Abstract
Political conservatism is associated with an increased negativity bias, including increased attention and reactivity toward negative and threatening stimuli. Although the human amygdala has been implicated in the response to threatening stimuli, no studies to date have investigated whether conservatism is associated with altered amygdala function toward threat. Furthermore, although an influential theory posits that connectivity between the amygdala and bed nucleus of the stria terminalis (BNST) is important in initiating the response to sustained or uncertain threat, whether individual differences in conservatism modulate this connectivity is unknown. To test whether conservatism is associated with increased reactivity in neural threat circuitry, we measured participants’ self-reported social and economic conservatism and asked them to complete high-resolution fMRI scans while under threat of an unpredictable shock and while safe. We found that economic conservatism predicted greater connectivity between the BNST and a cluster of voxels in the left amygdala during threat vs safety. These results suggest that increased amygdala–BNST connectivity during threat may be a key neural correlate of the enhanced negativity bias found in conservatism.
Keywords: conservatism, bed nucleus of the stria terminalis, BNST, amygdala, 7T fMRI, threat bias
Introduction
It has been argued that an enhanced negativity bias—the tendency to give greater attention and react more strongly toward negative vs positive stimuli (Norris et al., 2010)—predisposes individuals to gravitate toward conservative ideology, because conservatism prioritizes stability and the minimization of tangible threats, while liberal ideology prioritizes social change and egalitarianism (Jost et al., 2009; Hibbing et al., 2014). This line of reasoning is based on research demonstrating an increased bias toward negative and threatening stimuli among those who endorse conservative views (Jost et al., 2003; Hibbing et al., 2014; Lilienfeld and Latzman, 2014). Conservatism is associated with a greater attentional bias toward negative words, images and angry faces (Carraro et al., 2011; Dodd et al., 2012; McLean et al., 2014), increased interpretation of ambiguous facial expressions as threatening (Vigil, 2010) and increased physiological reactivity to negative stimuli (Oxley et al., 2008; Smith et al., 2011; Dodd et al., 2012). Moreover, the link between conservative views and sensitivity to threat has been confirmed in multiple meta-analyses (Jost et al., 2003, 2017; Burke et al., 2013).
Despite this evidence, the link between conservatism and threat has not been universally accepted (Jost et al., 2017). For example, some have argued that threat sensitivity is associated with extremism on both sides of the political spectrum (Greenberg and Jonas, 2003; van Prooijen et al., 2015). Other theories predict that threat sensitivity is positively associated with greater social conservatism but also with greater economic liberalism (Duckitt and Sibley, 2010), particularly among those who are low on political engagement (Malka and Soto, 2015; Crawford, 2017).
Despite the wealth of research on conservatism and the negativity bias, little is known about the neural mechanisms underlying this association. Based on research demonstrating that the amygdala is involved in the detection of and response toward threat (Davis and Whalen, 2001; Sander et al., 2003; Öhman, 2005; Davis et al., 2010), studies have investigated how amygdala structure and function is related to individual differences in conservatism (Kanai et al., 2011; Schreiber et al., 2013). Kanai et al. (2011) found that conservatism was associated with greater gray matter volume in the amygdala, and suggested that this finding may be associated with the emotional and cognitive differences across political orientation, particularly those associated with ‘managing fear and uncertainty’ (p. 678). Schreiber et al. (2013) examined amygdala activation in Democrats and Republicans during a risk-taking task in which participants had the option of receiving a small, but guaranteed monetary reward, or taking a gamble that would sometimes result in a larger reward, and sometimes result in a commensurate monetary loss. They found that Republicans exhibited a larger amygdala response for trials in which they took the risky gamble and won a large reward vs trials in which they took the safe option and received a small reward.
Although these studies suggest that differences in amygdala structure and function are associated with political orientation, neither of these studies examined whether conservatism is associated with an increase in amygdala reactivity toward threat or toward negative stimuli more generally. Although Kanai et al. (2011) found greater gray matter volume in the amygdala of conservatives, they did not attempt to determine whether this increase in amygdala volume predicted a greater response toward threat. Similarly, Schreiber et al.’s (2013) finding that Republicans exhibit a greater amygdala response on winning risky trials vs winning safe trials could imply either that conservatism is associated with greater amygdala reactivity to the uncertainty accompanying risk taking or that conservatism is associated with greater amygdala reactivity to receiving a large (vs small) reward. As noted by Tritt et al. (2014), given that the amygdala has been implicated in the response to reward (Baxter and Murray, 2002; Murray, 2007; Cunningham and Brosch, 2012), neither of these studies definitively demonstrate that conservatism is associated with increased amygdala reactivity toward threat.
In addition to the amygdala, the bed nucleus of the stria terminalis (BNST) is another neural structure that is relevant for individual differences in threat reactivity. The BNST is a region of the basal forebrain, which is heavily connected, both structurally and functionally, with the amygdala. An influential model of the neural threat response states that connectivity between the amygdala and BNST is critical in the initiation of the response to sustained or uncertain threat (Davis et al., 2010). In support of this theory, rodent research has implicated amygdala–BNST connectivity in the response to sustained threat (Lee and Davis, 1997; Keen-Rhinehart et al., 2009; Cai et al., 2012; Flandreau et al., 2012; Asok et al., 2016). Although there is little human research examining amygdala–BNST connectivity, a growing body of literature indicates that the BNST responds to a variety of threat-related stimuli (Mobbs et al., 2010; Somerville et al., 2010; Choi et al., 2012; Klumpers et al., 2015; Pedersen et al., 2016). The BNST mediates this response via numerous pathways, including outputs which affect the functioning of the hypothalamic–pituitary-adrenal axis and the autonomic nervous system (Davis et al., 2010). Although past findings highlight altered amygdala–BNST connectivity as a likely candidate as a neural correlate for the increased threat bias associated with conservatism, no studies have investigated this possibility. In fact, the function and connectivity of the BNST is understudied more generally, in part because the small size of the BNST makes it difficult to study with standard neuroimaging techniques such as 3-Tesla fMRI scanning. Thus, high-resolution imaging is needed to investigate whether changes in amygdala–BNST connectivity are associated with individual differences in conservatism.
Additionally, while past research has investigated amygdala differences associated with conservatism, no studies have investigated the separable influences of social and economic conservatism on amygdala structure or activity. Social and economic conservatism are distinct, although correlated, constructs (Treier and Hillygus, 2009; Carmines et al., 2012; Feldman and Johnston, 2014; Ksiazkiewicz et al., 2016) and may differentially predict some personality and cognitive traits. Arguments that sensitivity to threat is associated with social conservatism and economic liberalism (Malka and Soto, 2015; Crawford, 2017) highlight the need to measure social and economic political attitudes separately, particularly when studying the threat bias.
To test whether social and economic conservatism are associated with increased reactivity in neural threat circuitry, we monitored participants’ resting brain activity via fMRI while under threat of an unpredictable shock and while safe. We used 7-Tesla magnetic resonance imaging (MRI) to enable the collection of high-resolution functional images (0.86 0.86 1 mm) to adequately detect amygdala–BNST connectivity. We predicted that conservatism would be associated with greater changes in resting-state connectivity between the BNST and the amygdala during periods of threat vs safety.
Materials and methods
Participants
Participants who were pregnant, reported a neurological disorder, had metallic implants or were under 18 years of age were excluded from participation. Thirty-five right-handed University of Wisconsin–Milwaukee undergraduate students participated in the study. Two participants withdrew from the study before completing the fMRI task, five participants were excluded from analysis due to excessive motion during the scan, three participants were excluded due to signal loss during the fMRI scan that affected the BNST region and one was excluded due to equipment failure during the scan. As a result, data from 24 participants (17 female) were included in the analysis. Participants included in the analysis had a mean age of 22.5 years old (s.d. = 6.35).
Measures of conservatism
Economic conservatism
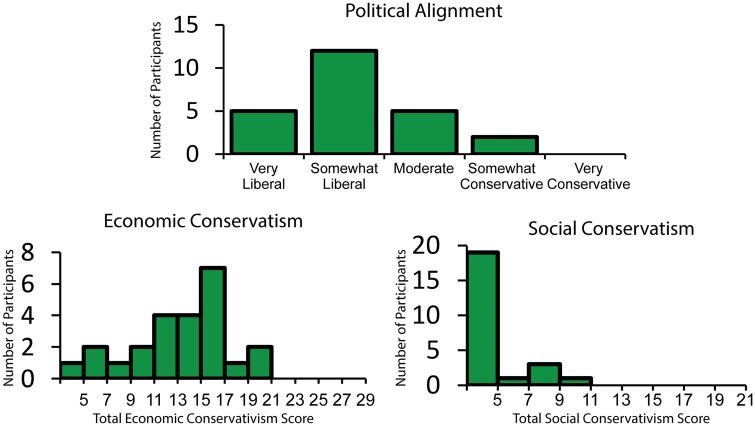
A summed score for four policy opinion questions taken from the American National Elections Studies (ANES, 2012) was used to measure economic conservatism. Each of these questions asked participants to report their opinion on a government policy issue on a 7-point Likert scale ranging from 1 to 7. These items were included based on the finding of Feldman and Johnston (2014) that these questions form a cohesive factor representing economic conservatism. Question topics included government spending, medical insurance, the government’s role in guaranteeing jobs and assistance to the poor. In our sample, these items had acceptable internal consistency (Cronbach’s α = 0.72) and a mean summed score of 13.75 (s.d. = 4.15). A histogram of summed economic conservatism scores can be seen in Figure 1.
Fig. 1.
Histograms for self-reported political alignment (top), economic conservatism (bottom left) and social conservatism (bottom right). Self-described liberals were overrepresented in our sample with the mode response to political alignment being ‘Somewhat Liberal’ (top). Although there was reasonable variation in economic conservatism within the sample, the lack of variation in self-reported social conservatism resulted in the exclusion of this variable from further analysis.
Social conservatism
Social conservatism was also measured using items from the ANES (2012) that Feldman and Johnston (2014) found to form a cohesive factor. This measure included three questions which asked about views on abortion, gay adoption and women’s roles in society, each on a 7-point Likert scale ranging from 1 to 7. The abortion question was altered to match the format of the other questions. However, inspection of the summed scores of these items revealed very little variation in our sample (M = 4.42, minimum = 3, maximum = 11, s.d. = 2.3), as well as poor internal consistency (Cronbach’s α = 0.62). As a result, this variable was not used as a predictor in our analysis, as initially planned. A histogram of summed social conservatism scores can be seen in Figure 1.
Political alignment
Although economic and social conservatism served as our predictors, participants were also asked to report their political leanings, to gauge participants’ overall self-identified political alignment. This was done with a single question asking, ‘what best describes your political views?’ Response options included ‘Very Liberal’, ‘Somewhat Liberal’, ‘Moderate’, ‘Somewhat Conservative’ and ‘Very Conservative’. Participants with liberal leanings were overrepresented in our sample, with the mode response being ‘Somewhat Liberal’ and no participants endorsing the ‘Very Conservative’ option. A histogram of participants’ self-reported political alignment can be seen in Figure 1.
Scanning procedures
Participants were asked to lie still with their eyes open during two 5 min task-free scans. Before the first scan, participants were shown the following instructions: ‘You will be under threat of shock during this scan, meaning that you may receive the electrical stimulus at any time during this scan. You may receive multiple electrical stimulations during this scan’. Before the second scan, the following instructions were shown to participants: ‘You will be safe from shock during this scan, meaning that you will not receive the electrical stimulus at any time during this scan’.
Participants did not receive any shocks during either the threat or safe scan. However, prior to these scans, participants did receive several presentations of shock during a task comparing working memory performance under threat of shock and safety. This task was included to address separate research questions, which are not discussed here. The electrodes were attached above participants’ right ankles before the working memory task and remained in place during the task-free threat of shock scan. We reasoned that having participants complete a task involving the administration of shocks before the task-free scan would increase the plausibility of receiving shocks during this scan, even though none were administered. This allowed us to examine connectivity specifically for anticipation—not receipt—of the shock. After the threat of shock scan, a researcher removed the electrodes from the participant’s leg, ensuring that the participant knew that they would not be shocked during the safe scan. The threat and safe scans were separated by a 6 min anatomical scan, as well as a brief single-volume echo-planar image (EPI) scan with reverse phase encode polarity. This design ensured that participants had ∼8–10 min for the anxious arousal elicited by the threat of shock to subside before the safe scan began. Order of threat and safe blocks was not counter-balanced, to eliminate anticipation effects, wherein participants may exhibit heightened anxiety during the safe scan if they know that a threat scan will follow shortly.
Immediately following each scan, participants used a button box to report how anxious they felt during the previous scan. Participants reported their anxiety on a 9-point Likert scale, with the anchor points ‘Not At All Anxious’ (1) and ‘Very Anxious’ (9). This was included as a manipulation check, as the threat of unpredictable shock manipulation was intended to induce state anxiety.
MRI data acquisition
MRI data were acquired on a 7-Tesla MR950 General Electric (GE Healthcare, Waukesha, WI) scanner. High-resolution T1-weighted whole-brain anatomical images were acquired using a BRAVO gradient-echo sequence (inversion time/repetition time/echo time/flip angle/field of view/matrix/slice thickness: 1050 ms/7.972 ms/3.776 ms/5°/220 mm/276 276 mm/0.8 mm).
Functional scans were acquired in the coronal plane, covering only the regions of interest. Single-shot gradient-echo EPI sequence was used for the functional scans (repetition time/echo time/flip angle/number of excitations/field of view/matrix: 2300 ms/24 ms/73°/1/220 mm/224 224; 28 1 mm coronal slices; gap: 0 mm; 131 volumes) with voxel resolution of 0.98 .98 1 mm. The scan coverage was determined for each participant by positioning the most anterior edge of the coverage just anterior to the amygdala, and then checking that coverage spanned at least 5 mm anterior to the anterior commissure to ensure coverage of the BNST. After the fMRI acquisitions, an additional single-volume EPI scan with reverse phase encode polarity was collected and used for susceptibility-related distortion correction.
fMRI data analysis
To avoid problems associated with global signal regression (Fox et al., 2009; Murphy et al., 2009), the fMRI data were analyzed using the Analysis of Functional NeuroImages (AFNI) software package’s ANATICOR processing stream (Cox, 1996). The first three volumes were discarded to allow for spins to achieve a steady state and volumes with excessive motion were censored (Euclidean norm > 0.2). Remaining EPI volumes were slice-time corrected and motion corrected. To create a distortion correction template, the third volume from the task EPI data and the reverse polarity EPI scan were aligned to each participant’s anatomical scan and warped together using the ‘plusminus’ option in AFNI’s 3dQwarp. Anatomical scans were corrected for intensity field bias (Advanced Normalization Tools 2.1; Avants et al., 2009) and non-linearly warped to the montreal neurological institute (MNI)-152 template. EPI data were aligned to the anatomical image, non-linearly warped to the distortion correction template and then registered to the MNI space. These transformations were calculated and applied in a single step to reduce the number of times the data were interpolated. A Gaussian blur with a kernel size of 2 mm full width half maximum (FWHM) was applied to the EPI data.
Regressors of no interest for six head motion parameters and their derivatives were modeled. Bandpass filter regressors (0.01–0.1 Hz) were also included in the model. Using the residual time series remaining after modeling these regressors, a correlation between the mean time series for the BNST and each voxel in the amygdalae was computed. This was done separately for the left and right BNST. These correlation values were converted to z-scores with a Fisher’s z-transformation for use in group-level statistics. All voxels that were outside of the amygdala or not included in at least 90% of participants were removed from the resulting Fisher’s z-score statistical maps. Doing so resulted in the loss of a small portion (12%) of the most anterior aspect of the amygdala.
AFNI’s (Cox, 1996) 3dLME was used to perform a Condition (threat vs safe) Side (left vs right BNST) Economic Conservatism linear mixed model, with participant as a random factor. The resulting statistical map was corrected for multiple comparison based on cluster extent (P = 0.001, family-wise α = 0.05), which was computed with Monte Carlo simulation via AFNI 3dClustSim’s autocorrelation function, which was developed to address concerns of inflated error rates (Cox et al., 2017; Eklund et al., 2016). Correction for multiple comparison was computed using a small-volume correction for bilateral amygdala (k = 12 voxels). For significant activation clusters, average Fisher’s z-score was extracted for each condition and used in follow-up tests.
Defining the ROIs
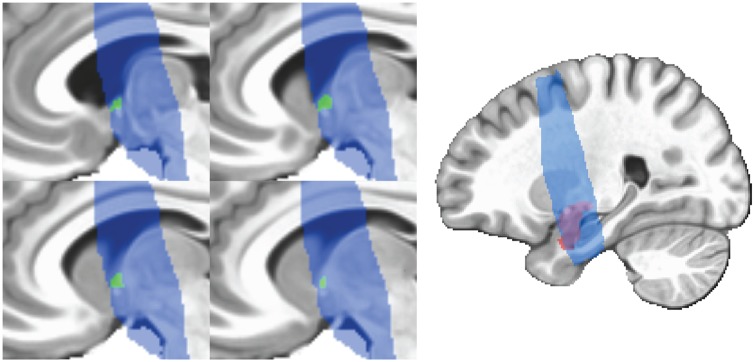
BNST ROIs were traced by hand in AFNI using the anatomical boundaries detailed by Avery et al. (2014). The ROIs were visually inspected by overlaying them onto the EPI data and adjusted when necessary, for example if the region of interest (ROI) encroached onto lateral ventricle. The ROIs were then transformed to MNI space, using the transform matrix computed while warping the anatomical images. Average sizes were 90.85 mm3 for the left BNST and 97.67 mm3 for the right BNST. Amygdala ROIs were defined using AFNI’s (Cox, 1996) CA_MPM_18_MNIA atlas, based on the Eickhoff–Zilles atlas (Eickhoff et al., 2005). Examples of BNST and amygdala ROIs can be found in Figure 2.
Fig. 2.
Example of single subject BNST ROI (green) after warping to MNI space and amygdala ROI (red) taken from AFNI (Cox, 1996) atlas. BNST ROIs were drawn on each subject’s anatomy according to the boundaries described by Avery et al. (2014) and then warped to MNI space. Blue voxels represent the area covered by partial brain scan in at least 90% of participants. MNI, montreal neurological institute.
Results
Anxiety ratings
Participants reported more state anxiety during the threat of shock scan (M = 3.58, s.d. = 1.89) than during the safe scan (M = 1.96, s.d. = 1.68), t(23) = 4.39, P < 0.001. This suggests that the threat of shock manipulation successfully induced state anxiety. Anxiety ratings for threat minus safe did not correlate with economic conservatism scores, r = 0.2, P = 0.36.
Relationships between measures of conservatism
Participants identifying as more conservative on our political affiliation measure scored higher on economic conservatism, r = 0.501, P = 0.01. There was no relationship between social conservatism and either economic conservatism, r = 0.243, P = 0.252 or political affiliation, r = 0.105, P = 0.625. This is likely due to the lack of variation in social conservatism scores, caused by a floor effect (Figure 1).
Amygdala–BNST connectivity analysis
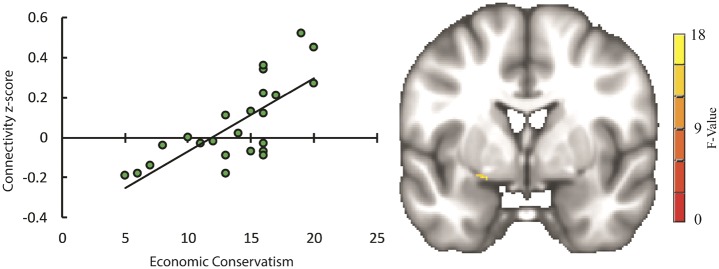
The results of the Condition (threat vs safe) Side (left vs right) Economic Conservatism linear mixed model were small-volume corrected within the amygdala. As stated in the Materials and Methods section, Social Conservatism was not included in this analysis, due to a lack of variation on this measure in our sample. After correction for multiple comparisons, there were no clusters of connectivity with the BNST for any of the main effects or for any interaction involving Side. There was, however, a cluster of connectivity in the left amygdala for the Condition Economic Conservatism interaction (RAI MNI coordinates: x = 20, y = 1, z = −12; Figure 3). Running simple linear regression follow-up tests using Fisher’s z-scores extracted from this cluster revealed that Economic Conservatism positively predicted connectivity between this cluster and the BNST during the threat scan, b = 0.019, β = 0.556, P = 0.01, as well as negatively predicting connectivity during the safe scan, b = −0.017, β = −0.511, P = 0.02 (Bonferroni-corrected). These coefficients remained significant when age and gender were included as covariates in their respective regressions. Standardized DFBETA was calculated for each observation for each regression to check for influential data points. None of the observations had a standardized DFBETA with an absolute value above 1 (max = 0.79), a common cutoff for small data sets (Cohen et al., 2013, p. 405). It should be noted that running follow-up tests using data extracted from a cluster that was the result of a voxel-wise analysis may produce inflated effect sizes. Although these follow-up regressions are helpful for describing the direction of the simple effects and inspecting the data for outliers, they may not be reliable indicators of the strength of the effect (Lieberman et al., 2009; Poldrack and Mumford, 2009). Figure 3 depicts the location of the cluster of connectivity in the left amygdala, as well as the linear relationship between Economic Conservatism and threat minus safe scan connectivity in this cluster.
Fig. 3.
Scatter plot (left) depicting degree of connectivity between bilateral BNST and left amygdala cluster (right) for the threat minus safe contrast on the vertical axis, and self-reported economic conservatism on horizontal axis, with trendline depicting simple linear regression, b = 0.036, β = 0.73, P < 0.001. It should be noted that statistics using data extracted from a cluster that was the result of a voxel-wise analysis may produce inflated effect sizes. Although visualizing this data is useful for inspecting outliers, the strength of the relationship implied by the scatter plot and the accompanying regression may not be reliable (Lieberman et al., 2009; Poldrack and Mumford, 2009).
Discussion
Based on past research demonstrating that conservatism is associated with an increased bias toward negative or threatening stimuli (Jost et al., 2003; Oxley et al., 2008; Vigil, 2010; Carraro et al., 2011; Smith et al., 2011; Dodd et al., 2012; Hibbing et al., 2014; Lilienfeld and Latzman, 2014; McLean et al., 2014), and that conservatism is related to altered amygdala structure (Kanai et al., 2011) and function (Schreiber et al., 2013), we sought to test whether conservatism is associated with an increased neural threat response. Based on an influential model (Davis et al., 2010) stating that connectivity between the amygdala and BNST is a critical component of the response to prolonged or uncertain threats, we predicted that economic and social conservatism would be associated with greater changes in connectivity between these regions across conditions of potential threat and safety. We tested this by having participants complete task-free scans, one while under threat of shock and one while safe. We assessed connectivity between the amygdala and BNST by calculating a correlation between the blood-oxygen-level dependent (BOLD) response of the BNST and that of the amygdala during these respective scans. This gave us a measure of the degree to which the activity in these regions was coupled, suggesting connectivity.
Although we were unable to include social conservatism as a variable in our analysis due to lack of variation in our sample, our results were congruent with our hypothesis—economic conservatism predicted greater connectivity between a cluster in the left amygdala and the BNST for a threat minus safe contrast. This suggests that greater reactivity in threat-related neural circuitry is associated with economic conservatism. Critically, this is the first study to demonstrate that conservatism is associated with changes in connectivity between the amygdala and BNST. This is particularly important, given that no past studies have tied conservatism to altered amygdala function in the context of threat.
Follow-up tests revealed that the increased amygdala–BNST connectivity for threat vs safe conditions in economically conservative participants was driven by both increased connectivity during threat and reduced connectivity during the safe condition. The former finding suggests that economic conservatism is associated with increased sensitivity in neural circuitry associated with the threat response. However, the latter finding, that economic conservatism is associated with a decrease in amygdala–BNST connectivity during conditions of safety was unexpected. This finding suggests that individuals who exhibit more reactivity to threat also exhibit a reduced baseline in amygdala–BNST connectivity, possibly due to some negative feedback mechanism.
A growing body of literature links conservatism to an increased threat bias (Hibbing et al., 2014). Participants who are high in conservatism exhibit increased capture of attention by task-irrelevant aversive stimuli (Carraro et al., 2011; McLean et al., 2014), have better memory for negative vs positive scenes (Mills et al., 2016), and when shown a variety of images, spend more time viewing negative ones (Dodd et al., 2012). High conservatism is also related to greater skin conductance responses to aversive stimuli (Oxley et al., 2008; Smith et al., 2011; Dodd et al., 2012), and increased levels of self-reported phobic-fears (Hatemi et al., 2013). Furthermore, participants who are high in conservatism anticipate experiencing more negative emotional reactions when imagining a future negative event, and report experiencing a more negative emotional reaction toward receiving a lower than expected exam grade (Joel et al., 2014). This study found that conservatism is associated with a greater increase in connectivity between the amygdala and BNST during conditions of unpredictable threat compared with safety. As this connectivity is thought to be important in initiating the response to sustained threat (Davis et al., 2010), changes in this connectivity may be an important neural mechanism underlying the increased threat bias accompanying high conservatism.
Greenberg and Jonas (2003) have argued that sensitivity to threat is associated with extreme views on either side of the political spectrum. Our results run contrary to this argument, demonstrating a linear relationship between economic conservatism and threat sensitivity in an important component of neural threat circuitry. Van Prooijen et al. (2015) suggest that there may be both an overall linear relationship between conservatism and sensitivity to threat, and an underlying quadratic trend, with individuals at both political extremes exhibiting greater threat sensitivity than their more moderate counterparts. Future research with larger, more politically diverse samples is needed to test whether sensitivity in neural threat circuitry follows this pattern.
Some have proposed a model of the personality differences underlying political ideology that predicts that sensitivity to threat is associated with social conservatism but also economic liberalism (Duckitt and Sibley, 2010). Our findings, however, suggests that economic conservatism is associated with greater reactivity in an important component of neural threat circuitry. Malka and Soto (2015) have proposed that individuals with traits like threat sensitivity may be heavily predisposed toward social conservatism and more modestly toward economic liberalism but that this pattern may only manifest in participants who are low in political engagement. In this view, those who are sensitive to threat are drawn toward social conservatism. However, those who are high in political engagement may be motivated to adopt views that are consistently conservative across both social and economic domains. This may cause individuals who are high in threat sensitivity to adopt economic conservatism to maintain consistency with their socially conservative views. Because of this, economic conservatism may predict sensitivity to threat among the highly politically engaged but not because of a direct relationship between the two. One would expect that among our socially liberal sample, those who endorsed economically conservative views were relatively unaffected by motivations to maintain consistency across social and economic domains. As such, our findings seem inconsistent with Malka and Soto’s (2015) model. However, future research should more directly test whether the relationship between economic conservatism and sensitivity in neural threat circuitry is dependent on political engagement.
Although our results demonstrate an association between neural function and economic conservatism, these results do not address causality. The structure and function of the brain can be shaped by experience (Mechelli et al., 2004; Ceccarelli et al., 2009; Fu and Zuo, 2011; Woollett and Maguire, 2011; Klimecki et al., 2014). As such, the functional differences in neural threat reactivity associated with conservatism that we have observed could either be a heritable trait that predisposes individuals toward economic conservatism or a neural change that has developed because of the adoption of conservative economic views. In practice, political ideology and neural structure and function likely influence one another in a dynamic process that unfolds over time (Jost et al., 2014).
It is important to note that while these results add to current research suggesting an increased negativity bias associated with conservatism (Hibbing et al., 2014), this line of research should not be construed as implying that one ideology is superior to another. As past researchers have noted, the finding that conservatives exhibit an enhanced negativity bias has sometimes been used to paint conservatives in a negative light (Dodd et al., 2012; Motyl and Iyer, 2014). Although stronger in conservatives on average, the negativity bias—the tendency to attend and react more strongly to negative vs positive stimuli—is a general characteristic of human psychology (Norris et al., 2010). Furthermore, the negativity bias is thought to be an evolutionarily adaptive trait that helps individuals avoid danger, and individual variation in this trait is not associated with decreased life satisfaction (Norris et al., 2011). Surely attending to threat is necessary not only on a personal level but also on a societal level as well. The specifics of what should be considered a threat, the amount of attention each threat should be given and the best way to mitigate threats are matters that warrant careful consideration along with thoughtful public debate.
Although the finding that economic conservatism is accompanied by increased neural reactivity toward threat is a valuable starting point for future research, readers should also consider the limitations of this study. To avoid anticipation effects during the safe condition—in which participants may exhibit heightened anxiety during the safe scan if they know that a threat scan will follow shortly—we did not counterbalance the order of the threat and safe scans. As such, it is possible that the neural differences associated with conservatism observed were the result of an altered response to the order of the blocks, rather than an altered response to threat. Additionally, this study involved a small sample size (n = 24), and a relatively large number of participants whose data were excluded due to both artifacts in the data and participant attrition. Future research should seek to replicate these results while counterbalancing the order of conditions in a larger sample.
In addition, conservatives were underrepresented in our sample. The most common response to our political alignment question was ‘Somewhat Liberal’ with no participants endorsing the ‘Very Conservative’ option. This issue is not unique to this study. For example, the past studies investigating changes in the amygdala associated with conservatism also involved left-leaning samples. Of the 90 participants who took part in Kanai et al.’s (2011) studies, none identified as ‘Very Conservative’, while 60 Democrats and 22 Republicans took part in Schreiber et al.’s (2013) study. Thus, while existing research demonstrates that variations in conservatism is accompanied by altered amygdala structure and function in somewhat liberal samples, future research is needed to confirm that a similar pattern exists within samples that are distributed more evenly along the liberal-conservative spectrum.
Our results suggest that increased reactivity to potential threat in the amygdala and BNST may be an important neural correlate of the increased reactivity to threat that accompanies conservatism (Hibbing et al., 2014). However, although we did find increased neural reactivity to threat associated with conservatism, conservatives did not report greater changes in state anxiety from threat to safety. Given the high face validity of our threat of shock manipulation, it is very likely that participants were aware that the purpose of the manipulation was to induce state anxiety. As such, reports of state anxiety may have been colored by expectation or good-participant effects that may have washed out individual differences in state anxiety associated with conservatism. Alternatively, our small sample size may not have enabled adequate power to detect a relationship between conservatism and self-reported anxiety. Future research should seek to tie alterations in the function of the amygdala and BNST to behavioral and self-reported indicators of the enhanced negativity bias in conservatism.
Although future research is needed to further examine the neural mechanisms underlying the increased negativity bias in conservatism, this study adds to existing literature suggesting that conservatism is associated with altered amygdala function (Schreiber et al., 2013). This is the first study to show altered amygdala function in conservatism during threat by employing high-resolution 7T fMRI to demonstrate that conservatism is associated with increased amygdala–BNST connectivity during the anticipation of threat vs safety. This is critical, because connectivity between the amygdala and BNST is thought to be an important component of the neural circuitry that coordinates the response to sustained and uncertain threat (Davis et al., 2010). As such, increased amygdala–BNST connectivity during threat may be a key neural correlate of the enhanced negativity bias found in conservatism.
Funding
This research was supported by a Medical College of Wisconsin Daniel M. Soref Research Award (FP8072), and an award from the National Institute of Mental Health of the National Institute of Health (MH086809).
Conflict of interest. None declared.
References
- American National Election Studies. (2012). The ANES Guide to Public Opinion and Electoral Behavior Ann Arbor: University of Michigan, Center for Political Studies [producer and distributor]. Available: www.electionstudies.org [January 17, 2017].
- Asok A., Schulkin J., Rosen J.B. (2016). Corticotropin releasing factor type-1 receptor antagonism in the dorsolateral bed nucleus of the stria terminalis disrupts contextually conditioned fear, but not unconditioned fear to a predator odor. Psychoneuroendocrinology, 70, 17–24.http://dx.doi.org/10.1016/j.psyneuen.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Tustison N., Song G. (2009). Advanced normalization tools (ANTS). Insight Journal, 2, 1–35. [Google Scholar]
- Avery S.N., Clauss J.A., Winder D.G., Woodward N., Heckers S., Blackford J.U. (2014). BNST neurocircuitry in humans. Neuroimage, 91(0), 311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M.G., Murray E.A. (2002). The amygdala and reward. Nature Reviews Neuroscience, 3(7), 563–73.http://dx.doi.org/10.1038/nrn875 [DOI] [PubMed] [Google Scholar]
- Burke B.L., Kosloff S., Landau M.J. (2013). Death goes to the polls: a meta‐analysis of mortality salience effects on political attitudes. Political Psychology, 34(2), 183–200. [Google Scholar]
- Cai L., Bakalli H., Rinaman L. (2012). Yohimbine anxiogenesis in the elevated plus maze is disrupted by bilaterally disconnecting the bed nucleus of the stria terminalis from the central nucleus of the amygdala. Neuroscience, 223, 200–8.http://dx.doi.org/10.1016/j.neuroscience.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmines E.G., Ensley M.J., Wagner M.W. (2012). Political ideology in American politics: one, two, or none? The Forum, 10(3), 4. [Google Scholar]
- Carraro L., Castelli L., Macchiella C., Avenanti A. (2011). The automatic conservative: ideology-based attentional asymmetries in the processing of valenced information. PLoS One, 6(11), e26456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli A., Rocca M.A., Pagani E., Falini A., Comi G., Filippi M. (2009). Cognitive learning is associated with gray matter changes in healthy human individuals: a tensor-based morphometry study. Neuroimage, 48(3), 585–9. [DOI] [PubMed] [Google Scholar]
- Choi J.M., Padmala S., Pessoa L. (2012). Impact of state anxiety on the interaction between threat monitoring and cognition. Neuroimage, 59(2), 1912–23.http://dx.doi.org/10.1016/j.neuroimage.2011.08.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Cohen P., West S.G., Aiken L.S. (2013). Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences: Third Edition. Mahwah, New Jersey: Lawrence Erlbaum Associates Inc. [Google Scholar]
- Cox R.W., Chen G., Glen D.R., Reynolds R.C., Taylor P.A. (2017). FMRI clustering in AFNI: False-positive rates redux. Brain Connectivity, 7(3), 152–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73.http://dx.doi.org/10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Crawford J.T. (2017). Are conservatives more sensitive to threat than liberals? it depends on how we define threat and conservatism. Social Cognition, 35(4), 354–73.http://dx.doi.org/10.1521/soco.2017.35.4.354 [Google Scholar]
- Cunningham W.A., Brosch T. (2012). Motivational salience: amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science, 21(1), 54–9.http://dx.doi.org/10.1177/0963721411430832 [Google Scholar]
- Davis M., Walker D.L., Miles L., Grillon C. (2010). Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology, 35(1), 105–35.http://dx.doi.org/10.1038/npp.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. (2001). The amygdala: vigilance and emotion. Molecular Psychiatry, 6(1), 13–34.http://dx.doi.org/10.1038/sj.mp.4000812 [DOI] [PubMed] [Google Scholar]
- Dodd M.D., Balzer A., Jacobs C.M., Gruszczynski M.W., Smith K.B., Hibbing J.R. (2012). The political left rolls with the good and the political right confronts the bad: connecting physiology and cognition to preferences. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 367(1589), 640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckitt J., Sibley C.G. (2010). Personality, ideology, prejudice, and politics: a dual‐process motivational model. Journal of Personality, 78(6), 1861–94. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H.. et al. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage, 25(4), 1325–35. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, 113(28), 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S., Johnston C. (2014). Understanding the determinants of political ideology: implications of structural complexity. Political Psychology, 35(3), 337–58.http://dx.doi.org/10.1111/pops.12055 [Google Scholar]
- Flandreau E.I., Ressler K.J., Owens M.J., Nemeroff C.B. (2012). Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology, 37(1), 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Zhang D., Snyder A.Z., Raichle M.E. (2009). The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology, 101(6), 3270–83.http://dx.doi.org/10.1152/jn.90777.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M., Zuo Y. (2011). Experience-dependent structural plasticity in the cortex. Trends in Neurosciences, 34(4), 177–87.http://dx.doi.org/10.1016/j.tins.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J., Jonas E. (2003). Psychological motives and political orientation–the left, the right, and the rigid: Comment on Jost et al. (2003). Psychological Bulletin, 129(3), 376–82. [DOI] [PubMed] [Google Scholar]
- Hatemi P.K., McDermott R., Eaves L.J., Kendler K.S., Neale M.C. (2013). Fear dispositions and their relationship to political preferences. American Journal of Political Science, 57(2), 279–93. [Google Scholar]
- Hibbing J.R., Smith K.B., Alford J.R. (2014). Differences in negativity bias underlie variations in political ideology. Behavioral and Brain Sciences, 37(3), 297–307.http://dx.doi.org/10.1017/S0140525X13001192 [DOI] [PubMed] [Google Scholar]
- Joel S., Burton C.M., Plaks J.E. (2014). Conservatives anticipate and experience stronger emotional reactions to negative outcomes. Journal of Personality, 82(1), 32–43.http://dx.doi.org/10.1111/jopy.12031 [DOI] [PubMed] [Google Scholar]
- Jost J.T., Federico C.M., Napier J.L. (2009). Political ideology: its structure, functions, and elective affinities. Annual Review of Psychology, 60(1), 307–37.http://dx.doi.org/10.1146/annurev.psych.60.110707.163600 [DOI] [PubMed] [Google Scholar]
- Jost J.T., Glaser J., Kruglanski A.W., Sulloway F.J. (2003). Political conservatism as motivated social cognition. Psychological Bulletin, 129(3), 339–75. [DOI] [PubMed] [Google Scholar]
- Jost J.T., Nam H.H., Amodio D.M., Van Bavel J.J. (2014). Political neuroscience: the beginning of a beautiful friendship. Political Psychology, 35(S1), 3–42. [Google Scholar]
- Jost J.T., Stern C., Rule N.O., Sterling J. (2017). The politics of fear: is there an ideological asymmetry in existential motivation? Social Cognition, 35(4), 324–53.http://dx.doi.org/10.1521/soco.2017.35.4.324 [Google Scholar]
- Kanai R., Feilden T., Firth C., Rees G. (2011). Political orientations are correlated with brain structure in young adults. Current Biology, 21(8), 677–80.http://dx.doi.org/10.1016/j.cub.2011.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen-Rhinehart E., Michopoulos V., Toufexis D.J., Martin E.I., Nair H., Ressler K.J., Davis M. (2009). Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Molecular Psychiatry, 14(1), 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimecki O.M., Leiberg S., Ricard M., Singer T. (2014). Differential pattern of functional brain plasticity after compassion and empathy training. Social Cognitive and Affective Neuroscience, 9(6), 873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F., Kroes M.C., Heitland I., et al. (2015). Dorsomedial prefrontal cortex mediates the impact of serotonin transporter linked polymorphic region genotype on anticipatory threat reactions. Biological Psychiatry, 78(8), 582–9.http://dx.doi.org/10.1016/j.biopsych.2014.07.034 [DOI] [PubMed] [Google Scholar]
- Ksiazkiewicz A., Ludeke S., Krueger R. (2016). The role of cognitive style in the link between genes and political ideology. Political Psychology, 37(6), 761–76.http://dx.doi.org/10.1111/pops.12318 [Google Scholar]
- Lee Y., Davis M. (1997). Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 17(16), 6434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M.D., Berkman E.T., Wager T.D. (2009). Correlations in social neuroscience aren’t voodoo: commentary on vul et al. (2009). Perspectives on Psychological Science, 4(3), 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld S.O., Latzman R.D. (2014). Threat bias, not negativity bias, underpins differences in political ideology. Behavioral and Brain Sciences, 37(3), 318–9.http://dx.doi.org/10.1017/S0140525X1300263X [DOI] [PubMed] [Google Scholar]
- Malka A., Soto C.J. (2015). Rigidity of the economic right? menu-independent and menu-dependent influences of psychological dispositions on political attitudes. Current Directions in Psychological Science, 24(2), 137–42.http://dx.doi.org/10.1177/0963721414556340 [Google Scholar]
- McLean S.P., Garza J.P., Wiebe S.A., et al. (2014). Applying the flanker task to political psychology: a research note. Political Psychology, 35(6), 831–40.http://dx.doi.org/10.1111/pops.12056 [Google Scholar]
- Mechelli A., Crinion J.T., Noppeney U., et al. (2004). Neurolinguistics: structural plasticity in the bilingual brain. Nature, 431(7010), 757..http://dx.doi.org/10.1038/431757a [DOI] [PubMed] [Google Scholar]
- Mills M., Gonzalez F.J., Giuseffi K., et al. (2016). Political conservatism predicts asymmetries in emotional scene memory. Behavioural Brain Research, 306, 84–90.http://dx.doi.org/10.1016/j.bbr.2016.03.025 [DOI] [PubMed] [Google Scholar]
- Mobbs D., Yu R., Rowe J.B., Eich H., FeldmanHall O., Dalgleish T. (2010). Neural activity associated with monitoring the oscillating threat value of a tarantula. Proceedings of the National Academy of Sciences, 107(47), 20582–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyl M., Iyer R. (2014). Will the real fundamental difference underlying ideology please stand up? Behavioral and Brain Sciences, 37(3), 322–3.http://dx.doi.org/10.1017/S0140525X13002677 [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage, 44(3), 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E.A. (2007). The amygdala, reward and emotion. Trends in Cognitive Sciences, 11(11), 489–97.http://dx.doi.org/10.1016/j.tics.2007.08.013 [DOI] [PubMed] [Google Scholar]
- Norris C.J., Gollan J., Berntson G.G., Cacioppo J.T. (2010). The current status of research on the structure of evaluative space. Biological Psychology, 84(3), 422–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris C.J., Larsen J.T., Crawford L.E., Cacioppo J.T. (2011). Better (or worse) for some than others: individual differences in the positivity offset and negativity bias. Journal of Research in Personality, 45(1), 100–11. [Google Scholar]
- Öhman A. (2005). The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology, 30(10), 953–8. [DOI] [PubMed] [Google Scholar]
- Oxley D.R., Smith K.B., Alford J.R., et al. (2008). Political attitudes vary with physiological traits. Science, 321(5896), 1667–70. [DOI] [PubMed] [Google Scholar]
- Pedersen W.S., Balderston N.L., Miskovich T.A., Belleau E.L., Helmstetter F.J., Larson C.L. (2016). The effects of stimulus novelty and negativity on BOLD activity in the amygdala, hippocampus, and bed nucleus of the stria terminalis. Social Cognitive and Affective Neuroscience, 12(5), 748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A., Mumford J.A. (2009). Independence in ROI analysis: where is the voodoo? Social Cognitive and Affective Neuroscience, 4(2), 208–13.http://dx.doi.org/10.1093/scan/nsp011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander D., Grafman J., Zalla T. (2003). The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences, 14(4), 303–16. [DOI] [PubMed] [Google Scholar]
- Schreiber D., Fonzo G., Simmons A.N., et al. (2013). Red brain, blue brain: evaluative processes differ in democrats and republicans. PLoS One, 8(2), e52970..http://dx.doi.org/10.1371/journal.pone.0052970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.B., Oxley D., Hibbing M.V., Alford J.R., Hibbing J.R. (2011). Disgust sensitivity and the neurophysiology of left-right political orientations. PLoS One, 6(10), e25552.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Whalen P.J., Kelley W.M. (2010). Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry, 68(5), 416–24.http://dx.doi.org/10.1016/j.biopsych.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier S., Hillygus D.S. (2009). The nature of political ideology in the contemporary electorate. Public Opinion Quarterly, 73(4), 679–703.http://dx.doi.org/10.1093/poq/nfp067 [Google Scholar]
- Tritt S.M., Inzlicht M., Peterson J.B. (2014). Commentary on Hibbing, Smith, & Alford: confounding valence and arousal: what really underlies political orientation? Behavioral and Brain Sciences, 37(3), 301–31. [DOI] [PubMed] [Google Scholar]
- van Prooijen J., Krouwel A.P., Boiten M., Eendebak L. (2015). Fear among the extremes: how political ideology predicts negative emotions and outgroup derogation. Personality and Social Psychology Bulletin, 41(4), 485–97. [DOI] [PubMed] [Google Scholar]
- Vigil J.M. (2010). Political leanings vary with facial expression processing and psychosocial functioning. Group Processes & Intergroup Relations, 13(5), 547–58.http://dx.doi.org/10.1177/1368430209356930 [Google Scholar]
- Woollett K., Maguire E.A. (2011). Acquiring “the knowledge” of London’s layout drives structural brain changes. Current Biology, 21(24), 2109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]