Abstract
Background
Previous studies have shown that a low dose of scopolamine produces rapid-acting antidepressant-like actions in rodents. Understanding the mechanisms underlying this effect and the dose-dependent variations of drug responses remains an important task. L-type voltage-dependent calcium channels were found to mediate rapid-acting antidepressant effects of certain medications (e.g., ketamine). Therefore, it is of great interest to determine the involvement of L-type voltage-dependent calcium channels in the action of scopolamine.
Methods
Herein, we investigated the mechanisms underlying behavioral responses to various doses of scopolamine in mice to clarify the involvement of L-type voltage-dependent calcium channels in its modes of action. Open field test, novel object recognition test, and forced swimming test were performed on mice administered varied doses of scopolamine (0.025, 0.05, 0.1, 1, and 3 mg/kg, i.p.) alone or combined with L-type voltage-dependent calcium channel blocker verapamil (5 mg/kg, i.p.). Then, the changes in brain-derived neurotrophic factor and neuropeptide VGF (nonacronymic) levels in the hippocampus and prefrontal cortex of these mice were analyzed.
Results
Low doses of scopolamine (0.025 and 0.05 mg/kg) produced significant antidepressant-like effects in the forced swimming test, while higher doses (1 and 3 mg/kg) resulted in significant memory deficits and depressive-like behaviors. Moreover, the behavioral changes in responses to various doses may be related to the upregulation (0.025 and 0.05 mg/kg) and downregulation (1 and 3 mg/kg) of brain-derived neurotrophic factor and VGF in the hippocampus and prefrontal cortex in mice. We further found that the rapid-acting antidepressant-like effects and the upregulation on brain-derived neurotrophic factor and VGF produced by a low dose of scopolamine (0.025 mg/kg) were completely blocked by verapamil.
Conclusions
These results indicate that L-type voltage-dependent calcium channels are likely involved in the behavioral changes in response to various doses of scopolamine through the regulation of brain-derived neurotrophic factor and VGF levels.
Keywords: L-type voltage-dependent calcium channel, scopolamine, brain-derived neurotrophic factor, neuropeptide VGF, cognitive deficits and depression-like behaviors
Significance Statement
Previous studies demonstrate that a low dose of nonselective muscarinic receptor antagonist scopolamine produces rapid antidepressant actions relative to conventional antidepressants, while high doses of this drug result in potential memory impairment. Herein, we demonstrate that low and high doses of scopolamine produce different effects on depression-like and cognitive behaviors along with opposite-directional changes in BDNF and VGF levels in the hippocampus and prefrontal cortex. We further reveal that L-VDCC blocker verapamil significantly blocked the effects of low-dose scopolamine, suggesting that L-VDCC may be involved in the changes of behavior as well as BDNF and VGF levels produced by scopolamine treatment.
Introduction
Conventional antidepressants lack efficacy in many patients (treatment-resistant depression) and generally take weeks to produce a full therapeutic response in others. This is particularly concerning given the high suicide risk in major depressive disorder (MDD) (Trivedi et al., 2006). Previous studies have demonstrated that specific drugs such as ketamine are rapidly acting antidepressants for resistant MDD patients (Bartoli et al., 2017; Duncan et al., 2017). Recently, clinical (Janowsky et al., 1972; Furey et al., 2015) and preclinical (Podkowa et al., 2016; Martin et al., 2017) studies suggest that scopolamine was also reported to produce rapid and long-lasting antidepressant effects and has shown similarities in the underlying mechanisms with ketamine. However, scopolamine antagonizes muscarinic receptors and may cause cognitive deficits at high doses (Klinkenberg and Blokland, 2010). The current study therefore aims to reveal the mechanisms underlying the varied effects of scopolamine at different doses on cognitive changes and depressive-like behaviors in mice.
Previous neuropharmacology studies indicate that brain derived neurotrophic factor (BDNF) and neuropeptide VGF (nonacronymic) related signaling pathways play important roles in the action of multiple antidepressants. For example, murine studies have demonstrated the putative roles of BDNF and neuropeptide VGF (nonacronymic) in the prefrontal cortex and/or hippocampus in the rapid-acting antidepressant-like actions of ketamine (Li et al., 2010; Duman and Aghajanian, 2012; Voleti et al., 2013; Pazini et al., 2016; Girgenti et al., 2017) and GLYX-13 (Lu et al., 2014). Previous mechanistic studies have shown that the rapid onset of antidepressant-like effect at lower doses of scopolamine shares the same signaling cascades with ketamine by activating glutamate cycling activation and the mammalian target of rapamycin complex 1 (Chowdhury et al., 2017; Wohleb et al., 2017; Voleti et al., 2013). Therefore, it was hypothesized that ketamine-associated BDNF and/or VGF pathways might also mediate the action of scopolamine. Indeed, our recent study suggested that BDNF/tropomyosin-related kinase receptor B (TrkB) signaling likely mediated antidepressant-like activities of muscarinic acetylcholine receptor antagonists (Zhou et al., 2017), supporting the above hypothesis. Notably, the stimulation of L-type voltage-dependent calcium channel (L-VDCC) plays a critical role in the release of BDNF (Jourdi et al., 2009) and the rapid-acting antidepressant effects of ketamine (Li et al., 2010; Duman and Aghajanian, 2012; Voleti et al., 2013; Pazini et al., 2016; Girgenti et al., 2017). However, whether L-VDCC is involved in scopolamine’s regulation of BDNF and neuropeptide VGF (nonacronymic) remains unknown. Herein, we sought to investigate whether pretreatment with verapamil, a phenylakylamine calcium channel antagonist, can modulate behavioral changes induced by various doses of scopolamine and neurotrophins (e.g., BDNF and VGF) in the hippocampus and prefrontal cortex of mice.
Materials and Methods
Animals
Adult male C57BL/6J mice (10–12 weeks old upon arrival) were born and reared in the animal facility of Ningbo University Medical School, China. All animals were maintained at 22°C ±3°C and 60%±5% relative humidity under a 12-hour-light/-dark cycle (lights on at 7:00 am) with ad libitum access to food and water. All procedures involving animals were conducted following the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996) as well as the European Community Council Directive for the Care and Use of Laboratory Animals of 22 September 2010 (2010/63/EU). All the experiments were approved by the Institutional Animal Care and Use Committee of the Medical School of Ningbo University.
Drugs
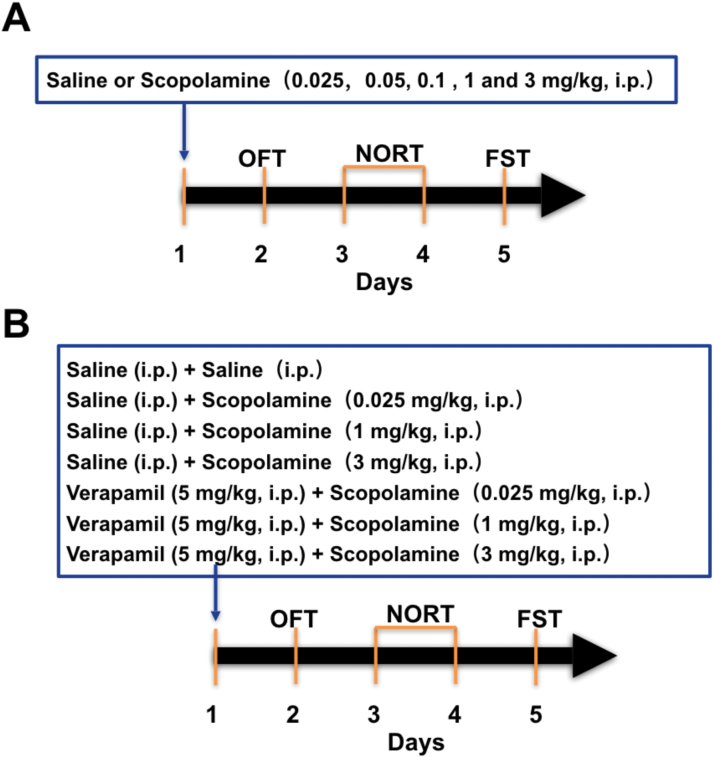
Scopolamine hydrochloride (Sigma-Aldrich) and verapamil hydrochloride were dissolved in 0.9% saline and injected i.p. with an injection volume of 10 mL/kg body weight at doses of 0.025, 0.05, 0.1, 1, and 3 mg/kg. Controlgroups received i.p. saline injections with matched volumes. The experimental regimen is shown in Figure 1. To explore the behavioral changes induced by scopolamine, mice weregrouped and received a single injection of the drug at various doses (0.025, 0.05, 0.1, 1, and 3 mg/kg, i.p.). After 24 hours, behavioral changes were examined using the open field test (OFT), novel object recognition test (NORT), and forced swimming test (FST) (Figure 1A). To test the involvement of L-VDCC in the rapid-acting antidepressant-like effects of scopolamine, L-VDCC blocker verapamil was administered 60 minutes before treatment of scopolamine (0.025, 1, and 3 mg/kg, i.p.) or saline (i.p.) on day 1, and then the OFT, NORT, and FST were conducted from day 2 to day 5 successively (Figure 1B).
Figure 1.
Schematic timeline of drug administration and behavioral testing. (A) Experimental procedure for the various scopolamine dose groups and cognitive and depression-like behaviors in the mice. (B) Experimental procedure for the assessment of the effects of L-type voltage-dependent calcium channel (L-VDCC) blocker verapamil in the regulation of behavioral changes and brain derived neurotrophic factor (BDNF) and VGF levels produced by various doses of scopolamine.
Behavioral Tests
OFT and NORT
OFT was performed to estimate the possible effects of drug treatment on locomotor activity in mice. An apparatus made of a white Plexiglas box (50×50×39 cm) with the floor divided into 4 identical squares in a dim room was used for the test, and mice were habituated to an empty apparatus for 5 minutes to test locomotor activity. Specifically, each mouse was placed individually into the center of the arena and free exploration was recorded. The number of rearings (forepaws elevated from the floor) and line crossings (with all 4 paws placed into a new square) were considered to be an index of exploratory behaviors. After each trial, the instrument was cleaned with 1% ethanol.
Twenty-four hours after the habituation and OFT, the NORT was conducted to quantify intact recognition memory of the mice. This test is based on the tendency of mice to spend more time exploring a novel object than a familiar one. First, an acquisition trial was conducted by leaving the animals in the apparatus containing 2 identical objects (A and A’). Twenty-four hours later, recognition memory was evaluated, and a different pair of dissimilar objects (a familiar and a novel one; A and B, respectively) was presented. Each mouse was placed in the apparatus facing the wall and allowed to explore the objects for 5 minutes, after which the mouse was returned to its home cage. Their behavior was recorded by a video camera mounted vertically above the test arena and analyzed using appropriated video-tracking software (Duoyi). Object exploration was defined as when the mouse touched or sniffed the object from no more than 2 cm away, and the duration spent exploring each object was scored by an observer blinded to the treatmentgroup. Exploration duration recorded was converted to a recognition index (RI) defined as RI=Tn/(Tn+Tf), where Tn is time spent exploring the novel object, and Tf is the time spent exploring the familiar object. Between trials, the apparatus was cleaned with 1% ethanol solution to hide animal clues. The light and sound inside the apparatus were maintained at a minimum level to reduce anxiety behavior induced by environmental factors.
FST
The FST is one of the most commonly used assays for the study of depressive-like behavior in rodents. The FST was conducted in a sound-attenuated room eliminated by white light (40 luminous flux). Briefly, mice were placed individually in a clear plastic cylinder (height: 25 cm; diameter: 10 cm) containing 10 cm of fresh water at 23°C±2°C for 6 minutes, and the duration of immobility was scored during the last 4 minutes. Immobility was recognized when a mouse stopped struggling and continued with only slight movements to keep its head above water.
Western-Blot Analysis
Total proteins of the hippocampal and prefrontal cortex tissues of each mice (n=3/group) were extracted using ice-cold radio immunoprecipitation assay lysis buffer (Pierce) containing 50 mM Tris-HCl (pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS; Upstate). The lysates were then centrifuged (15000×g, 30 minutes, 4°C), and the protein concentration of each sample lysate was determined using the BCA kit (Thermo Scientific). The sample lysates (20 μg total protein) were separated on 10% SDS-polyacrylamide gel electrophoresis gels and transferred to PVDF membranes (0.22 μm; Millipore). Membranes were then incubated with anti-BDNF (1:800; Millipore), anti-VGF (1:1000; Cell Signaling), and rabbit anti-β-actin (1:2000; Millipore) at 4°C overnight. The membranes were then incubated with Alexa Fluor 700 (for VGF) or 800 (for BDNF and β-actin)-conjugated antibody (1:10000; Invitrogen) for 60 minutes. Target bands were detected and quantified using a fluorescence scanner (Odyssey Infrared Imaging System, LI-COR Biotechnology).
Immunohistochemistry
Immunohistochemistry was performed to quantify the densities of VGF and BDNF (the cells showing positive staining). At killing time point, animals (n=5/group) under pentobarbital anesthesia were transcardially perfused with 250 mL cold saline solution followed by 250 mL of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS, pH 7.4). Brains were removed and postfixed in the same fixative overnight and stored in 10%, 20%, and 30% sucrose solution at 4◦C for dehydration. The brains were then quenched in cold N-hexane at −60◦C for 20 seconds and stored at −80◦C until required. Serial coronal sections of the prefrontal cortex and hippocampus (30 μm thick; Paxinos and Watson, 2005) were collected on a cryostat (Leica). Free-floating sections were permeabilized with 0.2% TritonX-100 in PBS for 15 minutes, blocked in PBS containing 5% donkey serum for 1 hour at room temperature, and then incubated with anti-VGF (1:400, Abcam) and anti-BDNF (1:800, Abcam) antibodies overnight at 4◦C. The next day, all slices were rinsed and washed in the PBS and then incubated with fluorescent secondary antibodies, donkey anti rabbit conjugated with Alexa Fluor 488 (1:1000; Invitrogen), and donkey anti goat conjugated with Alexa Fluor 594 (1:1000; Invitrogen) for 1 hour at room temperature. DNA (nuclei) was stained with DAPI for 15 minutes, mounted onto slides, and coverslipped with ProLong (Invitrogen). The images were captured using the Olympus system and analyzed with ImageJ software.
Statistical Analysis
All measurements were performed by an independent investigator blinded to the experimental conditions. Data are presented as the mean ± SEM. One-way ANOVA followed by Newman-Keuls posthoc test using GraphPad Prism software (Version 6.0, Prism software for PC) was performed to analyze the data, and P<.05 was considered significant.
Results
Effects of Different Doses of Scopolamine on Locomotor Activity, Recognition, and Depression-Like Behaviors in Mice
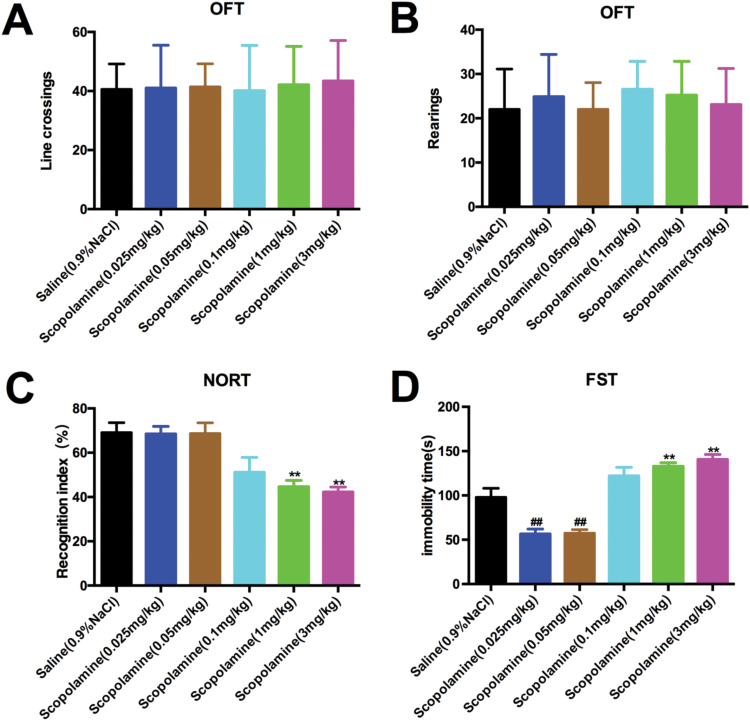
The OFT was conducted first to explore whether various doses of scopolamine (0.025, 0.05, 0.1, 1, and 3 mg/kg, i.p.) produced alterations of locomotor activity in mice. We found that scopolamine treatments had no effect on line crossings [F (5, 48)=0.08283, P=.9946; Figure 2A] or rearings [F (5, 48)=0.5072, P=.7694; Figure 2B] compared with the saline-treated group, indicating that behavioral changes observed in the subsequent NORT and FST were not attributed to alterations in locomotor activity. Following the OFT, NORT was conducted to examine the novel object recognition. We saw significant reduction of the object recognition index in the retention session in mice treated with 1 and 3 mg/kg scopolamine compared with the saline-treatedgroup [F (5, 48)=8.613, P<.01; Figure 2C], suggesting that scopolamine at these 2 doses induced cognitive impairment. However, no significant alterations in cognition were observed in these 2groups during the training session (data not shown).
Figure 2.
Various doses of scopolamine-induced behavioral changes in mice. To measure general locomotor activity, we used the open field test (OFT). Animals were placed in a novel environment during a 5-minute session. (A) Line crossings and (B) rearing measurement of total distance traveled in time segments of 5 minutes. (C) Novel object recognition test (NORT). 24 hours after acquisition trial, the recognition index was measured while the animals were allowed to explore the familiar and novel objects for 5 minutes. (D) The increase in time spent immobile during the forced swimming test (FST) was also defined as depression-like behavior. Data are expressed as the mean±SEM (n=9/group); comparisons were made using the 1-way ANOVA followed by Newman-Keuls posthoc test. Significantly different from vehicle-treated group (saline plus saline). **P<.01 for the drug-treatment group vs the control group.
In the FST for examination of depression-like behaviors, mice treated with low doses of scopolamine (0.025 and 0.05 mg/kg, i.p.) demonstrated significantly shorter periods of immobility than those treated with saline [F (5, 48)=28.69, P<.01 for both doses] (Figure 2D), indicating antidepressant-like effects of scopolamine at these 2 doses in mice. However, high doses scopolamine (1 and 3 mg/kg, i.p.)-treated mice exhibited significantly increased immobility time compared with the saline-treated mice, thereby showing depression-like behaviors [F (5, 48)=28.69, P<.01 for both doses] (Figure 2D).
Effects of Different Doses of Scopolamine on the Levels of BDNF and VGF in the Murine Hippocampus and Prefrontal Cortex
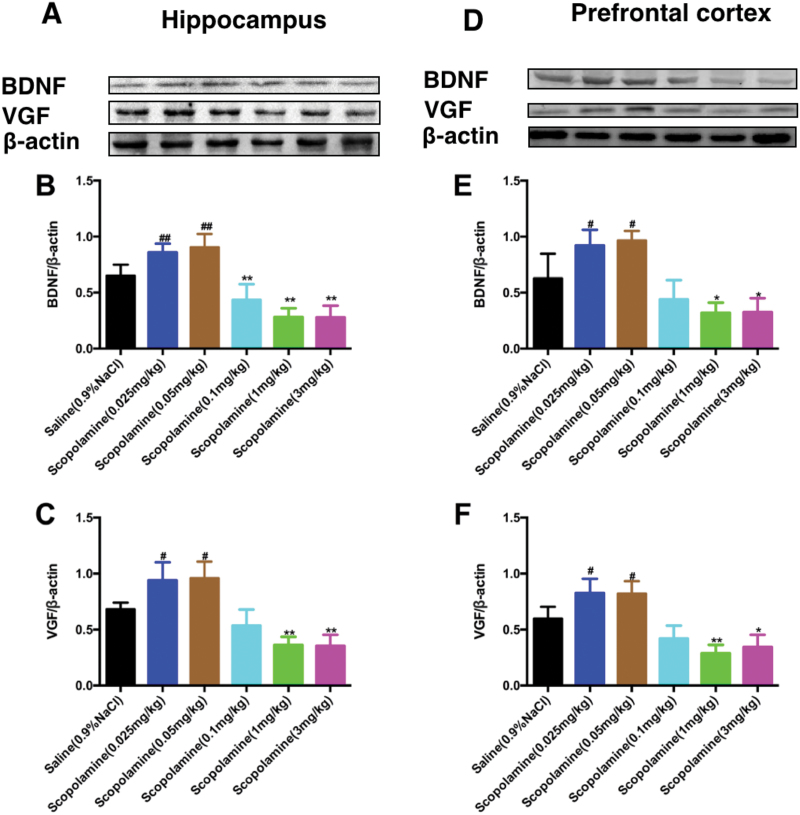
As shown in Figure 3, high doses of scopolamine significantly decreased the expression of BDNF in the hippocampus [F (5, 24)=34.43, 0.1 mg/kg, P<.01, 1 mg/kg, P<.01 and 3 mg/kg, P<.01; Figure 3B] and prefrontal cortex [F (5, 24)=19.50, 1 mg/kg, P<.05 and 3 mg/kg, P<.05; Figure 3E] compared with the saline treatment. However, the levels of BDNF in the hippocampus [F (5, 24)=34.43, 0.025 mg/kg, P<.01, 0.05 mg/kg, P<.01; Figure 3B] and prefrontal cortex [F (5, 24)=19.50, 0.025 mg/kg, P<.05 and 0.05 mg/kg, P<.05; Figure 3E] were significantly increased by low doses of scopolamine treatment compared with the saline treatment.
Figure 3.
Effects of various doses of scopolamine on the brain derived neurotrophic factor (BDNF) and VGF levels in brain regions. (A) and (D) representative immunoblots of BDNF and VGF detected by western blotting with tissues from the hippocampus (A) and prefrontal cortex (D); the panels (B,C,E,F) are the quantification of the immunoblotting bands of BDNF (B,E) and VGF (C,F) in the hippocampus or prefrontal cortex of the mice. The data are expressed as the mean±SEM (n=5/group). *P<.05, **P<.01, compared with control (saline plus saline)group.
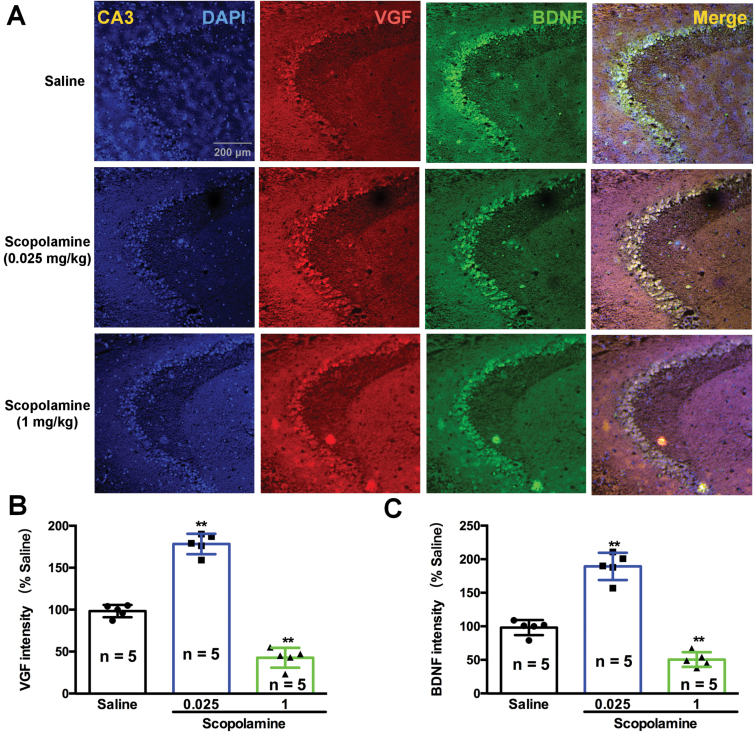
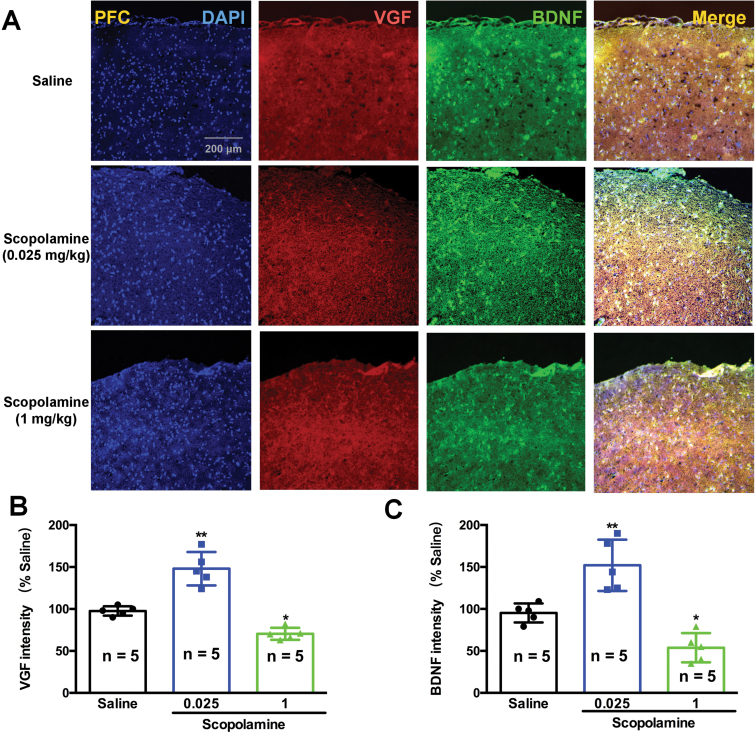
Consistent with the changes in BDNF, the levels of VGF were also significantly decreased by high doses of scopolamine in the hippocampus [F (5, 24)=25.05, 1 mg/kg, P<.01 and 3 mg/kg, P<.01; Figure 3C] and prefrontal cortex [F (5, 24)=24.06, 1 mg/kg, P<.01 and 3 mg/kg, P<.05; Figure 3F] compared with the saline-treated mice. In contrast, low doses of scopolamine resulted in a significant increase in VGF expression in the hippocampus [F (5, 24)=25.05, 0.025 mg/kg, P<.05, 0.05 mg/kg, P<.05; Figure 3B] and prefrontal cortex [F (5, 24)=24.06, 0.025 mg/kg, P<.05, 0.05 mg/kg, P<.05; Figure 3F] compared with the salinegroup. The expression levels of VGF and BDNF in the prefrontal cortex (Figure 4) and hippocampus (Figure 5) of mice were visualized using immunofluorescence. Co-staining of VGF with BDNF in prefrontal cortex and hippocampus revealed overlapped colocalization of these 2 proteins, suggesting that VGF might exert its function with the same process as BDNF in the prefrontal cortex and hippocampus. Our fluorescence immunohistochemistry showed that low doses of scopolamine (0.025 mg/kg) significantly increased the expression of VGF [F (2, 12)=203.8, P<.01, Figure 4B; F (2, 12)=48.34, P<.01, Figure 5B] and BDNF [F (2, 12)=113.2, P<.01, Figure 4C; F (2, 12)=26.59, P<.01, Figure 5C] in the hippocampal CA3 region and prefrontal cortex, respectively. However, the higher dose of scopolamine (1 mg/kg) significantly decreased the expression of VGF (Figure 4B and 5B) and BDNF (Figure 4C and 5C) in the hippocampal CA3 and prefrontal cortex regions.
Figure 4.
Effects of various doses of scopolamine on VGF and brain derived neurotrophic factor (BDNF) immunoreactivity and colocalization in the hippocampal CA3 regions of mice. (A) VGF and BDNF protein expression was examined using immunofluorescence of frozen mice hippocampal sections. Anti-VGF was labeled with an Alex Fluor 594 conjugated secondary antibody (red). Anti-BDNF antibody was labeled with an Alex Fluor 488 conjugated secondary antibody (green). The densities of both VGF and BDNF were significantly increased by low doses of scopolamine (0.025 mg/kg) and were significantly decreased by a high dose of scopolamine (1 mg/kg). The cellular localization of VGF and BDNF after scopolamine treatment was characterized by colocalization studies with markers of neurons (DAPI). n=5/group. **P<.01 compared with the control (saline plus saline) group.
Figure 5.
Effects of various doses of scopolamine on VGF and brain derived neurotrophic factor (BDNF) immunoreactivity and colocalization in the prefrontal cortex of mice. (A) VGF and BDNF protein expression was examined using immunofluorescence of frozen mice prefrontal cortex sections. Anti-VGF was labeled with an Alex Fluor 594 conjugated secondary antibody (red). Anti-BDNF antibody was labeled with an Alex Fluor 488 conjugated secondary antibody (green). The densities of both VGF and BDNF were significantly increased by a low dose of scopolamine (0.025 mg/kg) and were significantly decreased by a high dose of scopolamine (1 mg/kg). The cellular localization of VGF and BDNF after scopolamine treatment was characterized by colocalization studies with markers of neurons (DAPI). n=5/group. *P<.05, **P<.01 compared with the control (saline plus saline)group.
Inhibition of L-VDCC Blocked the Rapid Onset of Antidepressant-Like Effects by Scopolamine at Low Doses in Mice
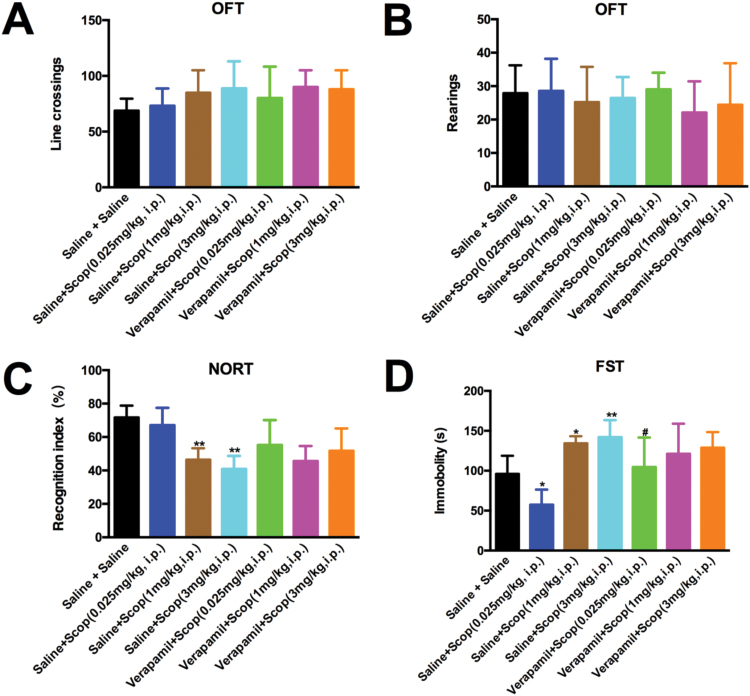
To determine the involvement of L-VDCC in behavioral changes induced by various doses of scopolamine, mice were divided into several groups and treated as follows: (1) mice treated with saline (vehicle for verapamil, i.p.) plus saline (vehicle for scopolamine, i.p.); (2) mice treated with saline (i.p.) plus scopolamine (0.025 mg/kg, i.p.); (3) mice treated with saline (i.p.) plus scopolamine (1 mg/kg, i.p.); (4) mice treated with saline (i.p.) plus scopolamine (3 mg/kg, i.p.); (5) mice treated with verapamil (5 mg/kg, i.p.) plus scopolamine (0.025 mg/kg, i.p.); (6) mice treated with verapamil (5 mg/kg, i.p.) plus scopolamine (1 mg/kg, i.p.); (7) mice treated with verapamil (5 mg/kg, i.p.) plus scopolamine (3 mg/kg, i.p.). As described earlier, mice received i.p. administration of verapamil (5 mg/kg) 60 minutes before scopolamine, and the OFT followed with NORT were performed 24 hours after the last treatment. We did not observe significant changes in line crossing [F (6, 56)=1.623, P=1.623; Figure 6A] or rearings [F (6, 56)=0.6704, P=.6739; Figure 6B]. However, the recognition index in NORT exhibited a significant reduction in mice treated with high doses of scopolamine [F (6, 56)=11.17, 1 mg/kg, P<.01 and 3 mg/kg, P<.01] (Figure 6C) compared with those treated with saline plus saline. However, treatment with verapamil plus scopolamine (1 or 3 mg/kg) did not affect the recognition deficits in mice induced by high doses of scopolamine compared with that of the saline plus scopolamine (1 or 3 mg/kg)groups. By further examination of the depression-like behaviors in FST, we confirmed that a low dose (0.025 mg/kg) of scopolamine [F (6, 56)=11.65, P<.05] (Figure 6D) significantly decreased the immobility time compared with the saline plus saline treatment, suggesting a potent antidepressant-like effect of scopolamine at this concentration in mice. Intriguingly, pretreatment with verapamil before scopolamine (0.025 mg/kg) injection significantly blocked the aforementioned antidepressant-like effects (P<.05; Figure 6D). Additionally, mice treated with high doses of scopolamine (1 and 3 mg/kg) consistently showed significant increase in the immobility time compared with those receiving saline plus saline (1 mg/kg, P<.05 and 3 mg/kg, P<.01) (Figure 6D). However, verapamil did not modulate the immobility time alterations associated with scopolamine treatment (data not shown).
Figure 6.
L-type voltage-dependent calcium channel (L-VDCC) involved in the antidepressant-like effects of low-dose scopolamine in mice. Mice were pre-injected with verapamil (5 mg/kg, i.p.) followed by scopolamine (0.025, 1 and 3 mg/kg, i.p.), and the open field test (OFT) was conducted 24 hours after the last drug treatment. None of the treatments affected locomotor activity, reflected by the line crossings (A) and rearings (B) in the mice. (C) The novel object recognition index was examined in the novel object recognition test (NORT). (D) Immobility time of mice was measured in the forced swimming test (FST). Pretreatment with verapamil significantly reversed the antidepressant-like effects of scopolamine (0.025 mg/kg). The data are expressed as the mean±SEM (n=9/group). **P<.05 and P<.01, compared with saline plus saline group; #P<.05, compared with saline plus scopolamine (0.025 mg/kg)group.
Inhibition of L-VDCC Prevents the Upregulation of VGF and BDNF by Scopolamine at Low Doses in Mice
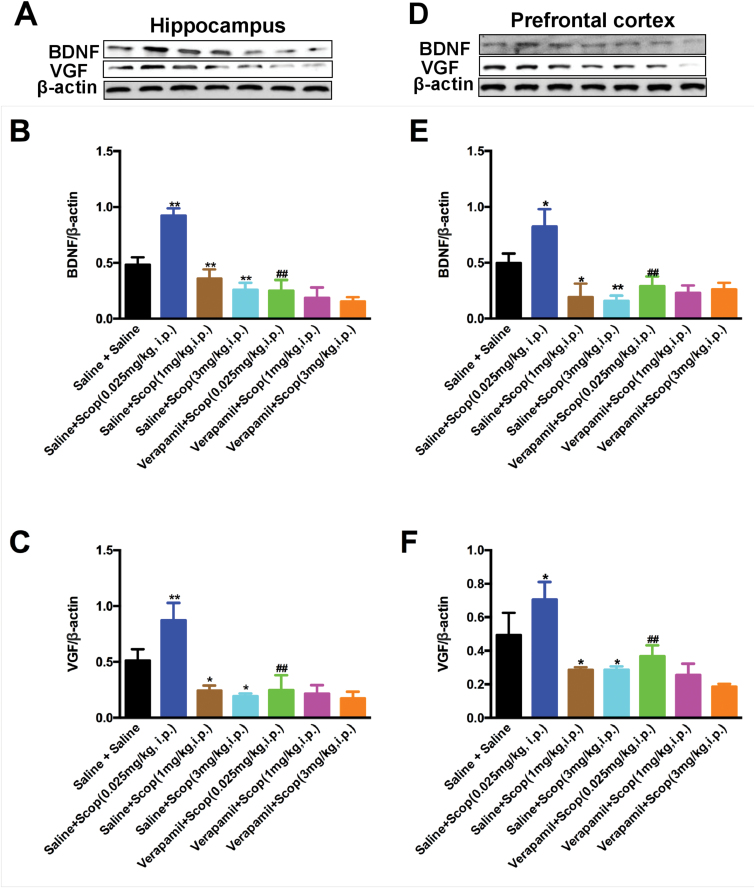
BDNF and VGF were found to play critical roles in rapid-acting antidepressant-like actions of certain drugs (Li et al. 2010; Voleti et al., 2013; Lepack et al. 2014; Lu et al., 2014; Pazini et al., 2016; Girgenti et al., 2017). To clarify whether BDNF and VGF activation also facilitates the mechanistic action of scopolamine and whether L-VDCC participated in this process, we performed a western-blot analysis of BDNF and VGF in select murine brain regions (prefrontal cortex and hippocampus) 4 days after treatment with scopolamine (0.025, 0.05, 0.1, 1, and 3 mg/kg, i.p.) with and without verapamil (5 mg/kg). The 1-way ANOVA of BDNF [hippocampus, F (6, 14)=38.45, P<.01, Figure 7B; prefrontal cortex, F (6, 14)=18.45, P<.01, Figure 7E] and VGF [hippocampus, F (6, 14)=21.62, P<.01, Figure 7C; prefrontal cortex, F (6, 14)=17.25, P<.01, Figure 7F] data showed significant changes in any test group for any brain region. Specifically, a low dose of scopolamine (0.025 mg/kg) significantly increased levels of BDNF [hippocampus, P<.01; prefrontal cortex, P<.05] and VGF [hippocampus, P<.01; prefrontal cortex, P<.05] proteins in the hippocampus and prefrontal cortex compared with the saline plus saline treatment. On the contrary, the high doses of scopolamine (1 and 3 mg/kg) significantly decreased the BDNF [1 mg/kg, hippocampus, P<.01; prefrontal cortex, P<.05; 3 mg/kg, both P<.01] and VGF [all P<.05] levels in all brain regions of the mice compared with the saline plus saline treatment. Notably, verapamil injection prevented the induction of brain BDNF and VGF expression by low-dose scopolamine (0.025 mg/kg), as the BDNF [hippocampus, both P<.01; prefrontal cortex, both P<.01] and VGF levels in the hippocampus and prefrontal cortex of mice treated with verapamil plus scopolamine were significantly lower than those of the saline plus scopolamine (0.025 mg/kg)-treated mice.
Figure 7.
Pretreatment with verapamil blocked the upregulation of scopolamine (0.025 mg/kg) on brain derived neurotrophic factor (BDNF) and VGF in the brains of mice. (A) and (D) representative immunoblots of BDNF and VGF detected by western blotting with tissues from the hippocampus (A) and prefrontal cortex (D); the remaining panels are the quantification of the immunoblotting bands of BDNF (B,E) and VGF (C,F). The data are expressed as the mean±SEM (n=3/group). *P<.05, **P<.01, compared with saline plus salinegroup; ##P<.01, compared with verapamil plus scopolamine (0.025 mg/kg) group.
Discussion
Deficits in the cholinergic system in the brain have been associated with dysfunction of cognitive ability (Blake and Boccia, 2017; Newhouse and Dumas, 2015), leading to the so-called “cholinergic hypothesis of Alzheimer’s disease (AD)” (Francis et al., 1999). The inhibitors of acetylcholinesterase, which works to increase acetylcholine (ACh) levels by inhibiting ACh degradation, have therefore been used clinically as first-line therapy in memory dysfunction (Lleó et al., 2006). Recently, scopolamine, a nonselective muscarinic receptor antagonist that is used to induce memory impairment in rodents (Pitsikas and Gravanis, 2017; Hwang et al., 2017) based on the cholinergic hypothesis, was generated (Francis et al., 1999; Klinkenberg and Blokland 2010) and used as a standard-reference drug for inducing age- and dementia-related cognitive deficits in the animals. Interestingly, the cholinergic system has been currently under investigation as a target for rapid-acting antidepressant interventions (Drevets et al., 2013), with the evidence suggesting that hyper-sensitivity of the cholinergic system plays a role in the pathophysiology of depression (Janowsky et al., 1972). Several randomized, double-blind, placebo-controlled studies have been conducted with i.v. injection of the anticholinergic agent scopolamine as an adjunctive or monotherapy in subjects with depressive disorders (Khajavi et al., 2012; Yang and Hashimoto, 2015; Drevets et al., 2013). However, the undesirable effects induced by scopolamine, especially cognitive deficits (Klinkenberg and Blokland, 2010), have partially limited the use of this drug in psychiatric disorders. Preclinical models suggest that these memory deficits and rapid antidepressant-like effects can be recapitulated with a blockade of M-type muscarinic acetylcholine receptors and downregulation of BDNF and VGF in the brain (Ghumatkar et al., 2015; Jeon et al., 2017; Zhou et al., 2017); however, the underlying cellular mechanisms of BDNF and VGF changes and behavioral responses to various doses of scopolamine have not been determined. Herein, we report different regulatory effects on memory and depression-like behaviors as well as changes in BDNF and VGF produced by various doses of scopolamine treatment in mice.
It has been well demonstrated that high doses of scopolamine produce cognitive deficits in various behavioral tests in animal models (Klinkenberg and Blokland, 2010). In the current study, scopolamine (1 and 3 mg/kg, i.p.) significantly decreased the recognition index in the NORT and increased the immobility time in the FST, with no changes in the total number of line crossings and rearings, suggesting that high doses of scopolamine (1 and 3 mg/lg, i.p.) impair memory and induce depression-like behaviors without affecting locomotion. Our results were consistent with previous reports in general, in which high doses of scopolamine impair cognitive ability in rodents with or without increased locomotor activity (Hiramatsu and Inoue, 2000; Kwon et al., 2009; Jeon et al., 2017). It is important to note that our results showed that lower doses of scopolamine at 25 and 50 μg/kg (i.p.) produced significant antidepressant-like effects, which is consistent with previous studies (Wohleb et al., 2016) and contrast to the high-dose studies that typically use 1- to 3-mg/kg doses of scopolamine to produce psychiatric disorders (Almasi-Nasrabadi et al., 2012; Busquet et al., 2012; Lin et al., 2016).
Given the involvement of BDNF and VGF in the rapid-acting antidepressant-like and memory-enhancing effects (Lu et al., 2014; Ma et al., 2016; Yang et al., 2016), it is likely that BDNF and VGF may also play a key role in the actions of various doses of scopolamine in mice. Notably, BDNF is a member of the neurotrophin family and plays a critical role in the cognitive processes (de Assis and de Almondes, 2017;) and depression-like behaviors (Jiang et al., 2017). Additionally, BDNF plays a critical role in the synaptic activity activation directly or by exerting a positive feedback on synaptic activity (Arancio and Chao, 2007). Recently, previous studies reported a marked downregulation of the BDNF-mediated signaling in the prefrontal cortex and hippocampus of various depression and memory deficit models (Zhou et al., 2017; Xiang et al., 2017). However, upregulation of BDNF promoted rapid and sustained antidepressant-like and memory-enhancing effects, thus implicating the BDNF-mediated signaling in the prefrontal cortex and hippocampus, which plays an important role in regulating the effects of various doses of scopolamine. Herein, we confirmed that low doses of scopolamine (25 and 50 μg/kg) significantly increased BDNF levels in the prefrontal cortex and hippocampus in addition to rapid-acting antidepressant-like action in mice. Growing evidence suggests that the antidepressant-like effects of ketamine and scopolamine in rodent models are caused by an influx of extracellular glutamate, elevated BDNF, and activation of the BDNF/tropomyosin-related kinase receptor B signaling (Lepack et al., 2014; Wohleb et al., 2016, 2017), which may explain the BDNF upregulation by low doses of scopolamine in our current work. Recently, VGF, a secreted neuropeptide was found to be downregulated in the brain of animal models of depression (Hunsberger et al., 2007; Thakker-Varia et al., 2007; Lin et al., 2014). Additionally, VGF involves in the antidepressant-like effects dependent on the BDNF/TrkB/CREB signaling in mice (Hunsberger et al., 2007; Thakker-Varia et al., 2007; Lin et al., 2014; Lu et al., 2014). Consistent with our expectations in our current work, the levels of VGF were significantly increased in the hippocampus and prefrontal cortex by low doses of scopolamine, indicating the similar manner with the BDNF changes regulated by low doses of scopolamine in mice.
In contrast to the effects of low doses of scopolamine, our data showed that the high doses of scopolamine (1 and 3 mg/kg) significantly produced the memory deficits and depression-like behaviors in mice. Our present data are consistent with previous studies that demonstrated that the high doses of scopolamine induced memory impairments and anxiety-like and depression-like behaviors in rats (Aydin et al., 2016; Bagci et al., 2016). Additionally, our biochemical studies found that the levels of BDNF and VGF in the hippocampus and prefrontal cortex were significantly decreased after high doses of scopolamine (1 and 3 mg/kg) treatment, which may explain the depression-like behaviors and memory deficits in mice. Previous studies have revealed that scopolamine (1 or 3 mg/kg) induced an increase in acetylcholinesterase activity as well as decreases in BDNF protein expression and cAMP response element-binding protein, extracellular regulated kinase 1/2, and protein kinase B phosphorylation levels in the hippocampus and cortex (Chen et al., 2016; Park et al., 2016), which may explain the downregulation mechanisms of BDNF and VGF expressions at high doses of scopolamine.
The activation of synaptic plasticity has also been proposed as an underlying biological effect for scopolamine’s rapid-acting antidepressant-like action at a low dose. Similar to ketamine, scopolamine treatment acutely enhances extracellular glutamate production in the striatum of rodents (Rawls and McGinty, 1998), which may activate synaptic plasticity pathways. Increased glutamate release activates α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, resulting in depolarization of the cell and an influx of calcium through L-VDCC in the rapid-acting antidepressant-like actions of ketamine (Lepack et al., 2014; Chowdhury et al., 2017; Wohleb et al., 2017). Interestingly, scopolamine’s antidepressant-like effects were blocked by pretreatment with mammalian target of rapamycin complex 1 and AMPA antagonists (Voleti et al., 2013), similar to ketamine (Li et al., 2010). As noted above, scopolamine’s mechanism of action goes beyond simple M-type muscarinic acetylcholine receptor antagonism or even activation of the AMPA receptor. Importantly, L-VDCC may play crucial roles in the actions of scopolamine. To further validate the specificity of L-VDCC-induced regulation of BDNF and VGF expression after various doses of scopolamine treatment in mice, the L-VDCC blocker verapamil (5 mg/kg, i.p.) was administered as a pretreatment before scopolamine. When effective doses of scopolamine (0.025, 1 and 3 mg/kg, i.p.) were administered with the verapamil, the low dose of scopolamine (0.025 mg/kg) plus verapamil showed significant memory deficits and depression-like behaviors in the NORT and FST compared with the scopolamine (0.025 mg/kg) single treatment. Specifically, the low dose of scopolamine (0.025 mg/kg) produced increases in BDNF and VGF, which were completely blocked by verapamil pretreatment. These findings demonstrate a requirement of L-VDCC in the rapid-acting antidepressant-like effects of low doses of scopolamine in mice.
Conclusions
Taken together, our findings suggest that the different changes of behaviors produced by different doses of scopolamine are dependent on the opposing regulation of BDNF and VGF levels in the hippocampus and prefrontal cortex of mice. Additionally, our results further implicate that L-VDCC in the hippocampus and prefrontal cortex may be involved in the behavioral and molecular responses to scopolamine.
Statement of Interest
None.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 81671337; No. 81201050; No. 81541087); the Natural Science Foundation of Zhejiang Province (No. LQ12H09001), Natural Science Foundation of Ningbo (No. 2012A610251; No. 2017A610212), and the Ningbo municipal innovation team of life science and health (2015C110026). This project is also sponsored by K.C. Wong Magna funded in Ningbo University and the Li Dak Sum YiP Yio Chin Kenneth Li Marine Biopharmaceutical Development Fund, National 111 Project of China.
References
- Almasi-Nasrabadi M, Javadi-Paydar M, Mahdavian S, Babaei R, Sharifian M, Norouzi A, Dehpour AR(2012)Involvement of NMDA receptors in the beneficial effects of pioglitazone on scopolamine-induced memory impairment in mice. Behav Brain Res 231:138–145. [DOI] [PubMed] [Google Scholar]
- Arancio O, Chao MV(2007)Neurotrophins, synaptic plasticity and dementia. Curr Opin Neurobiol 17:325–330. [DOI] [PubMed] [Google Scholar]
- Aydin E, Hritcu L, Dogan G, Hayta S, Bagci E(2016)The effects of inhaled Pimpinella peregrina essential oil on scopolamine-induced memory impairment, anxiety, and depression in laboratory rats. Mol Neurobiol 53:6557–6567. [DOI] [PubMed] [Google Scholar]
- Bagci E, Aydin E, Ungureanu E, Hritcu L(2016)Anthriscus nemorosa essential oil inhalation prevents memory impairment, anxiety and depression in scopolamine-treated rats. Biomed Pharmacother 84:1313–1320. [DOI] [PubMed] [Google Scholar]
- Bartoli F, Riboldi I, Crocamo C, Di Brita C, Clerici M, Carrà G(2017)Ketamine as a rapid-acting agent for suicidal ideation: a meta-analysis. Neurosci Biobehav Rev 77:232–236. [DOI] [PubMed] [Google Scholar]
- Blake MG, Boccia MM(2017)Basal forebrain cholinergic system and memory. Curr Top Behav Neurosci doi: 10.1007/7854_2016_467. [DOI] [PubMed] [Google Scholar]
- Busquet P, Capurro V, Cavalli A, Piomelli D, Reggiani A, Bertorelli R(2012)Synergistic effects of galantamine and memantine in attenuating scopolamine-induced amnesia in mice. J Pharmacol Sci 120:305–309. [DOI] [PubMed] [Google Scholar]
- Chen Z, Huang C, Ding W(2016)Z-guggulsterone improves the scopolamine-induced memory impairments through enhancement of the BDNF signal in C57BL/6J mice. Neurochem Res 41:3322–3332. [DOI] [PubMed] [Google Scholar]
- Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, Bristow L, Schaeffer E, Duman RS, Rothman DL, Behar KL, Sanacora G(2017)Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry 22:120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Assis GG, de Almondes KM(2017)Exercise-dependent BDNF as a modulatory factor for the executive processing of individuals in course of cognitive decline. A systematic review. Front Psychol 8:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Zarate CA Jr, Furey ML(2013)Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry 73:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK(2012)Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC Jr, Slonena E, Hejazi NS, Brutsche N, Yu KC, Park L, Ballard ED, Zarate CA Jr(2017)Motor-Activity markers of circadian timekeeping are related to ketamine’s rapid antidepressant properties. Biol Psychiatry pii:S0006–3223(17)31368–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis PT, Palmer AM, Snape M, Wilcock GK(1999)The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry 66:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Drevets WC, Szczepanik J, Khanna A, Nugent A, Zarate CA Jr(2015)Pretreatment differences in BOLD Response to emotional faces correlate with antidepressant response to scopolamine. Int J Neuropsychopharmacol 18:pyv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghumatkar PJ, Patil SP, Jain PD, Tambe RM, Sathaye S(2015)Nootropic, neuroprotective and neurotrophic effects of phloretin in scopolamine induced amnesia in mice. Pharmacol Biochem Behav 135:182–191. [DOI] [PubMed] [Google Scholar]
- Girgenti MJ, Ghosal S, LoPresto D, Taylor JR, Duman RS(2017)Ketamine accelerates fear extinction via mTORC1 signaling. Neurobiol Dis 100:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu M, Inoue K(2000)Improvement by low doses of nociceptin on scopolamine-induced impairment of learning and/or memory. Eur J Pharmacol 395:149–156. [DOI] [PubMed] [Google Scholar]
- Hunsberger JG, Newton SS, Bennett AH, Duman CH, Russell DS, Salton SR, Duman RS(2007)Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 13:1476–1482. [DOI] [PubMed] [Google Scholar]
- Hwang ES, Kim HB, Lee S, Kim MJ, Lee SO, Han SM, Maeng S, Park JH(2017)Loganin enhances long-term potentiation and recovers scopolamine-induced learning and memory impairments. Physiol Behav 171:243–248. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ(1972)A cholinergic-adrenergic hypothesis of mania and depression. Lancet 2:632–635. [DOI] [PubMed] [Google Scholar]
- Jeon SJ, Lee HJ, Lee HE, Park SJ, Gwon Y, Kim H, Zhang J, Shin CY, Kim DH, Ryu JH(2017)Oleanolic acid ameliorates cognitive dysfunction caused by cholinergic blockade via TrkB-dependent BDNF signaling. Neuropharmacology 113:100–109. [DOI] [PubMed] [Google Scholar]
- Jiang H, Chen S, Li C, Lu N, Yue Y, Yin Y, Zhang Y, Zhi X, Zhang D, Yuan Y(2017)The serum protein levels of the tPA-BDNF pathway are implicated in depression and antidepressant treatment. Transl Psychiatry 7:e1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M(2009)Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci 29:8688–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajavi D, Farokhnia M, Modabbernia A, Ashrafi M, Abbasi SH, Tabrizi M, Akhondzadeh S(2012)Oral scopolamine augmentation in moderate to severe major depressive disorder: a randomized, double–blind, placebo-controlled study. J Clin Psychiatry 73:1428–1433. [DOI] [PubMed] [Google Scholar]
- Klinkenberg I, Blokland A(2010)The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev 34:1307–1350. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Kim HC, Lee SY, Jang CG(2009)Loganin improves learning and memory impairments induced by scopolamine in mice. Eur J Pharmacol 619:44–49. [DOI] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS(2014)BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 18:pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS(2010)mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Huang L, Yu J, Xiang S, Wang J, Zhang J, Yan X, Cui W, He S, Wang Q(2016)Fucoxanthin, a marine carotenoid, reverses scopolamine-induced cognitive impairments in mice and inhibits acetylcholinesterase in vitro. Mar Drugs 14:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Wang C, Xu B, Gao S, Guo J, Zhao X, Huang H, Zhang J, Chen X, Wang Q, Zhou W(2014)The VGF-derived peptide TLQP62 produces antidepressant-like effects in mice via the BDNF/TrkB/CREB signaling pathway. Pharmacol Biochem Behav 120:140–148. [DOI] [PubMed] [Google Scholar]
- Lleó A, Greenberg SM, Growdon JH(2006)Current pharmacotherapy for Alzheimer’s disease. Annu Rev Med 57:513–533. [DOI] [PubMed] [Google Scholar]
- Lu Y, Wang C, Xue Z, Li C, Zhang J, Zhao X, Liu A, Wang Q, Zhou W(2014)PI3K/AKT/mTOR signaling-mediated neuropeptide VGF in the hippocampus of mice is involved in the rapid onset antidepressant-like effects of GLYX-13. Int J Neuropsychopharmacol 18(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Ren Q, Yang C, Zhang JC, Yao W, Dong C, Ohgi Y, Futamura T, Hashimoto K(2016)Adjunctive treatment of brexpiprazole with fluoxetine shows a rapid antidepressant effect in social defeat stress model: role of BDNF-TrkB signaling. Sci Rep 6:39209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AE, Schober DA, Nikolayev A, Tolstikov VV, Anderson WH, Higgs RE, Kuo MS, Laksmanan A, Catlow JT, Li X, Felder CC, Witkin JM(2017)Further evaluation of mechanisms associated with the antidepressant-like signature of scopolamine in mice. CNS Neurol Disord Drug Targets doi: 10.2174/1871527316666170309142646. [DOI] [PubMed] [Google Scholar]
- Newhouse P, Dumas J(2015)Estrogen-cholinergic interactions: implications for cognitive aging. Horm Behav 74:173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HR, Lee H, Park H, Cho WK, Ma JY(2016)Fermented Sipjeondaebo-tang alleviates memory deficits and loss of hippocampal neurogenesis in scopolamine-induced amnesia in mice. Sci Rep 6:22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazini FL, Cunha MP, Rosa JM, Colla AR, Lieberknecht V, Oliveira Á, Rodrigues AL(2016)Creatine, similar to ketamine, counteracts depressive-like behavior induced by corticosterone Via Pi3k/Akt/MTOR Pathway. Mol Neurobiol 53:6818–6834. [DOI] [PubMed] [Google Scholar]
- Pitsikas N, Gravanis A(2017)The novel dehydroepiandrosterone (DHEA) derivative BNN27 counteracts delay-dependent and scopolamine–induced recognition memory deficits in rats. Neurobiol Learn Mem 140:145–153. [DOI] [PubMed] [Google Scholar]
- Podkowa K, Podkowa A, Sałat K, Lenda T, Pilc A, Pałucha-Poniewiera A(2016)Antidepressant-like effects of scopolamine in mice are enhanced by the group II mGlu receptor antagonist LY341495. Neuropharmacology 111:169–179. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Mcginty JF(1998)Muscarinic receptors regulate extracellular glutamate levels in the rat striatum: an in vivo microdialysis study. J Pharmacol Exp Ther 28:91–98. [PubMed] [Google Scholar]
- Thakker-Varia S, Krol JJ, Nettleton J, Bilimoria PM, Bangasser DA, Shors TJ, Black IB, Alder J(2007)The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus. J Neurosci 27:12156–12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M, STAR*D Study Team (2006)Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163:28–40. [DOI] [PubMed] [Google Scholar]
- Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, Sanacora G, Eid T, Aghajanian G, Duman RS(2013)Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry 74:742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, Duman RS(2016)GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest 126:2482–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Gerhard D, Thomas A, Duman RS(2017)Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol 15:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang S, Liu F, Lin J, Chen H, Huang C, Chen L, Zhou Y, Ye L, Zhang K, Jin J, Zheng J, Wang C, He S, Wang Q, Cui W, Zhang J(2017)Fucoxanthin inhibits β-amyloid assembly and attenuates β-amyloid oligomer-induced cognitive impairments. J Agric Food Chem doi: 10.1021/acs.jafc.7b00805. [DOI] [PubMed] [Google Scholar]
- Yang B, Zhang JC, Han M, Yao W, Yang C, Ren Q, Ma M, Chen QX, Hashimoto K(2016)Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 233:3647–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Hashimoto K(2015)Combination of nitrous oxide with isoflurane or scopolamine for treatment-resistant major depression. Clin Psychopharmacol Neurosci 13:118–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Zhang Z, Liu L, Li C, Li M, Yu H, Cai X, Sun X, Shen X, Wang J, Geng J, Wang C, Shi Y(2017)The antidepressant-like effects of biperiden may involve BDNF/TrkB signaling-mediated BICC1 expression in the hippocampus and prefrontal cortex of mice. Pharmacol Biochem Behav 157:47–57. [DOI] [PubMed] [Google Scholar]