for analyses of gene expression and oxidative stress markers. Depending on availability, a male and female from each litter were also collected at P90 for tissue collection intended for gene expression analysis. Rats were anesthetized using CO2 and immediately decapitated. Brains were quickly removed with the mPFC and hypothalamus separated and snap frozen in liquid nitrogen prior to storing in a –80°C freezer where they remained until further analyses.
The mPFC was dissected with conservative boundaries: from the rostral start of frontal white matter to the caudal end in which the genu corpus callosum appears. The dorsal and ventral borders of the mPFC were delineated in reference to the underlying white matter (49). The hypothalamus was dissected as the area surrounding the third ventricle that contains the paraventricular nucleus.
Total RNA was isolated using TRI Reagent (Sigma, St. Louis, MO) followed by Direct-zol RNA MiniPrep (Zymo Research, Irvine, CA) according to the manufacturer’s instructions. Reverse transcription was performed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative real-time polymerase chain reaction was performed using the StepOnePlus RT-PCR System with Power SYBR Green PCR Master Mix and the respective forward and reverse primers for each gene (Table 1), which were designed by Vector NTI software (Invitrogen Corporation, Carlsbad, CA) and synthesized by Integrated DNA Technologies (www.idtdna.com).
Table 1.
Primers Used for Quantitative Polymerase Chain Reaction of mPFC and Hypothalamic Tissue Samples From Rats
| Gene | Common Name | Position | Primers (5′ → 3′) | Efficiency |
|---|---|---|---|---|
| Ahr | Aryl hydrocarbon receptor | Forward +582 | CACAAGGAGTAGACGAGA | 95.07% |
| Reverse +661 | GGTATTCTCTGGAGGAAG | |||
| Ar | Androgen receptor | Forward +2508 | GAAATGGGACCTTGGATGGAGA | 100.01% |
| Reverse +2585 | TAAAACGTGGTCCCTGGTACTGTC | |||
| Avp | Vasopressin | Forward +31 | TGATGCTCAACACTACGCTC | 105.69% |
| Reverse +111 | TTGGGCAGTTCTGGAAGTAG | |||
| Avpr1a | Arginine vasopressin receptor 1A | Forward +1092 | TTACACCTTGTGTCAGCAGCGTGA | 98.73% |
| Reverse +1160 | CAAAGGTCATCTTCACAGTGCGGA | |||
| Avpr1b | Arginine vasopressin receptor 1B | Forward + 1521 | TGGTAGAATCTTCATTGGGGGC | 99.85% |
| Reverse + 1588 | TGTAAGGCAGGGGCTGAGGAAGT | |||
| Bad | BCL2-associated agonist of cell death | Forward +108 | GAGACCAGCAGCCCAGAGTATGTTC | 103.15% |
| Reverse +168 | GTCTTCCTGCTCACTCGGCTCAA | |||
| Bcl2 | B-cell CLL/lymphoma 2 | Forward +708 | AGCGTCAACAGGGAGATGTCACC | 99.81% |
| Reverse +770 | CAGGTACTCAGTCATCCACAGAGCG | |||
| Casp3 | Caspase 3 | Forward +448 | ACGGACCTGTGGACCTGAAAAAA | 98.49% |
| Reverse +529 | AAGAGTTTCGGCTTTCCAGTCAGAC | |||
| Cat | Catalase | Forward +220 | TTGACAGAGAGCGGATTCCTGAGA | 105.06% |
| Reverse +297 | CGTGGGTGACCTCAAAGTATCCAA | |||
| Cd38 | CD38 | Forward +188 | CACTTCGCTGACATCATC | 105.09% |
| Reverse +263 | AGTCCTGATCTCTCATCTCC | |||
| Cyp11a1 | Cytochrome P450 family 11 subfamily A member 1 | Forward +164 | AATGAGATCCCTTCCCCTGGTG | 99.40% |
| Reverse +239 | TTCTGTGTGTGCCGTTCTCCCT | |||
| Cyp19a1 | Aromatase | Forward +430 | GAGACACATCATGCTGGACACTTC | 103.79% |
| Reverse +496 | AATAGAACTTTCGTCCAGGGGG | |||
| Drd1 | Dopamine receptor 1 | Forward +333 | GGGTCCTTTTGTAACATCTGG | 101.36% |
| Reverse +407 | GATCACGCAGAGGTTCAGAAT | |||
| Drd2 | Dopamine receptor 2 | Forward +1128 | AGTTTCCCAGTGAACAGG | 98.22% |
| Reverse +1202 | GCTTGACAGCATCTCCAT | |||
| Esr1 | Estrogen receptor α | Forward +664 | CCGCCTTCTACAGGTCCAATTC | 106.73% |
| Reverse +736 | TTCTCGCTGCTGCTGGAGAG | |||
| Esr2 | Estrogen receptor β | Forward +1172 | CTCACTAAGCTGGCGGACAAG | 93.01% |
| Reverse +1241 | CCACAAAGCCAGGGATTTTC | |||
| Esrrg | Estrogen-related receptor γ | Forward +1030 | GGTGGTTATCATTGGATGG | 103.81% |
| Reverse +1107 | TGGAGAAGGCTCATCTGAT | |||
| Glrx | Glutaredoxin | Forward +262 | CGGAGCAAGAACAGTTCCTCGG | 97.80% |
| Reverse +331 | GGAGAGTAGATCACTGCATCCGCC | |||
| Ifng | Interferon γ | Forward +116 | CTCAAGTAGCATGGATGCTATGGA | 96.64% |
| Reverse +182 | CTTTTGCCAGTTCCTCCAGATATC | |||
| Il1b | Interleukin-1β | Forward +793 | CACCTCTCAAGCAGAGCACAG | 95.15% |
| Reverse +871 | GGGTTCCATGGTGAAGTCAAC | |||
| Il6 | Interleukin 6 | Forward +559 | TCCTACCCCAACTTCCAATGCTC | 98.82% |
| Reverse +637 | TTGGATGGTCTTGGTCCTTAGCC | |||
| Ldlr | Low-density lipoprotein receptor | Forward +823 | CAAGGACAAGTCGGACGAGGAGA | 108.87% |
| Reverse +909 | CCGTGAATACAGGAGCCATCTGC | |||
| Oxt | Oxytocin | Forward +7 | ACGGTGGATCTCGGACTGAACA | 99.60% |
| Reverse +70 | CAAGCAGGCAGCAAGCAAGACT | |||
| Oxtr | Oxytocin receptor | Forward +886 | TGCTGGACACCTTTCTTCTTCGTG | 100.78% |
| Reverse +970 | TGGCAATGATGAAGGCAGAAGC | |||
| Rpl7a | Ribosomal protein L7a | Forward +64 | GAGGCCAAAAAGGTGGTCAATCC | 99.33% |
| Reverse +127 | CCTGCCCAATGCCGAAGTTCT | |||
| Sod1 | Superoxide dismutase 1 | Forward +239 | CAGCGGATGAAGAGAGGCA | 104.24% |
| Reverse +310 | ACACATTGGCCACACCGTC | |||
| Sod2 | Superoxide dismutase 2 | Forward +738 | GTTTGCAAGAAGTGAAGC | 106.66% |
| Reverse +801 | ACTACAAAACACCCACCA | |||
| Sod3 | Superoxide dismutase 3 | Forward +37 | CAGAGGCTCTTTCTCAGG | 103.60% |
| Reverse +125 | GTTCCACACCTGACAAGC | |||
| Star | Steroidogenic acute regulatory protein | Forward +358 | GGAAGAAGGAAAGCCAGCAGG | 97.53% |
| Reverse +434 | TCGGAACACCTTGCCCACA | |||
| Thra | Thyroid hormone receptor α | Forward +490 | ATCACTACCGCTGTATCACTTG | 108.64% |
| Reverse +559 | TGGAGGTTCTTCTGGATTGT | |||
| Tnf | Tumor necrosis factor | Forward +364 | GCCCAGACCCTCACACTCAGAT | 107.46% |
| Reverse +439 | GGTTTGCTACGACGTGGGCT |
Standard curves with amplification efficiencies between 90% and 110% and r2 ≥ 0.99 were accepted. The housekeeping gene 60S ribosomal protein (Rpl7a) was used to normalize the gene expression data. Genes related to social behaviors (Avp, Avpr1a, Avpr1b, CD38, Oxt, Oxtr), endocrine receptors (Ahr, Ar, Esr1, Esr2, Esrrg, Thra), steroid synthesis (Cyp11a1, Cyp19a1, Ldlr, Star), oxidative stress (Cat, Sod1, Sod2, Sod3, Glrx), inflammation (Ifng, Il1b, Il6, Tnf), apoptosis (Bad, Bcl2, Casp3), and dopamine receptors (Drd1, Drd2) were investigated (abbreviations defined in Table 1). The expression of the six genes related to social behaviors was examined in the hypothalamic tissue, and all of the genes except for Avp and Oxt (i.e., 28) were examined in the mPFC. There was an average n of six to nine pups within each group for analyses of each gene at either P10 or P90.
Oxidative stress markers
Using a colorimetric oxidative stress enzyme-linked immunosorbent assay strip (EA-1501; Signosis, Santa Clara, CA), we tested (total n = 89; n = 6 to 10/group) for eight cytokines that are known to increase in response to oxidative stress: tumor necrosis factor α (TNFα), transforming growth factor β (TGFβ), monocyte chemotactic protein-1 (MCP-1), interleukin-1α (IL-1α), IL-1β, IL-6, IL-15, and vascular endothelial growth factor (VEGF).
In accordance with the protocol from Signosis, samples were introduced to 1× cell lysis buffer (EA-0001), homogenized, and centrifuged to collect the supernatant. Using a NanoDrop fluorospectrometer (Thermo Scientific), protein concentrations were determined and appropriately diluted with diluent buffer to a final concentration of 50 µg/100 µL per well. A total of 16 96-well plates were incubated with samples in duplicates for 16 to 17 hours, a biotin-labeled antibody for 3 hours, streptavidin–horseradish peroxidase for 45 minutes, and substrate for 30 minutes, all with gentle shaking prior to being stopped with stop solution. Between incubation periods, each well was aspirated and washed thrice with 200 µL of 1× assay wash buffer. The optical density of each well was read at 450 nm with a microplate reader and its associated Gen5 software (BioTek Instruments). The duplicates were averaged, and all values were represented as percentage change from vehicle-exposed CON rats for each sex.
Periadolescent social behavior
For all remaining pups, social play behavior was observed before the start of the dark cycle for 4 consecutive days between P32 and P40, which is an age period characterized by the highest levels of social play (41). Each day, the periadolescent pups were isolated in cages in a separate room for 1 hour prior to the 20-minute observation session to increase motivation to play. For the daily observations, periadolescent pups were paired with another rat from a different litter of the same age, sex, exposure, and diet. The same pairings occurred across the 4 consecutive days, and each pair was designated to a litter prior to observations. For each pair, a behavioral observation was made at every 1 minute for a total of 20 minutes by the observer, who was blind to the exposure group. Given that there was a varying number of pairings for each sex within a litter across litters, the pairings of each sex within a specific litter were averaged to avoid overrepresentation of any given litter. The behavioral categories observed were wrestling, sniffing, chasing, alone (i.e., no contact), and in contact (i.e., “passive contact behavior,” as they were in contact but sniffing elsewhere or laying down next to each other). Active social behaviors were defined as social play, which was the sum of wrestling, sniffing, and chasing behaviors. Additionally, because maternal nursing and licking are known to have a long-term impact on the offspring’s behavior (32), correlations between these maternal behaviors and social play were run.
Statistical analyses
All analyses were performed using SPSS and included a two-way (diet × phthalate exposure) analysis of variance (ANOVA) with cohort as a cofactor, except for oxidative stress markers where the plate was used as a cofactor in lieu of cohort. Each sex was analyzed separately because we expected that the sexes would react differently to the endocrine-disrupting properties of the phthalate mixture. Additionally, a covariate (litter size) was used in the gestational weight gain measurement. Furthermore, litter was the experimental unit for all the developmental and social behavior measurements within a sex (i.e., each measurement from a given sex within a litter was averaged). Post hoc tests were performed using Bonferroni comparisons with each exposure dose (200 and 1000 µg/kg) only compared with the vehicle-exposed (0 µg/kg) group and additionally for significant interactions, within each diet group. Additionally, another post hoc test was performed for significant interactions using Bonferroni comparisons to assess diet effects within each exposure group. Bivariate correlations with two-tailed tests of significance were run for maternal licking and nursing behaviors with social play in both males and females separately.
Results
Gestation
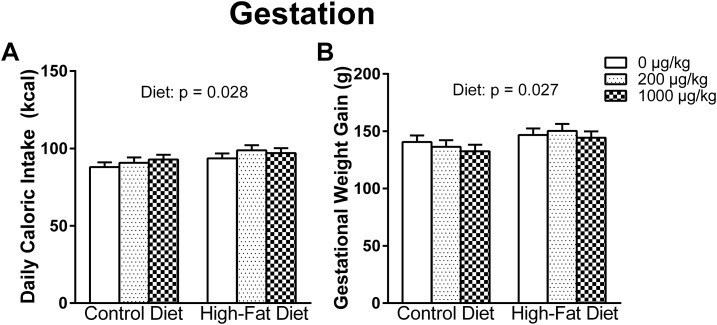
Almost all dams reached parturition on GD22, with two giving birth on GD21 and one on GD23. For the average daily caloric intake, there was a main effect of diet [F(1, 50) = 5.089, P = 0.028], indicating that the HFD group consumed more daily calories than the CON group (Fig. 1A). Accordingly, there was a main effect of diet [F(1, 50) = 5.200, P = 0.027] on gestational weight gain, with HFD dams gaining more gestational weight than CON dams, even when controlling for litter size (Fig. 1B).
Figure 1.
(A) Daily caloric intake from GD0 through P10 and (B) the gestational weight gain. HFD dams consumed more daily calories and gained more weight during gestation in comparison with CON dams.
Litter
There were no significant effects or interactions with litter size; however, there was a trend for phthalate-exposed litters to be larger than control litters [F(2, 51) = 3.081, P = 0.055] and a trend for an interaction [F(2, 51) = 2.723, P = 0.075]. Furthermore, there were no significant effects or interactions with sex ratio of the litter.
Effects in offspring
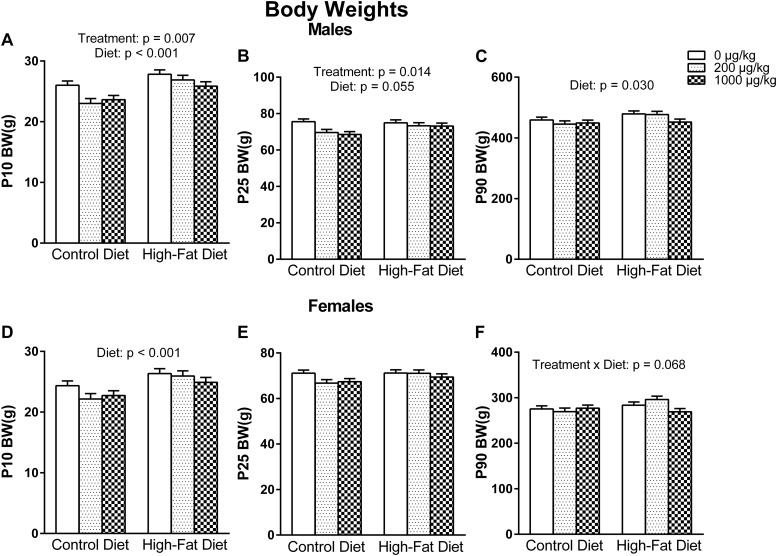
In the pups, there were no significant effects or interactions in body weight, anogenital distance, or anogenital index at P1. However, at P10, there was a main effect of diet on body weight in both males [F(1, 50) = 18.632, P < 0.001] and females [F(1, 50) = 15.337, P < 0.001], indicating that HFD pups weighed more at P10 than CON pups. Although not significant at P25, there was a trend in the same direction for both males [F(1, 50) = 3.856, P = 0.055] and females [F(1, 50) = 3.237, P = 0.078], and at P90, the effect of diet reappeared only in males [F(1, 50) = 5.001, P = 0.030].
There was an exposure effect on body weight found only in males at P10 [F(2, 50) = 5.438, P = 0.007] that persisted to P25 [F(2, 50) = 4.672, P = 0.014], indicating that phthalate-exposed males at either dose had lower body weights at P10 (P = 0.024, P = 0.008) and P25 (P = 0.046, P = 0.012) compared with control males. Lastly, at P90 in females, there was a trend for a diet-by-exposure interaction [F(2, 50) = 2.832, P = 0.068] (Fig. 2).
Figure 2.
Male pup body weights at (A) P10, (B) P25, and (C) P90. Phthalate-exposed males had lower prepubertal body weights. Furthermore, a perinatal HFD tended to result in higher body weights that persisted into adulthood. Female pup body weights at (D) P10, (E) P25, and (F) P90. A perinatal HFD increased female pup body weight at P10.
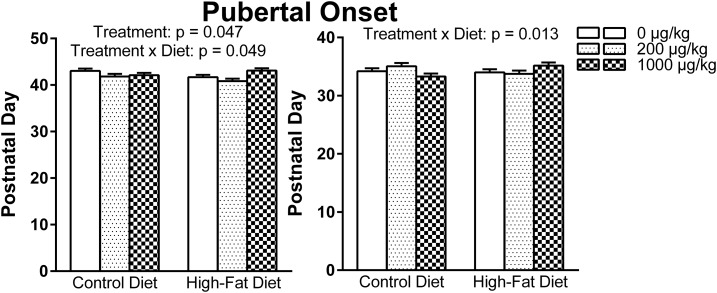
Analysis of age at pubertal onset in males resulted in a significant exposure effect [F(2, 50) = 3.252, P = 0.047] and a significant exposure-by-diet interaction [F(2, 50) = 3.196, P = 0.049]; however, post hoc tests revealed no significant findings. In females, there was a significant exposure-by-diet interaction [F(2, 50) = 4.705, P = 0.013] that revealed a diet effect within the 1000 µg phthalates/kg–exposed females (P = 0.037), such that a perinatal HFD delayed pubertal onset compared with a CON within the relatively high phthalate–exposed females (Fig. 3).
Figure 3.
Pubertal onset in (left) males and (right) females. In males, there was a significant exposure effect and a significant exposure-by-diet interaction, and in females, there was a significant exposure-by-diet interaction.
Maternal behavior
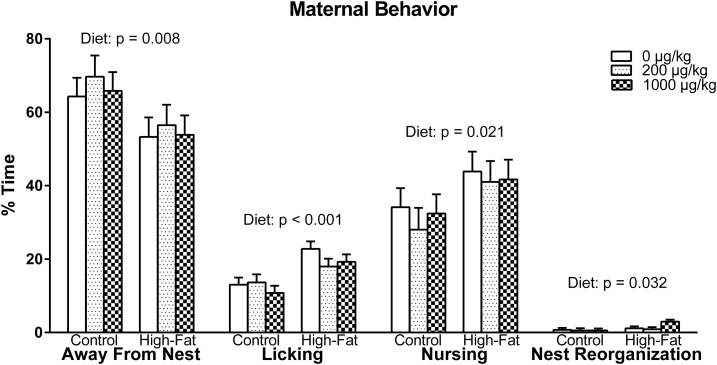
Overall, there was a main effect of diet in maternal care behavior [F(1, 50) = 7.536, P = 0.008], indicating that HFD dams spent more time caring for their pups compared with CON dams (Fig. 4). This is attributed to HFD dams spending more time licking [F(1, 50) = 19.869, P < 0.001], nursing [F(1, 50) = 5.645, P = 0.021], and, to a lesser extent, reorganizing nests [F(1, 50) = 4.882, P = 0.032] compared with CON dams. In concordance with an HFD resulting in better maternal care, the HFD dams spent less time away from the nest compared with CON dams [F(1, 50) = 7.536, P = 0.008].
Figure 4.
Maternal behavior observations. Dams fed an HFD spent less time away from the nest and therefore cared more for their pups compared with those fed a CON. This was seen in HFD dams spending more time licking, nursing, and reorganizing nests in comparison with CON dams. Note: when licking and nursing behaviors occurred simultaneously, they were recorded in both behavioral categories.
Periadolescent social behavior
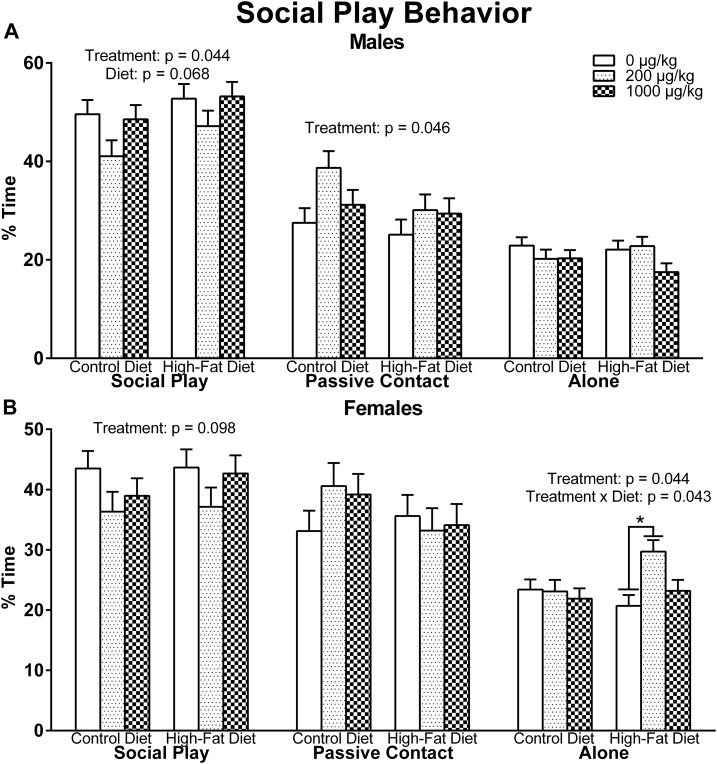
In males, there was a main effect of exposure in social play [F(2, 50) = 3.323, P = 0.044], such that Bonferroni comparisons revealed that the 200 µg phthalates/kg males had a close to significant decrease in social play compared with control males (P = 0.051) (Fig. 5A). This decrease in social play was offset by the exposure effect in passive contact behavior [F(2, 50) = 3.287, P = 0.046], with the 200 µg phthalates/kg males displaying more passive contact behavior than control males (P = 0.027). There were no significant effects or interactions in alone behavior. Diet showed a trend for social play [F(1, 50) = 3.485, P = 0.068] in which HFD males appeared to show more social play than CON males.
Figure 5.
Periadolescent social play behavior in (A) males and (B) females. Males that were perinatally exposed to the relatively low concentration of the phthalate mixture had a near to significant tendency to engage in less social play (P = 0.051) but an increase in passive contact. Relative to males, females showed a similar, but not significant, pattern in social play in response to phthalate exposure However, the relatively low phthalate–exposed females with a perinatal HFD displayed more withdrawn behaviors during social play (P = 0.004).
Likewise in females, there was a weak trend for an exposure effect in social play [F(2, 50) = 2.434, P = 0.098] that appeared in the same pattern as the significant difference in males (Fig. 5B). There was a main effect of exposure in alone behavior [F(2, 50) = 3.322, P = 0.044], such that the 200 µg phthalates/kg females demonstrated more alone behavior than control females (P = 0.043). However, this effect was driven by the HFD females, as there was a significant exposure-by-diet interaction [F(2, 50) = 3.353, P = 0.043] revealing that, within HFD females, the 200 µg phthalates/kg group demonstrated more alone behavior than control females (P = 0.004). This also resulted in a significant diet effect within the 200 µg phthalates/kg–exposed females (P = 0.049), such that a perinatal HFD increased withdrawn behaviors compared with CON within the relatively low phthalate–exposed females. There were no significant effects or interactions in passive contact behavior
In separate analyses of males and females, maternal licking was significantly correlated with social play (R = 0.258, P = 0.047 and R = 0.351, P = 0.006, respectively), such that pups with mothers that licked more engaged in more social play during adolescence. Additionally, in females only, maternal nursing was significantly correlated with social play (R = 0.259, P = 0.046) so that increased time nursing correlated with more social play during adolescence (Table 2).
Table 2.
Correlations [Pearson’s Coefficient (R), P Value] Between Periadolescent Social Play and Select Maternal Behaviors in Both Male and Female Rats
| Maternal Behavior Category | Males | Females |
|---|---|---|
| Licking | R = 0.258, P = 0.047a | R = 0.351, P = 0.006a |
| Nursing | R = 0.222, P = 0.089 | R = 0.259, P = 0.046a |
n = 60/sex.
P < 0.05.
Gene expression
Overall, there were no systematic effects on gene expression within the classes of genes examined in the mPFC (Tables 3 and 4) or hypothalamus (Tables 5 and 6) of either males or females at P10 or P90, except for a diet effect that was observed in the mPFC of P90 males across the estrogen receptors (Esr1, Esr2, and Esrrg). Of the 28 genes examined in the mPFC and 6 in the hypothalamus, only 3 genes at P10 in males and 3 in females were statistically significant between groups with independent tests. At P90, only five genes in males and three in females were statistically significant between relevant groups with independent tests.
Table 3.
P10 (Top Number) and P90 (Bottom Number) mPFC Gene Expression in Males
| Gene |
CON |
HFD |
ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Oil | 200 | 1000 | Oil | 200 | 1000 | ||
| Endocrine system | |||||||
| Ldlr | 0.94 ± 0.13 | 0.90 ± 0.14 | 0.95 ± 0.11 | 1.10 ± 0.12 | 1.17 ± 0.12 | 1.13 ± 0.12 | Diet, P = 0.050 |
| 1.57 ± 0.15 | 1.32 ± 0.14 | 1.28 ± 0.12 | 1.17 ± 0.13 | 1.40 ± 0.17 | 1.23 ± 0.15 | ||
| Cyp11a1 | 2.25 ± 0.05 | 1.10 ± 0.52 | 1.72 ± 0.41 | 2.32 ± 0.43 | 1.22 ± 0.46 | 1.61 ± 0.43 | |
| 0.39 ± 0.17 | 0.41 ± 0.15 | 0.35 ± 0.12 | 0.56 ± 0.14 | 0.36 ± 0.18 | 0.37 ± 0.16 | ||
| Star | 0.32 ± 0.05 | 0.30 ± 0.05 | 0.35 ± 0.04 | 0.35 ± 0.05 | 0.33 ± 0.05 | 0.29 ± 0.05 | |
| 0.56 ± 0.31 | 1.04 ± 0.27 | 0.89 ± 0.22 | 0.81 ± 0.24 | 0.84 ± 0.32 | 0.66 ± 0.28 | ||
| Ar | 1.57 ± 0.51 | 1.27 ± 0.54 | 1.81 ± 0.42 | 1.45 ± 0.45 | 2.73 ± 0.48 | 1.06 ± 0.45 | Trt × diet, P = 0.006 |
| 1.34 ± 0.08 | 1.10 ± 0.08 | 1.00 ± 0.07 | 0.97 ± 0.07 | 1.14 ± 0.10 | 1.13 ± 0.09 | ||
| Cyp19a1 | 1.51 ± 0.35 | 1.81 ± 0.37 | 2.10 ± 0.29 | 1.58 ± 0.30 | 1.98 ± 0.32 | 1.37 ± 0.30 | |
| 2.08 ± 0.33 | 1.83 ± 0.30 | 1.27 ± 0.24 | 1.88 ± 0.29 | 1.47 ± 0.35 | 1.44 ± 0.31 | ||
| Esr1 | 0.84 ± 0.14 | 0.80 ± 0.14 | 0.75 ± 0.11 | 0.87 ± 0.11 | 0.58 ± 0.12 | 1.08 ± 0.11 | Diet, P = 0.001 |
| 1.72 ± 0.16 | 1.57 ± 0.14 | 1.30 ± 0.12 | 1.02 ± 0.14 | 1.11 ± 0.18 | 1.18 ± 0.16 | ||
| Esr2 | 2.82 ± 0.53 | 2.82 ± 0.56 | 4.13 ± 0.43 | 3.29 ± 0.46 | 3.48 ± 0.50 | 3.28 ± 0.45 | Diet, P = 0.046 |
| 1.77 ± 0.20 | 1.20 ± 0.20 | 1.22 ± 0.17 | 0.90 ± 0.17 | 1.20 ± 0.23 | 1.08 ± 0.20 | ||
| Esrrg | 1.62 ± 0.27 | 1.63 ± 0.28 | 1.87 ± 0.22 | 1.70 ± 0.23 | 2.32 ± 0.25 | 1.69 ± 0.25 | Trt, P = 0.033; diet, P = 0.016 |
| 1.29 ± 0.14 | 1.52 ± 0.12 | 1.10 ± 0.10 | 1.01 ± 0.11 | 1.18 ± 0.15 | 0.92 ± 0.13 | ||
| Cd38 | 1.17 ± 0.09 | 1.15 ± 0.10 | 1.19 ± 0.08 | 1.20 ± 0.08 | 1.14 ± 0.09 | 1.18 ± 0.08 | |
| 1.33 ± 0.12 | 1.11 ± 0.11 | 1.00 ± 0.10 | 1.00 ± 0.11 | 1.22 ± 0.14 | 1.17 ± 0.14 | ||
| Oxtr | 3.02 ± 0.63 | 3.00 ± 0.75 | 3.49 ± 0.52 | 2.68 ± 0.65 | 1.87 ± 0.58 | 3.27 ± 0.54 | |
| 1.28 ± 0.22 | 1.59 ± 0.22 | 1.09 ± 0.19 | 0.77 ± 0.21 | 1.29 ± 0.26 | 1.14 ± 0.23 | ||
| Avpr1a | 0.19 ± 0.03 | 0.22 ± 0.03 | 0.20 ± 0.02 | 0.18 ± 0.02 | 0.17 ± 0.02 | 0.24 ± 0.02 | |
| 1.16 ± 0.17 | 0.98 ± 0.17 | 0.98 ± 0.13 | 0.90 ± 0.15 | 0.96 ± 0.20 | 0.93 ± 0.17 | ||
| Avpr1b | 1.72 ± 0.25 | 1.13 ± 0.29 | 1.26 ± 0.21 | 1.67 ± 0.22 | 1.39 ± 0.25 | 1.03 ± 0.22 | |
| 1.44 ± 0.34 | 1.03 ± 0.29 | 0.75 ± 0.24 | 0.75 ± 0.24 | 1.21 ± 0.31 | 1.12 ± 0.31 | ||
| Thra | 1.98 ± 0.19 | 2.22 ± 0.20 | 1.97 ± 0.16 | 2.11 ± 0.17 | 2.08 ± 0.17 | 2.38 ± 0.16 | Trt, P = 0.030 |
| 1.79 ± 0.15 | 1.69 ± 0.15 | 1.32 ± 0.12 | 1.35 ± 0.13 | 1.75 ± 0.17 | 1.35 ± 0.15 | ||
| Defense system | |||||||
| Ahr | 0.44 ± 0.16 | 0.61 ± 0.16 | 0.45 ± 0.13 | 0.57 ± 0.13 | 0.37 ± 0.14 | 0.60 ± 0.13 | |
| 0.77 ± 0.14 | 0.80 ± 0.13 | 0.78 ± 0.10 | 0.70 ± 0.11 | 0.76 ± 0.15 | 0.69 ± 0.13 | ||
| Cat | 0.26 ± 0.02 | 0.25 ± 0.03 | 0.23 ± 0.02 | 0.27 ± 0.02 | 0.30 ± 0.02 | 0.30 ± 0.02 | Diet, P = 0.022 |
| 1.37 ± 0.11 | 1.19 ± 0.10 | 1.12 ± 0.08 | 1.11 ± 0.09 | 1.33 ± 0.11 | 1.17 ± 0.10 | ||
| Glrx | 1.64 ± 0.14 | 1.89 ± 0.15 | 1.54 ± 0.12 | 1.58 ± 0.12 | 1.48 ± 0.13 | 1.61 ± 0.12 | |
| 1.64 ± 0.19 | 1.53 ± 0.17 | 1.31 ± 0.13 | 1.26 ± 0.15 | 1.59 ± 0.19 | 1.04 ± 0.17 | ||
| Sod1 | 1.60 ± 0.19 | 1.52 ± 0.20 | 1.69 ± 0.16 | 1.40 ± 0.17 | 1.81 ± 0.20 | 1.88 ± 0.17 | |
| 1.36 ± 0.12 | 1.20 ± 0.11 | 1.21 ± 0.10 | 1.10 ± 0.11 | 1.38 ± 0.14 | 1.42 ± 0.12 | ||
| Sod2 | 1.83 ± 0.29 | 1.72 ± 0.30 | 2.08 ± 0.26 | 2.06 ± 0.23 | 1.97 ± 0.30 | 2.10 ± 0.24 | |
| 1.00 ± 0.12 | 1.28 ± 0.11 | 0.98 ± 0.09 | 0.86 ± 0.10 | 0.98 ± 0.13 | 0.89 ± 0.12 | ||
| Sod3 | 1.76 ± 0.54 | 1.73 ± 0.57 | 2.15 ± 0.44 | 1.59 ± 0.47 | 2.65 ± 0.55 | 1.46 ± 0.47 | Trt, P = 0.029 |
| 2.31 ± 0.25 | 2.09 ± 0.23 | 1.50 ± 0.20 | 1.62 ± 0.22 | 2.14 ± 0.29 | 1.49 ± 0.26 | ||
| Inflammation | |||||||
| Ifng | 1.04 ± 0.29 | 1.22 ± 0.28 | 1.13 ± 0.23 | 1.32 ± 0.25 | 0.81 ± 0.26 | 1.01 ± 0.27 | |
| 0.46 ± 0.22 | 0.47 ± 0.19 | 0.65 ± 0.15 | 0.11 ± 0.19 | 0.39 ± 0.25 | 0.25 ± 0.20 | ||
| Il1b | 0.37 ± 0.09 | 0.20 ± 0.10 | 0.36 ± 0.08 | 0.27 ± 0.08 | 0.25 ± 0.08 | 0.20 ± 0.08 | |
| 1.56 ± 0.70 | 2.84 ± 0.55 | 3.18 ± 0.48 | 2.53 ± 0.47 | 2.09 ± 0.64 | 2.14 ± 0.56 | ||
| Il6 | 1.23 ± 0.70 | 2.20 ± 0.69 | 2.57 ± 0.53 | 1.86 ± 0.57 | 2.95 ± 0.60 | 1.23 ± 0.60 | |
| 0.76 ± 0.09 | 0.66 ± 0.08 | 0.68 ± 0.07 | 0.60 ± 0.07 | 0.56 ± 0.10 | 0.64 ± 0.09 | ||
| Tnf | 1.20 ± 0.16 | 1.23 ± 0.17 | 1.30 ± 0.13 | 1.48 ± 0.14 | 1.39 ± 0.15 | 1.25 ± 0.14 | |
| 0.32 ± 0.19 | 0.62 ± 0.17 | 0.76 ± 0.16 | 0.85 ± 0.15 | 0.81 ± 0.22 | 0.61 ± 0.18 | ||
| Apoptosis | |||||||
| Bad | 1.38 ± 0.11 | 1.39 ± 0.11 | 1.37 ± 0.09 | 1.33 ± 0.09 | 1.36 ± 0.10 | 1.49 ± 0.09 | |
| 1.60 ± 0.15 | 1.40 ± 0.13 | 1.45 ± 0.10 | 1.02 ± 0.11 | 1.46 ± 0.15 | 1.34 ± 0.13 | ||
| Bcl2 | 1.49 ± 0.24 | 1.28 ± 0.26 | 1.50 ± 0.20 | 1.32 ± 0.21 | 1.59 ± 0.25 | 1.29 ± 0.21 | |
| 1.83 ± 0.24 | 1.34 ± 0.19 | 1.31 ± 0.15 | 1.02 ± 0.19 | 1.36 ± 0.22 | 1.13 ± 0.20 | ||
| Casp3 | 1.36 ± 0.18 | 1.24 ± 0.19 | 1.26 ± 0.15 | 1.33 ± 0.16 | 1.17 ± 0.17 | 1.60 ± 0.16 | |
| 1.46 ± 0.15 | 1.36 ± 0.14 | 1.18 ± 0.11 | 1.11 ± 0.12 | 1.45 ± 0.16 | 1.16 ± 0.14 | ||
| Dopamine receptors | |||||||
| Drd1 | 1.13 ± 0.28 | 1.44 ± 0.29 | 1.53 ± 0.23 | 0.96 ± 0.26 | 1.54 ± 0.31 | 1.53 ± 0.24 | |
| 1.15 ± 0.18 | 1.38 ± 0.15 | 1.15 ± 0.13 | 1.25 ± 0.14 | 1.28 ± 0.19 | 1.01 ± 0.19 | ||
| Drd2 | 0.66 ± 0.30 | 0.81 ± 0.32 | 1.43 ± 0.25 | 0.92 ± 0.28 | 1.76 ± 0.30 | 1.72 ± 0.28 | Trt, P = 0.025; diet, P = 0.038 |
| 0.79 ± 0.28 | 1.18 ± 0.23 | 0.94 ± 0.20 | 0.79 ± 0.22 | 0.84 ± 0.29 | 0.91 ± 0.26 | ||
Values are means ± standard error of the mean. Data are expressed as the ratio to L7a.
Abbreviation: Trt, treatment.
Table 4.
P10 (Top Number) and P90 (Bottom Number) mPFC Gene Expression in Females
| Gene |
CON |
HFD |
ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Oil | 200 | 1000 | Oil | 200 | 1000 | ||
| Endocrine system | |||||||
| Ldlr | 0.91 ± 0.15 | 0.95 ± 0.17 | 1.09 ± 0.13 | 1.07 ± 0.16 | 0.98 ± 0.17 | 1.02 ± 0.18 | |
| 1.40 ± 0.16 | 1.34 ± 0.20 | 1.23 ± 0.15 | 1.38 ± 0.19 | 1.30 ± 0.23 | 1.25 ± 0.19 | ||
| Cyp11a1 | 2.50 ± 0.42 | 1.66 ± 0.47 | 1.85 ± 0.37 | 2.15 ± 0.45 | 1.00 ± 0.47 | 1.43 ± 0.50 | |
| 0.39 ± 0.13 | 0.47 ± 0.15 | 0.39 ± 0.12 | 0.73 ± 0.16 | 0.36 ± 0.17 | 0.44 ± 0.14 | ||
| Star | 0.39 ± 0.07 | 0.41 ± 0.08 | 0.32 ± 0.07 | 0.39 ± 0.08 | 0.24 ± 0.08 | 0.29 ± 0.09 | |
| 0.61 ± 0.09 | 0.55 ± 0.12 | 0.86 ± 0.09 | 0.70 ± 0.11 | 0.69 ± 0.13 | 0.60 ± 0.12 | ||
| Ar | 1.69 ± 0.68 | 2.17 ± 0.75 | 1.11 ± 0.60 | 2.17 ± 0.73 | 0.57 ± 0.76 | 1.60 ± 0.92 | |
| 1.18 ± 0.12 | 1.06 ± 0.15 | 1.20 ± 0.11 | 1.17 ± 0.14 | 1.20 ± 0.16 | 1.01 ± 0.14 | ||
| Cyp19a1 | 1.34 ± 0.29 | 2.14 ± 0.32 | 1.87 ± 0.25 | 1.82 ± 0.31 | 1.23 ± 0.32 | 1.45 ± 0.34 | |
| 2.01 ± 0.32 | 1.10 ± 0.37 | 1.60 ± 0.30 | 1.23 ± 0.38 | 1.37 ± 0.41 | 1.14 ± 0.34 | ||
| Esr1 | 1.16 ± 0.13 | 0.88 ± 0.15 | 0.95 ± 0.12 | 0.80 ± 0.14 | 0.99 ± 0.15 | 0.78 ± 0.16 | |
| 1.23 ± 0.11 | 0.93 ± 0.13 | 1.15 ± 0.10 | 1.22 ± 0.12 | 1.17 ± 0.15 | 1.03 ± 0.12 | ||
| Esr2 | 2.56 ± 0.51 | 3.20 ± 0.63 | 2.75 ± 0.43 | 3.07 ± 0.63 | 2.28 ± 0.51 | 2.76 ± 0.56 | |
| 1.38 ± 0.16 | 1.02 ± 0.20 | 0.90 ± 0.15 | 1.14 ± 0.18 | 1.45 ± 0.22 | 1.11 ± 0.18 | ||
| Esrrg | 1.17 ± 0.30 | 1.76 ± 0.30 | 1.54 ± 0.24 | 1.98 ± 0.28 | 1.38 ± 0.30 | 1.10 ± 0.35 | |
| 1.35 ± 0.16 | 0.99 ± 0.19 | 1.21 ± 0.14 | 1.09 ± 0.19 | 1.09 ± 0.21 | 1.03 ± 0.17 | ||
| Cd38 | 1.40 ± 0.16 | 1.07 ± 0.17 | 1.09 ± 0.14 | 1.20 ± 0.17 | 1.05 ± 0.17 | 1.17 ± 0.19 | |
| 1.24 ± 0.12 | 1.14 ± 0.15 | 1.19 ± 0.12 | 0.92 ± 0.14 | 1.32 ± 0.17 | 1.07 ± 0.14 | ||
| Oxtr | 2.53 ± 0.62 | 3.34 ± 0.69 | 4.04 ± 0.61 | 2.57 ± 0.66 | 3.93 ± 0.77 | 2.12 ± 0.75 | Diet, P = 0.027 |
| 1.20 ± 0.20 | 1.34 ± 0.25 | 1.73 ± 0.20 | 1.16 ± 0.24 | 0.87 ± 0.28 | 0.89 ± 0.24 | ||
| Avpr1a | 0.20 ± 0.03 | 0.22 ± 0.03 | 0.21 ± 0.02 | 0.16 ± 0.03 | 0.24 ± 0.03 | 0.16 ± 0.03 | |
| 0.82 ± 0.10 | 0.73 ± 0.13 | 0.89 ± 0.09 | 0.94 ± 0.11 | 0.84 ± 0.13 | 0.86 ± 0.11 | ||
| Avpr1b | 1.81 ± 0.31 | 1.08 ± 0.34 | 2.10 ± 0.29 | 0.93 ± 0.33 | 1.27 ± 0.37 | 1.90 ± 0.37 | Trt, P = 0.045 |
| 1.41 ± 0.23 | 0.95 ± 0.33 | 1.42 ± 0.22 | 1.61 ± 0.27 | 1.45 ± 0.35 | 0.74 ± 0.22 | ||
| Thra | 1.91 ± 0.15 | 2.15 ± 0.17 | 2.12 ± 0.13 | 1.97 ± 0.16 | 2.14 ± 0.17 | 1.96 ± 0.18 | |
| 1.62 ± 0.16 | 1.34 ± 0.19 | 1.49 ± 0.14 | 1.34 ± 0.19 | 1.51 ± 0.21 | 1.29 ± 0.17 | ||
| Antioxidant defense systems | |||||||
| Ahr | 0.56 ± 0.16 | 0.54 ± 0.18 | 0.37 ± 0.14 | 0.49 ± 0.17 | 0.39 ± 0.18 | 0.31 ± 0.19 | |
| 0.93 ± 0.12 | 0.44 ± 0.15 | 0.57 ± 0.11 | 0.65 ± 0.15 | 0.79 ± 0.16 | 0.77 ± 0.14 | ||
| Cat | 0.28 ± 0.03 | 0.27 ± 0.03 | 0.25 ± 0.02 | 0.28 ± 0.03 | 0.25 ± 0.03 | 0.25 ± 0.03 | |
| 1.45 ± 0.14 | 1.15 ± 0.16 | 1.20 ± 0.14 | 0.95 ± 0.17 | 1.41 ± 0.18 | 1.15 ± 0.15 | ||
| Glrx | 1.44 ± 0.14 | 1.63 ± 0.16 | 1.92 ± 0.12 | 1.61 ± 0.15 | 1.57 ± 0.16 | 1.76 ± 0.17 | Trt × diet, P = 0.016 |
| 1.74 ± 0.17 | 1.14 ± 0.22 | 1.23 ± 0.17 | 0. 92 ± 0.21 | 1.50 ± 0.23 | 1.27 ± 0.19 | ||
| Sod1 | 1.36 ± 0.20 | 1.87 ± 0.23 | 1.34 ± 0.18 | 1.50 ± 0.22 | 1.58 ± 0.23 | 1.53 ± 0.24 | |
| 1.39 ± 0.13 | 1.22 ± 0.17 | 1.31 ± 0.13 | 1.06 ± 0.16 | 1.27 ± 0.19 | 1.09 ± 0.15 | ||
| Sod2 | 1.44 ± 0.23 | 1.86 ± 0.28 | 1.48 ± 0.21 | 2.20 ± 0.31 | 1.52 ± 0.25 | 1.46 ± 0.30 | |
| 1.14 ± 0.13 | 0.91 ± 0.16 | 0.97 ± 0.12 | 1.01 ± 0.15 | 0.97 ± 0.18 | 1.00 ± 0.15 | ||
| Sod3 | 1.61 ± 0.48 | 1.58 ± 0.54 | 1.23 ± 0.43 | 2.36 ± 0.52 | 1.31 ± 0.54 | 1.66 ± 0.58 | |
| 2.06 ± 0.26 | 1.53 ± 0.32 | 1.68 ± 0.24 | 1.79 ± 0.30 | 2.18 ± 0.36 | 1.22 ± 0.30 | ||
| Inflammation | |||||||
| Ifng | 1.36 ± 0.25 | 1.59 ± 0.26 | 1.52 ± 0.21 | 1.02 ± 0.25 | 1.19 ± 0.26 | 0.58 ± 0.31 | Diet, P = 0.012 |
| 0.44 ± 0.13 | 0.28 ± 0.16 | 0.46 ± 0.12 | 0.73 ± 0.17 | 0.32 ± 0.17 | 0.26 ± 0.14 | ||
| Il1b | 0.34 ± 0.06 | 0.28 ± 0.06 | 0.28 ± 0.05 | 0.35 ± 0.06 | 0.23 ± 0.06 | 0.31 ± 0.08 | |
| 3.60 ± 0.49 | 1.29 ± 0.62 | 2.30 ± 0.47 | 2.78 ± 0.60 | 2.46 ± 0.65 | 3.22 ± 0.58 | ||
| Il6 | 2.32 ± 0.46 | 0.75 ± 0.65 | 1.31 ± 0.43 | 2.05 ± 0.49 | 1.13 ± 0.51 | 1.90 ± 0.55 | |
| 0.89 ± 0.15 | 0.74 ± 0.17 | 0.70 ± 0.14 | 0.88 ± 0.18 | 0.70 ± 0.19 | 0.69 ± 0.16 | ||
| Tnf | 1.24 ± 0.16 | 1.20 ± 0.18 | 1.21 ± 0.14 | 1.48 ± 0.17 | 1.00 ± 0.18 | 1.34 ± 0.19 | |
| 0.91 ± 0.17 | 0.60 ± 0.22 | 0.63 ± 0.15 | 0.58 ± 0.21 | 0.67 ± 0.22 | 0.76 ± 0.19 | ||
| Apoptosis | |||||||
| Bad | 1.25 ± 0.08 | 1.45 ± 0.09 | 1.45 ± 0.07 | 1.37 ± 0.09 | 1.47 ± 0.09 | 1.32 ± 0.10 | Trt × diet, P = 0.050 |
| 1.49 ± 0.15 | 1.16 ± 0.19 | 1.32 ± 0.14 | 1.01 ± 0.18 | 1.61 ± 0.22 | 1.28 ± 0.16 | ||
| Bcl2 | 1.02 ± 0.29 | 1.65 ± 0.29 | 1.21 ± 0.23 | 1.60 ± 0.28 | 1.12 ± 0.29 | 1.17 ± 0.31 | |
| 1.49 ± 0.29 | 0.98 ± 0.37 | 1.21 ± 0.31 | 1.45 ± 0.38 | 1.40 ± 0.42 | 1.35 ± 0.31 | ||
| Casp3 | 1.25 ± 0.16 | 1.30 ± 0.18 | 1.39 ± 0.15 | 1.15 ± 0.18 | 1.69 ± 0.18 | 1.47 ± 0.20 | |
| 1.56 ± 0.15 | 1.25 ± 0.19 | 1.29 ± 0.15 | 1.01 ± 0.18 | 1.33 ± 0.20 | 1.17 ± 0.16 | ||
| Dopamine receptors | |||||||
| Drd1 | 1.08 ± 0.30 | 0.98 ± 0.30 | 1.30 ± 0.24 | 1.27 ± 0.32 | 0.91 ± 0.30 | 1.61 ± 0.32 | |
| 1.23 ± 0.19 | 1.57 ± 0.22 | 1.32 ± 0.17 | 1.16 ± 0.23 | 1.11 ± 0.25 | 1.18 ± 0.20 | ||
| Drd2 | 0.87 ± 0.25 | 0.42 ± 0.30 | 1.09 ± 0.20 | 0.97 ± 0.26 | 0.86 ± 0.25 | 1.23 ± 0.30 | |
| 0.77 ± 0.10 | 0.61 ± 0.11 | 0.78 ± 0.09 | 0.66 ± 0.12 | 0.82 ± 0.13 | 0.67 ± 0.11 | ||
Values are means ± standard error of the mean. Data are expressed as the ratio to L7a.
Abbreviation: Trt, treatment.
Table 5.
P10 (Top Number) and P90 (Bottom Number) Hypothalamic Gene Expression in Males
| Gene |
CON |
HFD |
ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Oil | 200 | 1000 | Oil | 200 | 1000 | ||
| Endocrine system | |||||||
| Avp | 0.38 ± 0.19 | 0.25 ± 0.16 | 0.49 ± 0.14 | 0.31 ± 0.16 | 0.78 ± 0.16 | 0.42 ± 0.16 | |
| 0.84 ± 0.21 | 0.72 ± 0.21 | 1.23 ± 0.17 | 1.19 ± 0.17 | 1.16 ± 0.21 | 1.03 ± 0.18 | ||
| Avpr1a | 1.01 ± 0.23 | 0.79 ± 0.17 | 0.98 ± 0.16 | 1.13 ± 0.17 | 0.80 ± 0.17 | 1.15 ± 0.16 | |
| 1.01 ± 0.11 | 0.98 ± 0.11 | 0.86 ± 0.09 | 0.85 ± 0.09 | 0.90 ± 0.11 | 0.91 ± 0.10 | ||
| Avpr1b | 1.38 ± 0.27 | 1.10 ± 0.23 | 1.16 ± 0.23 | 1.03 ± 0.23 | 1.06 ± 0.27 | 0.87 ± 0.21 | |
| 2.14 ± 0.39 | 1.31 ± 0.40 | 1.80 ± 0.32 | 1.33 ± 0.36 | 1.63 ± 0.45 | 1.18 ± 0.34 | ||
| Cd38 | 0.49 ± 0.12 | 0.40 ± 0.11 | 0.44 ± 0.09 | 0.31 ± 0.11 | 0.48 ± 0.11 | 0.32 ± 0.10 | |
| 1.02 ± 0.09 | 1.22 ± 0.09 | 1.15 ± 0.07 | 1.10 ± 0.07 | 1.06 ± 0.09 | 1.12 ± 0.08 | ||
| Oxt | 0.34 ± 0.19 | 0.16 ± 0.16 | 0.43 ± 0.14 | 0.28 ± 0.16 | 0.53 ± 0.15 | 0.32 ± 0.14 | |
| 1.11 ± 0.17 | 1.00 ± 0.17 | 1.26 ± 0.14 | 1.17 ± 0.14 | 1.23 ± 0.17 | 1.10 ± 0.15 | ||
| Oxtr | 1.41 ± 0.22 | 1.24 ± 0.19 | 1.39 ± 0.16 | 1.51 ± 0.19 | 1.41 ± 0.18 | 1.38 ± 0.17 | |
| 1.22 ± 0.31 | 1.60 ± 0.32 | 1.42 ± 0.26 | 1.45 ± 0.26 | 1.95 ± 0.35 | 1.61 ± 0.27 | ||
Values are means ± standard error of the mean. Data are expressed as the ratio to L7a.
Table 6.
P10 (Top Number) and P90 (Bottom Number) Hypothalamic Gene Expression in Females
| Gene |
CON |
HFD |
ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Oil | 200 | 1000 | Oil | 200 | 1000 | ||
| Endocrine system | |||||||
| Avp | 0.36 ± 0.23 | 0.41 ± 0.26 | 0.43 ± 0.20 | 0.59 ± 0.23 | 0.20 ± 0.26 | 0.56 ± 0.23 | |
| 1.32 ± 0.14 | 0.69 ± 0.16 | 0.87 ± 0.13 | 1.07 ± 0.13 | 1.01 ± 0.15 | 1.05 ± 0.15 | ||
| Avpr1a | 1.04 ± 0.10 | 0.70 ± 0.11 | 1.03 ± 0.09 | 0.81 ± 0.10 | 0.98 ± 0.12 | 1.12 ± 0.10 | Diet, P = 0.015 |
| 1.02 ± 0.10 | 1.04 ± 0.11 | 0.97 ± 0.09 | 0. 87 ± 0.09 | 0.86 ± 0.10 | 0.71 ± 0.10 | ||
| Avpr1b | 1.32 ± 0.28 | 1.89 ± 0.34 | 1.07 ± 0.25 | 0.97 ± 0.28 | 1.35 ± 0.31 | 1.25 ± 0.28 | |
| 1.72 ± 0.34 | 1.66 ± 0.38 | 1.94 ± 0.36 | 1.24 ± 0.30 | 1.73 ± 0.37 | 1.55 ± 0.37 | ||
| Cd38 | 0.37 ± 0.11 | 0.51 ± 0.12 | 0.34 ± 0.09 | 0.48 ± 0.11 | 0.38 ± 0.11 | 0.44 ± 0.11 | |
| 1.07 ± 0.09 | 1.01 ± 0.10 | 0.86 ± 0.09 | 0.96 ± 0.08 | 0.79 ± 0.10 | 0.91 ± 0.09 | ||
| Oxt | 0.23 ± 0.19 | 0.32 ± 0.21 | 0.10 ± 0.18 | 0.45 ± 0.19 | 0.02 ± 0.20 | 0.51 ± 0.19 | |
| 1.34 ± 0.16 | 1.04 ± 0.17 | 1.35 ± 0.15 | 1.14 ± 0.14 | 1.40 ± 0.17 | 1.42 ± 0.16 | ||
| Oxtr | 1.52 ± 0.20 | 1.24 ± 0.24 | 1.51 ± 0.17 | 1.19 ± 0.20 | 2. 20 ± 0.22 | 1.48 ± 0.20 | Trt × diet, P = 0.012 |
| 1.22 ± 0.21 | 1.22 ± 0.23 | 1.68 ± 0.20 | 1.16 ± 0.19 | 1.00 ± 0.25 | 1.25 ± 0.21 | ||
Values are means ± standard error of the mean. Data are expressed as the ratio to L7a.
Abbreviation: Trt, treatment.
In males at P10, there were significant diet effects in Ldlr [F(1, 34) = 4.133, P = 0.050], Cat [F(1, 34) = 5.747, P = 0.022], and Drd2 [F(1, 31) = 4.684, P = 0.038] expression within the mPFC, such that a perinatal HFD increased the expression of these genes. There was also a significant exposure effect in Drd2 [F(2, 31) = 4.183, P = 0.025] expression within the mPFC, such that the relatively high phthalate–exposed males had a greater gene expression of Drd2 than vehicle-exposed males (P = 0.014). There were no significant effects in gene expression within the hypothalamus of males at P10 or P90.
In the male mPFC at P90, there was a significant diet-by-exposure interaction in Ar expression [F(2, 33) = 5.989, P = 0.006], such that within the vehicle-exposed males, a perinatal HFD, compared with CON, lowered Ar expression (P = 0.005). Additionally, within the CON males, the relatively high phthalate–exposed males had lower Ar expression than vehicle-exposed males (P = 0.010). At P90, there were also diet effects in Esr1 [F(1, 33) = 12.120, P = 0.001], Esr2 [F(1, 31) = 4.341, P = 0.046], and Esrrg [F(1, 32) = 6.443, P = 0.016], such that the perinatal HFD group had lower expressions of these receptors in comparison with the CON groups. There were significant exposure effects in the expression of Essrg [F(2, 32) = 3.795, P = 0.033] and Thra [F(2, 33) = 3.933, P = 0.030], but nothing was significant with further analysis. There was also a significant exposure effect in Sod3 expression [F(2, 33) = 3.946, P = 0.029], which revealed that the relatively high phthalate–exposed males had lower Sod3 expression than vehicle-exposed males (P = 0.096).
In females at P10, there was a diet effect in Ifng [F(1, 28) = 4.684, P = 0.038] expression within the mPFC, such that females perinatally exposed to CON had increased Ifng gene expression. Although there was a significant exposure effect in AVPr1b within the mPFC, neither phthalate dose differed from controls. Within the hypothalamus, there was only a significant diet-by-exposure interaction in Oxtr gene expression [F(2, 32) = 5.065, P = 0.012], such that, within the HFD group, the relatively low phthalate–exposed females had greater Oxtr expression than vehicle-exposed females (P = 0.006). There was also a significant diet effect within the 200 µg phthalates/kg–exposed females (P = 0.013), such that a perinatal HFD increased Oxtr expression compared with CON within the relatively low phthalate–exposed females.
In the female mPFC at P90, there was a significant diet effect in Oxtr expression [F(1, 34) = 5.364, P = 0.027], such that a perinatal HFD lowered the expression of Oxtr in comparison with CON. There were also significant diet-by-exposure interactions in the expression of Glrx [F(2, 30) = 4.763, P = 0.016] and Bad [F(2, 29) = 3.317, P = 0.050], such that further analyses only revealed a diet effect in Glrx expression in vehicle-exposed females, where a perinatal HFD lowered expression in comparison with an HFD (P = 0.011). In the hypothalamus at P90, there was only a diet effect on the expression of Avpr1a [F(1, 33) = 6.561, P = 0.015], such that the CON group displayed higher levels of expression compared with an HFD.
Oxidative stress markers
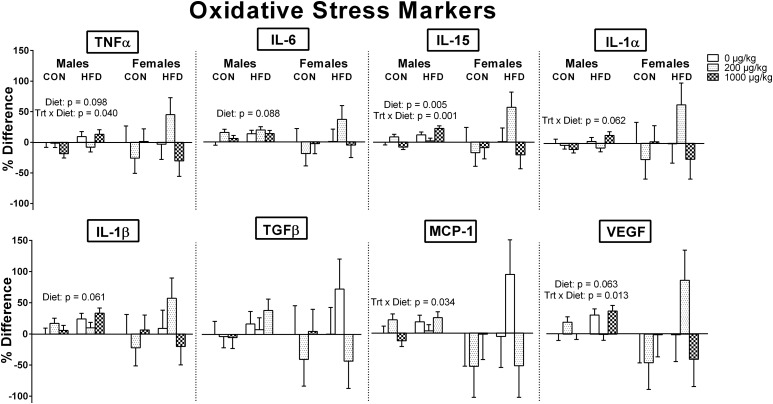
Although oxidative stress markers were numerically increased in HFD groups within the mPFC, only in males were there indications of significance (Fig. 6). In particular, an HFD in males significantly increased IL-15 [F(1, 24) = 9.443, P = 0.005], and there were nonsignificant trends for an increase in TNFα [F(1, 24) = 2.967, P = 0.098], IL-1β [F(1, 24) = 3.858, P = 0.061], IL-6 [F(1, 24) = 3.153, P = 0.088], and VEGF [F (1, 24) = 3.807, P = 0.063]. The other three oxidative stress markers in males (TGFβ, MCP-1, and IL-1α) and all of the oxidative stress markers for females were also in the same direction, but not significant.
Figure 6.
Oxidative stress markers within the mPFC of males and females at P10. In general, oxidative stress markers were numerically increased in HFD groups within the mPFC, but only in males were there actual indications, as seen in IL-15 and in the nonsignificant trends in TNFα, IL-1β, IL-6, and VEGF. There were no significant exposure effects in either sex, but there were, in males only, significant exposure-by-diet interactions in TNFα, MCP-1, IL-15, and VEGF that indicated a perinatal HFD had a significant effect on increasing TNFα (P = 0.012), MCP-1 (P = 0.029), IL-15 (P < 0.001), and VEGF (P = 0.017) only within the relatively high phthalate–exposed males. Trt, treatment.
There were no significant phthalate exposure effects in either sex, but there were, in males only, significant exposure-by-diet interactions in TNFα [F(2, 24) = 3.676, P = 0.040], MCP-1 [F(2, 24) = 3.921, P = 0.034], IL-15 [F(2, 24) = 9.824, P = 0.001], and VEGF [F(2, 24) = 5.194, P = 0.013]. Post hoc tests revealed that a perinatal HFD had a significant effect on increasing TNFα (P = 0.012), MCP-1 (P = 0.029), IL-15 (P < 0.001), and VEGF (P = 0.017) only within the relatively high phthalate–exposed males.
Discussion
This is, to our knowledge, the first study to assess the effects of low doses of an environmentally relevant mixture of phthalates crossed with an HFD. Here, perinatal exposure to phthalates resulted in a small, dose-dependent decrease in periadolescent social play behavior in males as well as a modest decrease in the prepubertal body weight of males across phthalate doses. These effects were independent of maternal care, as phthalate exposure did not affect maternal behaviors. Overall, a maternal HFD did not exacerbate phthalate-induced effects; however, an HFD generally increased oxidative stress markers within males exposed to the higher dose of the phthalate mixture. A maternal HFD also had effects of its own. Dams fed an HFD consumed more calories, had greater gestational weight gain, displayed more maternal care, and nourished pups more, such that an early postnatal HFD generally increased pup body weight that persisted only in males into adulthood. Additionally, similar to what is observed in the periphery, an HFD tended to increase oxidative stress markers within the mPFC at P10 in both males and females.
The current study indicates that perinatal exposure to phthalates can have a modest but lasting impact on social behaviors in both males and females. In particular, perinatal phthalate exposure resulted in a small, dose-specific reduction in active social play behaviors in males, whereas females at the lower phthalate dose in the HFD group showed more isolated behaviors during social interaction testing. These results are compatible with associations made in the human literature, which indicate an impact of prenatal phthalate exposure on social deficits (18) and withdrawn behavior (50).
The only other study in rodents that explored the effects of perinatal phthalate exposure on social behaviors has also shown an effect. Quinnies et al. (33) found that, in both males and females, only the higher doses (i.e., 40 and 400 µg/kg) of DEHP affected social interactions in juvenile mice. Specifically, the higher doses resulted in less sitting, both alone and side by side, as well as more investigative sniffing and independent exploring. Comparing these effects to ours is difficult given the species and age differences, as well as a single phthalate to a mixture. However, the doses of DEHP that affected behavior are similar to the levels of DEHP in our mixture: 42 and 211 µg/kg DEHP at the relatively low and high doses of the mixture, respectively. Given that the high dose of the mixture did not affect social behaviors, it may suggest that the higher doses of other phthalates within the mixture may compete with or counteract the effects of DEHP alone. Moreover, nonmonotonic responses from phthalate exposure are not uncommon in the rodent literature (51, 52).
Social play is known to occur more frequently in males and is affected by androgen exposure during the neonatal period. For example, prepubertal castration decreases male social play, whereas masculinization of females from neonatal exposure to androgens increased later rates of social play (53). In addition, neonatal exposure to an androgen receptor (AR) antagonist blocks the masculinization of social play (40). Although androgens play an important role in the organization of male-typical social play, other hormones can affect social play. For instance, lowering endogenous levels of progesterone neonatally can increase play in males (54), presumably because of the weak androgenic actions of progestins, which can compete with other androgens (55). Also, dose-dependent effects on social play are seen with estrogen, where neonatal exposure to higher doses (100 µg) of estradiol benzoate can increase social play in females (56). Although this suggests that estrogens have an influence on social play, estrogens are known to increase AR expression in the brain (57), even during the neonatal period (58), and result in more AR protein in adulthood (59).
Considering the influence of perinatal hormone exposure on social play, we speculate that the relatively low dose of the phthalate mixture may generally be antiandrogenic. DiBP, benzyl butyl phthalate, and dibutyl phthalate are the only parent phthalates from the mixture that directly antagonize the AR (8); however, DEHP also is commonly known for its antiandrogenic effects (60), presumably due to secondary metabolites disrupting steroidogenesis (61) and antagonizing ARs (62). Although considerably less is known about the actions of DiNP and DEP, there are indications that DiNP may have antiandrogenic activity (63) and DEP may have estrogenic activity (64). Still, the specific cellular mechanisms behind these endocrine-disrupting activities are not clear. Furthermore, precisely why the low, but not the high, dose of our phthalate mixture affected social play is unknown; however, dose-dependent effects are not uncommon in the literature on phthalates (28, 33). Also, other mechanisms, such as the capacity of some phthalates to antagonize cannabinoid receptor type 1 (12), may be involved, as the neonatal endocannabinoid system has recently been shown to contribute to the development of sex differences in social play (65).
The current study also found that an environmentally relevant mixture of phthalates did not affect maternal behaviors, which is consistent with Quinnies et al. (33), who found no differences in maternal care in mice exposed to DEHP. This suggests that the observed effects in offspring were directly due to phthalates and not to alterations in maternal care. Perinatal phthalate exposure also reduced prepubertal body weight in males. This is similar to what is found in single phthalate exposure studies, though it is generally observed across sexes (28, 45, 66). The lack of a body weight difference in prepubertal females may be due to the mixture, as opposed to a single phthalate, as well as the relatively low doses in the current study. This also may be why we did not observe distinct phthalate effects on pubertal onset in either males or females, unlike other single phthalate studies (67–69); however, in females, we observed that a perinatal HFD delayed pubertal onset compared with a CON within the relatively high phthalate–exposed females.
An HFD generally did not exacerbate phthalate-induced effects but had effects of its own. Here, HFD-fed dams consumed more calories and had greater gestational weight gain, which is commonly observed in the literature (70). However, this finding is not always observed, possibly due to the crude nature of body weight in comparison with adiposity, as well as differences in the duration of HFD exposure, percentage of calories from the three primary macronutrients, and fatty acid composition (71). In the current study, HFD dams nursed and licked their pups more than CON dams. This finding is also congruent with the existing literature. In fact, similar to our findings, other studies have shown that a maternal HFD increases nursing (34) and licking behaviors (35, 72). Additionally, this is similar to findings in our laboratory involving an HFD and another endocrine disruptor (bisphenol A), where dams fed an HFD spent more time in the nest with indications of more nursing (73). It is unclear why dams fed an HFD spent more time tending to their pups, but it could be as simple as being more quickly sated, decreasing the time spent away from the nest.
Maternal care during the perinatal period has a long-term influence on the behavior of the offspring (31), including social behaviors with cage mates (32). In spite of the lack of significant effects of diet on social play, we found that maternal licking positively correlated with periadolescent social play in both males and females. This is consistent with van Hasselt et al. (74) who found that the maternal licking and grooming received by male rats during the first postnatal week positively correlated with the frequency and duration of pouncing and pinning, two of the most distinct behaviors in social play. Although their correlation was only found in males, it is worth mentioning that dams have been found to lick male pups more frequently than female pups (75).
It is well-established that a maternal HFD can developmentally program offspring toward an increased adiposity phenotype due to dysregulated metabolism and behavioral changes (76). Here, given that HFD dams displayed more total nursing and have been shown to produce milk with higher fat content (34), it is unsurprising that their pups had higher postnatal body weights; however, this persisted only in males into adulthood. This effect was most prominent at P10 and waned at P25 in both sexes, which is likely due to the shorter duration and less severe HFD used in the current study, as many studies introduce an HFD, with 60% calories from fat, weeks before conception and continue it until weaning. Why the effect persisted into adulthood in males, but not females, is unknown, but it is possible that there may have been differences in maternal care between the sexes, as we did not identify pups during maternal behaviors. Additionally, the diet effect on male body weight at P90 was small (4%), compared with 11% at P10.
In this study, we demonstrated that there were generally no systematic or consistent effects on P10 or P90 gene expression within the mPFC or hypothalamus of males and females, with the exception of a diet effect that was observed in the mPFC of P90 males across the estrogen receptors (Esr1, Esr2, and Esrrg), such that a perinatal HFD decreased the expression of these receptors. This is consistent with a study that showed that HFD alters Esr1 and Esr2 expression in the cortex of male Wistar rats (77); however, there are differences compared with our study in the composition of the HFD, the age at exposure and duration, and the time of tissue collection. The lack, or direction, of effects in other genes may, in part, be due to the inclusion of several hypothalamic nuclei within the dissection, which could have diluted effects in specific nuclei.
Maternal HFD has been shown to augment both the expression of oxidative/inflammatory markers in the cortex of male offspring (7) and Oxtr in the prefrontal cortex of males but not females (36). Here, in contrast, of the 10 genes related to the cellular defense system and inflammation examined within the mPFC, only 3 were influenced by a maternal HFD at one of the time points (i.e., P10 or P90) in a sex-specific manner. Cat expression was increased due to a maternal HFD in males at P10, whereas in females, the expression of Ifng and, within the vehicle-exposed group, Glrx were actually decreased at P10 and P90, respectively. Moreover, there were no effects on the expression of Oxtr in the mPFC in males, whereas in females, there was a decrease in its expression at P90 due to a maternal HFD. Additionally, a perinatal HFD in mice has been shown to reduce the expression of dopamine receptors in the prefrontal cortex while increasing expression of the dopamine reuptake transporter and opioid-related genes in adulthood (78), although we found no effects in adulthood on dopamine receptor expression.
Consistent with the genetic expression data, the protein levels of oxidative stress markers did not show robust differences between groups in either sex; however, an HFD tended to increase oxidative stress markers within the mPFC of both males and females, although not as reliably as in the periphery (reviewed in 79). Additionally, there were significant exposure-by-diet interactions in males with respect to oxidative stress markers, indicating that an HFD increased these markers when combined with the high dose of the phthalate mixture. The significant interactions and diet effects were only found in males, which is consistent with the well-documented sex difference in offspring outcomes due to perinatal stressors, like a maternal HFD (79) or immune challenge (80).
Overall, perinatal exposure to an environmentally relevant mixture of phthalates and a maternal HFD had independent effects that only sporadically interacted other than in oxidative stress markers in males. The effects due to the mixture of phthalates were often small and either dose- or sex-specific. This study demonstrates that perinatal exposure to an environmentally relevant mixture of phthalates can modestly influence later behavior, without regard to diet.
Acknowledgments
We thank the Animal Care Staff in Psychology, Kaitlyn Wehrheim, and Kaanan Raja for help with dosing the animals and Dr. Amogh Belagodu for help with the enzyme-linked immunosorbent assay analysis.
Financial Support: This work was supported by National Institute of Environmental Health Sciences Grant P01 ES002848-Project 3 (to J.M.J.), US Environmental Protection Agency Grant 83543401 Project 3 (to J.M.J.), and National Institute of Environmental Health Sciences Grant T32 ES007326 (to D.G.K.).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANOVA
- analysis of variance
- AR
- androgen receptor
- AVPr1a
- arginine vasopressin receptor 1a
- Bad
- BCL2-associated agonist of cell death
- Cat
- catalase
- CON
- control diet
- DEHP
- di-(2-ethylhexyl) phthalate
- DEP
- diethyl phthalate
- DiBP
- diisobutyl phthalate
- DiNP
- diisononyl phthalate
- Drd1
- dopamine receptor 1
- Esr1
- estrogen receptor α
- Esr2
- estrogen receptor β
- Esrrg
- estrogen-related receptor γ
- GD
- gestational day
- Glrx
- glutaredoxin
- HFD
- high-fat diet
- Ifng
- interferon γ
- IL-1α
- interleukin-1α
- Ldlr
- low-density lipoprotein receptor
- MCP-1
- monocyte chemotactic protein-1
- mPFC
- medial prefrontal cortex
- Oxtr
- oxytocin receptor
- P
- postnatal day
- Sod1
- superoxide dismutase 1
- TGFβ
- transforming growth factor β
- Thra
- thyroid hormone receptor α
- TNFα
- tumor necrosis factor α
- VEGF
- vascular endothelial growth factor.
References
- 1.Pereira LC, de Souza AO, Franco Bernardes MF, Pazin M, Tasso MJ, Pereira PH, Dorta DJ. A perspective on the potential risks of emerging contaminants to human and environmental health. Environ Sci Pollut Res Int. 2015;22(18):13800–13823. [DOI] [PubMed] [Google Scholar]
- 2.Weschler CJ, Bekö G, Koch HM, Salthammer T, Schripp T, Toftum J, Clausen G. Transdermal uptake of diethyl phthalate and di(n-butyl) phthalate directly from air: experimental verification. Environ Health Perspect. 2015;123(10):928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210(5):623–634. [DOI] [PubMed] [Google Scholar]
- 4.Lyche JL, Gutleb AC, Bergman A, Eriksen GS, Murk AJ, Ropstad E, Saunders M, Skaare JU. Reproductive and developmental toxicity of phthalates. J Toxicol Environ Health B Crit Rev. 2009;12(4):225–249. [DOI] [PubMed] [Google Scholar]
- 5.Andersen RE. The spread of the childhood obesity epidemic. CMAJ. 2000;163(11):1461–1462. [PMC free article] [PubMed] [Google Scholar]
- 6.Kasahara E, Sato EF, Miyoshi M, Konaka R, Hiramoto K, Sasaki J, Tokuda M, Nakano Y, Inoue M. Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)phthalate. Biochem J. 2002;365(Pt 3):849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim SO, Hise TL, Keller JN, Ingram DK, Morrison CD, Bruce-Keller AJ. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol Dis. 2009;35(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi S, Iida M, Kobayashi S, Jin K, Matsuda T, Kojima H. Differential effects of phthalate esters on transcriptional activities via human estrogen receptors alpha and beta, and androgen receptor. Toxicology. 2005;210(2-3):223–233. [DOI] [PubMed] [Google Scholar]
- 9.David RM. Proposed mode of action for in utero effects of some phthalate esters on the developing male reproductive tract. Toxicol Pathol. 2006;34(3):209–219. [DOI] [PubMed] [Google Scholar]
- 10.Kim HS, Kim TS, Shin JH, Moon HJ, Kang IH, Kim IY, Oh JY, Han SY. Neonatal exposure to di(n-butyl) phthalate (DBP) alters male reproductive-tract development. J Toxicol Environ Health A. 2004;67(23-24):2045–2060. [DOI] [PubMed] [Google Scholar]
- 11.Liu PS, Tseng FW, Liu JH. Comparative suppression of phthalate monoesters and phthalate diesters on calcium signalling coupled to nicotinic acetylcholine receptors. J Toxicol Sci. 2009;34(3):255–263. [DOI] [PubMed] [Google Scholar]
- 12.Bisset KM, Dhopeshwarkar AS, Liao C, Nicholson RA. The G protein-coupled cannabinoid-1 (CB1) receptor of mammalian brain: inhibition by phthalate esters in vitro. Neurochem Int. 2011;59(5):706–713. [DOI] [PubMed] [Google Scholar]
- 13.Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol. 2012;355(2):240–248. [DOI] [PubMed] [Google Scholar]
- 14.Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebæk NE, Hegedüs L, Hilsted L, Juul A, Main KM. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect. 2010;118(10):1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang T, Saxena AR, Isganaitis E, James-Todd T. Gender and racial/ethnic differences in the associations of urinary phthalate metabolites with markers of diabetes risk: National Health and Nutrition Examination Survey 2001-2008. Environ Health. 2014;13(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smerieri A, Testa C, Lazzeroni P, Nuti F, Grossi E, Cesari S, Montanini L, Latini G, Bernasconi S, Papini AM, Street ME. Di-(2-ethylhexyl) phthalate metabolites in urine show age-related changes and associations with adiposity and parameters of insulin sensitivity in childhood. PLoS One. 2015;10(2):e0117831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mose T, Knudsen LE, Hedegaard M, Mortensen GK. Transplacental transfer of monomethyl phthalate and mono(2-ethylhexyl) phthalate in a human placenta perfusion system. Int J Toxicol. 2007;26(3):221–229. [DOI] [PubMed] [Google Scholar]
- 18.Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, Calafat AM, Wolff MS. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32(2):261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ejaredar M, Nyanza EC, Ten Eycke K, Dewey D. Phthalate exposure and childrens neurodevelopment: A systematic review. Environ Res. 2015;142:51–60. [DOI] [PubMed] [Google Scholar]
- 20.Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, Wolff MS. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect. 2010;118(4):565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lien YJ, Ku HY, Su PH, Chen SJ, Chen HY, Liao PC, Chen WJ, Wang SL. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan Maternal and Infant Cohort Study. Environ Health Perspect. 2015;123(1):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobrosly RW, Evans S, Miodovnik A, Barrett ES, Thurston SW, Calafat AM, Swan SH. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6-10 years of age. Environ Health Perspect. 2014;122(5):521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoshi H, Ohtsuka T. Adult rats exposed to low-doses of di-n-butyl phthalate during gestation exhibit decreased grooming behavior. Bull Environ Contam Toxicol. 2009;83(1):62–66. [DOI] [PubMed] [Google Scholar]
- 24.Dai Y, Yang Y, Xu X, Hu Y. Effects of uterine and lactational exposure to di-(2-ethylhexyl) phthalate on spatial memory and NMDA receptor of hippocampus in mice. Horm Behav. 2015;71:41–48. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Yang Y, Wang R, Wang Y, Ruan Q, Lu Y. Perinatal exposure to di-(2-ethylhexyl) phthalate affects anxiety- and depression-like behaviors in mice. Chemosphere. 2015;124:22–31. [DOI] [PubMed] [Google Scholar]
- 26.Carbone S, Ponzo OJ, Gobetto N, Samaniego YA, Reynoso R, Scacchi P, Moguilevsky JA, Cutrera R. Antiandrogenic effect of perinatal exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate increases anxiety-like behavior in male rats during sexual maturation. Horm Behav. 2013;63(5):692–699. [DOI] [PubMed] [Google Scholar]
- 27.Wang DC, Chen TJ, Lin ML, Jhong YC, Chen SC. Exercise prevents the increased anxiety-like behavior in lactational di-(2-ethylhexyl) phthalate-exposed female rats in late adolescence by improving the regulation of hypothalamus-pituitary-adrenal axis. Horm Behav. 2014;66(4):674–684. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Zhuang M, Li T, Shi N. Neurobehavioral toxicity study of dibutyl phthalate on rats following in utero and lactational exposure. J Appl Toxicol. 2009;29(7):603–611. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Li T, Zhuang M, Wang K, Zhang J, Shi N. High-dose dibutyl phthalate improves performance of F1 generation male rats in spatial learning and increases hippocampal BDNF expression independent on p-CREB immunocontent. Environ Toxicol Pharmacol. 2010;29(1):32–38. [DOI] [PubMed] [Google Scholar]
- 30.Boberg J, Christiansen S, Axelstad M, Kledal TS, Vinggaard AM, Dalgaard M, Nellemann C, Hass U. Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reprod Toxicol. 2011;31(2):200–209. [DOI] [PubMed] [Google Scholar]
- 31.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA. 1998;95(9):5335–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parent CI, Meaney MJ. The influence of natural variations in maternal care on play fighting in the rat. Dev Psychobiol. 2008;50(8):767–776. [DOI] [PubMed] [Google Scholar]
- 33.Quinnies KM, Harris EP, Snyder RW, Sumner SS, Rissman EF. Direct and transgenerational effects of low doses of perinatal di-(2-ethylhexyl) phthalate (DEHP) on social behaviors in mice. PLoS One. 2017;12(2):e0171977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purcell RH, Sun B, Pass LL, Power ML, Moran TH, Tamashiro KL. Maternal stress and high-fat diet effect on maternal behavior, milk composition, and pup ingestive behavior. Physiol Behav. 2011;104(3):474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertino M. Effects of high fat, protein supplemented diets on maternal behavior in rats. Physiol Behav. 1982;29(6):999–1005. [DOI] [PubMed] [Google Scholar]
- 36.Hehar H, Ma I, Mychasiuk R. Effects of metabolic programming on juvenile play behavior and gene expression in the prefrontal cortex of rats. Dev Neurosci. 2016;38(2):96–104. [DOI] [PubMed] [Google Scholar]
- 37.Pellis SM, Pasztor TJ. The developmental onset of a rudimentary form of play fighting in C57 mice. Dev Psychobiol. 1999;34(3):175–182. [PubMed] [Google Scholar]
- 38.Blake BE, McCoy KA. Hormonal programming of rat social play behavior: standardized techniques will aid synthesis and translation to human health. Neurosci Biobehav Rev. 2015;55:184–197. [DOI] [PubMed] [Google Scholar]
- 39.Olioff M, Stewart J. Sex differences in the play behavior of prepubescent rats. Physiol Behav. 1978;20(2):113–115. [DOI] [PubMed] [Google Scholar]
- 40.Meaney MJ, Stewart J, Poulin P, McEwen BS. Sexual differentiation of social play in rat pups is mediated by the neonatal androgen-receptor system. Neuroendocrinology. 1983;37(2):85–90. [DOI] [PubMed] [Google Scholar]
- 41.Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14(4):327–332. [DOI] [PubMed] [Google Scholar]
- 42.Sadowski RN, Wise LM, Park PY, Schantz SL, Juraska JM. Early exposure to bisphenol A alters neuron and glia number in the rat prefrontal cortex of adult males, but not females. Neuroscience. 2014;279:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HC, Yamanouchi K, Nishihara M. Effects of perinatal exposure to phthalate/adipate esters on hypothalamic gene expression and sexual behavior in rats. J Reprod Dev. 2006;52(3):343–352. [DOI] [PubMed] [Google Scholar]
- 44.Gao N, Hu R, Huang Y, Dao L, Zhang C, Liu Y, Wu L, Wang X, Yin W, Gore AC, Sun Z. Specific effects of prenatal DEHP exposure on neuroendocrine gene expression in the developing hypothalamus of male rats [published online ahead of print September 4, 2017]. Arch Toxicol. [DOI] [PubMed]
- 45.Dostal LA, Weaver RP, Schwetz BA. Transfer of di(2-ethylhexyl) phthalate through rat milk and effects on milk composition and the mammary gland. Toxicol Appl Pharmacol. 1987;91(3):315–325. [DOI] [PubMed] [Google Scholar]
- 46.Corbasson I, Hankinson SE, Stanek EJ III, Reeves KW. Urinary bisphenol-A, phthalate metabolites and body composition in US adults, NHANES 1999-2006. Int J Environ Health Res. 2016;26(5-6):606–617. [DOI] [PubMed] [Google Scholar]
- 47.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. [DOI] [PubMed] [Google Scholar]
- 48.Koch HM, Drexler H, Angerer J. An estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int J Hyg Environ Health. 2003;206(2):77–83. [DOI] [PubMed] [Google Scholar]
- 49.Van De Werd HJ, Uylings HB. Comparison of (stereotactic) parcellations in mouse prefrontal cortex. Brain Struct Funct. 2014;219(2):433–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, Diaz D, Quinn J, Adibi J, Perera FP, Factor-Litvak P. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect. 2012;120(2):290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrade AJ, Grande SW, Talsness CE, Grote K, Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): non-monotonic dose-response and low dose effects on rat brain aromatase activity. Toxicology. 2006;227(3):185–192. [DOI] [PubMed] [Google Scholar]
- 52.Do RP, Stahlhut RW, Ponzi D, Vom Saal FS, Taylor JA. Non-monotonic dose effects of in utero exposure to di(2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reprod Toxicol. 2012;34(4):614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meaney MJ, Stewart J. Neonatal-androgens influence the social play of prepubescent rats. Horm Behav. 1981;15(2):197–213. [DOI] [PubMed] [Google Scholar]
- 54.Birke LI, Sadler D. Modification of juvenile play and other social behaviour in the rat by neonatal progestins: further studies. Physiol Behav. 1984;33(2):217–219. [DOI] [PubMed] [Google Scholar]
- 55.Birke LI, Sadler D. Effects of modulating neonatal progestins and androgens on the development of play and other social behavior in the rat. Horm Behav. 1988;22(2):160–171. [DOI] [PubMed] [Google Scholar]
- 56.Olesen KM, Jessen HM, Auger CJ, Auger AP. Dopaminergic activation of estrogen receptors in neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinology. 2005;146(9):3705–3712. [DOI] [PubMed] [Google Scholar]
- 57.Burgess LH, Handa RJ. Hormonal regulation of androgen receptor mRNA in the brain and anterior pituitary gland of the male rat. Brain Res Mol Brain Res. 1993;19(1-2):31–38. [DOI] [PubMed] [Google Scholar]
- 58.McAbee MD, Doncarlos LL. Estrogen, but not androgens, regulates androgen receptor messenger ribonucleic acid expression in the developing male rat forebrain. Endocrinology. 1999;140(8):3674–3681. [DOI] [PubMed] [Google Scholar]
- 59.Handa RJ, Roselli CE, Horton L, Resko JA. The quantitative distribution of cytosolic androgen receptors in microdissected areas of the male rat brain: effects of estrogen treatment. Endocrinology. 1987;121(1):233–240. [DOI] [PubMed] [Google Scholar]
- 60.Christiansen S, Boberg J, Axelstad M, Dalgaard M, Vinggaard AM, Metzdorff SB, Hass U. Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reprod Toxicol. 2010;30(2):313–321. [DOI] [PubMed] [Google Scholar]
- 61.Piché CD, Sauvageau D, Vanlian M, Erythropel HC, Robaire B, Leask RL. Effects of di-(2-ethylhexyl) phthalate and four of its metabolites on steroidogenesis in MA-10 cells. Ecotoxicol Environ Saf. 2012;79:108–115. [DOI] [PubMed] [Google Scholar]
- 62.Stroheker T, Cabaton N, Nourdin G, Régnier JF, Lhuguenot JC, Chagnon MC. Evaluation of anti-androgenic activity of di-(2-ethylhexyl)phthalate. Toxicology. 2005;208(1):115–121. [DOI] [PubMed] [Google Scholar]
- 63.Gray LE Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58(2):350–365. [DOI] [PubMed] [Google Scholar]
- 64.Kumar N, Sharan S, Srivastava S, Roy P. Assessment of estrogenic potential of diethyl phthalate in female reproductive system involving both genomic and non-genomic actions. Reprod Toxicol. 2014;49:12–26. [DOI] [PubMed] [Google Scholar]
- 65.Argue KJ, VanRyzin JW, Falvo DJ, Whitaker AR, Yu SJ, McCarthy MM. Activation of both CB1 and CB2 endocannabinoid receptors is critical for masculinization of the developing medial amygdala and juvenile social play behavior. eNeuro. 2017;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin Y, Wei J, Li Y, Chen J, Zhou Z, Song L, Wei Z, Lv Z, Chen X, Xia W, Xu S. Developmental exposure to di(2-ethylhexyl) phthalate impairs endocrine pancreas and leads to long-term adverse effects on glucose homeostasis in the rat. Am J Physiol Endocrinol Metab. 2011;301(3):E527–E538. [DOI] [PubMed] [Google Scholar]
- 67.Hu J, Du G, Zhang W, Huang H, Chen D, Wu D, Wang X. Short-term neonatal/prepubertal exposure of dibutyl phthalate (DBP) advanced pubertal timing and affected hypothalamic kisspeptin/GPR54 expression differently in female rats. Toxicology. 2013;314(1):65–75. [DOI] [PubMed] [Google Scholar]
- 68.Grande SW, Andrade AJ, Talsness CE, Grote K, Chahoud I. A dose-response study following in utero and lactational exposure to di(2-ethylhexyl)phthalate: effects on female rat reproductive development. Toxicol Sci. 2006;91(1):247–254. [DOI] [PubMed] [Google Scholar]
- 69.Andrade AJ, Grande SW, Talsness CE, Grote K, Golombiewski A, Sterner-Kock A, Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): effects on androgenic status, developmental landmarks and testicular histology in male offspring rats. Toxicology. 2006;225(1):64–74. [DOI] [PubMed] [Google Scholar]
- 70.White CL, Purpera MN, Morrison CD. Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1464–R1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sullivan EL, Smith MS, Grove KL. Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology. 2011;93(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Connor KL, Vickers MH, Beltrand J, Meaney MJ, Sloboda DM. Nature, nurture or nutrition? Impact of maternal nutrition on maternal care, offspring development and reproductive function. J Physiol. 2012;590(9):2167–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wise LM, Boas SM, Juraska JM Effects of perinatal bisphenol A and high fat diet on adult anxiety and social behavior. Program No. 730.03. Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2016. Online. 2016. [Google Scholar]
- 74.van Hasselt FN, Tieskens JM, Trezza V, Krugers HJ, Vanderschuren LJ, Joëls M. Within-litter variation in maternal care received by individual pups correlates with adolescent social play behavior in male rats. Physiol Behav. 2012;106(5):701–706. [DOI] [PubMed] [Google Scholar]
- 75.Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. J Comp Physiol Psychol. 1979;93(4):677–684. [DOI] [PubMed] [Google Scholar]
- 76.Frihauf JB, Fekete EM, Nagy TR, Levin BE, Zorrilla EP. Maternal Western diet increases adiposity even in male offspring of obesity-resistant rat dams: early endocrine risk markers. Am J Physiol Regul Integr Comp Physiol. 2016;311(6):R1045–R1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scudiero R, Verderame M. Gene expression profile of estrogen receptors alpha and beta in rat brain during aging and following high fat diet. C R Biol. 2017;340(8):372–378. [DOI] [PubMed] [Google Scholar]
- 78.Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151(10):4756–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bolton JL, Bilbo SD. Developmental programming of brain and behavior by perinatal diet: focus on inflammatory mechanisms. Dialogues Clin Neurosci. 2014;16(3):307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Howerton CL, Bale TL. Prenatal programing: at the intersection of maternal stress and immune activation. Horm Behav. 2012;62(3):237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]